1. Introduction
As a Gram-negative opportunistic pathogen,
Pseudomonas aeruginosa is one of the most prevalent pathogens that cause acute and chronic infections in various parts of our body, such as lung, wounds and urinary tract [
1,
2]. In recent years, the rate of infections induced by
P. aeruginosa among hospitalized patients increased significantly. For example, during the past 3 decades, the rates of hospital-acquired infections caused by
P.
aeruginosa was nearly 15%, and the rates for patients with cystic fibrosis (CF) or chronic obstructive pulmonary disease (COPD) was up to 50% [
3]. In addition,
P.
aeruginosa is the primary pathogen isolated from individuals with hospital-acquired pneumonia, and is associated with high morbidity and mortality [
4]. Moreover, with the increase in multi-drug resistant strains, eradicating
P. aeruginosa infections becomes more challenging.
It has been reported that iron dysregulation is an important factor in the maintenance of lung infection induced by
P. aeruginosa, for several reasons [
5]. The main reason is that iron is essential for multiple bacterial metabolic pathways and required for host colonization.
P. aeruginosa utilizes iron acquired from the host to promote growth and increase virulence, so as to aggravate the development of lung infection [
6]. Secondly, iron is necessary to produce reactive oxygen species (ROS) as part of the immune response to an infection. Excess iron can cause ROS overproduction, which can lead to damage of healthy cells and aggravation of inflammation [
6]. Thirdly, the cells of the immune system require iron to sustain its function, metabolism, and proliferation [
7]. However, iron overload can attenuate the phagocytosis of macrophages, and affect the function of T lymphocytes, leading to disruption of the immune system [
6]. It has been reported that enhancing macrophage iron accumulation promoted acute lung inflammation and oxidative stress, and macrophage ferroportin could serve as a therapeutic target in bacteria-induced acute lung injury [
8,
9]. Therefore, targeting iron metabolism, specifically iron overload, by using iron chelators is a potential supportive antimicrobial strategy to relieve lung infection caused by
P. aeruginosa.
Increasing evidence suggests that 3-hydroxypyridin-4-one (DIBI), through depriving microorganisms of bioavailable iron, has the potential to serve as a new anti-infective agent. Allan et al. reported that DIBI showed efficacy in reducing
Staphylococcus aureus (
S. aureus) burden in mouse nares comparable to mupirocin [
10]. Because of the high sensitivity of
S. aureus, DIBI was considered as an adjuvant to mupirocin to combat the natural colonization of
S. aureus isolates. Using minimum inhibitory concentration assay, Ang et al. also demonstrated that, DIBI had an inhibitory effect against representative reference strains for Gram-positive and Gram-negative bacteria, such as
S. aureus,
Acinetobacter baumannii, and the fungal pathogen
Candida albicans [
11]. In previous experiments we have demonstrated that DIBI has significant anti-inflammatory effects in lipopolysaccharides (LPS)-induced acute lung injury [
12].
However, it is unclear whether DIBI has the same effect against lung infections caused by P. aeruginosa. In order to reduce the risk of antimicrobial resistance and explore novel antimicrobial agents or potential adjuvant drugs that can be used in conjunction with existing antibiotics, we investigated the anti-inflammatory and anti-bacterial effects of DIBI in P. aeruginosa induced experimental lung infection in mice.
2. Materials and Methods
2.1. Bacterial Preparation
P. aeruginosa strain PA14 was kept in a freezing medium (50% Luria broth (LB), 50% glycerol) and stored at -80 ◦C until use. Three days prior to infection experiments, PA14 was streaked on LB agar plates to isolate single colonies and incubated at 37◦C for 16-24 hours. Following this, a single colony was selected from the plate and inoculated into 5 mL of LB broth, which was then cultured overnight in a rotating incubator (200 rpm, 37◦C) for at least 18 hours. On the third day, the bacterial optical density at 600 nm (OD600) was verified to be in the 5-6 range before the overnight culture was diluted 1:50 in 5 mL of fresh LB broth. The subculture was shaken for approximately 3 hours until OD600 reached 1.5-2, indicating that bacteria were in the exponential growth phase. Next, 1 mL of subculture was prepared by centrifugation (5000g, 5 min) and washing in phosphate-buffered saline (PBS). The final concentration was determined by the equation 1 OD600 = 1x109 CFU/mL. Bacteria were then diluted in PBS to deliver a dose of 5x105 CFU in 40 μL. Meanwhile, bacteria from the final concentration were serially diluted and plated to confirm the accuracy of the dose.
2.2. Animals
Age- and weight-matched C57BL/6 male and female mice were purchased from the Jackson Laboratory (Maine, USA), and were housed in ventilated plastic cage racks in a pathogen-free room of the Carleton Animal Care Facility, Dalhousie University, Halifax, NS, Canada. Animals were kept on a 12-h light/dark cycle at 21◦C and were acclimatized for one week prior to experiments. Animals were enrolled in experiments at 8-12 weeks of age. Experimental protocols were approved by the University Committee on Laboratory Animals at Dalhousie University under protocol number #21-090 and were performed following the guidelines and standards of the Canadian Council on Animal Care
2.3. Experimental Model
Mice were randomly allocated to four groups as follows: Control+PBS, PA14+PBS, PA14+DIBI X1, PA14+DIBI X2 (n = 12/group). Animals were weighed prior to anesthesia, and the induction of anesthesia was accomplished by inhalation of 4-5% isoflurane with oxygen (1L/min). After 2-3 min induction, once the animals reached the surgical plane of anesthesia (unresponsive to foot pinch), the animals were removed from the nose cone and placed onto a 45° angled platform hanging by the front incisors. The tongue was immobilized, and an otoscope was inserted into the mouth to visualize tracheal opening. Using otoscope guidance, 40 uL of bacterial culture, containing 5x105 CFU bacteria, was delivered by pipette just in front of the vocal folds of the tracheal opening. In order to maximize inhalation of inoculum, after withdrawing the tip, the otoscope was left in mouth for a few breaths to keep the airway open. After PA14 instillation, the animal was placed back into the cage on a 37 °C heating pad and monitored for 24 hours. In the control group (Control + PBS), the mice were given PBS to the tracheal at the same volume of bacterial culture. DIBI (80 mg/kg) was given by intraperitoneal (i.p) injection either as a single dose immediately after PA14 administration in the PA14+DIBI X1 group, or a double dose (second dose 4 h after PA14 administration) in the PA14+DIBI X2 group. The mice in PA14+PBS group were treated with i.p PBS once right after PA14 administration.
2.4. Clinical Scores and Body Weight Measurement
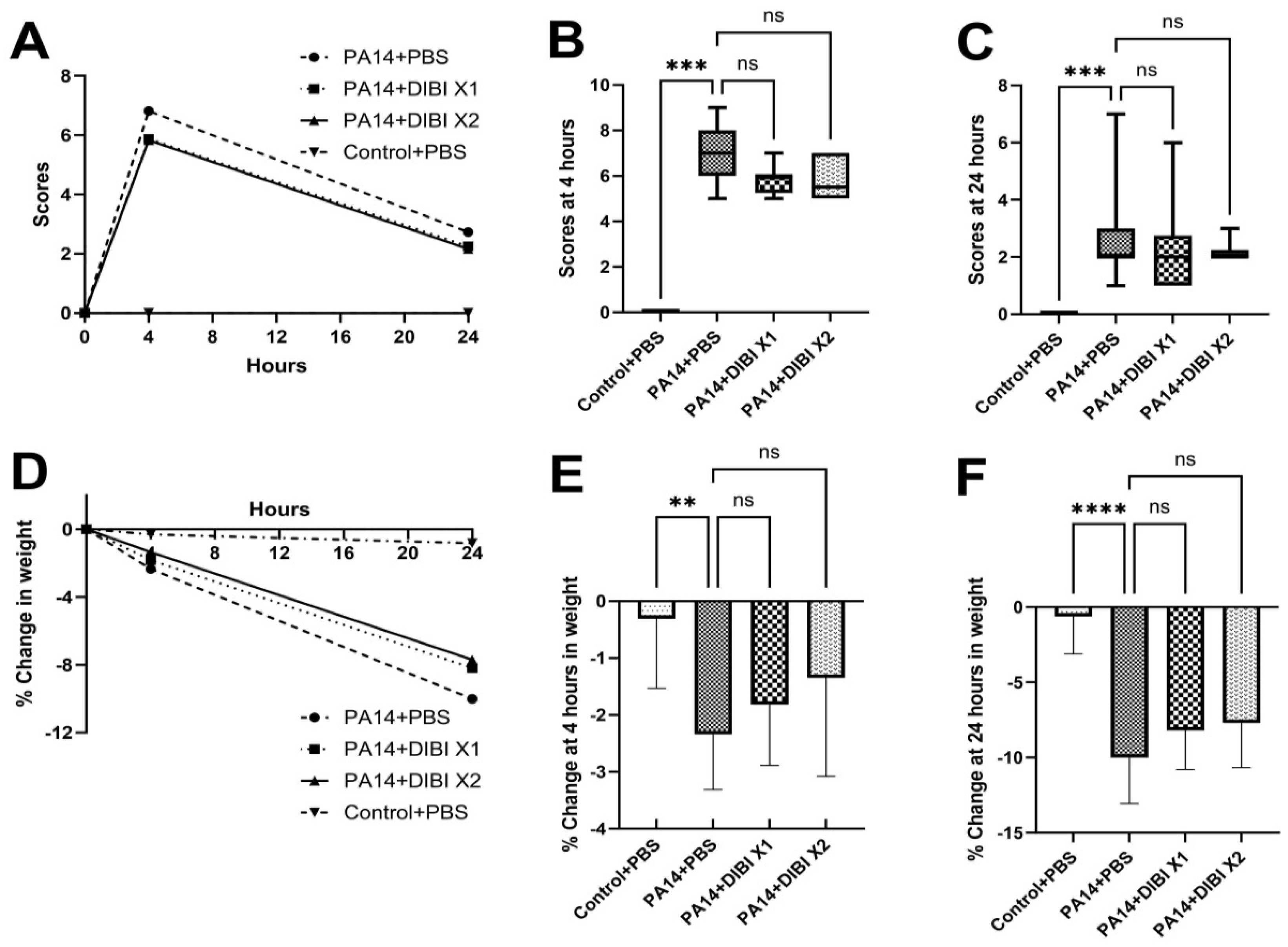
Mouse physical appearance, posture, righting reflex, respiration rate, activity/behaviour and body temperature were scored (each on a scale of 0-3) to assess morbidity after PA14 infection. The clinical scores were recorded 4h and 24h after PA14 administration. The weight changes were also recorded at each observation. The clinical scores >10 and/or weight loss > 15% were considered as criteria for immediate euthanasia.
2.5. Bronchoalveolar Lavage Fluid Collection
Twenty-four hours after PA14 infection, mice were anesthetized again. After reaching the surgical plane of anesthesia, blood was collected via cardiac puncture. Then a total of 1.4 mL of ice-cold PBS with protease inhibitors (Complete tablets, Roche Diagnostic, Basel, Switzerland) was used to perform the bronchoalveolar lavage fluid (BALF) collection. Briefly, after exposure of the trachea, a nick between two of the cartilage rings was created, and a 21-gauge catheter connected to a 3 ml syringe containing protease inhibitors was inserted and immobilized with a nylon string. Lungs were then flushed with 0.7 mL of ice-cold PBS with protease inhibitors. This process was repeated once more. Following BALF collection, lungs were harvested aseptically.
2.6. Lung Homogenates
Aseptically-removed lungs were collected in 1 mL sterile PBS with protease inhibitors and put on ice. In a biosafety cabinet, samples were homogenized using a TH115 homogenizer (Omni International, Kennesaw, GA) with sterile probes for 45 seconds on max speed (35000 rpm). Probes were washed in 70% ethanol and rinsed in sterile PBS between samples. Once all lungs were homogenized, 20 μL was removed for CFU plating, described below, while the remainder was centrifuged at 16,000 g for 30 min at 4◦C then stored at -80◦C for subsequent cytokine and western blot analysis.
2.7. Measurements of Bacterial Load in BALF and Lung Tissue
The obtained BALF and homogenized lung samples were aliquoted and serially diluted 1:10 in PBS. Lung homogenates were spot-plated on LB plates in 10 μL spots from 0 to 10-3 dilutions. Undiluted and 10-1 dilutions of BALF were spread-plated on LB plates. Afterward, plates were incubated upside-down at 37◦C overnight, and bacterial colonies were counted in the following morning. CFU was quantified using the calculation: CFU/mL = (colonies x dilution factor) / volume plated (mL).
2.8. Cytokine Analysis in Lung Tissue, BALF and Serum
The levels of inflammatory cytokines, including interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF), were assessed by enzyme-linked immunosorbent assay (ELISA) according to the instructions provided by the manufacturer (R&D Systems, Minneapolis, MN). Lung samples were diluted at 1:5 (IL-6, TNF) or 1:20 (IL-1β), and BALF and serum were diluted at 1:2.
2.9. Western Blotting
Western blotting analyses were performed as described previously [
12]. Protein content was quantified via BCA assay (Pierce
TM BCA Protein Assay kit, Thermo Fisher Scientific, Waltham, MA), and equal amounts of protein (40μg/lane) for all samples were separated by 12% SDS-PAGE and transferred onto a polyvinylidene fluoride membrane (Millipore, Billerico, Mass, US). Primary antibody against NF-κBp65 (1:1000, Cell Signaling Technology, Danvers, Mass, US) was added and incubated overnight. Blots were then incubated with horse radish peroxidase–linked anti-rabbit immunoglobulin G (diluted 1:3000) for 2h. With an enhanced chemiluminescence system (Chemidoc, Bio-Rad, Hercules, CA, US), the protein of interest was detected, and the intensity of each band was analyzed using Image J (NIH, Bethesda, MD). GAPDH (1:2000, Cell Signaling Technology, Danvers, MA) was defined as a loading control.
2.10. Histology
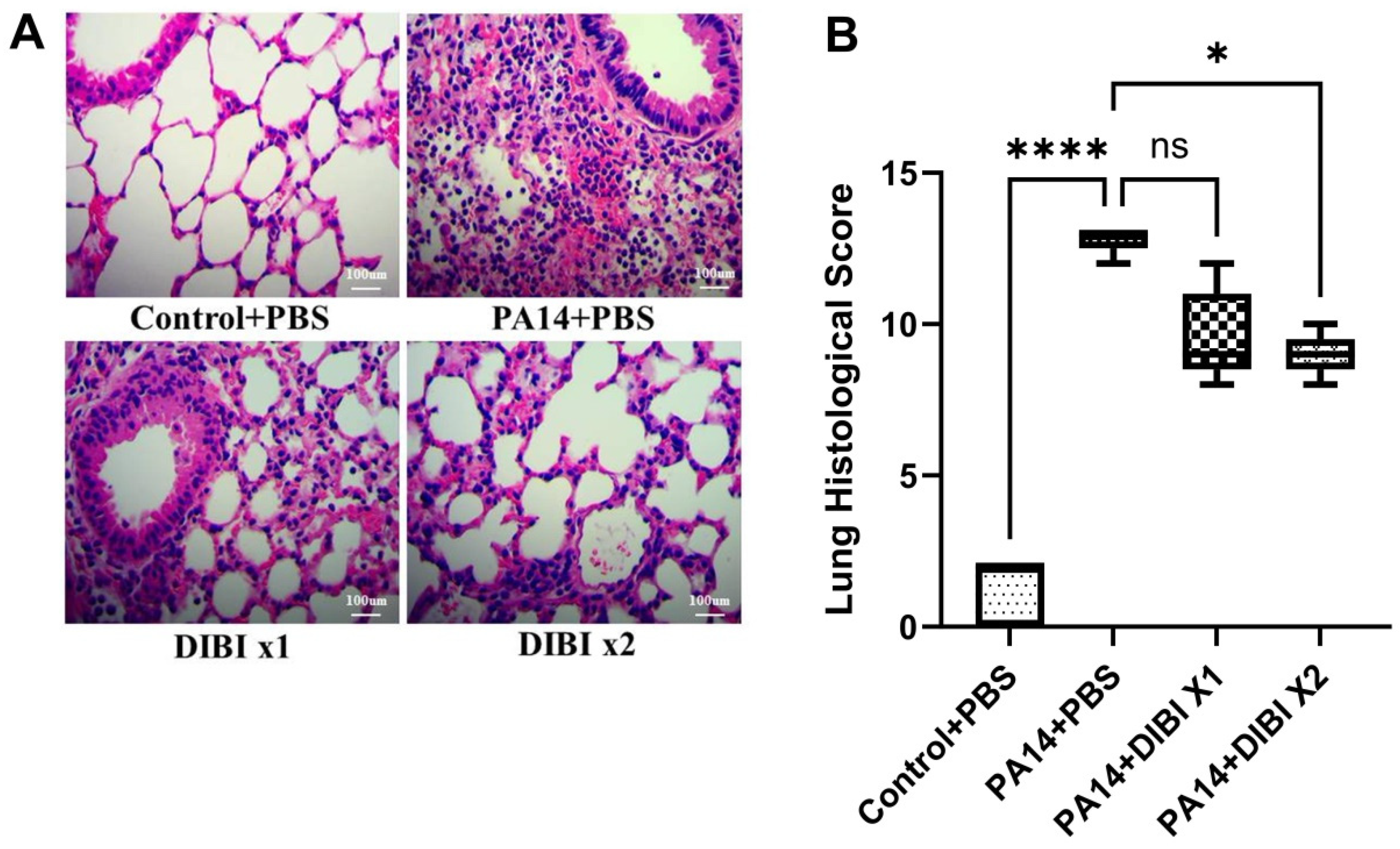
Mice were anesthetized by inhaled isoflurane 24h post-infection as previously described and sacrificed by cervical dislocation. Lungs were collected and fixed in 10% neutral-buffered formalin (NBF) for 24 h and then washed and stored in 70% ethanol until processing. The fixed lung samples were sent to the Department of Pathology, IWK Health Centre, Halifax, NS, Canada, for further processing, including paraffin embedding, sectioning, and staining with hematoxylin and eosin. A blinded histological analysis was performed using an established lung injury score, including the presence of edema, hemorrhage, immune cell infiltration, cell wall thickening, and presence of aggregates [
13]. Representative histology images were taken using Optika Microscopes (Ponteranica BG, Italy).
2.11. Statistical Analysis
All data were analyzed using the software Prism 10 (GraphPad Software, La Jolla, CA). To confirm normal distribution of data, the Kolmogorov-Smirnov Test was used. Pairwise comparisons were performed using Student’s t-test. One-way ANOVA or Kruskal-Wallis test was used to analyze multiple comparisons. Data was expressed as mean ± standard deviation (SD). Significance was assumed at p values less than 0.05 (p < 0.05).
4. Discussion
With the present study, we revealed the role of iron chelator DIBI treatment on relieving some disease symptoms in an acute lung infection in mouse model of P. aeruginosa. Specifically, systemic administration of DIBI decreased bacterial load in lung tissue and BALF, reduced pro-inflammatory cytokines production and improved bacterial lung injury in histology.
Based on the fact that weight loss in
P. aeruginosa infected mice was associated with the inflammatory process, we also observed the effect of DIBI on weight change at 4 and 24h after lung infection. Our results showed that the mice weight in PA14+PBS group reduced about 10% at the point of 24h, which is consistent with other reports [
14,
15], thus suggesting that the mice suffered from an acute inflammatory response. In addition, although there was no statistically significant difference in weight loss between DIBI treatment and PA14+PBS group, our study found that DIBI treated mice exhibited a tendency of less body weight decrease at both 4 and 24 hours (
Figure 1). After
P. aeruginosa infection in mice, it was reported that weight loss was greatest at day 3 [
15,
16,
17]. Rossi et al. reported in a model of
P. aeruginosa lung infection that the body weight of the mice was monitored continuously for 6 days, but until the 4th day β-sitosterol treatment exhibited significant faster body weight recovery [
14]. Therefore, it would be of great interest to study the potential effect of DIBI on weight loss after
P. aeruginosa lung infection at later time points.
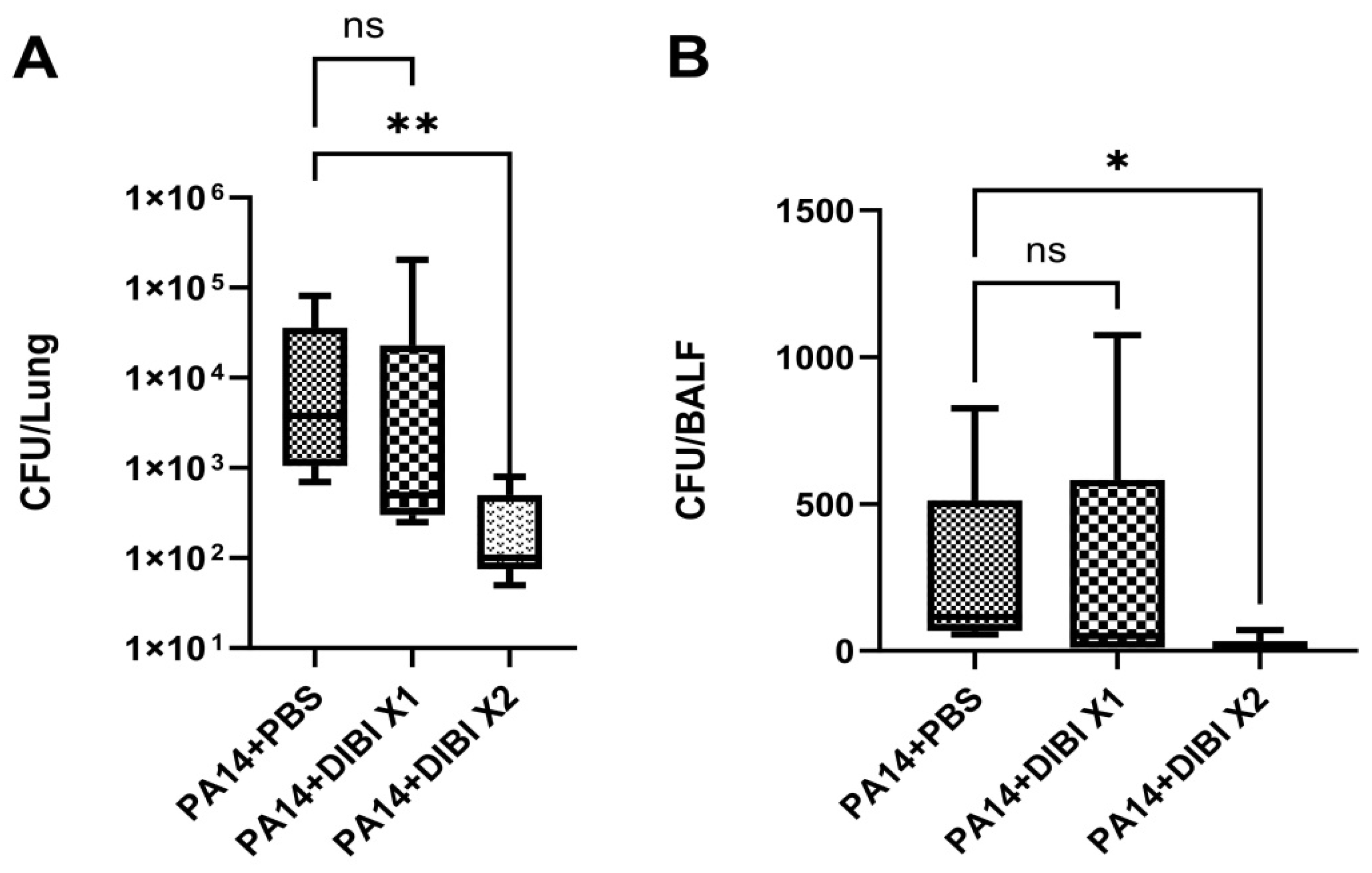
In our experiment, we showed that after 24 hours of lung infection induced by
P. aeruginosa, double dose i.p. DIBI treatment was efficient in reducing bacterial load in both lung homogenate and BALF. In agreement with our findings on DIBI-restricted
P. aeruginosa growth, it has previously been demonstrated that iron-withdrawal chelator DIBI has also been shown to disrupt the staphylococcal biofilm growth [
18]. In previous studies published by our group we found that in sepsis induced by colon ascendens stent peritonitis (CASP), DIBI treatment significantly decreased the bacterial count in blood and peritoneal lavage fluid [
19]. Consistently, it has been demonstrated that other iron chelators have the potential ability to combat
P. aeruginosa biofilms. For example, Sharareh et al. found that deferiprone (DFP) can be inhibitory to growth of
P. aeruginosa with high concentration [
20]. Similarly, gallium is also under consideration as an anti-pseudomonal agent, because it can inhibit
P. aeruginosa growth and biofilm formation by disrupting bacterial iron homeostasis [
21]. Taken together, our results indicate that with the anti-bacterial activity, DIBI could be a potential adjunct or alternative therapeutic approach for treating lung infections caused by
P. aeruginosa by effectively limiting bacterial growth
in vivo.
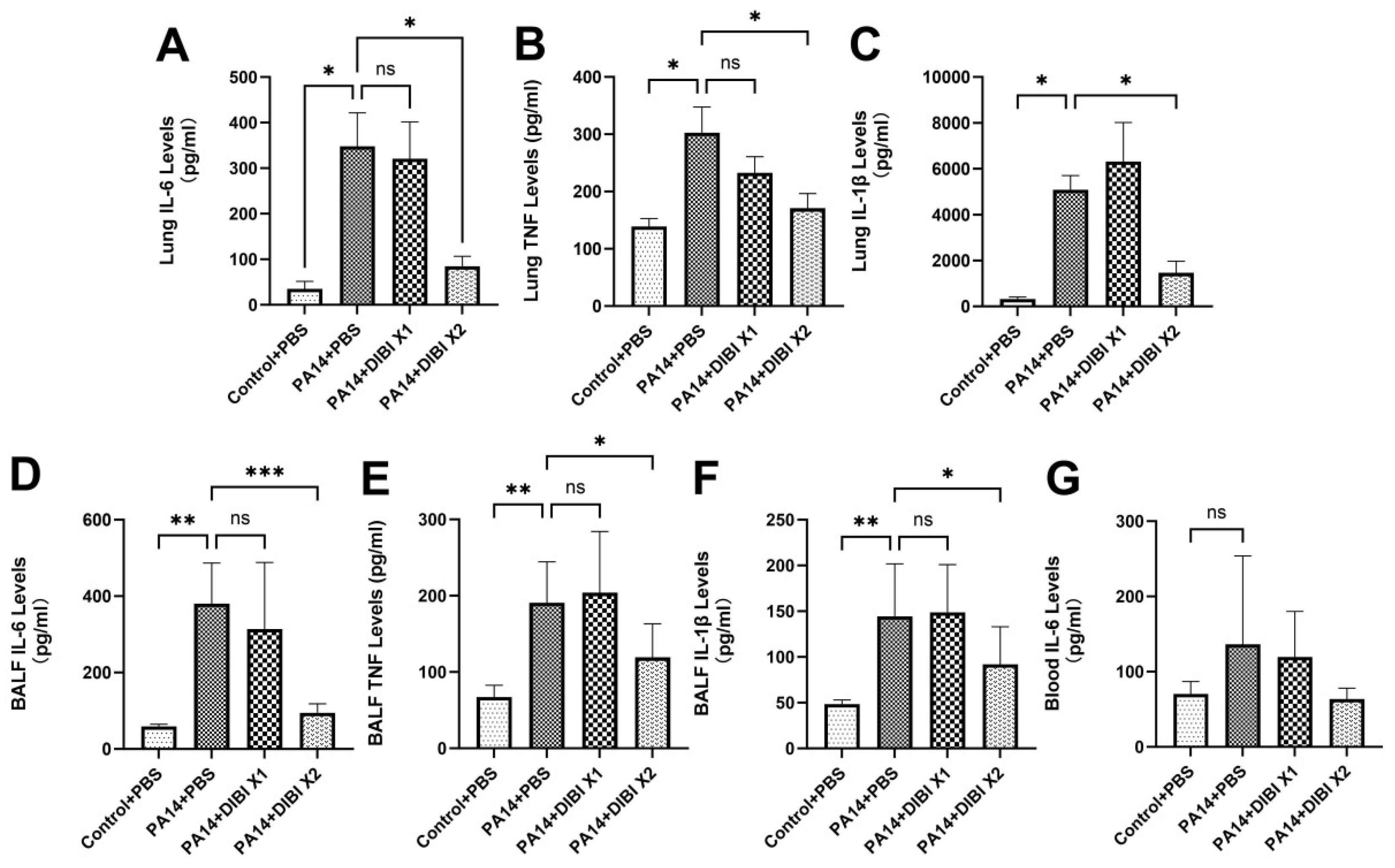
Because inflammation is a hallmark of lung infection, we also investigated the effect of DIBI administration on inflammatory mediators. The results demonstrated that treatment with two doses of DIBI significantly reduced cytokine levels including IL-6, TNF and IL-1β in both lung homogenates and BALF. Similar to our results, other studies also showed that iron chelators inhibit the production of inflammatory cytokines. For example, Cheon et al. demonstrated that IL-6 and TNF production were completely decreased by i.p. deferoxamine (DFO) injection in rats [
22]. In the model of lung ischemia reperfusion, Liu et al. showed that the levels of IL-6, TNF and IL-1β were dramatically inhibited after DFO administration [
23]. Interestingly, DFO has also been proven to alleviate viral replication and suppress consequent inflammatory cytokine storms, suggesting iron chelators can attenuate complications related to iron overload in diabetic patients with COVID-19 [
24,
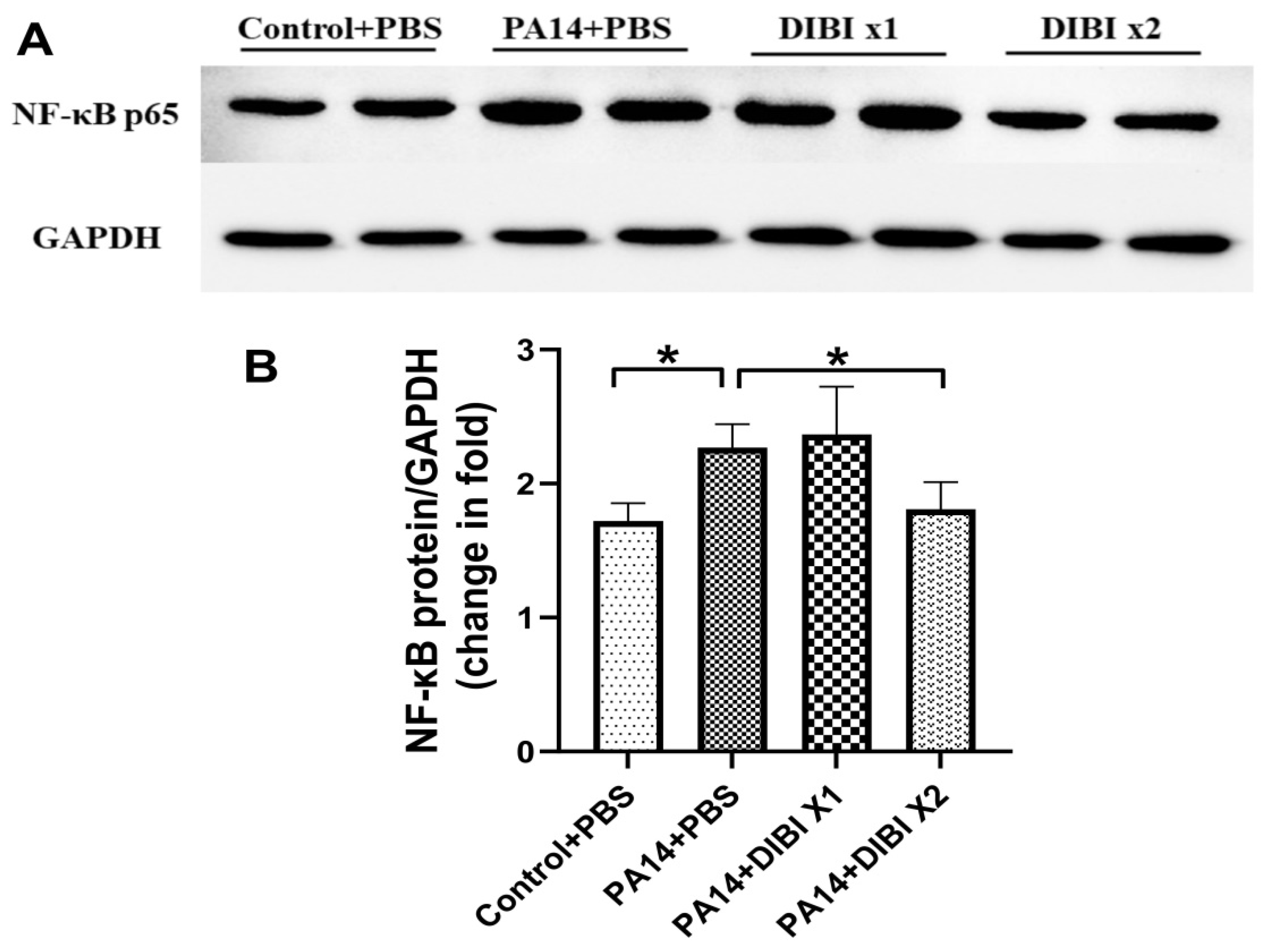
25]. In association with the anti-inflammatory activity of DIBI, our study also showed that after treatment with two doses of DIBI, the protein abundance of NF-kB in lung tissues was reduced significantly. Consistent with our research, it was reported in other studies that LPS-induced NF-kB expression could also be inhibited by iron chelators, such as DFP and DFO [
26,
27]. In our previous experiments
in vivo, it was confirmed that the levels of NF-κBp65 activation induced by LPS administration are reduced by DIBI treatment [
12]. Therefore, as with DFO and other chelators, DIBI exhibited anti-inflammatory effects so as to decrease the acute lung injury induced by
P. aeruginosa.
However, this study did not demonstrate a significant decrease in bacterial load and inflammatory mediator levels for treatment with one does of DIBI. It is likely that our protocol utilized a low dose of bacterial inoculum (5X10
5 CFU), as evidenced by our results showing that the highest clinical scores peaked at 7 (with 12 being the humane endpoint cutoff, for reference) at 4 hours after
P. aeruginosa administration. Therefore, the window for DIBI treatment is narrow to demonstrate a beneficial effect. Moreover, different from other reports [
14,
15,
28], the
P. aeruginosa inoculum was injected by way of the supraglottic region in our experiment, which would lead to more variability in the response to maximal inhalation of inoculum. Thirdly, the shorter half-life of DIBI has an effect on the duration of action of the drug, so without monitoring the blood concentration of DIBI, a single dose of DIBI may have limited efficacy.
For future studies, aerosolized or intravenous DIBI administration should be explored which could potentially be more effective in lung infection. In addition, as suggested by others [
29,
30], the effects of DIBI should be compared with standard antibiotics in lung infection induced by
P. aeruginosa. Allan et al. demonstrated that DIBI as an adjunct to ciprofloxacin and other antibiotics, could significantly improve antibiotic efficacy and reduce antibiotic resistance development [
10,
29,
31]. Our current study demonstrated great potential for using DIBI to reduce bacterial growth and host inflammation, so it is of great interest to evaluate the benefits of combination therapy with other antibiotics in the model of lung infection induced by
P. aeruginosa.
Author Contributions
Conceptualization, C.L., Z.Y.C. and B.H.; methodology, C.L., Z.Y.C. and J.Z.; formal analysis, C.L., Z.Y.C. and X.Y.Z.; investigation, X.Y.Z, R.N., L.B. and A.S.; writing-original draft preparation, X.Y.Z. and R.N.; writing—review and editing, R.N., C.L., Z.Y.C. and J.Z.; supervision, C.L., J.Z. and Z.Y.C.; project administration, J.Z.; All authors have read and agreed to the published version of the manuscript.