Submitted:
11 June 2024
Posted:
13 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material al Methods
3. Results
3.1. Patient and Tumor Characteristics
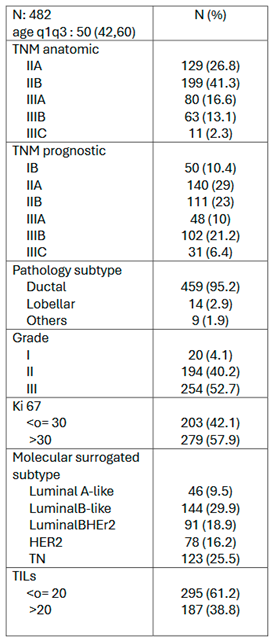
3.2. Neoadjuvant Chemotherapy Outcomes
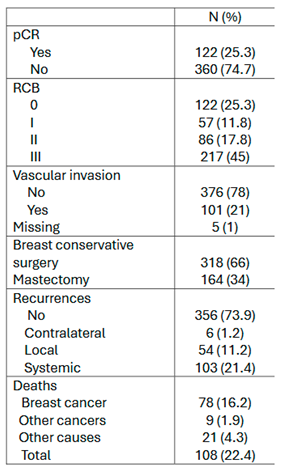
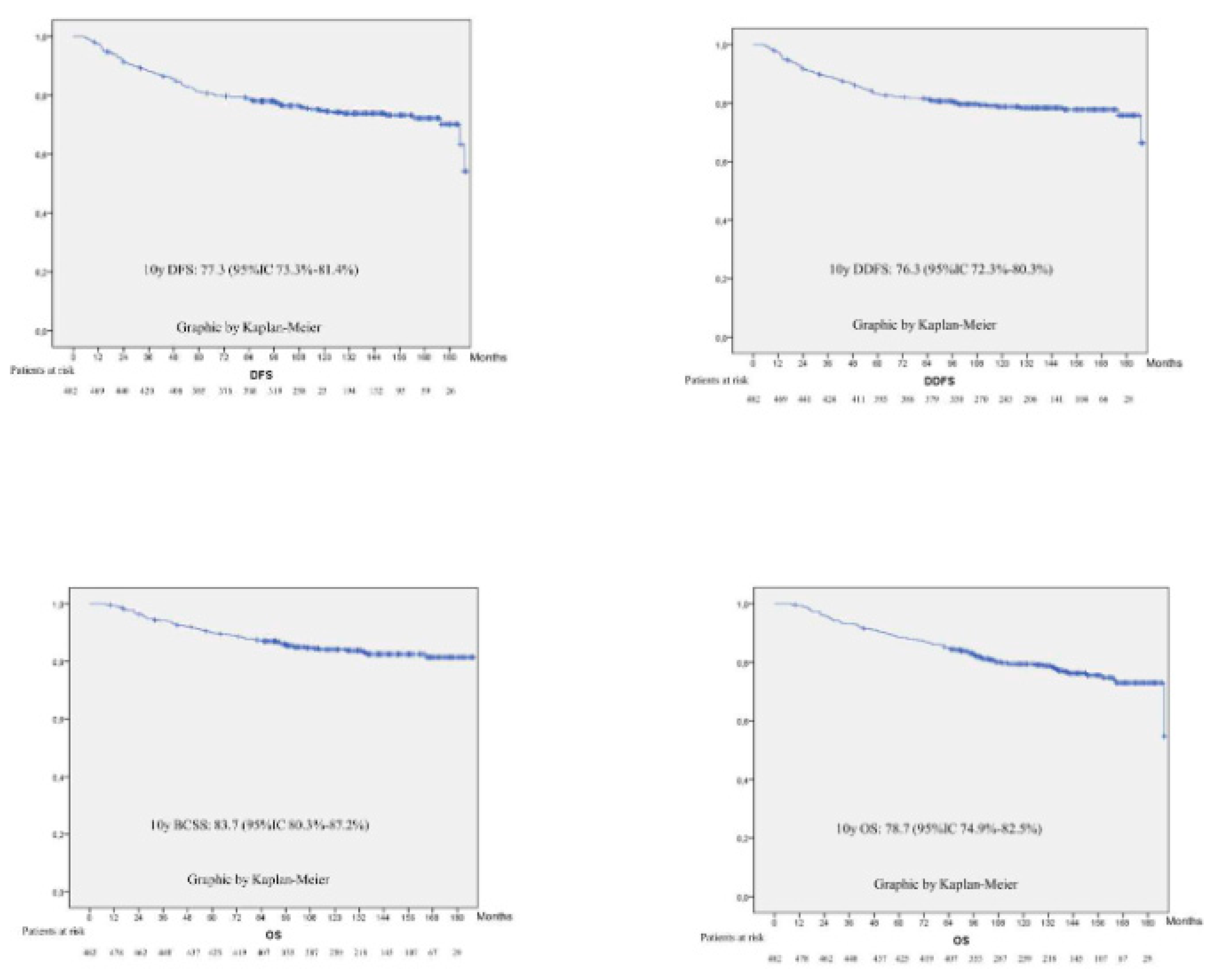
3.3. Survival Outcomes
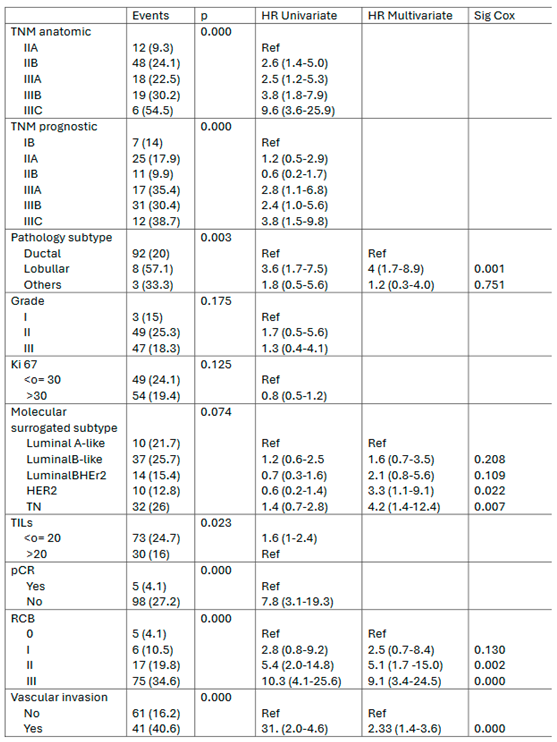
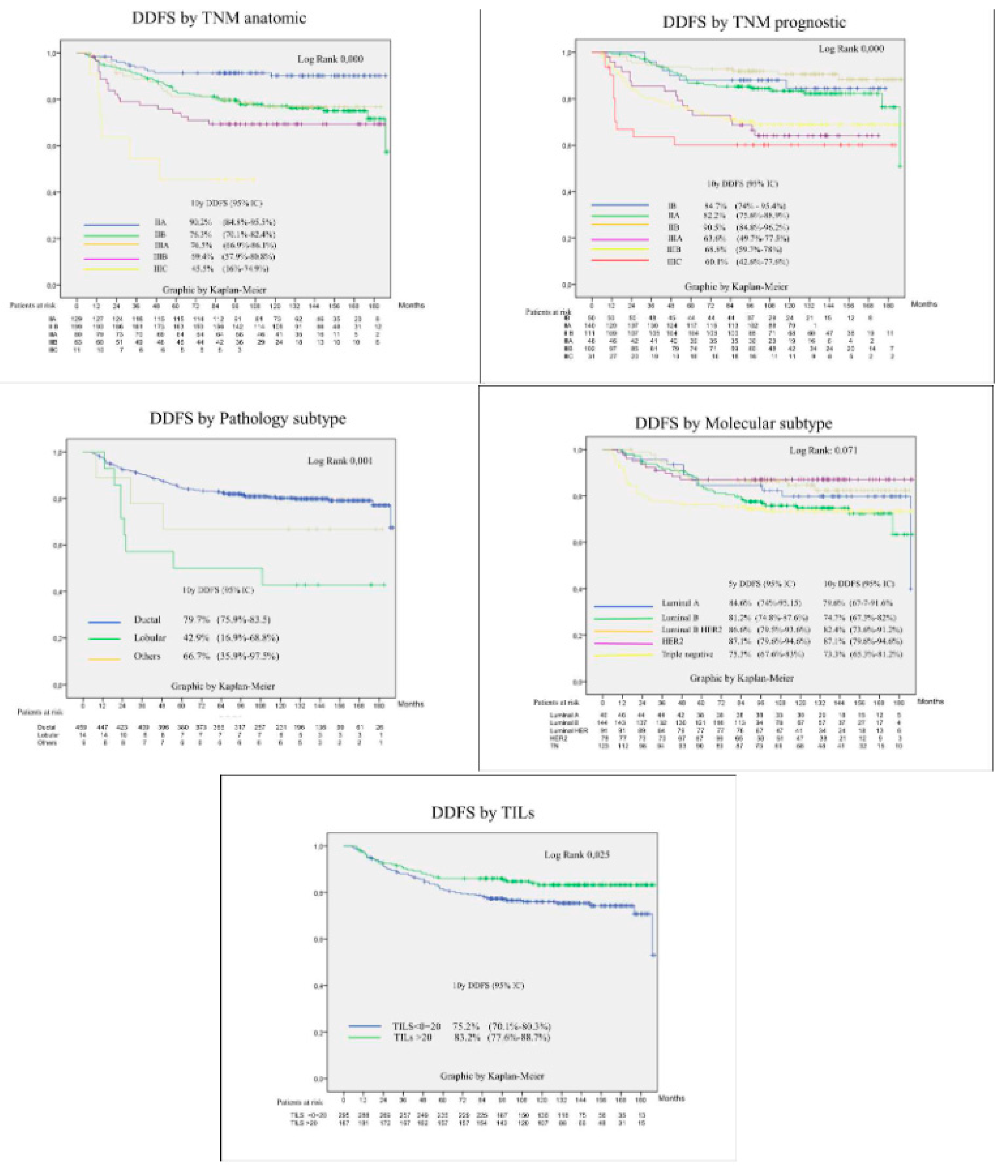
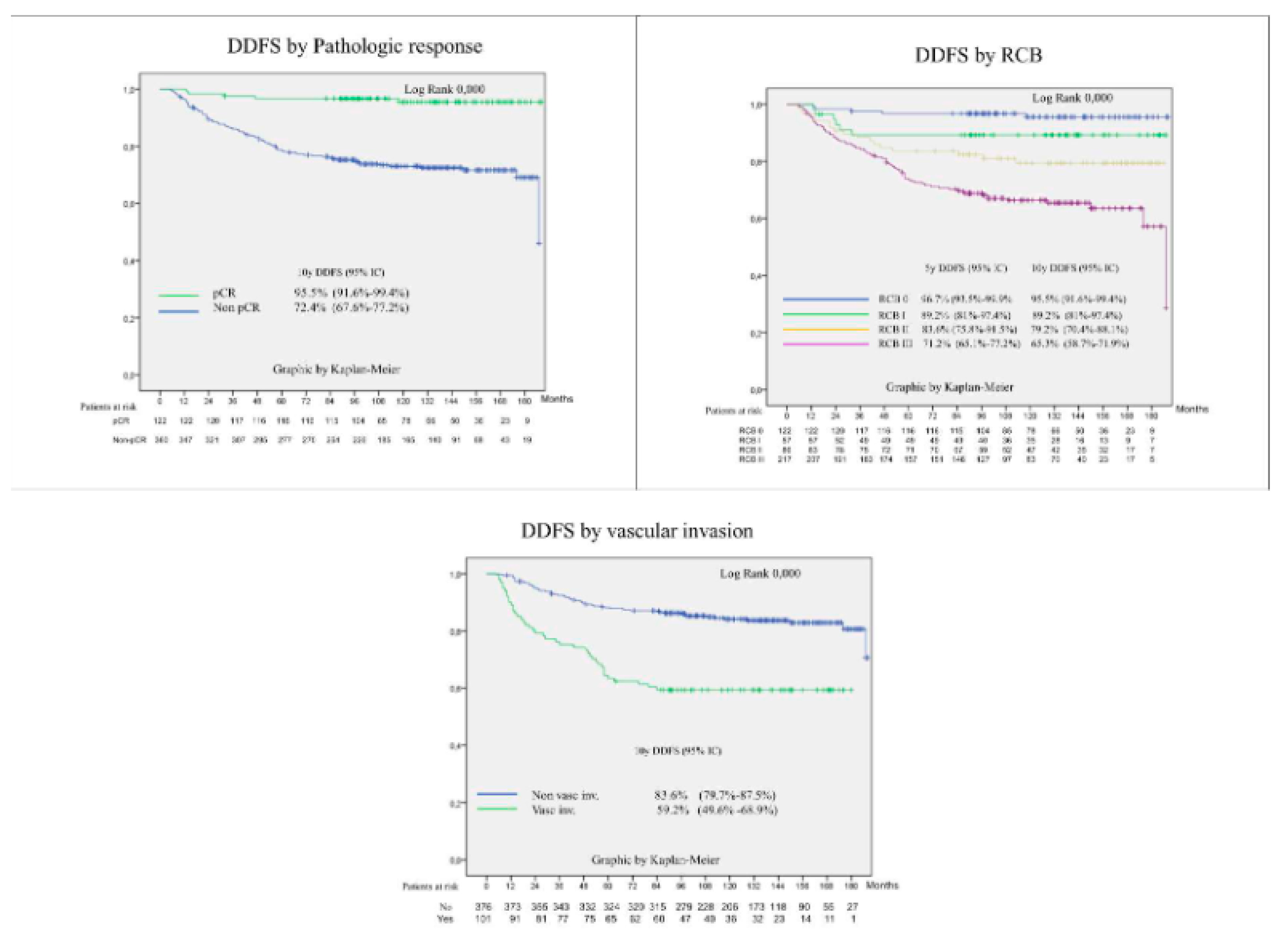
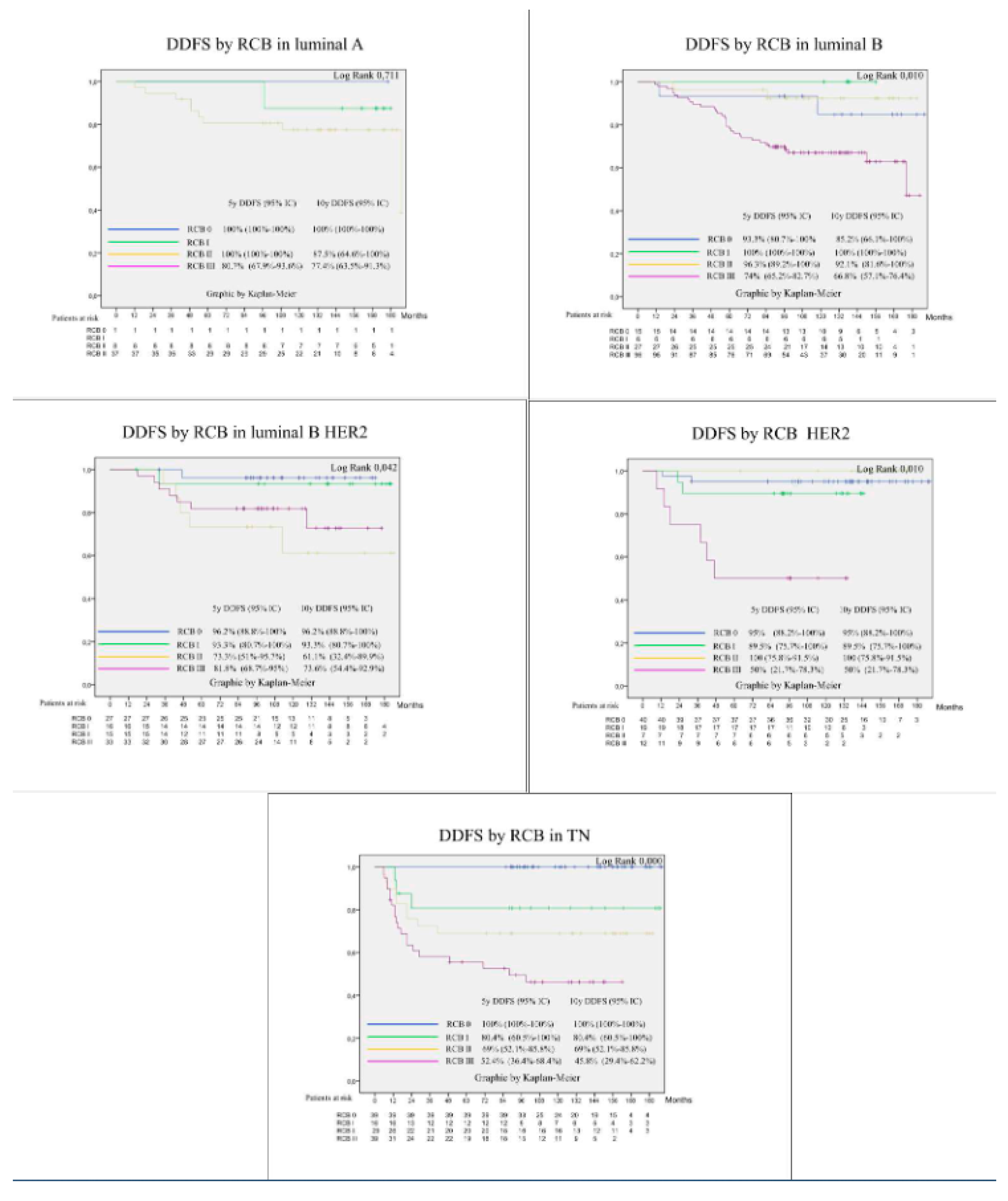
3.4. Prognostic Factors for Patient Survival
3.5. Discussion
Acknowledgments
Ethical Approval
Table of Abbreviations
| NATC | Neoadjuvant chemotherapy |
| pCR | Pathological complete response |
| NRI | Neoadjuvant response index |
| RCB | Residual cancer burden |
| TNBC | Triple negative breast cancer |
| AJCC | American Joint Committee on Cancer |
| ASCO | American Society of Clinical Oncology |
| cN0 | Clinical N0 |
| N+ | Node positive |
| RT | Radiotherapy |
| BMI | Body mass index |
| TILs | Tumor infiltrating lymphocytes |
| DFS | disease free survival |
| DDFS | Distant disease-free survival |
| OS | Overall survival |
| BCSS | Breast cancer specific survival |
| SD | Standard deviation |
| CI | Confidence intervals |
| HR | Hazard ratio |
References
- Bonadonna G, Veronesi U B et al. J Natl Cancer Inst. J Natl Cancer Inst. 1990;82:1539–45.
- Wolmark N, Wang J ME et al. No Title. J Natl Cancer Inst Monogr. 2001;30:96–102.
- Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: Highlights of the st gallen international expert consensus on the primary therapy of early breast Cancer 2013. Ann Oncol. 2013;24(9):2206–23. [CrossRef]
- Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Shelley Hwang E, et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J Clin Oncol. 2021;39(13):1485–505. [CrossRef]
- Masuda N, Lee S-J, Ohtani S, Im Y-H, Lee E-S, Yokota I, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147–59. [CrossRef]
- von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380(7):617–28. [CrossRef]
- Curigliano G, Burstein HJ, Gnant M, Loibl S, Cameron D, Regan MM, et al. Understanding breast cancer complexity to improve patient outcomes: The St Gallen International Consensus Conference for the Primary Therapy of Individuals with Early Breast Cancer 2023. Ann Oncol. 2023;34(11):970–86. [CrossRef]
- Fernandez-Gonzalez S, Falo C, Pla MJ, Pernas S, Bajen M, Soler T, et al. The Shift From Sentinel Lymph Node Biopsy Performed Either Before or After Neoadjuvant Systemic Therapy in the Clinical Negative Nodes of Breast Cancer Patients. Results, and the Advantages and Disadvantages of Both Procedures. Clin Breast Cancer [Internet]. 2018;18(1):71–7. Available from:. [CrossRef]
- Sheri A, Smith IE, Johnston SR, A’hern R, Nerurkar A, Jones RL, et al. Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann Oncol. 2015;26(1):75–80.
- Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med. 2015;373(4):307–16. [CrossRef]
- Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med. 2015;373(4):317–27.
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILS) in breast cancer: Recommendations by an International TILS Working Group 2014. Ann Oncol. 2015;26(2):259–71. [CrossRef]
- Gourgou-Bourgade S, Cameron D, Poortmans P, Asselain B, Azria D, Cardoso F, et al. Guidelines for time-to-event end point definitions in breast cancer trials: Results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials). Ann Oncol [Internet]. 2015;26(5):873–9. Available from:. [CrossRef]
- Asselain B, Barlow W, Bartlett J, Bergh J, Bergsten-Nordström E, Bliss J, et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. [CrossRef]
- Falo C, Moreno A, Benito E, Lloveras B, Varela M, Serra JM, et al. Primary chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil in operable breast carcinoma. Cancer. 2005;103(4):657–63.
- Fasching PA, Hartkopf AD, Gass P, Häberle L, Akpolat-Basci L, Hein A, et al. Efficacy of neoadjuvant pertuzumab in addition to chemotherapy and trastuzumab in routine clinical treatment of patients with primary breast cancer: a multicentric analysis. Breast Cancer Res Treat [Internet]. 2019;173(2):319–28. Available from: . [CrossRef]
- Poggio F, Bruzzone M, Ceppi M, Pondé NF, La Valle G, Del Mastro L, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann Oncol. 2018;29(7):1497–508. [CrossRef]
- Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382(9):810–21. [CrossRef]
- Johnston SRD, Toi M, O’Shaughnessy J, Rastogi P, Campone M, Neven P, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24(1):77–90. [CrossRef]
- Slamon D, Lipatov O, Nowecki Z, McAndrew N, Kukielka-Budny B, Stroyakovskiy D, et al. Ribociclib plus Endocrine Therapy in Early Breast Cancer. N Engl J Med. 2024;390(12):1080–91. [CrossRef]
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–85.
- Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol [Internet]. 2012;13(1):25–32. Available from:. [CrossRef]
- Loibl S, O’Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol [Internet]. 2018;19(4):497–509. Available from:. [CrossRef]
- Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13–21. [CrossRef]
- Von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014;15(7):747–56. [CrossRef]
- Geyer CE, Sikov WM, Huober J, Rugo HS, Wolmark N, O’Shaughnessy J, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol [Internet]. 2022;33(4):384–94. Available from:. [CrossRef]
- Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant Olaparib for Patients with BRCA1 - or BRCA2 -Mutated Breast Cancer . N Engl J Med. 2021;384(25):2394–405. [CrossRef]
- Prat A, Saura C, Pascual T, Hernando C, Muñoz M, Paré L, et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):33–43. [CrossRef]
- Johnston S, Puhalla S, Wheatley D, Ring A, Barry P, Holcombe C, et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor–positive early breast cancer: Pallet trial. J Clin Oncol. 2019;37(3):178–89. [CrossRef]
- Gil-Gil M, Alba E, Gavilá J, de la Haba-Rodríguez J, Ciruelos E, Tolosa P, et al. The role of CDK4/6 inhibitors in early breast cancer. Breast. 2021;58:160–9.
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. [CrossRef]
- Petrelli F, Barni S. Response to neoadjuvant chemotherapy in ductal compared to lobular carcinoma of the breast: A meta-analysis of published trials including 1,764 lobular breast cancer. Breast Cancer Res Treat. 2013;142(2):227–35. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





