Submitted:
16 June 2024
Posted:
17 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Cryoablation Treatment Procedure
2.1. Uses of Cryotherapy in Various Salvage Settings
- Post-radiation recurrence
- 2.
- Use of cryotherapy post-biochemical recurrence after radiotherapy
- Whole-gland salvage cryotherapy
- b.
- Focal salvage cryotherapy
- 3.
- Post-prior focal ablation recurrence
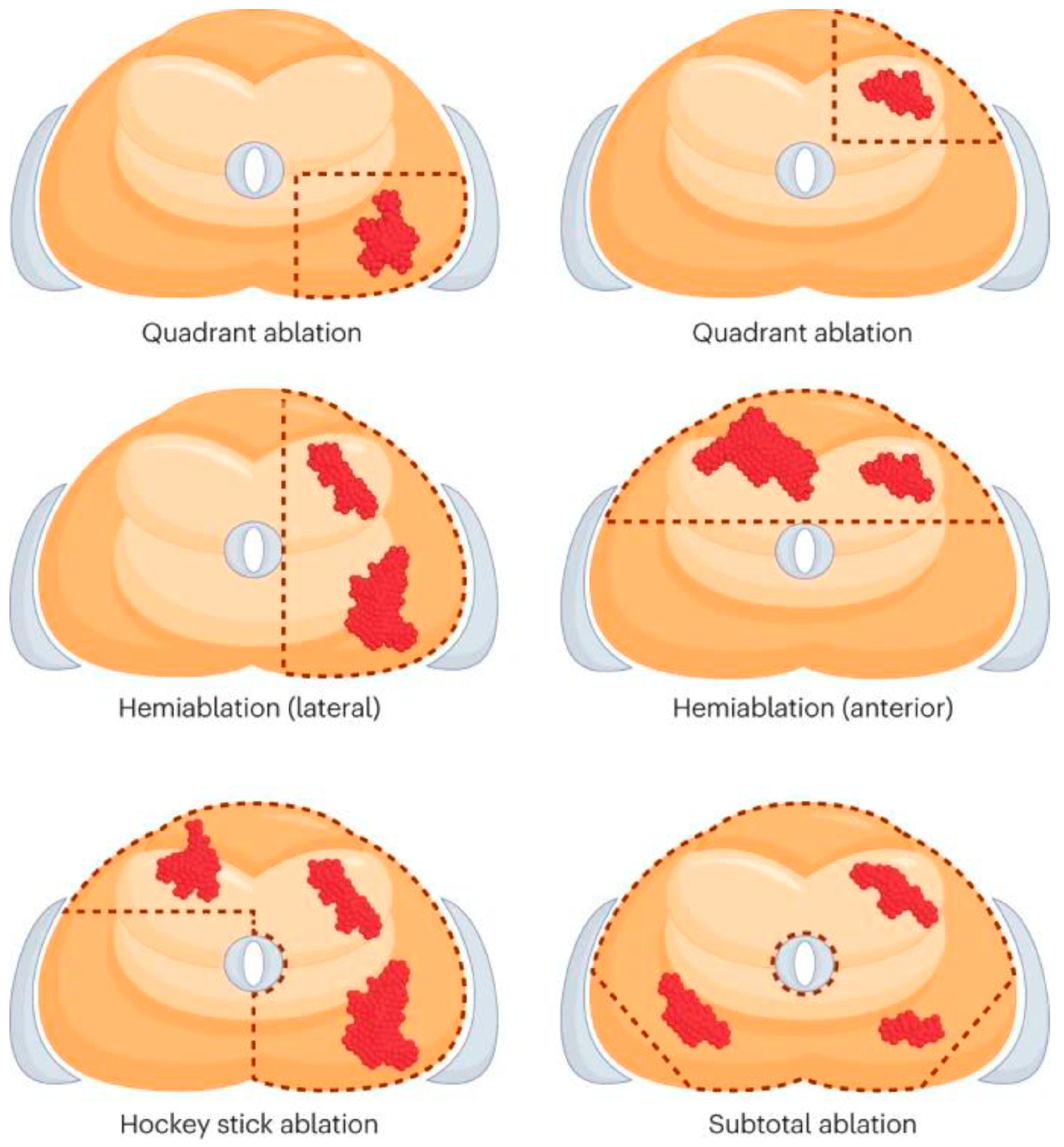
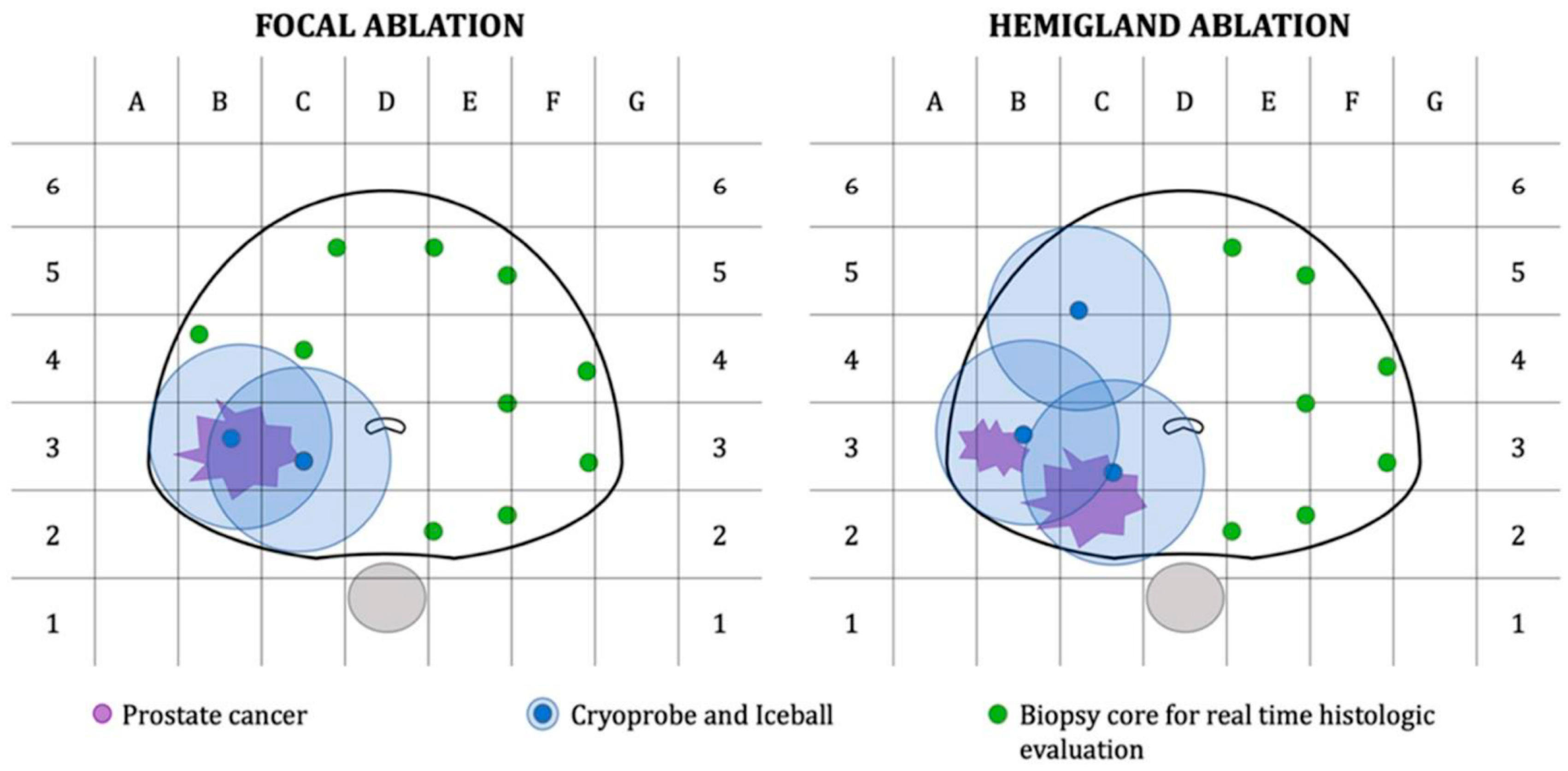
2.2. Salvage Cryoablation: Partial vs. Whole-Gland
3. Comparison of Salvage Cryotherapy vs. Salvage Radical Prostatectomy
4. Comparison of Salvage Cryotherapy vs. Salvage High-Intensity Focused Ultrasound
| References | Institution | Initial therapy (No. of Pts) | Risk Categories | Preproc-edural PSA (ng/ml), media-n (IQR) | Mos. Follow-up | Use of ADT, n (%) | Success/failure criteriaa | % Pos. Biopsy Rate | Urinary incontinence, n (%); erectile function, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Campbell et al. (2023) | Duke University Medical Center Durham, NC, USA | ERBT (419) | D’Amico Low: 120 (28.6%), Intermediate: 140 (33.4%), High: 159 (37.9%) | Mean 7.01 | Median72 (60-170) | 142 (33.9%) | Biochemical progression at 2-year: 55 (13.1%), and 5-year: 90 (21.5%) | NR | 67 (16%); 59 (14.1%) |
| Tan et al. (2023) | Duke University Medical Center, Durham, NC, USA | RT (110) | NR | Median 3.87 (2.48-5.86) | Median 71 (42.3-116) |
22 (20%) | BRFS at 12-months: 85%, 24-months: 81%, 36-months: 79%, 48-months: 75%, 60-months: 71%, and 72-months: 67% |
NR | 9%; NR |
| Spiess et al. (2013) | Department of Genitourinary Oncology, Tampa, FL |
NR (132) | D’Amico Low: 18 (14%), Intermediate: 82 (62%), High: 32 (24%) | Mean 6.2 (4.9–34.2) | Mean (range) 4.0 (0.9–12.7) | 0(0%) | bPFS at 1-year: 87.8%, 2-year: 72.4%, and 5-year: 45.5% | NR | NR |
| Wenske et al. (2013) | Multiple, USA | EBRT (259), BT (49), CR (20) |
NR | Median (range) 8 (0.6–290) | Median (range) 47 (1.6–203.5) | NR | BRFS at 5-year: 63%, 10-year: 37% |
NR | 7 (2.1); NR |
| Lian et al. (2016) | Department of Urology, Nanjing University, China | ERBT (4), BT (28) | NR | Median 7.9 (3.2–17.6) | Median 63 (38–92) | 5 (15.6%) | BRFS at 5-year: 43.5% | NR | |
| Ghafar et al. (2001) | Department of Urology, Columbia University, New York, USA |
EBRT (38) | NR | Mean 7.5 | Mean (range) 20.7 (3-37) | 38 (100%) | BRFS at 1-year: 86%, at 2-years: 74% | NR | 3 (7.9); NR |
| References | Institution | Initial therapy (No. of Pts) | Risk Categories | Preproce-dural PSA (ng/ml), median (IQR); Use of ADT, n (%) | Mos. Follow-up | Focal Template; Planned Treatment Margin | Success/ failure criteriaa |
% Post- ablative biopsy; % Pos. Biopsy Rate | Urinary incontinence, n (%); erectile function, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Li et al. (2014) | Cleveland Clinic, OH, USA | RT (91) | NR | 4.8 (0-92.6); 0 (0%) | Median 15 | NR; NR | BDFS at 1-year: 95.3%, 3-year: 72.4%, 5-year: 46.5% | 15.4%; 28.6% | 5 (5.5); 10 (50) |
| Kongnyuy et al. (2017) | Winthrop University Hospital, Garden City, NY, USA | CR - 8 (12.3%) SR - 5 (7.7) BT - 13 (20.0) PBR - 1 (1.5) RT/other - 37 (57.0) Unknown - 1 (1.5) (n=65) |
NR | 4.0 (0.01–19.0); 13 (20%) | Median 26.6 | Hemi; NR | BR: 52.3% | 52.3%; 20% | 4 (6.1); 14 (21.5) |
| Tan et al. (2021) | Duke University Medical Center, Durham, NC, USA | NR (11) | NR | 4.99 (2.23–7.86); 0 (0%) | Median 28 | Focal (n=6); NR, Hemi (n=2); NR, Subtotal (n=3); NR | FFS at 12-months: 100%, 24-months: 80%, and 36-months: 40% | NR; 27.3% | 1 (0.1); NR |
| Tan et al. (2020) | Multiple, USA | RT (72) | NR | 4 ( 2.7-5.6); 19 (26.4%) | Median 24.4 | NR; NR | BR: 16 of the 72 patients (22.2%) | 19.2%; 33.3% | 9.3%; 52.6% |
| Ismael et al. (2007) | The Royal Surrey County Hospital and St Luke’s Cancer Centre, Guildford, Surrey, UK. | RT (100) | High: 68, Intermediate: 20, Low: 12 | NR; NR | 33.5 | NR; NR | BRFS at 5-year: 73% (low-risk), 45% (intermediate), and 11% (high) | NR; NR | 13, ED: 86 |
| Chang et al. (2015) | The Affiliated Hospital of Nanjing University Medical School, Jiangsu, China. | CR (12) | NR | 2.5 (0.18–7.28); 3 (25%) | Median 33.5 | BRFS: 7 (58.3%) | 16.7; 16.7 | 1 (8.3), impotence: 2 (16.6) | |
| Bomers et al. (2013) | Multiple (the Netherlands) | CR (10) | NR | 3.6 (0.9-8.7); NR | 12 | NR; NR | BRFS at 3 months: 100%; BRFS at 6 months: 70%; BRFS at 12 months: 75% [3/4] | NR; NR | NR; NR |
| de Abreu Castro | Multiple (USA) | SFC (25), STC (25) | NR | 2.8 (SFC); 3.9 (STC) | 31 months (SFC); 53 months (STC) | NR; NR | BFS at 5 years: 54% (SFC), 86% (STC) | 48% (SFC), 28%( STC); 14.3% [1/7] (STC) | 0% (SFC), 13% (STC); 29% [2/7] (SFC), 0% [0/4] (STC) |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2024. Atlanta: American Cancer Society; 2024.
- Kishan AU, Karnes RJ, Romero T, et al. Comparison of Multimodal Therapies and Outcomes Among Patients With High-Risk Prostate Cancer With Adverse Clinicopathologic Features. JAMA Netw Open. 2021;4(7):e2115312. [CrossRef]
- Chin JL, Lavi A, Metcalfe MJ, et al. Long-Term Outcomes of Whole Gland Salvage Cryotherapy for Locally Recurrent Prostate Cancer following Radiation Therapy: A Combined Analysis of Two Centers. J Urol. 2021;206(3):646-654. [CrossRef]
- Zaorsky NG, Calais J, Fanti S, et al. Salvage therapy for prostate cancer after radical prostatectomy. Nat Rev Urol. 2021;18(11):643-668. [CrossRef]
- Safavy S, Jabaji RB, Lu SM, et al. Salvage Cryoablation for Radiorecurrent Prostate Cancer: Initial Experience at a Regional Health Care System. Perm J. 2019;23:18-153. [CrossRef]
- Khan A, Khan AU, Siref L, Feloney M. Focal cryoablation of the prostate: Primary treatment in 163 patients with localized prostate cancer. Cureus. 2023;15(4):e37172. [CrossRef]
- Tan WP, Wysock JS, Lepor H. Partial gland cryoablation for prostate cancer — Where are we? Nat Rev Urol. 2023;20(3):127-128. [CrossRef]
- Sonn GA, Margolis DJ, Marks LS. Target detection: magnetic resonance imaging-ultrasound fusion-guided prostate biopsy. Urol Oncol. 2014;32(6):903-911. [CrossRef]
- Valerio M, Shah TT, Shah P, et al. Magnetic resonance imaging-transrectal ultrasound fusion focal cryotherapy of the prostate: A prospective development study. Urol Oncol: Semin Orig. 2017;35(4): 150.e1-150.e7. [CrossRef]
- Priester A, Natarajan S, Khoshnoodi P, et al. Magnetic resonance imaging underestimation of prostate cancer geometry: Use of patient specific molds to correlate images with whole Mount Pathology. J Urol. 2017;197(2):320-326. [CrossRef]
- Littrup PJ, Jallad B, Vorugu V, et al. Lethal isotherms of cryoablation in a phantom study: Effects of heat load, probe size, and number. J Vasc Interv Radiol. 2009;20(10):1343-1351. [CrossRef]
- Shah TT, Arbel U, Foss S, et al. Modeling cryotherapy ice ball dimensions and isotherms in a novel gel-based model to determine optimal cryo-needle configurations and settings for potential use in clinical practice. Urology. 2016;91:234-240. [CrossRef]
- de Marini P, Cazzato RL, Garnon J, et al. Percutaneous MR-guided prostate cancer cryoablation technical updates and literature review. BJR Open. 2019;1(1):20180043. [CrossRef]
- Selvaggio O, Falagario UG, Bruno SM, et al. Intraoperative digital analysis of ablation margins (DAAM) by fluorescent confocal microscopy to improve partial prostate gland cryoablation outcomes. Cancers. 2021;13(17):4382. [CrossRef]
- Safavy S, Jabaji RB, Lu SM, et al. Salvage Cryoablation for Radiorecurrent Prostate Cancer: Initial Experience at a Regional Health Care System. Perm J. 2019;23:18-153. [CrossRef]
- Chin YF, Lynn N. Systematic review of focal and salvage cryotherapy for prostate cancer. Curēus. 2022;14(6):e26400-e26400. [CrossRef]
- Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Treatment failure after primary and salvage therapy for prostate cancer: Likelihood, patterns of care, and outcomes. Cancer. 2008;112(2):307-314. [CrossRef]
- Hruby G, Eade T, Kneebone A, et al. Delineating biochemical failure with 68Ga-PSMA-PET following definitive external beam radiation treatment for prostate cancer. Radiother Oncol. 2017;122(1):99-102. [CrossRef]
- Einspieler I, Rauscher I, Düwel C, et al. Detection Efficacy of Hybrid 68Ga-PSMA Ligand PET/CT in Prostate Cancer Patients with Biochemical Recurrence After Primary Radiation Therapy Defined by Phoenix Criteria. J Nucl Med. 2017;58(7):1081-1087. [CrossRef]
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021;27(13):3674-3682. [CrossRef]
- Perera M, Papa N, Roberts M, et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur Urol. 2020;77(4):403-417. [CrossRef]
- Mertan FV, Greer MD, Borofsky S, et al. Multiparametric Magnetic Resonance Imaging of Recurrent Prostate Cancer. Top Magn Reson Imaging. 2016;25(3):139-147. [CrossRef]
- Gaur S, Turkbey B. Prostate MR Imaging for Posttreatment Evaluation and Recurrence. Radiol Clin North Am. 2018;56(2):263-275. [CrossRef]
- Barchetti F, Panebianco V. Multiparametric MRI for recurrent prostate cancer post radical prostatectomy and postradiation therapy. Biomed Res Int. 2014;2014:316272. [CrossRef]
- Grant K, Lindenberg ML, Shebel H, et al. Functional and molecular imaging of localized and recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40 Suppl 1(Suppl 1):S48-S59. [CrossRef]
- Kim CK, Park BK, Lee HM. Prediction of locally recurrent prostate cancer after radiation therapy: incremental value of 3T diffusion-weighted MRI. J Magn Reson Imaging. 2009;29(2):391-397. [CrossRef]
- Kim CK, Park BK, Park W, Kim SS. Prostate MR imaging at 3T using a phased-arrayed coil in predicting locally recurrent prostate cancer after radiation therapy: preliminary experience. Abdom Imaging. 2010;35(2):246-252. [CrossRef]
- Tamada T, Sone T, Jo Y, et al. Locally recurrent prostate cancer after high-dose-rate brachytherapy: the value of diffusion-weighted imaging, dynamic contrast-enhanced MRI, and T2-weighted imaging in localizing tumors. AJR Am J Roentgenol. 2011;197(2):408-414. [CrossRef]
- Radzina M, Tirane M, Roznere L, et al. Accuracy of 68Ga-PSMA-11 PET/CT and multiparametric MRI for the detection of local tumor and lymph node metastases in early biochemical recurrence of prostate cancer. Am J Nucl Med Mol Imaging. 2020;10(2):106-118.
- Satapathy, S., Singh, H., Kumar, R., & Mittal, B. R. (2021). Diagnostic Accuracy of 68Ga-PSMA PET/CT for Initial Detection in Patients With Suspected Prostate Cancer: A Systematic Review and Meta-Analysis. AJR. American journal of roentgenology, 216(3), 599–607. [CrossRef]
- Petersen, L. J., & Zacho, H. D. (2020). PSMA PET for primary lymph node staging of intermediate and high-risk prostate cancer: an expedited systematic review. Cancer imaging : the official publication of the International Cancer Imaging Society, 20(1), 10. [CrossRef]
- Jannusch K, Bruckmann NM, Morawitz J, et al. Recurrent prostate cancer: combined role for MRI and PSMA-PET in 68Ga-PSMA-11 PET/MRI. Eur Radiol. Published online December 1, 2023. [CrossRef]
- Albisinni S, Aoun F, Marcelis Q, et al. Innovations in imaging modalities for recurrent and metastatic prostate cancer: a systematic review. Minerva Urol Nefrol. 2018;70(4):347-360. [CrossRef]
- Rasing M, van Son M, Moerland M, et al. Value of Targeted Biopsies and Combined PSMA PET/CT and mp-MRI Imaging in Locally Recurrent Prostate Cancer after Primary Radiotherapy. Cancers (Basel). 2022;14(3):781. Published 2022 Feb 3. [CrossRef]
- da Silva RD, Kim FJ. Prostate cancer - local treatment after radiorecurrence: Salvage cryoablation. Int Braz J Urol. 2018;44(3):435-439. [CrossRef]
- Bahn DK, Lee F, Badalament R, Kumar A, Greski J, Chernick M. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urology. 2002;60(2):3-11. [CrossRef]
- Miller RJ Jr, Cohen JK, Shuman B, Merlotti LA. Percutaneous, transperineal cryosurgery of the prostate as salvage therapy for post radiation recurrence of adenocarcinoma. Cancer. 1996;77(8):1510-1514. [CrossRef]
- Leibovici D, Chiong E, Pisters LL, et al. Pathological characteristics of prostate cancer recurrence after radiation therapy: Implications for focal salvage therapy. J Urol. 2012;188(1):98-102. [CrossRef]
- Finley DS, Belldegrun AS. Salvage cryotherapy for radiation-recurrent prostate cancer: outcomes and complications. Curr Urol Rep. 2011;12(3):209-215. [CrossRef]
- Spiess PE, Levy DA, Mouraviev V, et al. Biochemical failure predictors after prostate salvage cryotherapy. BJU Int. 2013;112(4):E256-E261. [CrossRef]
- Ward JF, Jones JS. Focal cryotherapy for localized prostate cancer: A report from the National Cryo on-line database (cold) registry. BJU Int. 2012;109(11):1648-1654. [CrossRef]
- Spiess PE, Levy DA, Pisters LL, Mouraviev V, Jones JS. Outcomes of salvage prostate cryotherapy stratified by pre-treatment PSA: update from the COLD registry. World J Urol. 2013;31(6):1321-1325. [CrossRef]
- Campbell, S. P., Deivasigamani, S., Arcot, R., Adams, E. S., Orabi, H., Elshafei, A., Tan, W. P., Davis, L., Wu, Y., Chang, A., Jones, J. S., & Polascik, T. J. (2023). Salvage Cryoablation for Recurrent Prostate Cancer Following Primary External Beam Radiotherapy or Primary Cryotherapy: A Propensity Score Matched Analysis of Mid-term Oncologic and Functional Outcomes. Clinical Genitourinary Cancer. 2023;21(5):555-562. [CrossRef]
- Ghafar MA, Johnson CW, De La Taille A, et al. Salvage cryotherapy using an argon based system for locally recurrent prostate cancer after radiation therapy: The Columbia experience. J Urol. 2001;166(4):1333-1338.
- Lian H, Yang R, Lin T, Wang W, Zhang G, Guo H. Salvage cryotherapy with third-generation technology for locally recurrent prostate cancer after radiation therapy. International urology and nephrology. 2016;48(9):1461-1466. [CrossRef]
- Wenske S, Scott Q, Katz AE. Salvage cryosurgery of the prostate for failure after primary radiotherapy or cryosurgery: Long-term clinical, functional, and oncologic outcomes in a large cohort at a tertiary referral centre. Eur Urol. 2013;64(1):1-7. [CrossRef]
- Tan WP, Kotamarti S, Ayala A, et al. Oncological and functional outcomes for men undergoing salvage whole-gland cryoablation for radiation-resistant prostate cancer. Eur Urol Oncol. 2023;6(3):289-294. [CrossRef]
- Babaian RJ, Donnelly B, Bahn D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008;180(5):1993-2004. [CrossRef]
- Izawa JI, Perrotte P, Greene GF, et al. Local tumor control with salvage cryotherapy for locally recurrent prostate cancer after external beam radiotherapy. J Urol. 2001;165(3):867-870. [CrossRef]
- Ismail M, Ahmed S, Kastner C, Davies J. Salvage cryotherapy for recurrent prostate cancer after radiation failure: a prospective case series of the first 100 patients. BJU Int. 2007;100(4):760-764. [CrossRef]
- Li YH, Elshafei A, Agarwal G, Ruckle H, Powsang J, Jones JS. Salvage focal prostate cryoablation for locally recurrent prostate cancer after radiotherapy: Initial results from the cryo on-line data registry. Prostate. 2015;75(1):1-7. [CrossRef]
- Bomers JG, Yakar D, Overduin CG, et al. MR imaging-guided focal cryoablation in patients with recurrent prostate cancer. Radiology. 2013;268(2):451-460. [CrossRef]
- Kongnyuy M, Berg CJ, Kosinski KE, et al. Salvage focal cryosurgery may delay use of androgen deprivation therapy in cryotherapy and radiation recurrent prostate cancer patients. Int J Hyperthermia. 2017;33(7):810-813. [CrossRef]
- Tan WP, Chang A, Sze C, Polascik TJ. Oncological and functional outcomes of patients undergoing individualized partial gland cryoablation of the prostate: A single-institution experience. J Endourol. 2021;35(9):1290–1299. [CrossRef]
- Kasivisvanathan V, Emberton M, Ahmed HU. Focal therapy for prostate cancer: rationale and treatment opportunities. Clin Oncol. 2013;25(8):461-473. [CrossRef]
- Aminsharifi A, Jibara G, Tsivian E, Tsivian M, Elshafei A, Polascik TJ. Salvage prostate cryoablation for the management of local recurrence after primary cryotherapy: A retrospective analysis of functional and intermediate-term oncological outcomes associated with a second therapeutic freeze. Clin Genitourin Cancer 2019;17(4):e831-e836. [CrossRef]
- Chang X, Liu T, Zhang F, et al. Salvage cryosurgery for locally recurrent prostate cancer after primary cryotherapy. Int Urol Nephrol. 2015;47(2):301-305. [CrossRef]
- Boissier R, Sanguedolce F, Territo A, et al. Partial salvage cryoablation of the prostate for local recurrent prostate cancer after primary radiotherapy: Step-by-step technique and outcomes. Urol Vid J. 2020;7:100040. [CrossRef]
- Tan WP, Chang A, Sze C, Polascik TJ. Oncological and functional outcomes of patients undergoing individualized partial gland cryoablation of the prostate: A single-institution experience. J Endourol. 2021;35(9):1290–1299. [CrossRef]
- Tan WP, ElShafei A, Aminsharifi A, et al. Salvage focal cryotherapy offers similar short-term oncologic control and improved urinary function compared with salvage whole gland cryotherapy for radiation-resistant or recurrent prostate cancer. Clin Genitourin Cancer. 2020;18(3):e260–e265. [CrossRef]
- de Castro Abreu AL, Bahn D, Leslie S, et al. Salvage focal and salvage total cryoablation for locally recurrent prostate cancer after primary radiation therapy. BJU Int. 2013;112(3):298-307. [CrossRef]
- Perera M, Vilaseca A, Tin AL, et al. Morbidity of salvage radical prostatectomy: limited impact of the minimally invasive approach. World J Urol. 2022;40(7):1637-1644. [CrossRef]
- Pisters LL, Leibovici D, Blute M, et al. Locally recurrent prostate cancer after initial radiation therapy: a comparison of salvage radical prostatectomy versus cryotherapy. J Urol. 2009;182(2):517-527. [CrossRef]
- Vora A, Agarwal V, Singh P, et al. Single-institution comparative study on the outcomes of salvage cryotherapy versus salvage robotic prostatectomy for radio-resistant prostate cancer. Prostate Int. 2016;4(1):7-10. [CrossRef]
- Friedlander DF, Gu X, Prasad SM, et al. Population-based Comparative Effectiveness of Salvage Radical Prostatectomy vs Cryotherapy. Urology. 2014;83(3):653-657. [CrossRef]
- Autran-Gomez AM, Scarpa RM, Chin J. High-intensity focused ultrasound and cryotherapy as salvage treatment in local radio-recurrent prostate cancer. Urol Int. 2012;89(4):373-379. [CrossRef]
- Uchida T, Illing RO, Cathcart PJ, Emberton M. To what extent does the prostate-specific antigen nadir predict subsequent treatment failure after transrectal high-intensity focused ultrasound therapy for presumed localized adenocarcinoma of the prostate?. BJU Int. 2006;98(3):537-539. [CrossRef]
- Ben Cheikh A, Girouin N, Ryon-Taponnier P, et al. Détection par IRM des récidives locales du cancer de prostate après traitement par ultrasons focalisés de haute intensité (HIFU) transrectaux: étude préliminaire [MR detection of local prostate cancer recurrence after transrectal high-intensity focused US treatment: preliminary results]. J Radiol. 2008;89(5 Pt 1):571-577. [CrossRef]
- Williams AK, Martínez CH, Lu C, Ng CK, Pautler SE, Chin JL. Disease-free survival following salvage cryotherapy for biopsy-proven radio-recurrent prostate cancer. Eur Urol. 2011;60(3):405-410. [CrossRef]
- Choyke PL, Bouchelouche K. Prostate specific membrane antigen (PSMA) imaging: the past is prologue. Transl Androl Urol. 2019;8(4):283-285. [CrossRef]
| References | Institution(s) | Prior Treatment (No. of patients) | Preprocedural PSA (ng/ml), median (IQR) | Site of Failure | Predictive Factors | PPV |
|---|---|---|---|---|---|---|
| Hruby et al. (2017) | Royal North Shore Hospital, Australia | EBRT (n=419) | Median (range) 10 (1–145) | Local only: 8 (16.7%) Local and distant: 15 (31.3%) Distant only: 25 (52%) |
Gleason scores ≥8 (17% vs. 9%, p = 0.011) and initial PSA >10 ng/ml (16% vs. 8%, p = 0.04) | NR |
| Einspieler et al. (2017) | Multiple (Germany, USA) | EBRT: 77 BT: 41 (n=118) |
Median 10.7 (6.9-24.7) | Local only: 43 (40.2%) Local and distant: 25 (23.4%) Distant only: 39 (36.4%) |
Increasing PSA and concomitant ADT (SUVmax: p = 0.018 and 0.004; SUVmean: p= 0.025 and 0.007, respectively) | NR |
| Morris et al. (2021) | Multiple (USA) | n=208 | Median (range) 0.8 (0.2–98.4) | NR | NR | Prostatic region: 75.0-83.3% (range) Pelvic lymph nodes: 67.2-72.7% Extra-pelvic region: 67.3-69.8% |
| MRI | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC (%) |
|---|---|---|---|---|---|
| Kim et al. (2009), Sungkyunkwan University School of Medicine, Republic of Korea | |||||
| T2W | 25 | 92 | 57 | 74 | 61.2 |
| T2W + DWI | 62 | 97 | 91 | 81 | 87.9 |
| Kim et al. (2010), Kangwon National University College of Medicine, Republic of Korea | |||||
| DWI | 49 | 93 | 72 | 84 | 78.2 |
| DCE | 49 | 92 | 67 | 84 | 73.7 |
| DWI + DCE | 59 | 91 | 69 | 87 | 86.3 |
| T2W | 27 | 80 | 32 | 76 | 59.4 |
| Tamada et al. (2011), Kawasaki Medical School, Japan | |||||
| mpMRI | 77 | 92 | 68 | 95 | NR |
| T2W | 27 | 99 | 86 | 87 | NR |
| DCE | 50 | 98 | 85 | 90 | NR |
| DWI | 68 | 95 | 75 | 94 | NR |
| Imaging | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Radzina et al. (2020), Multiple (Latvia, Germany) | |||||
| PSMA PET (local) | 63.6 | 73.7 | 58.3 | 77.8 | 77.8 |
| mpMRI (local) | 90.9 | 94.7 | 90.9 | 94.7 | 92.3 |
| PSMA PET (LN) | 83.3 | 80.0 | 80 | 100 | 90.6 |
| mpMRI (LN) | 41.7 | 94.4 | 83.3 | 70.8 | 72.0 |
| PSMA PET (bone) | 83.3 | 92.0 | 71.4 | 95.8 | 71.0 |
| mpMRI (bone) | 50.0 | 84.0 | 42.8 | 87.5 | 77.4 |
| Jannusch et al. (2023), Multiple, Germany | |||||
| PSMA PET (local) | 50 | 96 | 50 | 96 | 93 |
| mpMRI (local) | 100 | 100 | 100 | 100 | 100 |
| PSMA PET (LN) | 96 | 97 | 97 | 97 | 97 |
| mpMRI (LN) | 57 | 92 | 81 | 78 | 79 |
| PSMA PET (dLN) | 50 | 100 | 100 | 98 | 98 |
| mpMRI (dLN) | 0 | 100 | - | 97 | 97 |
| PSMA PET (bone) | 100 | 100 | 100 | 100 | 100 |
| mpMRI (bone) | 67 | 98 | 80 | 96 | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).