Submitted:
05 June 2024
Posted:
07 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
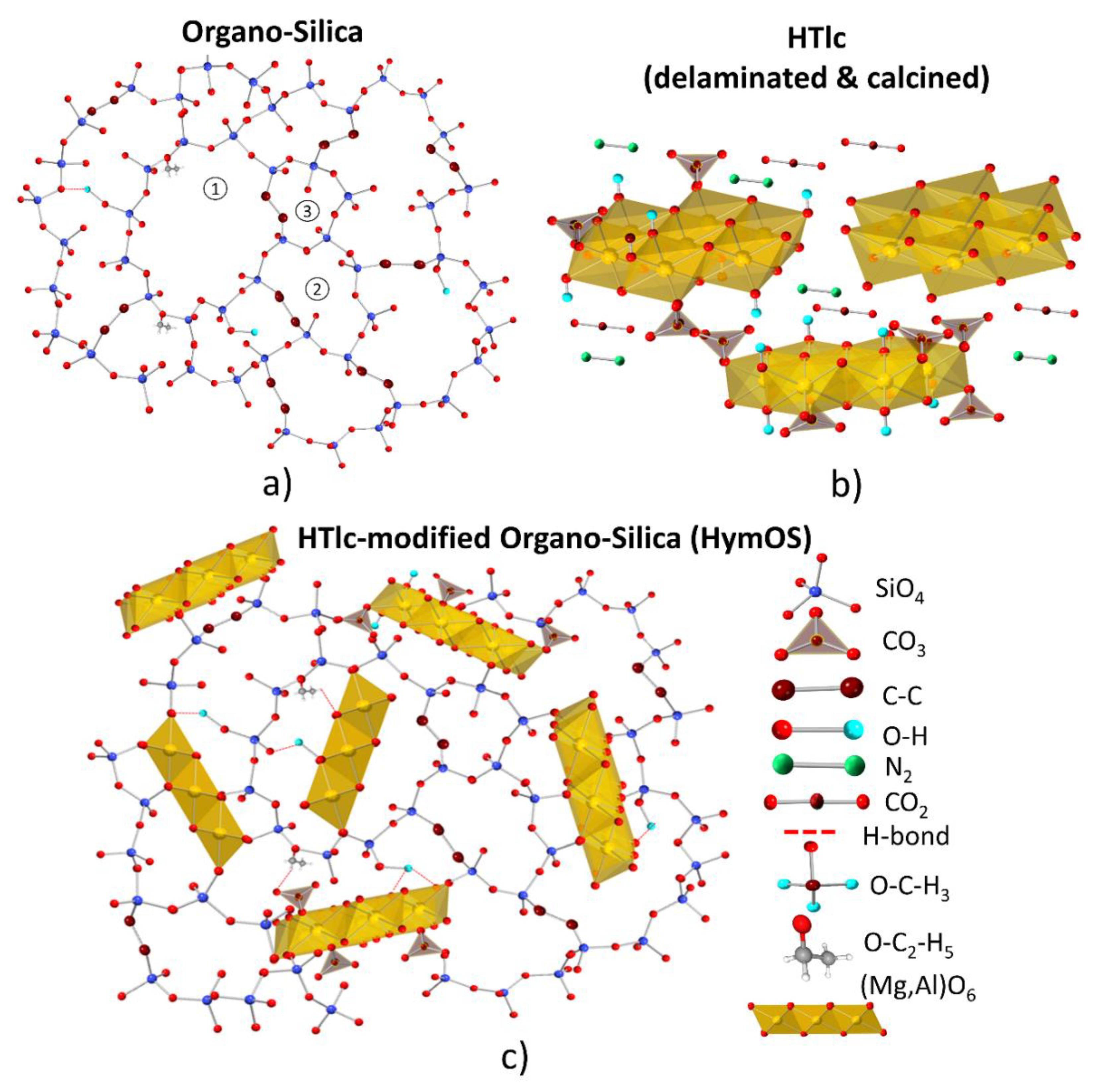
2. Materials and Methods
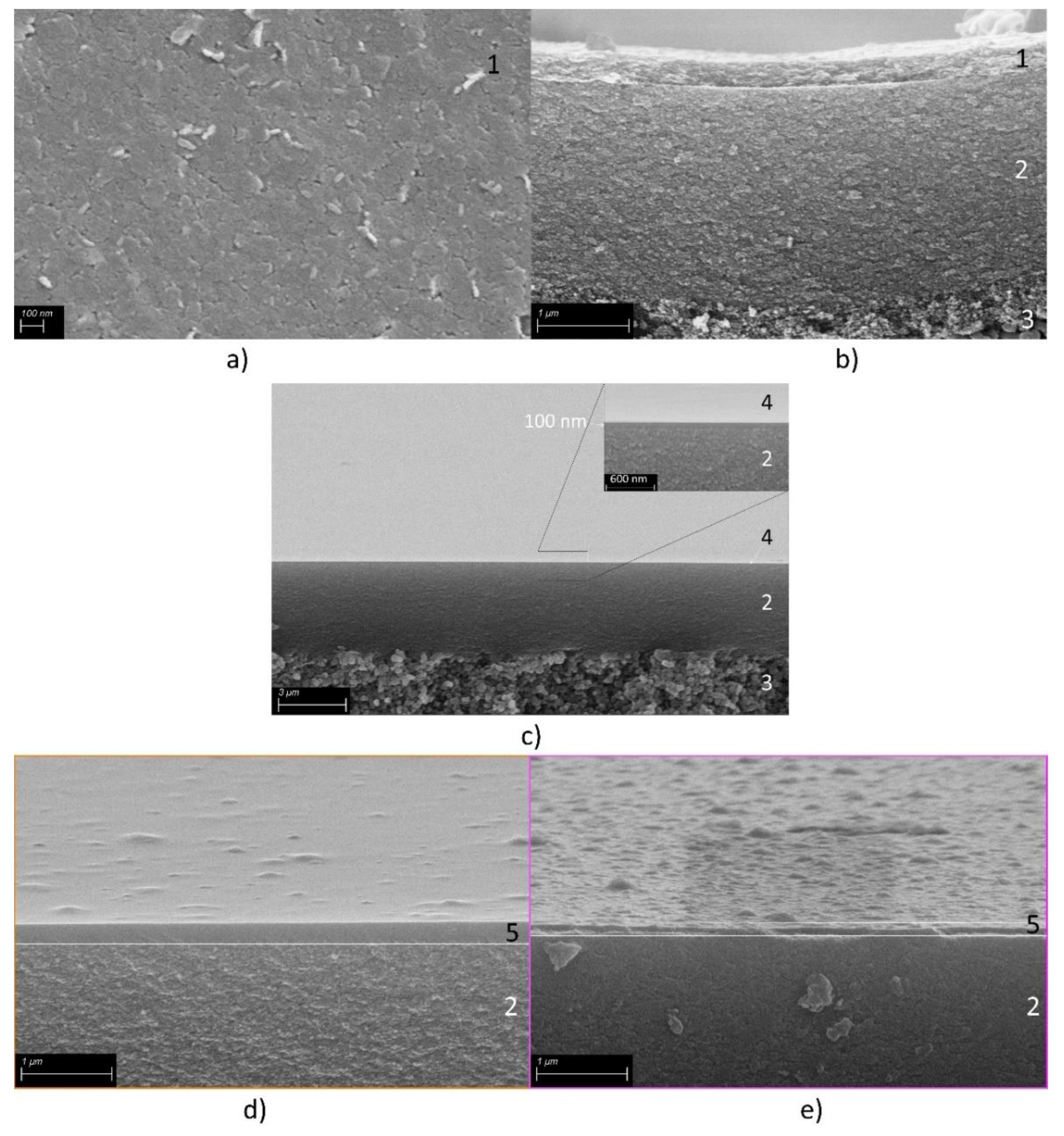
Membrane Fabrication
Structural Characterization
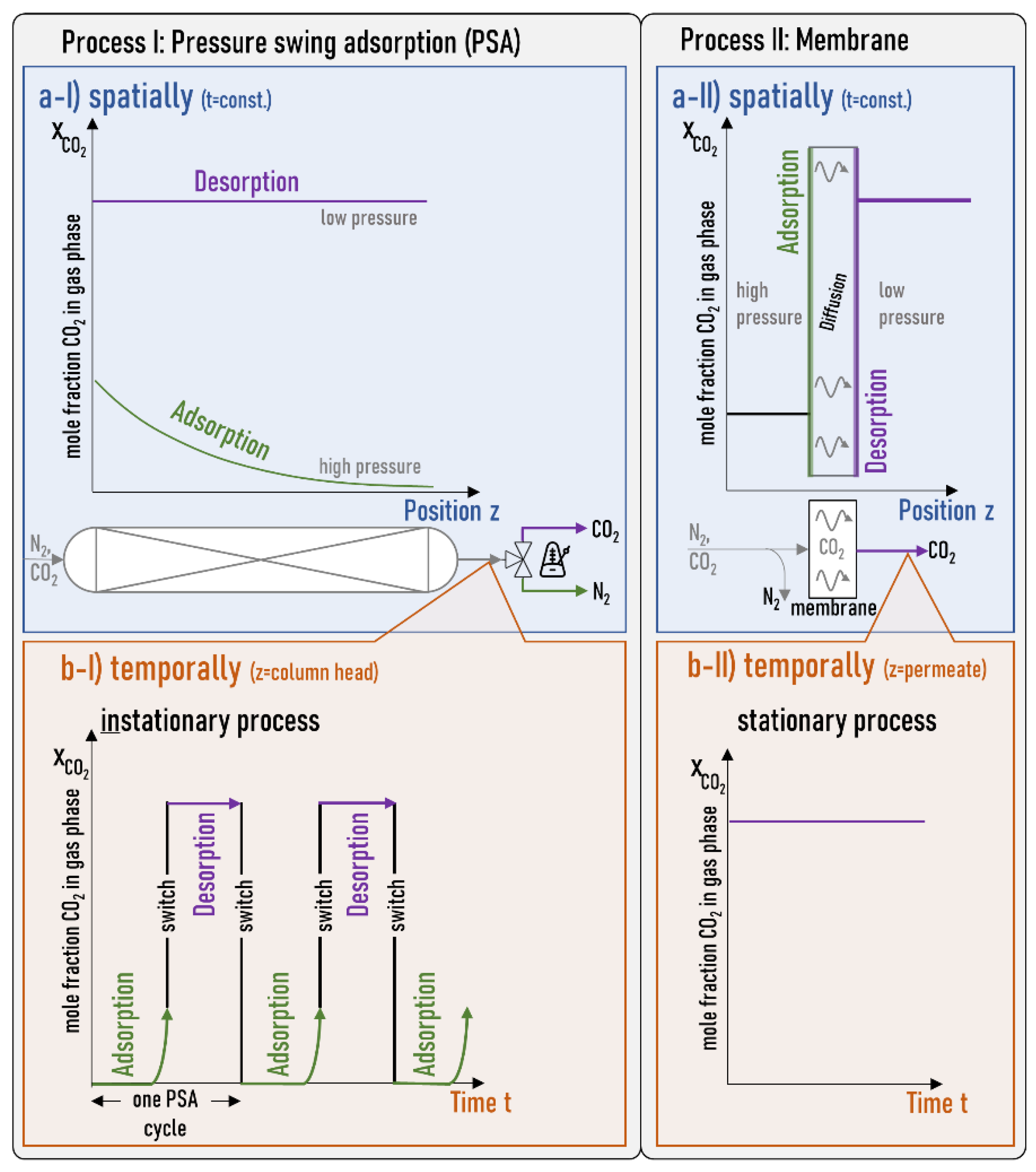
Gas Permeation and Separation Experiments
3. Results
3.1. HTlc Membrane
3.2. Pure Organo-Silica Membrane (Org-Sil)
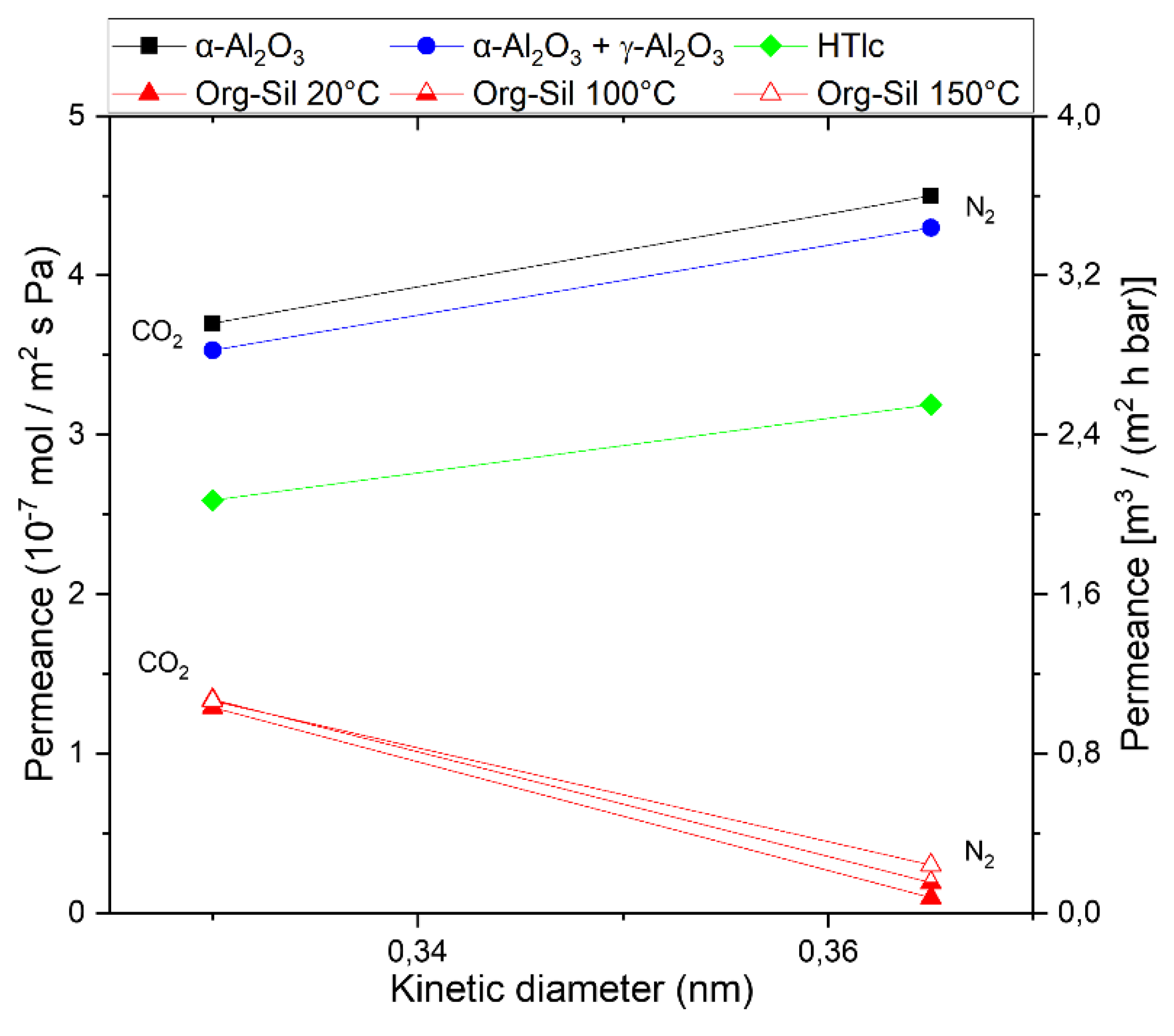
3.3. HTlc Modified Organo-Silica Membrane (HymOS)
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sircar, S. Pressure Swing Adsorption Technology. In Adsorption: Science and Technology; Rodrigues, A.E., LeVan, M.D., Tondeur, D., Eds.; Springer-Verlag: Dordrecht, 1989; pp 285–321.
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739. [CrossRef]
- Rezakazemi, M.; Sadrzadeh, M.; Matsuura, T. Thermally stable polymers for advanced high-performance gas separation membranes. Prog. Energy. Combust. Sci. 2018, 66, 1–41. [CrossRef]
- Baker, R.W. Membrane Transport Theory. In Membrane Technology and Applications; Baker, R.W., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2004; pp 15–87.
- Burggraaf, A.J. Transport and separation properties of membranes with gases and vapours. In Fundamentals of inorganic membrane science and technology; Burggraaf, A.J., Cot, L., Eds.; Elsevier: Amsterdam, 1996; pp 331–434.
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [CrossRef]
- Cerón, M.R.; Lai, L.S.; Amiri, A.; Monte, M.; Katta, S.; Kelly, J.C.; Worsley, M.A.; Merrill, M.D.; Kim, S.; Campbell, P.G. Surpassing the conventional limitations of CO2 separation membranes with hydroxide/ceramic dual-phase membranes. J. Membr. Sci. 2018, 567, 191–198. [CrossRef]
- Lu, B.; Lin, Y.S. Synthesis and characterization of thin ceramic-carbonate dual-phase membranes for carbon dioxide separation. J. Membr. Sci. 2013, 444, 402–411. [CrossRef]
- Anderson, M.; Lin, Y.S. Carbonate–ceramic dual-phase membrane for carbon dioxide separation. J. Membr. Sci. 2010, 357, 122–129. [CrossRef]
- Bernal, M.P.; Coronas, J.; Menéndez, M.; Santamaría, J. Separation of CO 2 /N 2 mixtures using MFI-type zeolite membranes. AIChE J. 2004, 50, 127–135. [CrossRef]
- Liu, Y.; Wang, N.; Diestel, L.; Steinbach, F.; Caro, J. MOF membrane synthesis in the confined space of a vertically aligned LDH network. Chem. Commun. (Camb ) 2014, 50, 4225–4227. [CrossRef]
- Richter, H.; Voss, H.; Kaltenborn, N.; Kämnitz, S.; Wollbrink, A.; Feldhoff, A.; Caro, J.; Roitsch, S.; Voigt, I. High-Flux Carbon Molecular Sieve Membranes for Gas Separation. Angew. Chem. Int. Ed Engl. 2017, 56, 7760–7763. [CrossRef]
- Vos, R.M. de; Verweij, H. High-selectivity, high-flux silica membranes for gas separation. Science 1998, 279, 1710–1711. [CrossRef]
- Elshof, J.E. ten. Hybrid Materials for Molecular Sieves. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, 2018; pp 1–27.
- Kanezashi, M.; Matsugasako, R.; Tawarayama, H.; Nagasawa, H.; Tsuru, T. Pore size tuning of sol-gel-derived triethoxysilane (TRIES) membranes for gas separation. J. Membr. Sci. 2017, 524, 64–72. [CrossRef]
- van Gestel, T.; Velterop, F.; Meulenberg, W.A. Zirconia-supported hybrid organosilica microporous membranes for CO2 separation and pervaporation. Sep. Purif. Technol. 2021, 259, 118114. [CrossRef]
- Ram Reddy, M.K.; Xu, Z.P.; Lu, G.Q.; Diniz da Costa, J.C. Layered Double Hydroxides for CO 2 Capture: Structure Evolution and Regeneration. Ind. Eng. Chem. Res. 2006, 45, 7504–7509. [CrossRef]
- León, M.; Díaz, E.; Bennici, S.; Vega, A.; Ordóñez, S.; Auroux, A. Adsorption of CO 2 on Hydrotalcite-Derived Mixed Oxides: Sorption Mechanisms and Consequences for Adsorption Irreversibility. Ind. Eng. Chem. Res. 2010, 49, 3663–3671. [CrossRef]
- Wang, Q.; Tay, H.H.; Ng, D.J.W.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. The effect of trivalent cations on the performance of Mg-M-CO(3) layered double hydroxides for high-temperature CO(2) capture. ChemSusChem 2010, 3, 965–973. [CrossRef]
- Bublinski, M. CO2-Abtrennung aus Synthesegasen mit Hydrotalciten unter Hochtemperatur-Hochdruckbedingungen. Dissertation; Universität Stuttgart, Stuttgart, 2016.
- Zhang, J.; Xu, Y.F.; Qian, G.; Xu, Z.P.; Chen, C.; Liu, Q. Reinvestigation of Dehydration and Dehydroxylation of Hydrotalcite-like Compounds through Combined TG-DTA-MS Analyses. J. Phys. Chem. C 2010, 114, 10768–10774. [CrossRef]
- Kim, T.W.; Sahimi, M.; Tsotsis, T.T. The Preparation and Characterization of Hydrotalcite Thin Films. Ind. Eng. Chem. Res. 2009, 48, 5794–5801. [CrossRef]
- Wook Kim, T.; Sahimi, M.; Tsotsis, T.T. The preparation and characterization of hydrotalcite micromembranes. Chem. Eng. Sci. 2009, 64, 1585–1590. [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO 2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [CrossRef]
- Fajrina, N.; Yusof, N.; Ismail, A.F.; Jaafar, J.; Aziz, F.; Salleh, W.; Nordin, N. MgAl-CO3 layered double hydroxide as potential filler in substrate layer of composite membrane for enhanced carbon dioxide separation. J. Environ. Chem. Eng 2021, 9, 106164. [CrossRef]
- Wiheeb, A.D.; Ahmad, M.A.; Murat, M.N.; Kim, J.; Othman, M. Identification of Molecular Transport Mechanisms in Micro-Porous Hydrotalcite–Silica Membrane. Transp Porous Med 2014, 104, 133–144. [CrossRef]
- Wiheeb, A.D.; Ahmad, M.A.; Murat, M.N.; Kim, J.; Othman, M. The declining affinity of microporous hydrotalcite-silica membrane for carbon dioxide. J Por Media 2014, 17, 159–167. [CrossRef]
- Wiheeb, A.D.; Ahmad, M.A.; Murat, M.N.; Kim, J.; Othman, M. The Effect of Hydrotalcite Content in Microporous Composite Membrane on Gas Permeability and Permselectivity. Sep. Sci. Technol. 2014, 49, 1309–1316. [CrossRef]
- Wiheeb, A.D.; Kim, J.; Othman, M. Highly Perm-Selective Micro-Porous Hydrotalcite-Silica Membrane for Improved Carbon Dioxide-Methane Separation. Sep. Sci. Technol. 2015, 50, 1701–1708. [CrossRef]
- Wiheeb, A.D.; Mohammed, T.E.; Abdel-Rahman, Z.A.; Othman, M. Flow dynamics of gases inside hydrotalcite-silica micropores. Microporous Microporous Mater. 2017, 246, 37–42. [CrossRef]
- Wiheeb, A.D.; Ahmad, M.A.; Murat, M.N.; Kim, J.; Othman, M. Predominant Gas Transport in Microporous Hydrotalcite–Silica Membrane. Transp Porous Med 2014, 102, 59–70. [CrossRef]
- Yoldas, B.E. Alumina Sol Preparation from Alkoxides. Amer. Ceram. Soc. Bull. 1975, 289–290.
- Gardner, E.; Huntoon, K.M.; Pinnavaia, T.J. Direct Synthesis of Alkoxide-Intercalated Derivatives of Hydrocalcite-like Layered Double Hydroxides: Precursors for the Formation of Colloidal Layered Double Hydroxide Suspensions and Transparent Thin Films. Adv. Mater. 2001, 13, 1263. [CrossRef]
- Gursky, J.A.; Blough, S.D.; Luna, C.; Gomez, C.; Luevano, A.N.; Gardner, E.A. Particle-particle interactions between layered double hydroxide nanoparticles. J. Am. Chem. Soc. 2006, 128, 8376–8377. [CrossRef]
- Castricum, H.L.; Sah, A.; Kreiter, R.; Blank, D.H.A.; Vente, J.F.; Elshof, J.E. ten. Hydrothermally stable molecular separation membranes from organically linked silica. J. Mater. Chem. 2008, 18, 2150. [CrossRef]
- Kanezashi, M.; Yada, K.; Yoshioka, T.; Tsuru, T. Organic–inorganic hybrid silica membranes with controlled silica network size: Preparation and gas permeation characteristics. J. Membr. Sci. 2010, 348, 310–318. [CrossRef]
- Iruretagoyena Ferrer, D. Supported Layered Double Hydroxides as CO2 Adsorbents for Sorption-enhanced H2 Production; Springer International Publishing: Cham, 2016.
- Radha, S.; Navrotsky, A. Energetics of CO 2 Adsorption on Mg–Al Layered Double Hydroxides and Related Mixed Metal Oxides. J. Phys. Chem. C 2014, 118, 29836–29844. [CrossRef]
- R.T. Yang. Rate Processes in Adsorbers. In Gas separation by adsorption processes, 1.th ed.; Yang, R.T., Ed.; Butterworths: London, 1987; pp 108–124.
- Alsyouri, H.M.; Lin, J.Y.S. Gas diffusion and microstructural properties of ordered mesoporous silica fibers. J. Phys. Chem. B 2005, 109, 13623–13629. [CrossRef]
- Lange, R. de; Keizer, K.; Burggraaf, A.J. Analysis and theory of gas transport in microporous sol-gel derived ceramic membranes. J. Membr. Sci. 1995, 104, 81–100. [CrossRef]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [CrossRef]
- Kim, T.W.; Sahimi, M.; Tsotsis, T.T. Preparation of Hydrotalcite Thin Films Using an Electrophoretic Technique. Ind. Eng. Chem. Res. 2008, 47, 9127–9132. [CrossRef]
- Lu, P.; Liu, Y.; Zhou, T.; Wang, Q.; Li, Y. Recent advances in layered double hydroxides (LDHs) as two-dimensional membrane materials for gas and liquid separations. J. Membr. Sci. 2018, 567, 89–103. [CrossRef]
- Xu, X.; Wang, J.; Zhou, A.; Dong, S.; Shi, K.; Li, B.; Han, J.; O'Hare, D. High-efficiency CO2 separation using hybrid LDH-polymer membranes. Nat. Commun. 2021, 12, 3069. [CrossRef]
- Monsalve-Bravo, G.; Bhatia, S. Modeling Permeation through Mixed-Matrix Membranes: A Review. Processes 2018, 6, 172. [CrossRef]
- Zimmerman, C.M.; Singh, A.; Koros, W.J. Tailoring mixed matrix composite membranes for gas separations. J. Membr. Sci. 1997, 137, 145–154. [CrossRef]
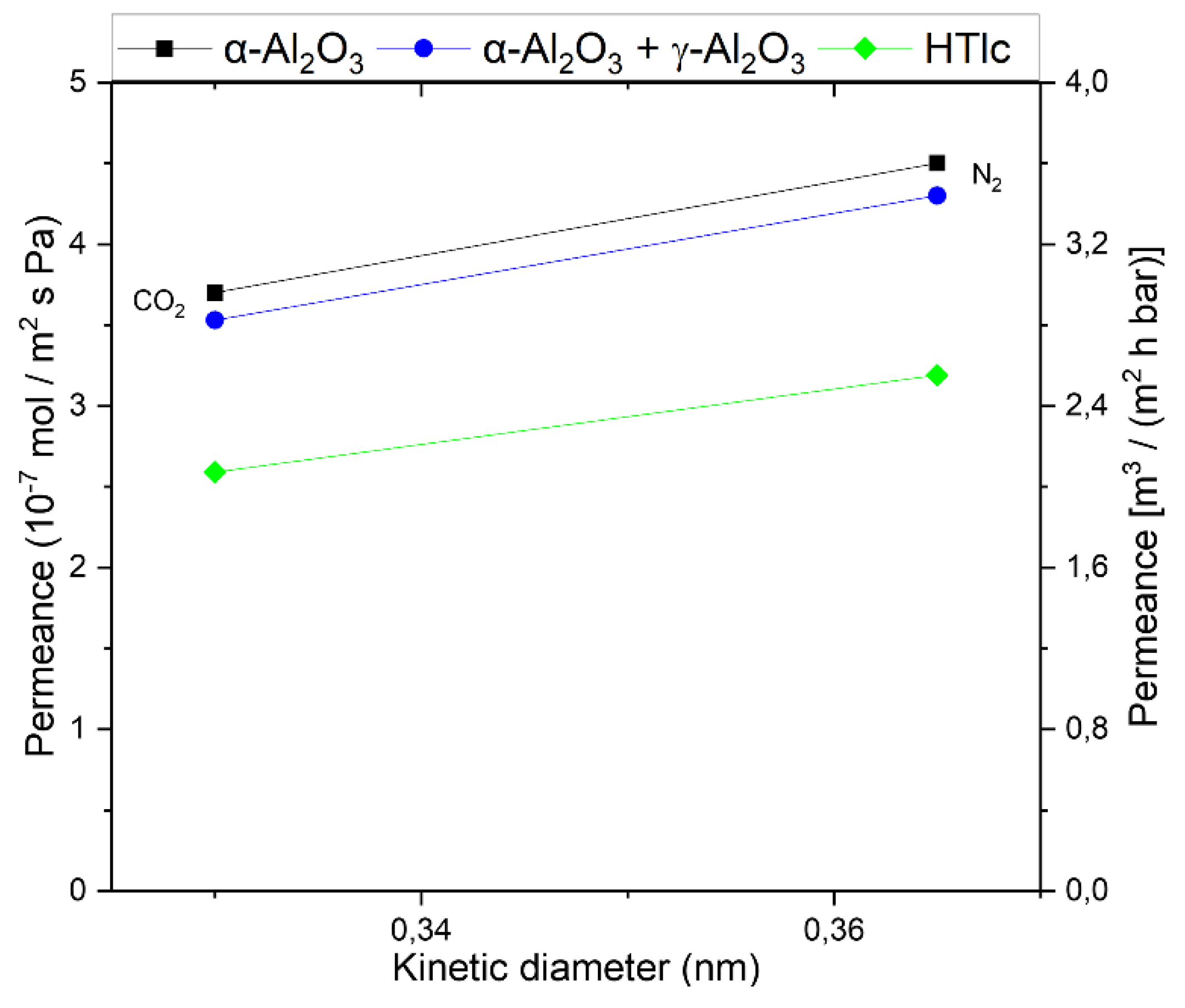
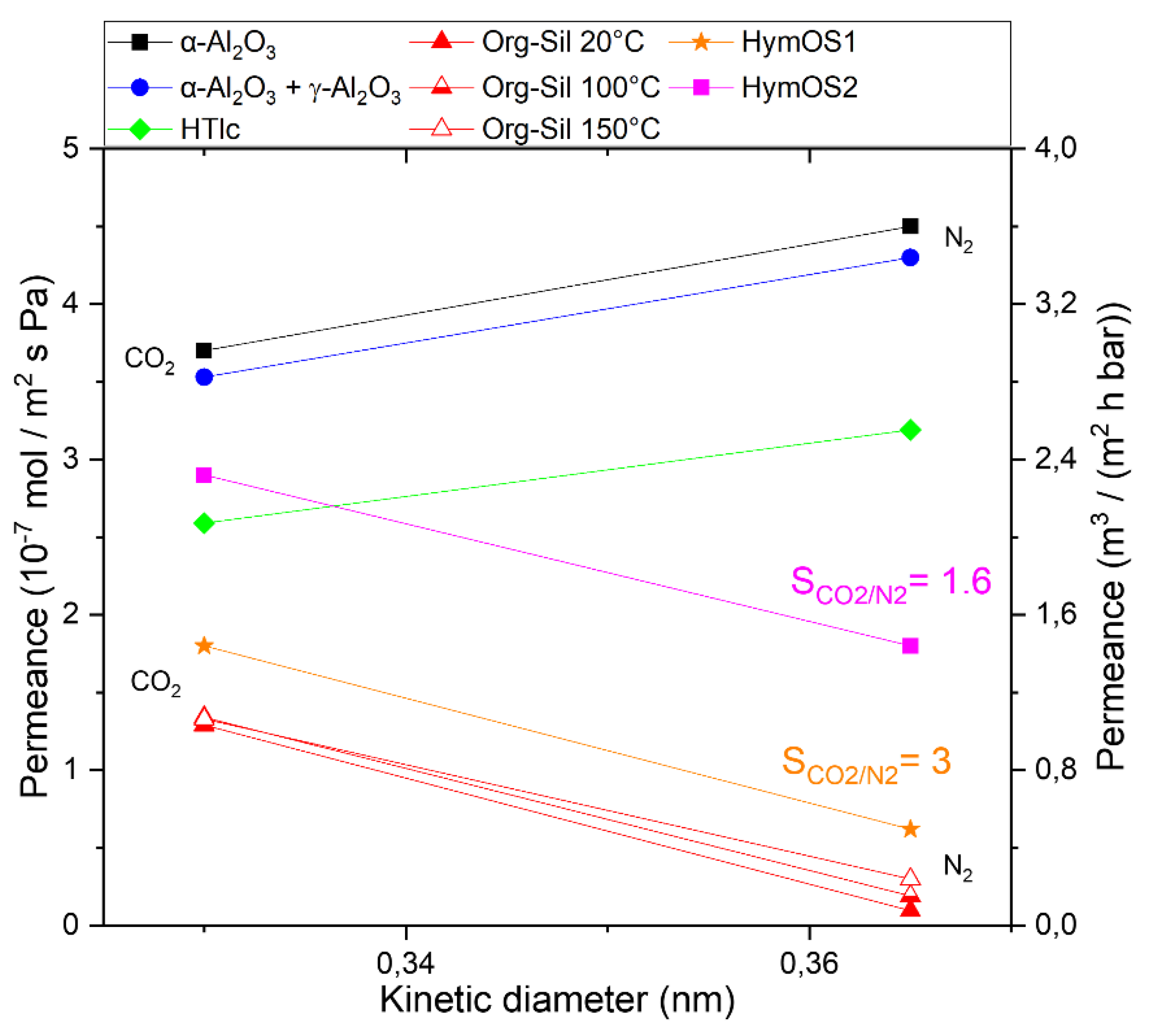
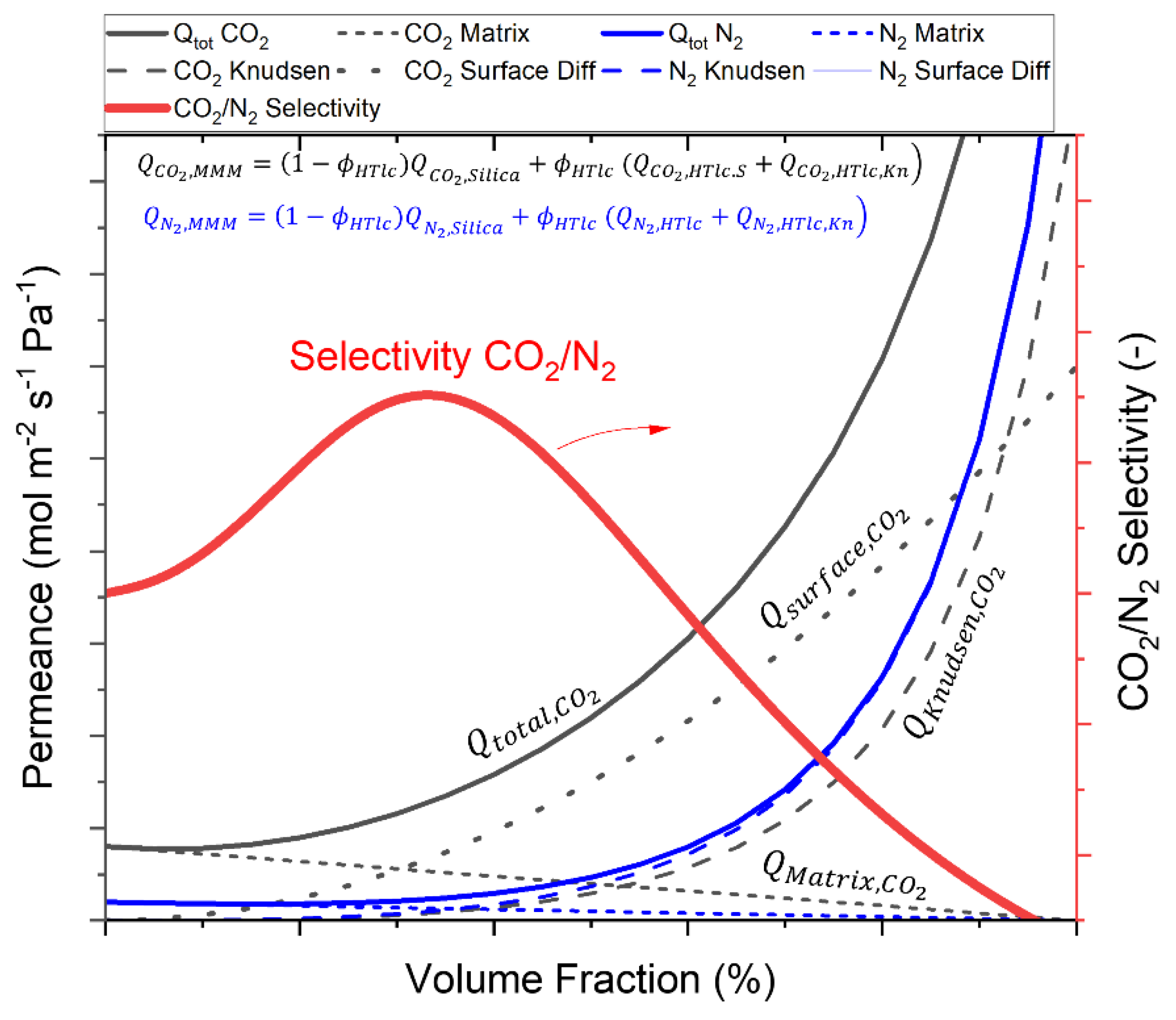
| Single Layer | Permeance (10-7 mol (m2 s Pa)-1) |
Permeability /10-14 mol (m s Pa)-1 |
Perm- Selectivity CO2/N2 |
||
|---|---|---|---|---|---|
| N2 | CO2 | N2 | CO2 | ||
| α-Al2O3 | 4.5 | 3.6 | 99000 | 79200 | 0.80 |
| γ-Al2O3 | 36 | 61.2 | 1440 | 2448 | 1.7 |
| HTlc | 14.7 | 12.3 | 29.52 | 24.6 | 0.83 |
| Organo- silica |
0.10 | 2.1 | 0.10 | 2.10 | 20.52 |
| HymOS1 | 0.73 | 3.83 | 0.73 | 3.83 | 5.2 |
| HymOS2 | 3.27 | 19.72 | 3.27 | 19.72 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





