Submitted:
04 June 2024
Posted:
06 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Dataset Preparation
Binding Constant Calculations
Docking Procedures
Molecular Descriptors Generation
Variable Selection and QSAR Modeling
Applicability Domain
Descriptor Significance Plot
3. Results and Discussion
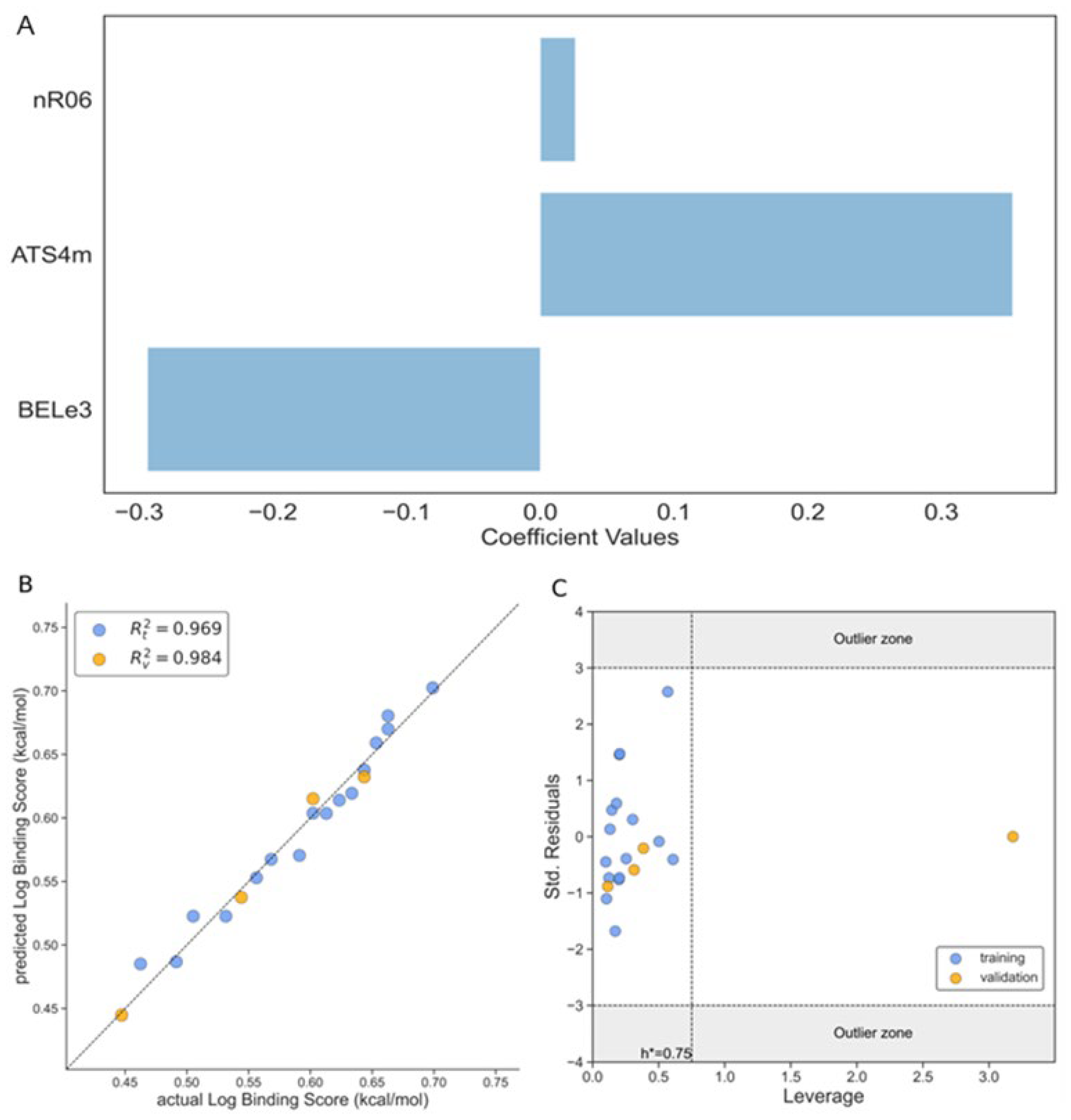
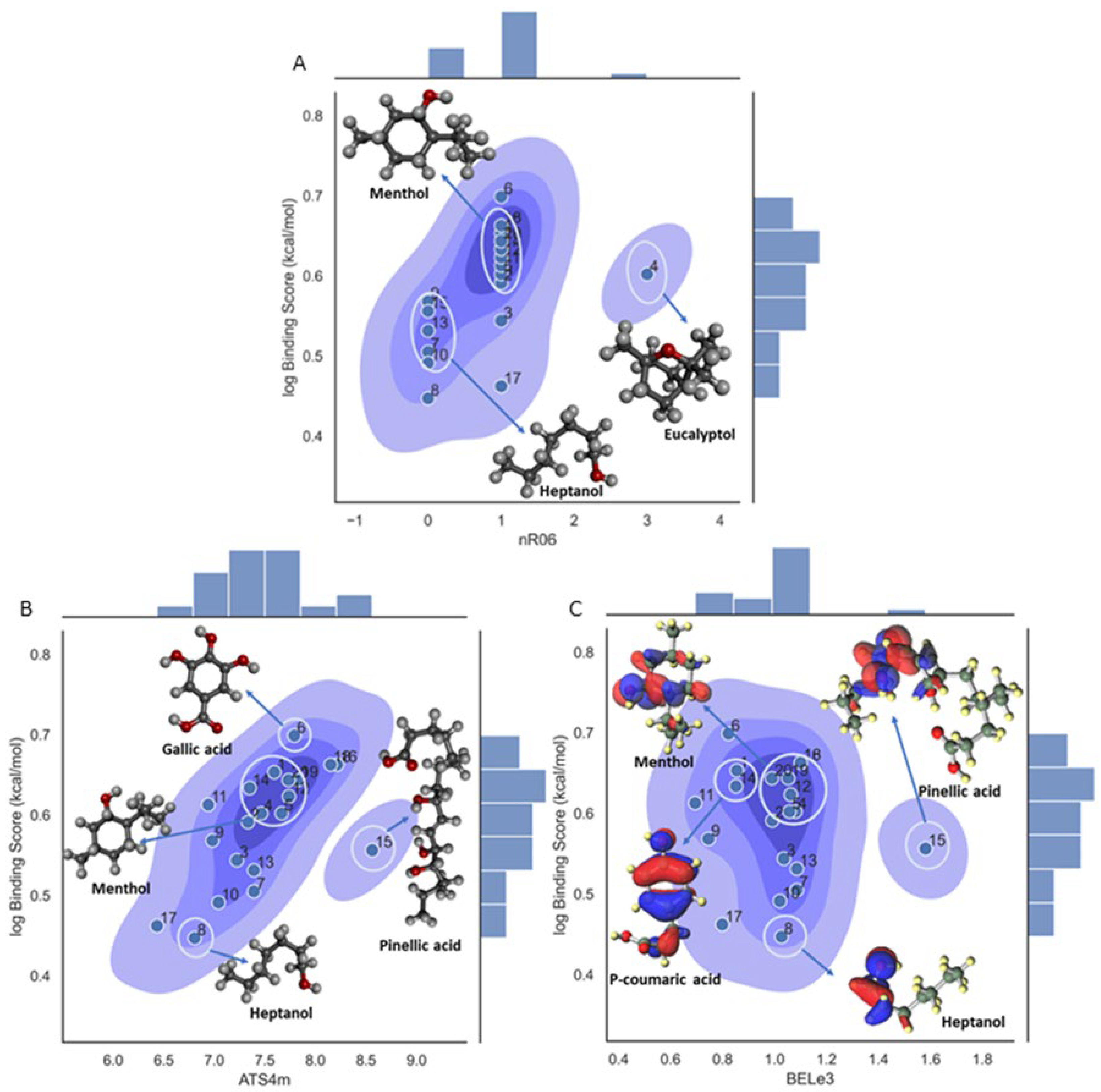
Binding Score/Affinity (Model 1)
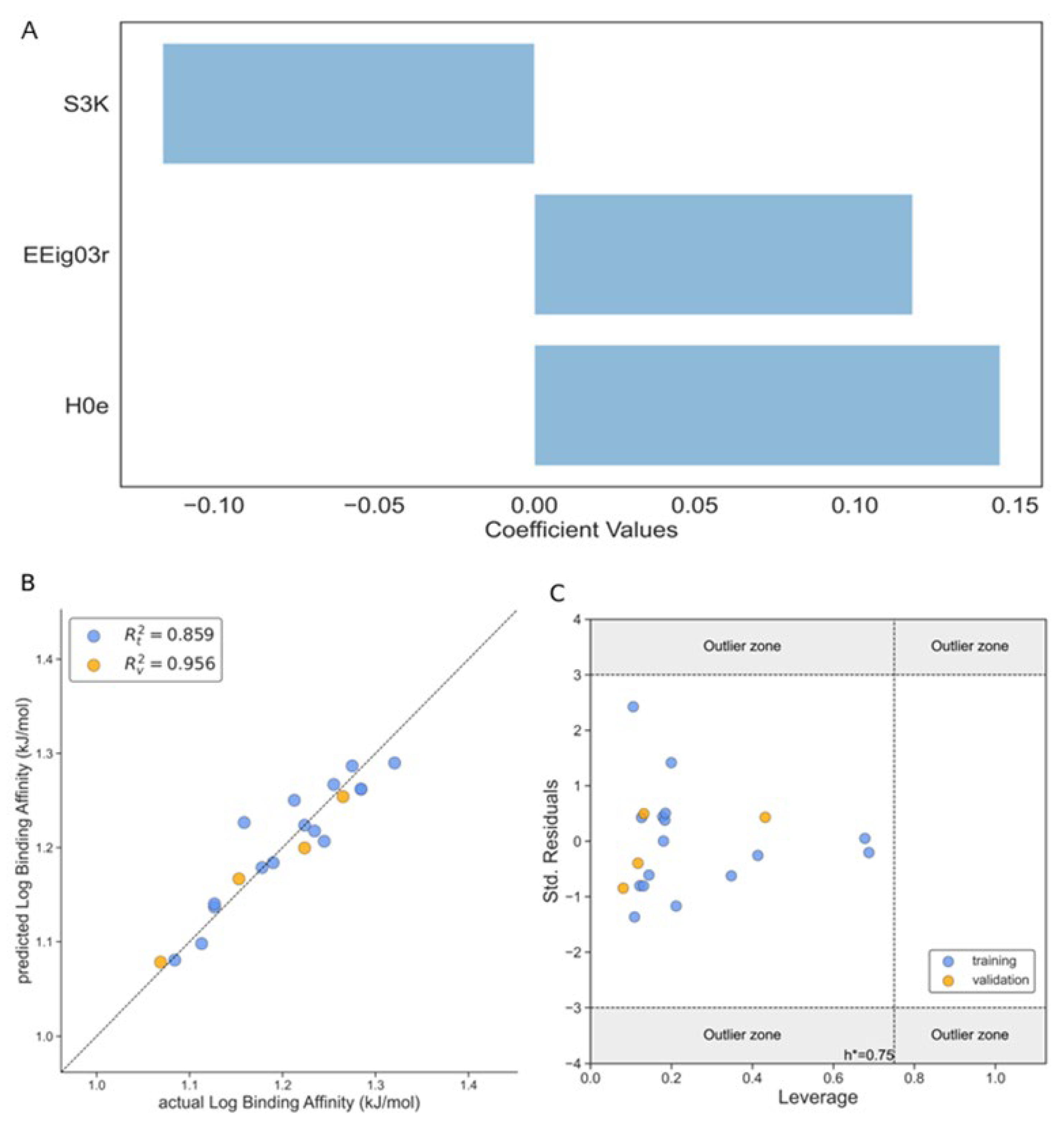
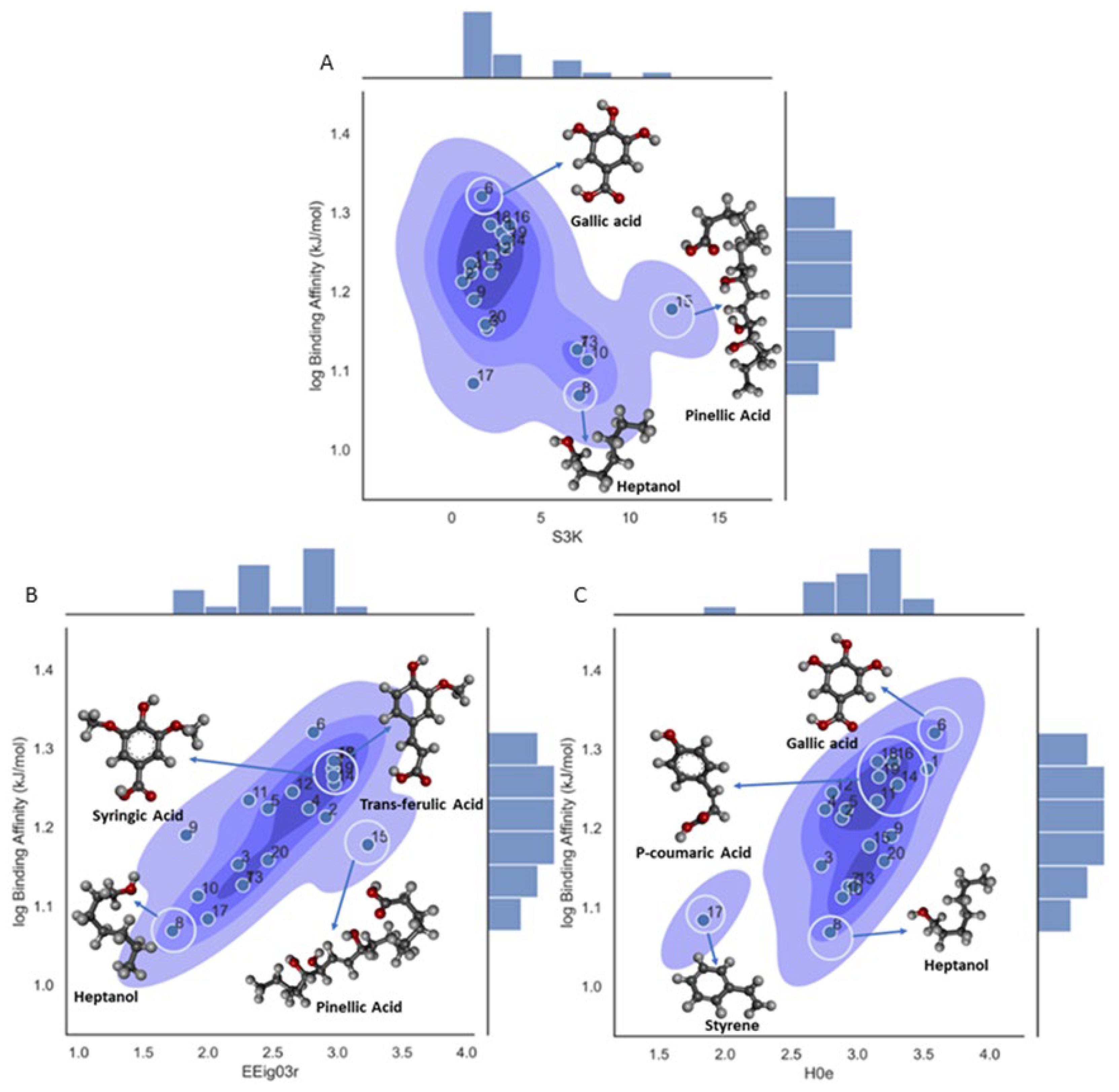
Binding Affinity (Model 2)
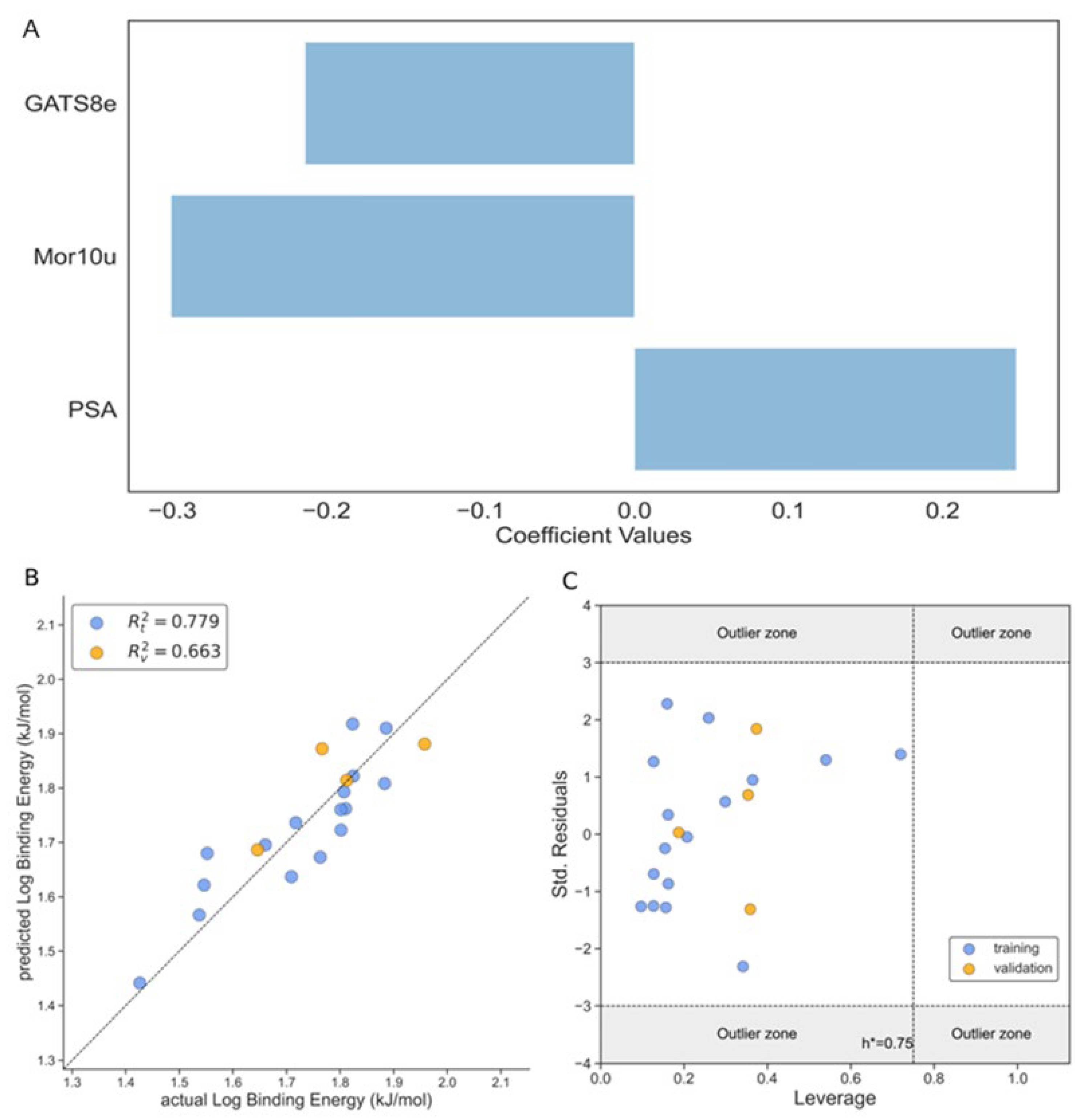
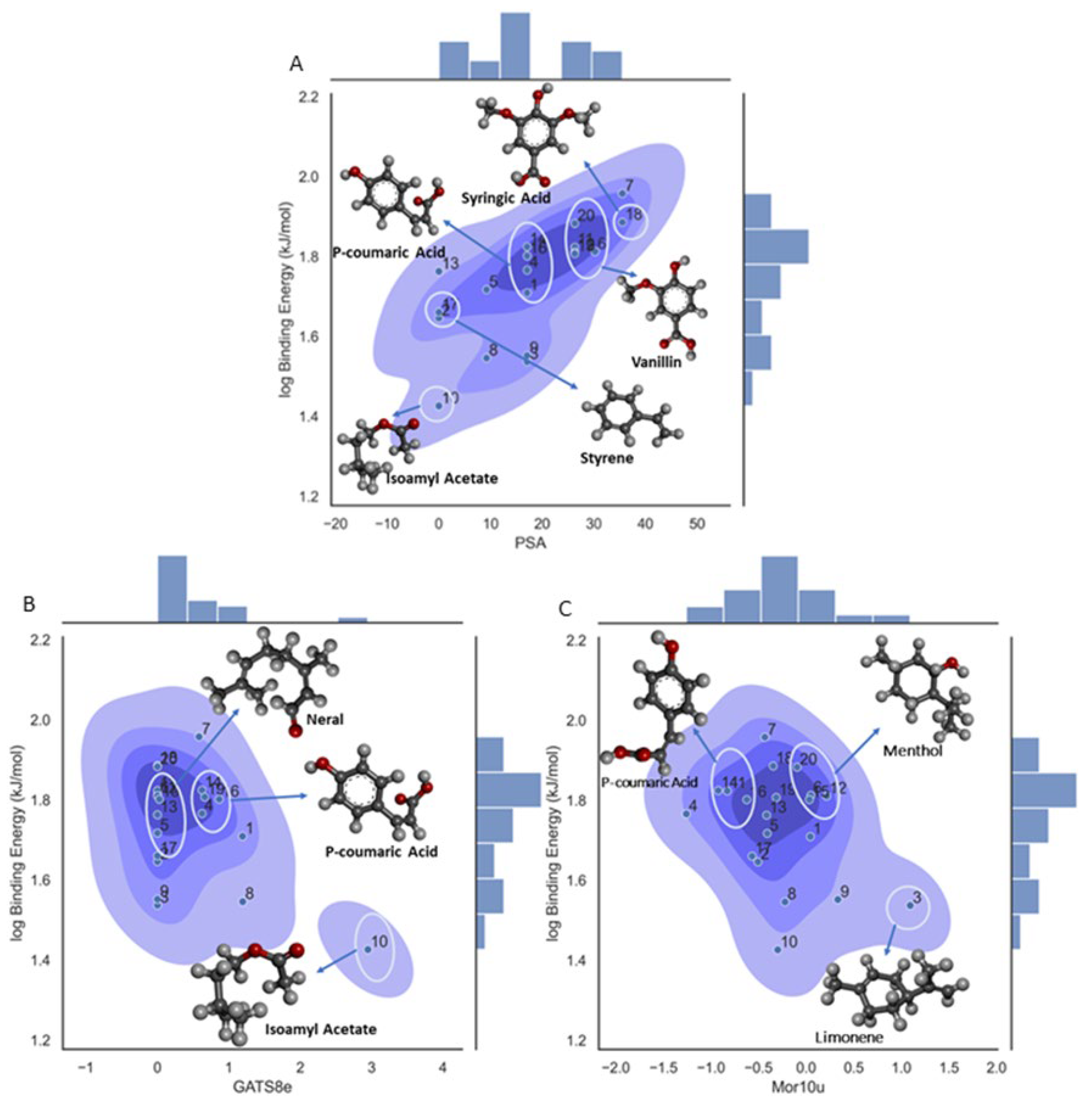
Binding Energy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, A. S.; Murtaza, B.; Hichami, A.; Khan, N. A. A cross-talk between fat and bitter taste modalities. Biochimie 2019, 159, 3-8. [CrossRef]
- Ardoin, R.; Smith, B.; Lea, J.; Boue, S.; Smolensky, D.; Santana, A. L.; Peterson, J. Consumer perceptions and antioxidant profiling of acidified cold-brewed sorghum bran beverages. Journal of Food Science 2023, 88 (6), 2301-2312, Article. Scopus. [CrossRef]
- Vuolo, M. M.; Lima, V. S.; Maróstica Junior, M. R. Chapter 2 - Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds, Campos, M. R. S. Ed.; Woodhead Publishing, 2019; pp 33-50.
- Taofiq, O.; González-Paramás, A. M.; Barreiro, M. F.; Ferreira, I. C. F. R. Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives, a Review. Molecules (Basel, Switzerland) 2017, 22 (2), 281. PubMed. [CrossRef]
- Heleno, S. A.; Martins, A.; Queiroz, M. J. R. P.; Ferreira, I. C. F. R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chemistry 2015, 173, 501-513. [CrossRef]
- Luna-Guevara, M. L.; Luna-Guevara, J. J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C. E. Chapter 3 - Phenolic Compounds: A Good Choice Against Chronic Degenerative Diseases. In Studies in Natural Products Chemistry, Atta ur, R. Ed.; Vol. 59; Elsevier, 2018; pp 79-108.
- Rosa, L. A.; Moreno-Escamilla, J. O.; Rodrigo-Gracia, J.; Haard, N. F. Postharvest Physiology and Biochemistry of Fruits and Vegetables, Phenolic Compounds, Chapter 12. UK: Woodhead Publishing 2019, 253-271.
- Combes, J.; Clavijo Rivera, E.; Clément, T.; Fojcik, C.; Athès, V.; Moussa, M.; Allais, F. Solvent selection strategy for an ISPR (In Situ/In stream product recovery) process: The case of microbial production of p-coumaric acid coupled with a liquid-liquid extraction. Separation and Purification Technology 2021, 259, 118170. [CrossRef]
- Furia, E.; Beneduci, A.; Malacaria, L.; Fazio, A.; La Torre, C.; Plastina, P. Modeling the Solubility of Phenolic Acids in Aqueous Media at 37 °C. In Molecules, 2021; Vol. 26.
- Rodrigues, J. F.; Soares, C.; Moreira, M. M.; Ramalhosa, M. J.; Duarte, N. F.; Delerue-Matos, C.; Grosso, C. Moringa oleifera Lam. Commercial Beverages: A Multifaceted Investigation of Consumer Perceptions, Sensory Analysis, and Bioactive Properties. Foods 2023, 12 (11), Article. Scopus. [CrossRef]
- Zhang, S.; Shan, X.; Niu, L.; Chen, L.; Wang, J.; Zhou, Q.; Yuan, H.; Li, J.; Wu, T. The Integration of Metabolomics, Electronic Tongue, and Chromatic Difference Reveals the Correlations between the Critical Compounds and Flavor Characteristics of Two Grades of High-Quality Dianhong Congou Black Tea. Metabolites 2023, 13 (7), Article. Scopus. [CrossRef]
- Issaoui, M.; Delgado, A. M.; Caruso, G.; Micali, M.; Barbera, M.; Atrous, H.; Ouslati, A.; Chammem, N. Phenols, Flavors, and the Mediterranean Diet. J AOAC Int 2020, 103 (4), 915-924. [CrossRef]
- Kim, J. S. Synthesis and Characterization of Phenolic Acid/Hydroxypropyl-β-Cyclodextrin Inclusion Complexes. Prev Nutr Food Sci 2020, 25 (4), 440-448. [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J. C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chemistry 2022, 384, 132467. [CrossRef]
- Gramage-Doria, R.; Armspach, D.; Matt, D. Metallated cavitands (calixarenes, resorcinarenes, cyclodextrins) with internal coordination sites. Coordination Chemistry Reviews 2013, 257 (3), 776-816. [CrossRef]
- Faisal, Z.; Fliszár-Nyúl, E.; Dellafiora, L.; Galaverna, G.; Dall’Asta, C.; Lemli, B.; Kunsági-Máté, S.; Szente, L.; Poór, M. Cyclodextrins Can Entrap Zearalenone-14-Glucoside: Interaction of the Masked Mycotoxin with Cyclodextrins and Cyclodextrin Bead Polymer. In Biomolecules, 2019; Vol. 9.
- Mathivet, T.; Méliet, C.; Castanet, Y.; Mortreux, A.; Caron, L.; Tilloy, S.; Monflier, E. Rhodium catalyzed hydroformylation of water insoluble olefins in the presence of chemically modified β-cyclodextrins: evidence for ligand-cyclodextrin interactions and effect of various parameters on the activity and the aldehydes selectivity. Journal of Molecular Catalysis A: Chemical 2001, 176 (1), 105-116. [CrossRef]
- Sandilya, A. A.; Natarajan, U.; Priya, M. H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5 (40), 25655-25667. [CrossRef]
- da Rocha Neto, A. C.; de Oliveira da Rocha, A. B.; Maraschin, M.; Di Piero, R. M.; Almenar, E. Factors affecting the entrapment efficiency of β-cyclodextrins and their effects on the formation of inclusion complexes containing essential oils. Food Hydrocolloids 2018, 77, 509-523. [CrossRef]
- Chodankar, D.; Vora, A.; Kanhed, A. β-cyclodextrin and its derivatives: application in wastewater treatment. Environmental Science and Pollution Research 2022, 29 (2), 1585-1604. [CrossRef]
- Tajbakhsh, M.; Naimi-Jamal, M. R. Copper-doped functionalized β-cyclodextrin as an efficient green nanocatalyst for synthesis of 1,2,3-triazoles in water. Scientific Reports 2022, 12 (1), 4948. [CrossRef]
- Hedges, A. Chapter 22 - Cyclodextrins: Properties and Applications. In Starch (Third Edition), BeMiller, J., Whistler, R. Eds.; Academic Press, 2009; pp 833-851.
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environmental Chemistry Letters 2018, 16 (4), 1361-1375. [CrossRef]
- Braga, S. S.; Barbosa, J. S.; Santos, N. E.; El-Saleh, F.; Paz, F. A. A. Cyclodextrins in Antiviral Therapeutics and Vaccines. In Pharmaceutics, 2021; Vol. 13.
- Jiayue, L.; Tian, B. Selective modifications at the different positions of cyclodextrins: a review of strategies. Turkish Journal of Chemistry 2020, 44, 261-278. [CrossRef]
- Stella, V. J.; He, Q. Cyclodextrins. Toxicologic Pathology 2008, 36 (1), 30-42. (acccessed 2023/05/20). [CrossRef]
- Toropov, A.; Toropova, A.; Rasulev, B.; Benfenati, E.; Gini, G.; Leszczynska, D.; Leszczynski, J. CORAL: Binary Classifications (Active/Inactive) for Liver-Related Adverse Effects of Drugs. Current Drug Safety 2012, 7, 257-261. [CrossRef]
- Karuth, A.; Alesadi, A.; Xia, W.; Rasulev, B. Predicting Glass Transition of Amorphous Polymers by Application of Cheminformatics and Molecular Dynamics Simulations. Polymer 2021, 218, 123495. [CrossRef]
- Chen, M.; Jabeen, F.; Rasulev, B.; Ossowski, M.; Boudjouk, P. A computational structure-property relationship study of glass transition temperatures for a diverse set of polymers. Journal of Polymer Science Part B: Polymer Physics 2018, 56. [CrossRef]
- Rasulev, B.; Jabeen, F.; Stafslien, S.; Chisholm, B.; Bahr, J.; Ossowski, M.; Boudjouk, P. Polymer Coating Materials and Their Fouling Release Activity: A Cheminformatics Approach to Predict Properties. ACS applied materials & interfaces 2016, 9. [CrossRef]
- Toropova, A.; Toropov, A.; Rasulev, B.; Benfenati, E.; Gini, G.; Leszczynska, D.; J, L. QSAR models for ACE-inhibitor activity of tri-peptides based on representation of the molecular structure by graph of atomic orbitals and SMILES. Structural Chemistry 2012, 23, 1873-1878. [CrossRef]
- Rasulev, B.; Kusic, H.; Leszczynska, D.; Leszczynski, J.; Koprivanac, N. QSAR modeling of acute toxicity on mammals caused by aromatic compounds: The case study using oral LD50 for rats. Journal of Environmental Monitoring 2010, 12, 1037-1044. [CrossRef]
- Gooch, A.; Sizochenko, N.; Rasulev, B.; Gorb, L.; Leszczynski, J. In vivo toxicity of nitroaromatics: A comprehensive quantitative structure–activity relationship study. Environmental Toxicology and Chemistry 2017, 36 (8), 2227-2233. https://doi.org/10.1002/etc.3761 (acccessed 2022/06/06). [CrossRef]
- Golmohammadi, M.; Aryanpour, M. Analysis and evaluation of machine learning applications in materials design and discovery. Materials Today Communications 2023, 35, Review. Scopus. [CrossRef]
- Ji, H.; Pu, D.; Yan, W.; Zhang, Q.; Zuo, M.; Zhang, Y. Recent advances and application of machine learning in food flavor prediction and regulation. Trends in Food Science and Technology 2023, 138, 738-751, Review. Scopus. [CrossRef]
- Kou, X.; Shi, P.; Gao, C.; Ma, P.; Xing, H.; Ke, Q.; Zhang, D. Data-Driven Elucidation of Flavor Chemistry. Journal of Agricultural and Food Chemistry 2023, 71 (18), 6789-6802, Review. Scopus. [CrossRef]
- Mirrahimi, F.; Salahinejad, M.; Ghasemi, J. B. QSPR approaches to elucidate the stability constants between β-cyclodextrin and some organic compounds: Docking based 3D conformer. Journal of Molecular Liquids 2016, 219, 1036-1043. [CrossRef]
- Rescifina, A.; Chiacchio, U.; Iannazzo, D.; Piperno, A.; Romeo, G. β-Cyclodextrin and Caffeine Complexes with Natural Polyphenols from Olive and Olive Oils: NMR, Thermodynamic, and Molecular Modeling Studies. Journal of Agricultural and Food Chemistry 2010, 58 (22), 11876-11882. [CrossRef]
- Simsek, T.; Rasulev, B.; Mayer, C.; Simsek, S. Preparation and Characterization of Inclusion Complexes of β-Cyclodextrin and Phenolics from Wheat Bran by Combination of Experimental and Computational Techniques. In Molecules, 2020; Vol. 25.
- MarvinView; www.chemaxon.com: 2016. (accessed.
- Hanwell, M. D.; Curtis, D. E.; Lonie, D. C.; Vandermeersch, T.; Zurek, E.; Hutchison, G. R. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. Journal of Cheminformatics 2012, 4 (1), 17. [CrossRef]
- HyperChem(TM) Professional 8.0; 2019. (accessed.
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem 2010, 31 (4), 671-690. [doi]. [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A. F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. Journal of Chemical Information and Modeling 2021, 61 (8), 3891-3898. [CrossRef]
- Górnas, P.; Neunert, G.; Baczyński, K.; Polewski, K. Beta-cyclodextrin complexes with chlorogenic and caffeic acids from coffee brew: Spectroscopic, thermodynamic and molecular modelling study. Food Chemistry 2009, 114 (1), 190-196. [CrossRef]
- Santos, C.; Buera, P.; Mazzobre, M. Phase solubility studies and stability of cholesterol/β-cyclodextrin inclusion complexes. Journal of the science of food and agriculture 2011, 91, 2551-2557. [CrossRef]
- Pinho, E.; Soares, G.; Henriques, M. Cyclodextrin modulation of gallic acid in vitro antibacterial activity. Journal of Inclusion Phenomena and Macrocyclic Chemistry 2015, 81 (1), 205-214. [CrossRef]
- Karathanos, V.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chemistry 2007, 101, 652-658. [CrossRef]
- Narayanasamy, R.; Thammanna, M.; J. Photophysics of Caffeic, Ferulic and Sinapic Acids with α- and β-Cyclodextrins: Spectral and Molecular Modeling Studies. International Letters of Chemistry, Physics and Astronomy 2017, 72, 37-51. [CrossRef]
- Liu, B.; Zeng, J.; Chen, C.; Liu, Y.; Ma, H.; Mo, H.; Liang, G. Interaction of cinnamic acid derivatives with β-cyclodextrin in water: Experimental and molecular modeling studies. Food Chemistry 2016, 194, 1156-1163. [CrossRef]
- Lukasiewicz, M.; Jakubowski, P. Determination of Complexation Parameters for β-Cyclodextrin and Randomly Methylated β-Cyclodextrin Inclusion Complexes of p-Cumaric Acid Using Reversed-Phase Liquid Chromatography; 2014. [CrossRef]
- Stewart, J. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. Journal of molecular modeling 2012, 19. [CrossRef]
- Stewart, J. J. P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. Journal of Molecular Modeling 2007, 13 (12), 1173-1213. [CrossRef]
- Hanson, R. M. Jmol SMILES and Jmol SMARTS: specifications and applications. Journal of Cheminformatics 2016, 8 (1), 50. [CrossRef]
- The PyMOL Molecular Graphics System; 2010. (accessed.
- Toddeschini; R.; Consonni, V.; Mauri, A.; Pavan, M. Dragon Software for the Calculation of Molecular Descriptors, Version 6 for Windows; Talete SRL: Milan, Italy. 2014.
- Cassani, S.; Kovarich, S.; Papa, E.; Roy, P. P.; van der Wal, L.; Gramatica, P. Daphnia and fish toxicity of (benzo)triazoles: Validated QSAR models, and interspecies quantitative activity-activity modelling. Journal of Hazardous Materials 2013, 258-259, 50-60, Article. Scopus. [CrossRef]
- Gramatica, P.; Chirico, N.; Papa, E.; Cassani, S.; Kovarich, S. QSARINS: A new software for the development, analysis, and validation of QSAR MLR models. Journal of Computational Chemistry 2013, 34, 2121 - 2132.
- Gramatica, P.; Cassani, S.; Chirico, N. QSARINS-chem: Insubria datasets and new QSAR/QSPR models for environmental pollutants in QSARINS. Journal of Computational Chemistry 2014, 35, 1036 - 1044.
- Katoch, S.; Chauhan, S. S.; Kumar, V. A review on genetic algorithm: past, present, and future. Multimedia tools and applications 2021, 80 (5), 8091-8126. PubMed. [CrossRef]
- Najafi, A.; Ardakani, S. S.; Marjani, M. Quantitative Structure-Activity Relationship Analysis of the Anticonvulsant Activity of Some Benzylacetamides Based on Genetic Algorithm-Based Multiple Linear Regression. Tropical Journal of Pharmaceutical Research 2011, 10, 483-490.
- MathWorks, I. MATLAB : the language of technical computing : computation, visualization, programming : installation guide for UNIX version 5; Natwick : Math Works Inc., 1996., 1996.
- Hunter, J. D. Matplotlib: A 2D graphics environment. Computing in Science and Engineering 2007, 9 (3), 99-104, Article. Scopus. [CrossRef]
- Tropsha, A. Best practices for QSAR model development, validation, and exploitation. Molecular Informatics 2010, 29 (6-7), 476-488, Review. Scopus. [CrossRef]
- Dieguez-Santana, K.; Pham-The, H.; Villegas-Aguilar, P. J.; Le-Thi-Thu, H.; Castillo-Garit, J. A.; Casañola-Martin, G. M. Prediction of acute toxicity of phenol derivatives using multiple linear regression approach for Tetrahymena pyriformis contaminant identification in a median-size database. Chemosphere 2016, 165, 434-441. [CrossRef]
- dos Passos Menezes, P.; Dória, G. A. A.; de Souza Araújo, A. A.; Sousa, B. M. H.; Quintans-Júnior, L. J.; Lima, R. N.; Alves, P. B.; Carvalho, F. M. S.; Bezerra, D. P.; Mendonça-Júnior, F. J. B.; et al. Docking and physico-chemical properties of α- and β-cyclodextrin complex containing isopulegol: a comparative study. Journal of Inclusion Phenomena and Macrocyclic Chemistry 2016, 85 (3), 341-354. [CrossRef]
- Talhout, R.; Villa, A.; Mark, A. E.; Engberts, J. B. F. N. Understanding Binding Affinity: A Combined Isothermal Titration Calorimetry/Molecular Dynamics Study of the Binding of a Series of Hydrophobically Modified Benzamidinium Chloride Inhibitors to Trypsin. Journal of the American Chemical Society 2003, 125 (35), 10570-10579. [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem 2000, 43 (20), 3714-3717. [CrossRef]
| ID | β -CD-Ligand Complex |
HOMO (eV) |
LUMO (eV) |
Gap (eV) |
Binding Score (kJ/mol) |
Binding Affinity (kJ/mol) |
|---|---|---|---|---|---|---|
| 1 | Caffeic acid-in | -9.1890 | -1.0514 | -8.1376 | -4.5 | -51.1831 |
| 2 | Camphor-in | -10.1147 | 0.0704 | -10.1851 | -3.9 | -34.4419 |
| 3 | D-Limonene-in | -9.3119 | 0.0092 | -9.3211 | -3.5 | -44.2580 |
| 4 | Eucalyptol-in | -9.9785 | 0.1605 | -10.139 | -4.0 | -52.1555 |
| 5 | Eugenol-up | -8.9658 | -0.1518 | -8.8140 | -4.0 | -35.1691 |
| 6 | Gallic acid-up | -9.5087 | -1.2760 | -8.2327 | -5.0 | -35.6459 |
| 7 | Geranial-up | -9.5651 | -0.2514 | -9.3137 | -3.2 | -58.3673 |
| 8 | Heptanol-up | -10.2024 | -0.1426 | -10.0598 | -2.8 | -26.6948 |
| 9 | Hydroxy Methyl Furfural-up | -9.2240 | -0.4026 | -8.8214 | -3.7 | -64.9081 |
| 10 | Isoamyl acetate-up | -10.1978 | -0.3113 | -9.8865 | -3.1 | -66.5904 |
| 11 | Maltol-up | -9.8792 | -1.3000 | -8.5792 | -4.1 | -64.6218 |
| 12 | Menthol-in | -10.1733 | -0.0167 | -10.1566 | -4.2 | -57.9313 |
| 13 | Neral-up | -9.7932 | -0.4443 | -9.3489 | -3.4 | -66.7781 |
| 14 | P-Coumaric acid-in | -9.3920 | -0.3048 | -9.0872 | -4.3 | -63.3347 |
| 15 | Pinellic acid-in | -9.8072 | 0.0819 | -9.8891 | -3.6 | -63.3198 |
| 16 | Sinapic acid-up | -9.1111 | -1.1979 | -7.9132 | -4.6 | -90.6849 |
| 17 | Styrene-up | -9.4684 | -0.4950 | -8.9734 | -2.9 | -45.7564 |
| 18 | Syringic acid-up | -9.2231 | -0.9025 | -8.3206 | -4.6 | -76.8800 |
| 19 | Trans Ferulic acid-up | -9.0647 | -1.4466 | -7.6181 | -4.4 | -64.1563 |
| 20 | Vanillic acid-in | -9.1477 | -0.9277 | -8.2200 | -4.4 | -76.3647 |
| Parameters | Log BSA | Log BA | Log BE |
|---|---|---|---|
| Model # Number of variables |
1 (Eq.3) 3 |
2(Eq.4) 3 |
3 (Eq.5) 3 |
| R [2] (training set) | 0.969 | 0.859 | 0.779 |
| RMSE (training set) | 0.0116 | 0.0256 | 0.0631 |
| MAE (training set) | 0.0095 | 0.0192 | 0.0527 |
| CCC (training set) | 0.985 | 0.924 | 0.876 |
| F | 126.902 | 24.349 | 14.117 |
| R [2] (cross-validation) | 0.925 | 0.805 | 0.634 |
| RMSE (cross-validation) | 0.0182 | 0.0302 | 0.0812 |
| MAE (cross-validation) | 0.0135 | 0.0236 | 0.0698 |
| CCC (cross-validation) | 0.961 | 0.897 | 0.790 |
| R [2] (external test) | 0.984 | 0.956 | 0.663 |
| RMSE (external test) | 0.0093 | 0.0156 | 0.0685 |
| MAE (external test) | 0.0082 | 0.0146 | 0.0563 |
| Descriptor | Description | Class |
|---|---|---|
| Model 1 – Binding Score Affinity | ||
| nR06 | Number of 6-membered rings | Ring Descriptors |
| ATS4m | Broto-Moreau autocorrelation of lag 4 (log function) weighted by mass | 2D Autocorrelations |
| BEle3 | Lowest eigenvalue n. 3 of Burden matrix / weighted by atomic Sanderson electronegativities | BCUT Descriptors |
| Model 2 – Binding Affinity | ||
| S3K | 3-path Kier alpha-modified shape index | Topological Indices |
| EEig03r | Eigenvalues | Edge adjacency indices |
| H0e | H autocorrelation of lag 0 / weighted by Sanderson electronegativity | GETAWAY Descriptors |
| Model 3 – Binding Energy | ||
| Descriptor | Description | Class |
| GATS8e | Geary autocorrelation of lag 8 weighted by mass | 2D Autocorrelations |
| Mor10u | Signal 10 / unweighted | 3D-MoRSE Descriptors |
| TPSA | Topological polar surface area using N,O polar contributions | Molecular properties |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





