1. Introduction
Malignant gallbladder lymphoma is particularly uncommon [
1,
2,
3,
4,
5]. Mostly, patients diagnosed with this disease are referred for surgery with the diagnostic hypothesis of gallbladder adenocarcinoma or cholecystitis. That is because preoperative diagnosis is exceedingly challenging. Although several reports have documented malignant gallbladder lymphomas, most of these malignancies are diffuse large B-cell lymphomas (DLBCL) or Marginal Zone Lymphomas (MZL) [
3,
5,
6], and only three reports have previously documented gallbladder Burkitt’s lymphoma (BL) [
1,
7,
8]. Herein, we report the first case of a child with gallbladder BL. It is also the first case of a patient living with HIV diagnosed with this type of lymphoma.
2. Detailed Case Description
2.1. Case Presentation
2.1.1. Chief Complaints
A five-year-old black female child, from Rio de Janeiro, RJ, Brazil, attended the hospital, Federal Hospital of Lagoa (HFL), Rio de Janeiro, Brazil, with a complaint of vomiting, abdominal pain, diarrhea, and jaundice for four days.
2.1.2. History of Present Illness
Symptoms started four days before presentation with recurrent vomiting, abdominal pain, diarrhea, and jaundice.
2.1.3. History of Past Illness
The patient was previously healthy. She was born on 09/03/94, delivered by normal birth, without complications. Prenatal care was complete. Exclusive breastfeeding was maintained until she was two years old. On 06/16/99, she started vomiting, presenting abdominal pain, diarrhea, jaundice and itching. Furthermore, the patient complained of B symptoms like fever, night sweats, and weight loss. She was admitted to the HFL on 06/20/99 and diagnosed with intestinal subocclusion caused by Ascaris lumbricoides. A clinical treatment was conducted with a single dose of albendazole 400 mg orally. Despite the treatment, she developed severe cholangitis and underwent an emergency cholecystectomy on 07/15/99. The postoperative pathological examination of the gallbladder revealed a diagnosis of BL. Due to the suspicion of possible immunodeficiency, on 07/28/99, HIV serology was performed, and returned positive.
2.1.4. Personal and Family History
The patient’s family denied any family history of malignant tumors or other chronic diseases. After the patient’s diagnosis, an HIV serology (enzyme-linked immunosorbent assay - ELISA) was performed on the patient’s mother on 07/28/99, with a positive result. There is no history of blood transfusion or physical/sexual abuse of the child. The diagnosis of vertical transmission (VT) of HIV was then established.
2.1.5. Physical Examination
The patient was presenting abdominal pain, dehydration, and jaundice in the physical examination on 06/20/99. She was afebrile at the moment. There were presence of abdominal pain on diffuse palpation, absence of Murphy’s sign, and absence of visceromegaly or masses. No swollen lymph nodes were found. No other changes were found on physical examination.
2.1.6. Laboratory Examination
On 07/28/99, HIV serology was performed, which returned positive. On 07/30/99, CD4
+, CD8
+, and viral load measurements were carried out, which showed the following values: CD4
+ 2.00%, CD8
+ 45.00%, and viral load 200,000 copies/mL (log 4.20). The immunological status of the patient was classified using the CDC clinical category and immunosuppression stage (N/A/B/C and 1/2/3/unknown, respectively) at the date of diagnosis. So, at this point, the patient was classified as C3 according to CDC clinical criteria [
9,
10].
2.1.7. Imaging Assessment
No imaging tests related to the condition in question were performed prior to surgery or to histopathological analysis.
2.2. Further Diagnostic Work-Up
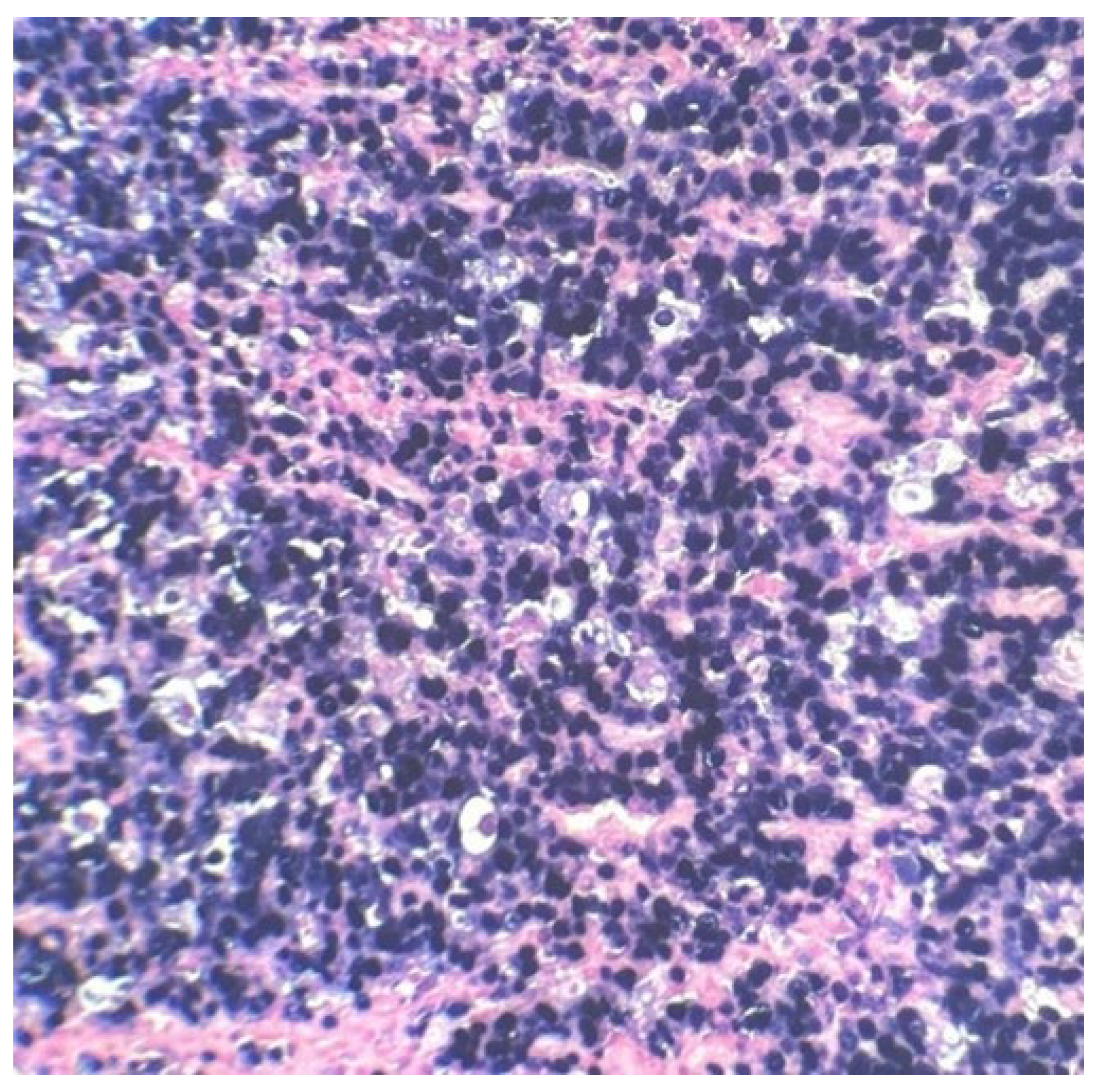
As previously said, the patient underwent an emergency cholecystectomy on 07/15/99 and the postoperative histopathological examination of the resected specimen, that occurred on 07/16/99 at the Department of Pathology of Clementino Fraga Filho University Hospital (HUCFF/UFRJ), revealed the diagnosis of BL.
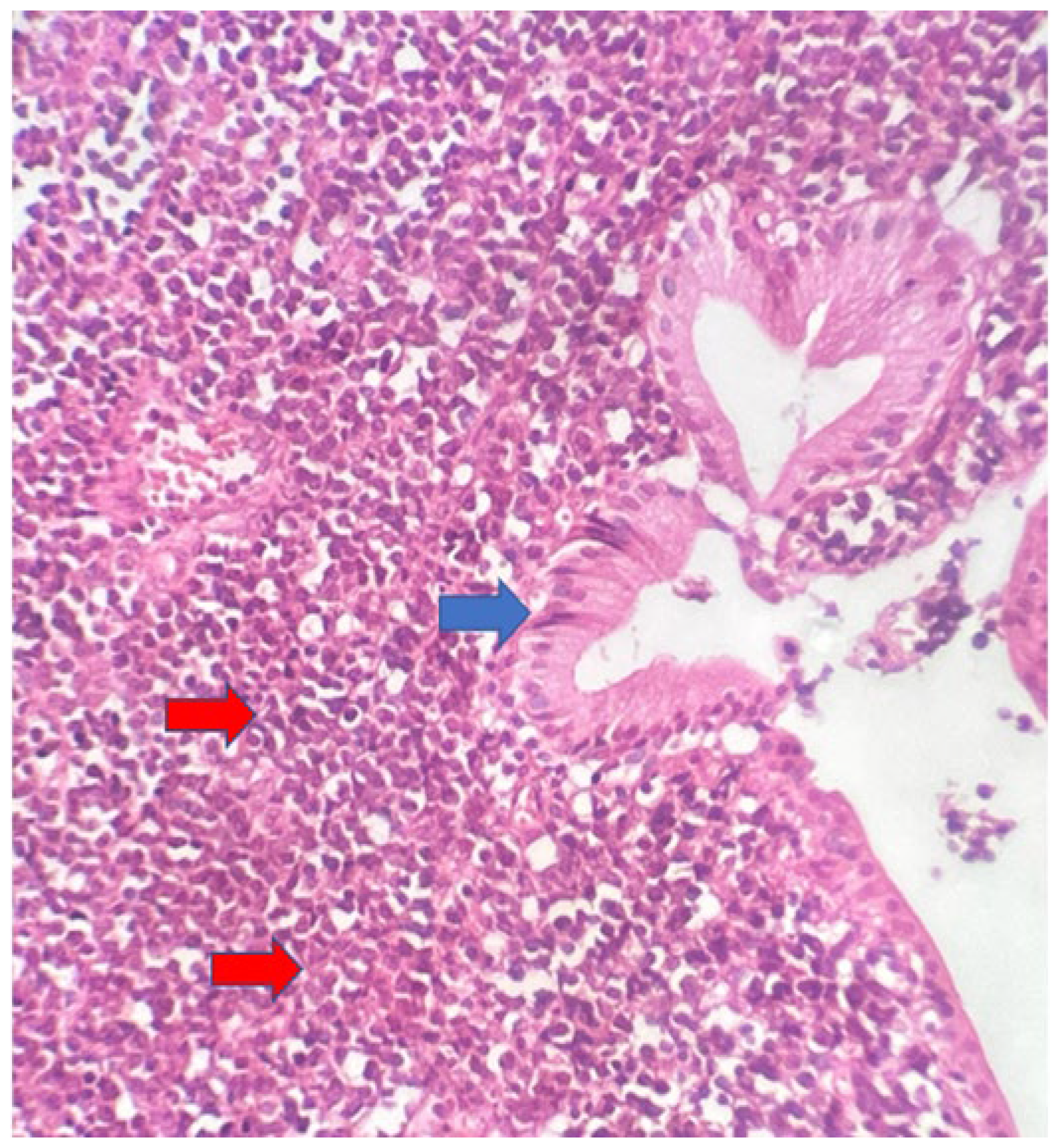
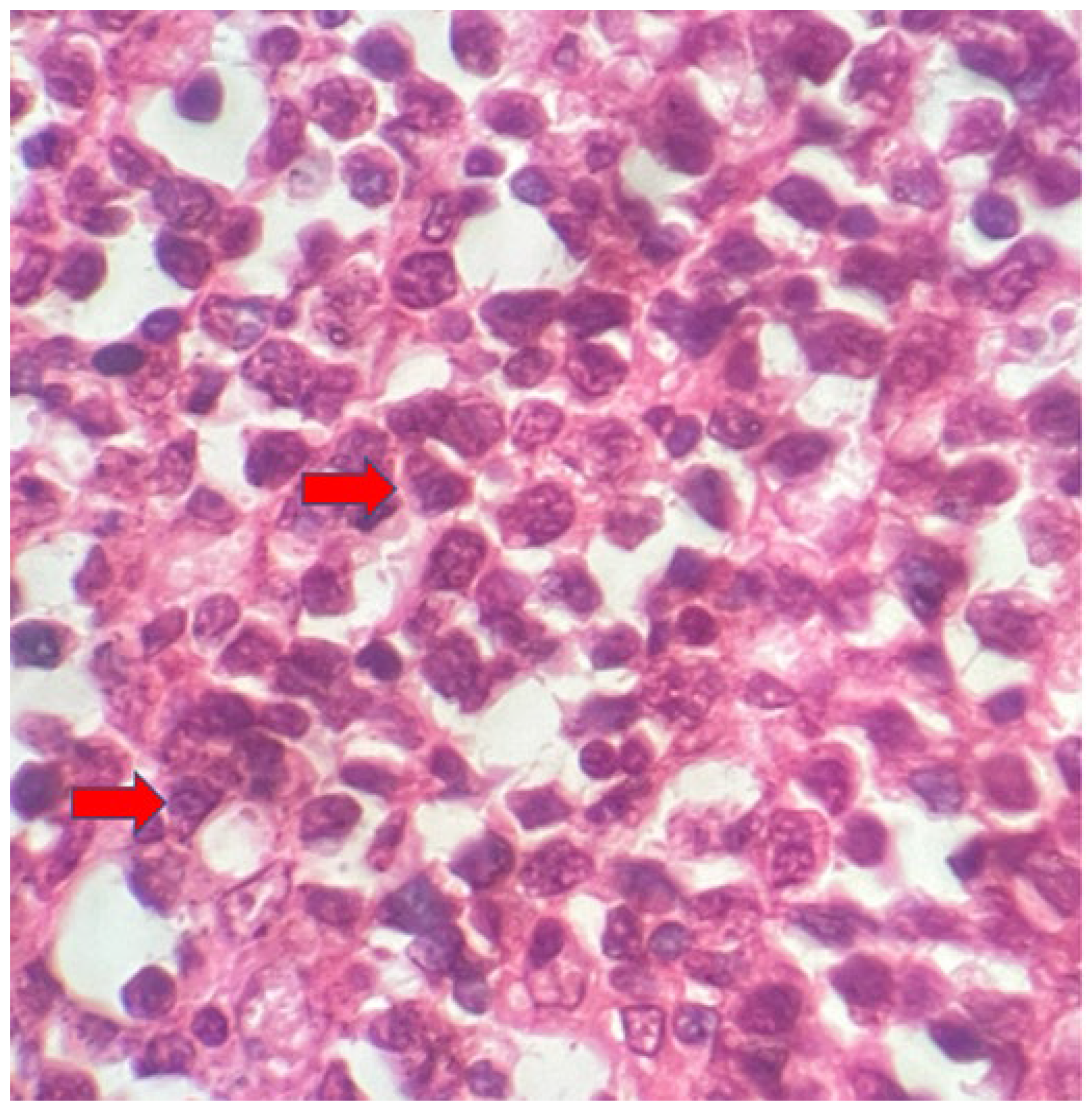
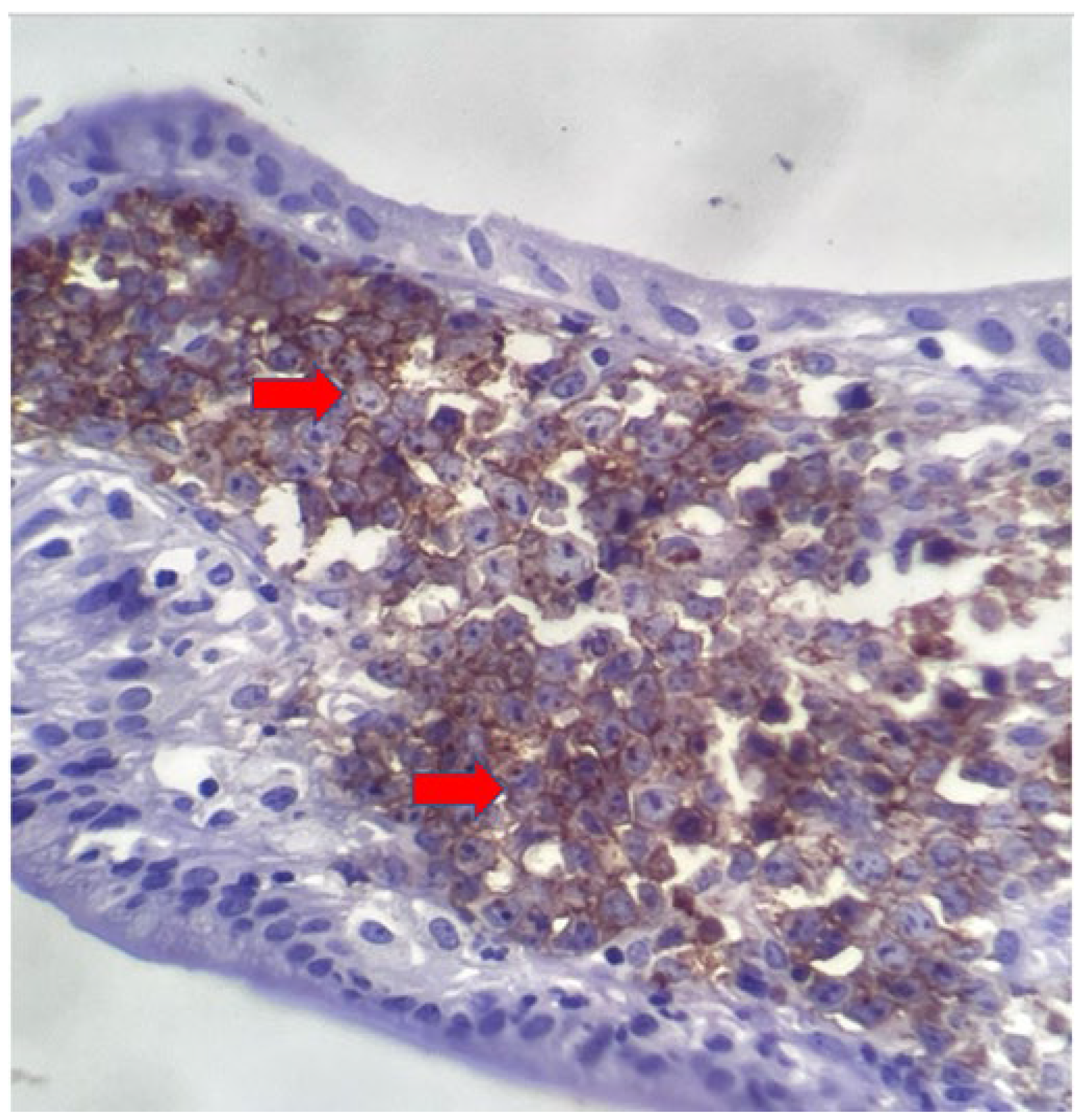
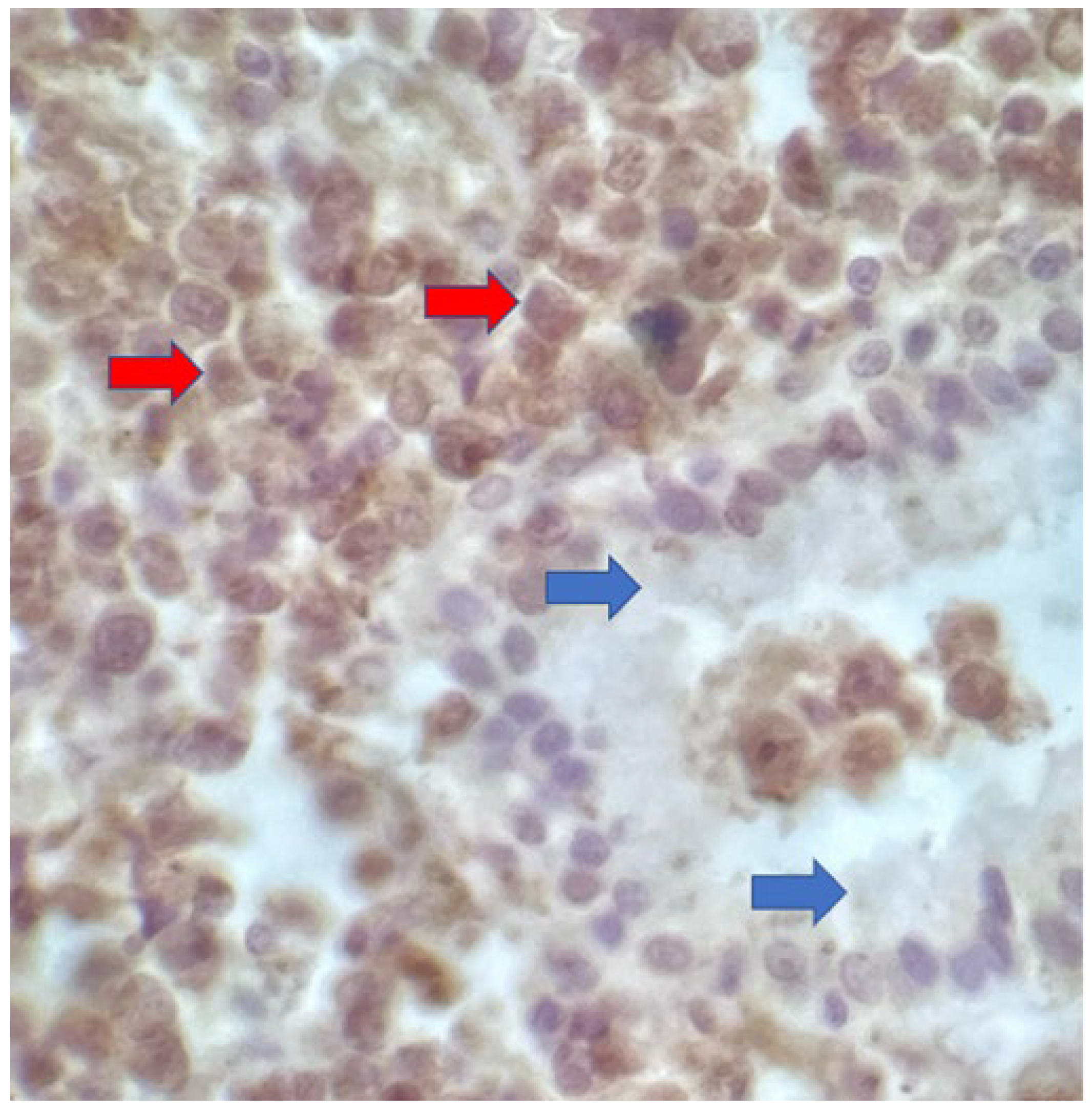
The macroscopic examination showed a gallbladder measuring 4cm x 1.0cm x 0.9cm. The serosa was opaque, and the wall thickened and white, with a firm consistency. The specimen was re-evaluated by a hematopathologist on 08/19/22 (C.B.M), who confirmed the diagnosis of BL through microscopic analysis and with immunohistochemical study according to WHO, 2022 [
11]. Morphological analysis revealed diffuse proliferation of intermediate-sized cells, with small nucleoli invading and destroying the bladder wall, with a high apoptotic and mitotic index. The neoplastic cells showed positivity for CD20, CD10, Ki67 99%, and EBER by in situ hybridization (EBER1) [
11,
12]. There are five photographs of the sample in question below (
Figure 1,
Figure 2,
Figure 3,
Figure 4 and
Figure 5).
2.3. Final Diagnosis
Combined with the patient’s medical history, the final diagnosis was gallbladder BL. This is an AIDS-defining malignancy (ADM), codified as 2A85.6 (BL) according to ICD-11 MMS [
13].
2.4. Treatment
During her hospitalization at HFL, she presented several infectious complications and probable a Pneumocystis jirovecii pneumonia with associated respiratory failure. Then, the use of corticosteroids and intravenous sulfamethoxazole/trimethoprim (SMX + TMP) was initiated.
She was transferred to the Martagão Gesteira Institute of Pediatrics and Childcare, the pediatric hospital of Federal University of Rio de Janeiro (IPPMG/UFRJ) on 07/31/99 for oncological treatment. Until that moment, she was not using prophylaxis or antiretroviral therapy/combined antiretroviral therapy (ART/cART). She arrived at IPPMG/UFRJ presenting hemodynamic instability, cutaneous-mucous parlor, sweating, grade III protein-energy malnutrition, hepatomegaly (palpable 8 cm below the right costal margin), and abdominal distension. The patient still had not swollen lymph nodes or other visceromegaly. Chest and abdominal x-ray showed no changes. Lastly, the patient remained without signs of neurological impairment. Current laboratory tests of the admission: hemoglobin (Hb) 7.3 g/dL, hematocrit (Ht) 19%, 3,000 leukocytes/mm
3 (43% neutrophils, 30% lymphocytes, and 17% monocytes) and 485,000/mm
3 platelets. On 08/03/99, bone marrow aspirate was performed, with infiltration by FAB (French-American-British Classification) L3 type blasts (>25%) and normal cerebrospinal fluid at the same date. A radiograph of long bones showed lytic lesions in the femur, bilaterally. A review of the bone marrow aspirate slide confirmed the diagnosis of L3 acute lymphoblastic leukemia (L3-ALL) at the time, now classified as BL according to the latest WHO review, 2022 [
11]. Lymphoma was classified as stage IVB according to Murphy classification [
14], and a Performance Status (PS) 4 was attributed to the patient.
On 08/04/99, after stabilization of the pulmonary infectious condition, the m-BACOD protocol [
15] was started with the use of granulocyte-colony stimulating factor (G-CSF), due to the patient’s poor PS. Lactate dehydrogenase (LDH) was 220 IU/L on 08/15/99. After four cycles of chemotherapy, with the disease progressing, NHL-BFM 95 [
16] was started, together with the G-CSF. An important point is that the patient was kept off ART/cART by a medical decision since this was the best strategy considered at that time for cases when the patient was also undergoing chemotherapy treatment [
17].
On 09/23/00, she was in clinical remission at the end of chemotherapy.
On 10/04/00, she had a relapse in the spinal cord and the central nervous system (CNS). A computerized tomography (CT) of the chest and abdomen scan revealed generalized lymphadenopathy. LDH dosage at this time was 998 Ul/L. The patient evolved with a progressive clinical deterioration, making it impossible to start a new chemotherapy protocol.
2.5. Outcome and Follow-Up
The patient died from sepsis and disease progression on 12/24/00.
3. Discussion
Malignant lymphoma of the gallbladder is a rare type of gallbladder malignancy, which encompasses 0.1%-0.2% of all gallbladder tumors [
1,
2,
3,
4]. In prior reports, the majority of them were described to be DLBCL or MZL [
3,
5]. In all medical literature, just three reports had documented Gallbladder BL [
1,
7,
8]. The present case report grants some interesting clinical information once it is the first Gallbladder BL described in the pediatric population and in an individual living with HIV.
Affording to the diagnostic criteria of gastrointestinal lymphoma, which was defined by Dawson et al. [
18] and Lewin et al. [
19], the case in question is considered as a “secondary” gallbladder BL, because it included extra-gallbladder lesions, which probably occurred before gallbladder infiltration. Some days after surgery (24 days), the patient underwent an investigation in a quaternary hospital, which diagnosed an L3-ALL. There was no CNS infiltration. Besides this, an x-ray of long bones showed bilateral lytic lesions in the femurs.
Gastrointestinal symptoms (vomiting, abdominal pain, diarrhea, and jaundice) were notable and led the patient to seek medical assistance. It is well known that gallbladder BL can be presented as a localized disease that mimics gallbladder cancer. Ono et al. [
4] reported imaging descriptions of malignant lymphomas, showing that high-grade malignant lymphomas can exhibit solid and bulky masses or unconventional wall thickening. Because BL is a highly aggressive and rapidly progressive disease, some extranodal sites are generally involved at the time of diagnosis, as in our case report [
20].
In children living with HIV (CLWH) the incidence of malignant neoplasms is higher. This substantial increase is related to a high frequency of non-Hodgkin’s lymphomas (NHL), particularly B-NHL (like BL), Kaposi’s sarcoma (KS), leiomyosarcoma and Hodgkin’s lymphoma (HL) [
9,
21,
22,
23,
24,
25].
When lymphomas and HIV occur simultaneously in children with immature immune systems, it can cause serious consequences. HIV-related lymphomas are typically linked to immune dysregulation, as HIV infection causes a depletion of both cellular and humoral immunity [
24,
26,
27,
28,
29,
30,
31,
32,
33]. In this population, lymphomas are often detected at a late stage with the involvement of tissues outside the lymph nodes, and usually with a rapid and aggressive progression [
22,
23,
25,
34]. It is widely acknowledged that the presence of HIV infection significantly worsens the prognosis for children and adolescents with lymphoma, even with the use of antiretroviral therapy and chemotherapy [
35,
36,
37,
38,
39,
40,
41].
The accurate antiretroviral therapy reduced morbidity and mortality in people living with HIV (PLWH) since it inhibits viral replication and restores immunological surveillance [
42,
43,
44,
45]. So, starting ART/cART as soon as possible is related to a better recuperation of CD4+ T-cell counts and, consequently, to the reduction of problems caused by HIV immunosuppression - such as opportunistic infections and malignant neoplasms [
42,
46].
In the context of immunosuppression due to HIV infection, ART/cART, in combination with chemotherapy, has the highest priority in BL treatment, even if higher than surgery [
1,
40,
41,
42,
46]. Unfortunately, the patient died from sepsis and disease progression about 18 months after the lymphoma diagnosis despite the early beginning of chemotherapy (around 20 days after surgery and histopathological analysis). It is noteworthy that the patient did not undergo ART/cART in any moment during the treatment, although she had been treated in a reference center for HIV and cancer treatment in Brazil (IPPMG/UFRJ). In reality, at this time (1999-2000), antiretroviral therapy was not yet recommended in similar cases by Brazilian Ministry of Health guidelines [
17]. In a recent work published by our research group, it was observed that patients who achieved complete remission, but did not recover CD4+ levels, had inferior survival rates with higher relapse occurrence and infections related to the rescue protocols instituted [
41]. Nowadays, the role of antiretroviral therapy is well-established for a better prognosis of PLWH precisely because it prevents the appearance and recurrence of malignant tumors, especially lymphomas [
10,
22,
40,
41,
42,
46].
Even though the precise preoperative diagnosis of gallbladder malignant lymphoma is difficult, some previous reports [
3,
4,
47,
48] have suggested the possibility of an accurate preoperative suspicion of lymphoma centered on imaging analysis. Unfortunately, in the case in question, we did not have access to modern imaging resources for evaluation and comparison with other reports. In addition, the patient had an emergency cholecystectomy due to an initial inaccurate diagnosis (intestinal subocclusion by Ascaris lumbricoides), with rapid progression to cholangitis probably because of an obstruction caused by the tumor. Furthermore, the correct mass location in the topography of the gallbladder was not documented in the medical records. As this is a case from 1999, some information were lost and valuable data for a contemporary case report were not collected. However, this did not harm the diagnosis and the focus of the discussion, once biopsy assessment is the gold standard diagnostic procedure in any lymphoma investigation [
1,
47,
49].
Another important point of our case is the presence of EBV in the sample, confirmed by EBER1 [
12]. Transcription of non-polyadenylated RNAs EBER1 and EBER2 is a constant feature of all EBV latent infection patterns and is, therefore, the best marker to demonstrate infection by this oncogenic virus [
50,
51,
52,
53]. It is known that EBV is linked to the development of a variety of HIV-related lymphomas [
54,
55,
56,
57] and it is present in 60% of BL cases in PLWH [
22]. In sub-Saharan Africa, LB is endemic, and its risk increases both with increasing anti-EBV antibody titers and with HIV infection, for example [
58]. Therefore, our finding corroborates the medical literature. However, the role of EBV in malignancies in CLWH across a range of Western countries in the post-cART era still is not fully recognized.
4. Conclusions
BL can occur in the gallbladder, especially in the context of immunosuppression - such as caused by HIV infection. As far as we concern, this is the first case report of a gallbladder BL in a pediatric patient. Furthermore, it is the first one reported in a patient living with HIV. It is already known that BL should be contemplated in the differential diagnosis of a gallbladder tumor, and biopsy is mandatory for diagnosis and early beginning of chemotherapy. Neoplasms may present themselves more aggressively in immunosuppressed patients. In this scenario, an early diagnosis can change the course of the disease. Furthermore, the case highlights the importance of an early initiation of ART/cART in PLWH. For this reason, this report is so important, since gallbladder BL should also be considered a differential diagnosis in this population.
Author Contributions
Nathalia Lopez Duarte, Ana Paula Silva Bueno, Bárbara Sarni Sanches, Gabriella Alves Ramos, Layanara Albino Batista, Thalita Fernandes de Abreu, Marcelo Gerardin Poirot Land and Cristiane Bedran Milito contributed to investigation, data collection, resources, manuscript writing, reviewing and editing; Nathalia Lopez Duarte, Marcelo Gerardin Poirot Land and Cristiane Bedran Milito contributed to conceptualization, project administration, data curation, methodology, validation, formal analysis, supervision and funding acquisition; all authors have approved the submitted version and the version substantially edited by journal staff that involves their contribution to this study; all authors agree to be personally accountable for their own contributions and for ensuring that questions related to the accuracy or integrity of any part of this work, even ones in which they were not personally involved, are appropriately investigated, resolved, and documented in the literature.
Funding
This research was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior — Brazil (CAPES) — Finance Code 001-88882.331795/2010-01 and from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro — Brazil (FAPERJ) — Finance Code SEI-260003/009300/2021-APQ1 — “Programa de Apoio a Projetos de Pesquisa e Desenvolvimento Tecnológico em Medicina de Precisão – 2021”. The APC was funded by all authors.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards of Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG/UFRJ) (Ethics Committee approval number: 2,504,586; date of approval: 21 February 2018) and Hospital Universitário Clementino Fraga Filho (HUCFF/UFRJ) (Ethics Committee approval number: 2,581,674; date of approval: 5 April 2018).
Informed Consent Statement
Patient consent was waived as this case report used data previously collected for medical care.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Acknowledgments
We would like to thank all clinicians and health professionals from the participating centers for their contributions to this study. We thank the Internal Medicine Postgraduate Program of the Faculty of Medicine, Federal University of Rio de Janeiro (FM/UFRJ), the Coordination for the Improvement of Higher Education Personnel (CAPES), and Carlos Chagas Filho Foundation for Research Support in the State of Rio de Janeiro (FAPERJ) for all the resources provided for the study. We also thank Elaine Sobral da Costa from the Transdisciplinary Center for Research in Child and Adolescent Health (NTISCA/IPPMG) for the academic support throughout the process of conducting this research. Finally, we gratefully thank José Carlos de Oliveira Morais from the Department of Pathology of HUCFF/UFRJ (in memoriam) for the technical and academic support.
Conflicts of Interest
The authors declare that they have no conflict of interest to disclose.
References
- Hosoda, K. et al. Gallbladder Burkitt’s lymphoma mimicking gallbladder cancer: A case report. World J Gastroenterol 2022, 28, 675. [Google Scholar] [CrossRef]
- Yasuma, T.; Yanana, M. Primary sarcoma of the gallbladder--report of three cases. Acta Pathol Jpn 1971, 28, 285–304. [Google Scholar] [CrossRef]
- Jelic, T.M. et al. Primary, extranodal, follicular non-Hodgkin lymphoma of the gallbladder: case report and a review of the literature. Leuk Lymphoma 2004, 45, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Ono, A. et al. Primary malignant lymphoma of the gallbladder: a case report and literature review. Br J Radiol 2009, 82, e15-e19. [CrossRef]
- Ayub, A. et al. Primary non-Hodgkin’s lymphoma of the gallbladder: A population-based analysis. Anticancer Res. 2017, 37, 2581–2586. [Google Scholar] [CrossRef]
- Mani, H. et al. Gall bladder and extrahepatic bile duct lymphomas: clinicopathological observations and biological implications. Am J Surg Pathol. 2010, 34, 1277-1286. [CrossRef]
- Doherty, B.; Palmer, W.; Cvinar, J.; Sadek, N. A Rare Case of Systemic Adult Burkitt Lymphoma Presenting as Acute Acalculous Cholecystitis. ACG Case Rep J 2019, 6, e00048. [Google Scholar] [CrossRef] [PubMed]
- Repine, T.B.; DeArmond, G.; López, J.D. Unusual sites of metastatic malignancy: Case 2. Burkitt’s lymphoma involving the gallbladder. J Clin Oncol. 2004, 22, 5014-5015. [CrossRef]
- Centers for Disease Control and Prevention. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Morb Mortal Wkly Rep. 1994, 43, 1–10. Available online: https://www.sandiegocounty.gov/content/dam/sdc/hhsa/programs/phs/documents/rr4312.pdf (accessed on 11 May 2024).
- Brasil, Ministério da Saúde, Coordenação Geral de Vigilância do HIV/AIDS e Hepatites Virais - CGAHV/DATHI/SVSA/MS. Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção Pelo HIV em Crianças e Adolescentes: Módulos 1 e 2. 2023. Available online: https://portaldeboaspraticas.iff.fiocruz.br/atencao-recem-nascido/pcdt-para-manejo-da-infeccao-pelo-hiv-em-criancas-e-adolescentes/ (accessed on 11 May 2024).
- Khoury, J.D. et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia 2022, 36, 1703–1719. [CrossRef]
- Gulley, M.L. et al. Guidelines for interpreting EBER in situ hybridization and LMP1 immunohistochemical tests for detecting Epstein-Barr virus in Hodgkin lymphoma. Am J Clin Pathol. 2002, 117, 259-267. [CrossRef]
- World Health Organization. International Classification of Diseases, 11th edition (ICD 11). Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 11 May 2024).
- Murphy, S.B. Classification, staging and end results of treatment of childhood non-Hodgkin’s lymphomas: Dissimilarities from lymphomas in adults. Semin. Oncol. 1980, 7, 332–339. [Google Scholar]
- Canellos, G.P. et al. The m-BACOD combination chemotherapy regimen in the treatment of diffuse large cell lymphoma. In: Seminars in Hematology. Semin Hematol. 1987, 24, 2–7. [Google Scholar]
- Reiter, A. et al. Non-Hodgkin’s lymphomas of childhood and adolescence: results of a treatment stratified for biologic subtypes and stage--a report of the Berlin-Frankfurt-Münster Group. J Clin Oncol. 1995, 13, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Ministério da Saúde, Coordenação Nacional de DST e AIDS. Guia de tratamento clínico da infecção pelo HIV em crianças. 2001. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/073_03Guia_tratamento.pdf (accessed on 11 May 2024).
- Dawson, I.M.; Cornes, J.S.; Morson, B.C. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961, 80–89. [Google Scholar] [CrossRef]
- Lewin, K.J.; Ranchod, M.; Dorfman, R.F. Lymphomas of the gastrointestinal tract. A study of 117 cases presenting with gastrointestinal disease. Cancer 1978, 42, 673–707. [Google Scholar] [CrossRef]
- Crombie, J.; LaCasce, A. The treatment of Burkitt lymphoma in adults. Blood 2021, 11, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Biggar, R.J.; Frisch, M.; Goedert, J.J. Risk of cancer in children with AIDS. JAMA 2000, 284, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Vaccher, E.; Gloghini, A. Hematologic cancers in individuals infected by HIV. Blood 2022, 139, 995–1012. [Google Scholar] [CrossRef] [PubMed]
- Caselli, D.; Klersy, C.; de Martino, M.; Gabiano, C.; Galli, L.; Tovo, P.; Aricò, M.; Italian register for HIV infection in children. Human immunodeficiency virus–related cancer in children: Incidence and treatment outcome—Report of the Italian register. J. Clin. Oncol. 2000, 18, 3854–3861. [CrossRef]
- Chiappini, E. et al. Pediatric human immunodeficiency virus infection and cancer in the highly active antiretroviral treatment (HAART) era. Cancer Lett. 2014, 347, 38–45. [Google Scholar] [CrossRef]
- Simard, E.P.; Shiels, M.S.; Bhatia, K.; Engels, E.A. Long-term cancer risk among people diagnosed with AIDS during childhood. Cancer Epidemiol Biomarkers Prev. 2012, 21, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.H. et al. Risk factors for pediatric human immunodeficiency virus–related malignancy. JAMA 2003, 289, 2393–2399. [CrossRef]
- Zicari, S. et al. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019, 11, 200. [CrossRef]
- Shindiapina, P. et al. Immunology of EBV-related lymphoproliferative disease in HIV-positive individuals. Front. Oncol. 2020, 10, 1723. [CrossRef]
- Verdu-Bou, M. et al. Clinical and Therapeutic Implications of Epstein–Barr Virus in HIV-Related Lymphomas. Cancers (Basel). 2021, 13, 5534. [CrossRef]
- Hatano, Y. et al. Virus-driven carcinogenesis. Cancers (Basel). 2021, 13, 2625. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, M.; Montagnier, L. Programmed Cell Death as a Mechanism of CD4 and CD8 T Cell Deletion in AIDS: Molecular Control and Effect of Highly Active Antiretroviral Therapy. Ann N. Y. Acad. Sci. 1999, 887, 199–212. [Google Scholar] [CrossRef]
- Chanock, S.J.; Pizzo, P.A. Infection prevention strategies for children with cancer and AIDS: Contrasting dilemmas. J. Hosp. Infect. 1995, 30, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Geel, J.A. et al. Prognostic factors affecting survival in children and adolescents with HIV and Hodgkin lymphoma in South Africa. Leuk Lymphoma 2021, 62, 2854–2863. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, E. et al. Cancer rates after year 2000 significantly decrease in children with perinatal HIV infection: A study by the Italian Register for HIV Infection in Children. J Clin Oncol. 2007, 25, 97–101. [CrossRef]
- Orem, J. et al. Clinical characteristics, treatment and outcome of childhood Burkitt’s lymphoma at the Uganda Cancer Institute. Trans R Soc Trop Med Hyg. 2011, 105, 717–726. [Google Scholar] [CrossRef]
- Stefan, D.C.; Stones, D.; Newton, R. Burkitt lymphoma in South African children: One or two entities? Transfus Apher Sci. 2011, 44, 191–194. [Google Scholar] [CrossRef]
- Abrahão, R. et al. Chronic medical conditions and late effects following non-Hodgkin lymphoma in HIV-uninfected and HIV-infected adolescents and young adults: A population-based study. Br J Haematol. 2020, 190, 371–384. [CrossRef]
- Singh, E. et al., J. HIV-associated malignancies in children. Curr Opin HIV AIDS 2017, 12, 77. [CrossRef]
- Katumba, R.G. et al. Cancer in Youth Living With HIV (YLWHIV): A Narrative Review of the Access to Oncological Services Among YLWHIV and the Role of Economic Strengthening in Child Health. Front Public Health 2020, 8, 409. [CrossRef]
- Duarte, N.L. et al. Incidence and Clinical Description of Lymphomas in Children and Adolescents with Vertical Transmission of HIV in Rio de Janeiro, Brazil, in Pre-and Post-Combined Antiretroviral Therapy Eras: A Multicentric Hospital-Based Survival Analysis Study. Cancers (Basel). 2022, 14, 6129. [CrossRef]
- Duarte, N.L. et al. Prognostic Factors in Children and Adolescents with Lymphomas and Vertical Transmission of HIV in Rio de Janeiro, Brazil: A Multicentric Hospital-Based Survival Analysis Study. Cancers (Basel). 2023, 15, 2292. [CrossRef]
- Chiappini, E. et al. Real-world analysis of survival and clinical events in a cohort of Italian perinatally HIV-1 infected children from 2001 to 2018. Front Pediatr. 2021, 9, 665764. [Google Scholar] [CrossRef] [PubMed]
- de Martino, M. et al. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. JAMA 2000, 284, 190–197. [CrossRef]
- Álvaro-Meca, A. et al. Epidemiologic trends of cancer diagnoses among HIV-infected children in Spain from 1997 to 2008. Pediatr. Infect. Dis. J. 2011, 30, 764–768. [CrossRef]
- Carrasco, I. et al. Innate and adaptive abnormalities in youth with vertically acquired HIV through a multicentre cohort in Spain. J. Int. AIDS Soc. 2021, 24, e25804. [Google Scholar] [CrossRef]
- Chhabra, S. et al. Malignancy and all-cause mortality; incidence in adolescents and young adults living with perinatally acquired HIV. J. Virus Erad. 2020, 6, 30–33.
- Kato, H. et al. Primary non-Hodgkin’s lymphoma of the gallbladder diagnosed by laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 2008, 15, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P. et al. Imaging-based algorithmic approach to gallbladder wall thickening. World J Gastroenterology 2020, 26, 6163–6181. [Google Scholar] [CrossRef]
- Gascoyne, R.D. Establishing the diagnosis of lymphoma: from initial biopsy to clinical staging. Oncology (Williston Park) 1998, 12, 11-16.
- Carbone, A. et al. HIV-associated Hodgkin lymphoma. Curr Opin HIV AIDS. 2009, 4, 3–10. [Google Scholar] [CrossRef]
- Carbone, A.; Gloghini, A. AIDS-related lymphomas: from pathogenesis to pathology. Br J Haematol. 2005, 130, 662–670. [Google Scholar] [CrossRef]
- Cesarman, E. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett. 2011, 305, 163–174. [Google Scholar] [CrossRef]
- Hijlkema, S.H. et al. A longitudinal and cross-sectional study of Epstein-Barr virus DNA load: a possible predictor of AIDS-related lymphoma in HIV-infected patients. Infect Dis (Lond). 2018, 50, 847-852. [CrossRef]
- Gloghini, A.; Dolcetti, R.; Carbone, A. Lymphomas occurring specifically in HIV-infected patients: from pathogenesis to pathology. Semin Cancer Biol. 2013, 23, 457–467. [Google Scholar] [CrossRef]
- Shindiapina, P. et al. Immunology of EBV-related lymphoproliferative disease in HIV-positive individuals. Front Oncol. 2020, 30, 1723. [CrossRef]
- Verdu-Bou, M. et al. Clinical and therapeutic implications of Epstein–Barr virus in HIV-related lymphomas. Cancers (Basel)., 2021, 13, 5534. [CrossRef]
- Westmoreland, K.D. et al. Hodgkin lymphoma, HIV, and Epstein–Barr virus in Malawi: Longitudinal results from the Kamuzu Central Hospital lymphoma study. Pediatr. Blood Cancer 2017, 64, e26302. [Google Scholar] [CrossRef]
- Newton, R. et al. A case-control study of human immunodeficiency virus infection and cancer in adults and children residing in Kampala, Uganda. Int J Cancer 2001, 92, 622–627. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).