Submitted:
06 June 2024
Posted:
10 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Methodology and Instrumentation
2.3. Digestion of CFA, CRM and Soil Prior to ICP-OES Analysis
3. Results and Discussion
3.1. Physical and Chemical Properties of Soil and CFA Samples
3.2. Trace Metal and Major Element Analysis
3.3. Chemical Speciation Modelling
4. Conclusions
Acknowledgements
References
- Assi, A., Bilo, F., Zanoletti, A., Ponti, J., Valsesia, A., La Spina, R., & Bontempi, E. (2020). Review of the reuse possibilities concerning ash residues from thermal process in a medium-sized urban system in Northern Italy. Sustainability, 12(10), 4193. [CrossRef]
- Kursun Unver, I., & Terzi, M. (2018). Distribution of trace elements in coal and coal fly ash and their recovery with mineral processing practices: A review. Journal of Mining and Environment, 9(3), 641-655. [CrossRef]
- Yadav, V. K., Modi, T., Alyami, A. Y., Gacem, A., Choudhary, N., Yadav, K. K., & Jeon, B. H. (2023). Emerging trends in the recovery of ferrospheres and plerospheres from coal fly ash waste and their emerging applications in environmental clean-up. Frontiers in Earth Science, 11, 1-18. [CrossRef]
- Alterary, S. S., & Marei, N. H. (2021). Fly ash properties, characterization, and applications: A review. Journal of King Saud University-Science, 33(6), 101536. [CrossRef]
- Musyoka, N. M. (2009). Hydrothermal synthesis and optimisation of zeolite Na-Pl from South African coal fly ash (MSc Dissertation, University of the Western Cape).
- Alegbe, J., Ayanda, O. S., Ndungu, P., Alexander, N., Fatoba, O. O., & Petrik, L. F. (2018). Chemical, mineralogical and morphological investigation of coal fly ash obtained from Mpumalanga province, South Africa. Research Journal of Environmental Sciences, 12 (3): 98-105, 2018. [CrossRef]
- Wardhono, A. (2018, January). Comparison study of class F and class C fly ashes as cement replacement material on strength development of non-cement mortar. In IOP Conference Series: Materials Science and Engineering (Vol. 288, p. 012019). IOP Publishing. [CrossRef]
- Panda, L., Dash, S., Kar, B., Panigrahi, S., & Mohanty, I. (2021). Alkaline Hydrothermal Synthesis of Zeolite from Class F Coal Fly Ash. The Journal of Solid Waste Technology and Management, 47(4), 674-681. [CrossRef]
- Kelechi, S. E., Adamu, M., Uche, O. A. U., Okokpujie, I. P., Ibrahim, Y. E., & Obianyo, I. I. (2022). A comprehensive review on coal fly ash and its application in the construction industry. Cogent Engineering, 9(1), 2114201. [CrossRef]
- Krishna, S., & Ahuja, P. (2023). Demographical Identification of Trace Metals Found in Soil Samples from India. In Trace Metals in the Environment. IntechOpen. [CrossRef]
- Munyengabe, A., Banda, M., Augustyn, W., Netshiongolwe, K., & Ramutshatsha-Makhwedzha, D. (2024). Application of coal fly ash for trace metal adsorption from wastewater: A review. Heliyon, e31494. [CrossRef]
- Ruwei, W., Jiamei, Z., Jingjing, L., & Liu, G. (2013). Levels and patterns of polycyclic aromatic hydrocarbons in coal-fired power plant bottom ash and fly ash from Huainan, China. Archives of environmental contamination and toxicology, 65, 193-202. [CrossRef]
- Rosselli, W., Keller, C., & Boschi, K. (2003). Phytoextraction capacity of trees growing on a metal contaminated soil. Plant and Soil, 256, 265-272. [CrossRef]
- Olaniran, A. O., Balgobind, A., & Pillay, B. (2013). Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. International Journal of Molecular Sciences, 14(5), 10197-10228. [CrossRef]
- Banda, M. F., Mokgalaka, N. S., Combrinck, S., & Regnier, T. (2021). Five-weeks pot trial evaluation of phytoremediation potential of Helichrysum splendidum Less. for copper-and lead-contaminated soils. International Journal of Environmental Science and Technology, 1-12. [CrossRef]
- Haider, U., Bittnar, Z., Kopecky, L., Šmilauer, V., Pokorny, J., Zaleska, M., & Hrbek, V. (2016). Determining the role of individual fly ash particles in influencing the variation in the overall physical, morphological, and chemical properties of fly ash. Acta Polytechnica, 56(4), 265-282. [CrossRef]
- Duffy, S. J. (2011). Environmental chemistry: a global perspective. Oxford University Press, USA.
- Komonweeraket, K., Cetin, B., Benson, C. H., Aydilek, A. H., & Edil, T. B. (2015). Leaching characteristics of toxic constituents from coal fly ash mixed soils under the influence of pH. Waste Management, 38, 174-184. [CrossRef]
- Zhang, Y., Cetin, B., Likos, W. J., & Edil, T. B. (2016). Impacts of pH on leaching potential of elements from MSW incineration fly ash. Fuel, 184, 815-825. [CrossRef]
- Cheng, S., Gao, X., Cao, L., Wang, Q., & Qiao, Y. (2020). Quantification of total organic carbon in ashes from smouldering combustion of sewage sludge via a thermal treatment-TGA method. ACS Omega, 5(51), 33445-33454. [CrossRef]
- Baran, P., Nazarko, M., Włosińska, E., Kanciruk, A., & Zarębska, K. (2021). Synthesis of geopolymers derived from fly ash with an addition of perlite. Journal of Cleaner Production, 293, 126112. [CrossRef]
- European Council Decision (EU) 2003/33/EC of 19th December 2002. (2003). Establishing criteria and procedures for acceptance of waste at landfills, pursuant to Article 16 of and Annex II to Directive 1999/31/EC. Official Journal of the European Communities, 11, 27-49.
- Altıkulaç, A., Turhan, S., Kurnaz, A., Gören, E., Duran, C., Hançerlioğulları, A., & Uğur, F. A. (2022). Assessment of the enrichment of heavy metals in coal and its combustion residues. ACS Omega, 7(24), 21239-21245. [CrossRef]
- Verma, C., Madan, S., & Hussain, A. (2016). Heavy metal contamination of groundwater due to fly ash disposal of coal-fired thermal power plant, Parichha, Jhansi, India. Cogent Engineering, 3(1), 1179243. [CrossRef]
- Arias-Arce, V., Lovera-Dávila, D., Guerrero-Rojas, J. J., Blas-Rodriguez, F., & Molina-Pereyra, I. (2023). Analysis of the Oxidation: Reduction Potential and Bacterial Population of Acidithiobacillus ferrooxidans during the Bioleaching Study of Sulfide Ores. [CrossRef]
- Li, Q., Wang, Y., Li, Y., Li, L., Tang, M., Hu, W., & Ai, S. (2022). Speciation of heavy metals in soils and their immobilization at micro-scale interfaces among diverse soil components. Science of the Total Environment, 825, 153862. [CrossRef]
- Nikolić, D., Bosnić, D., & Samardžić, J. (2023). Silicon in action: Between iron scarcity and excess copper. Frontiers in Plant Science, 14, 1039053. [CrossRef]
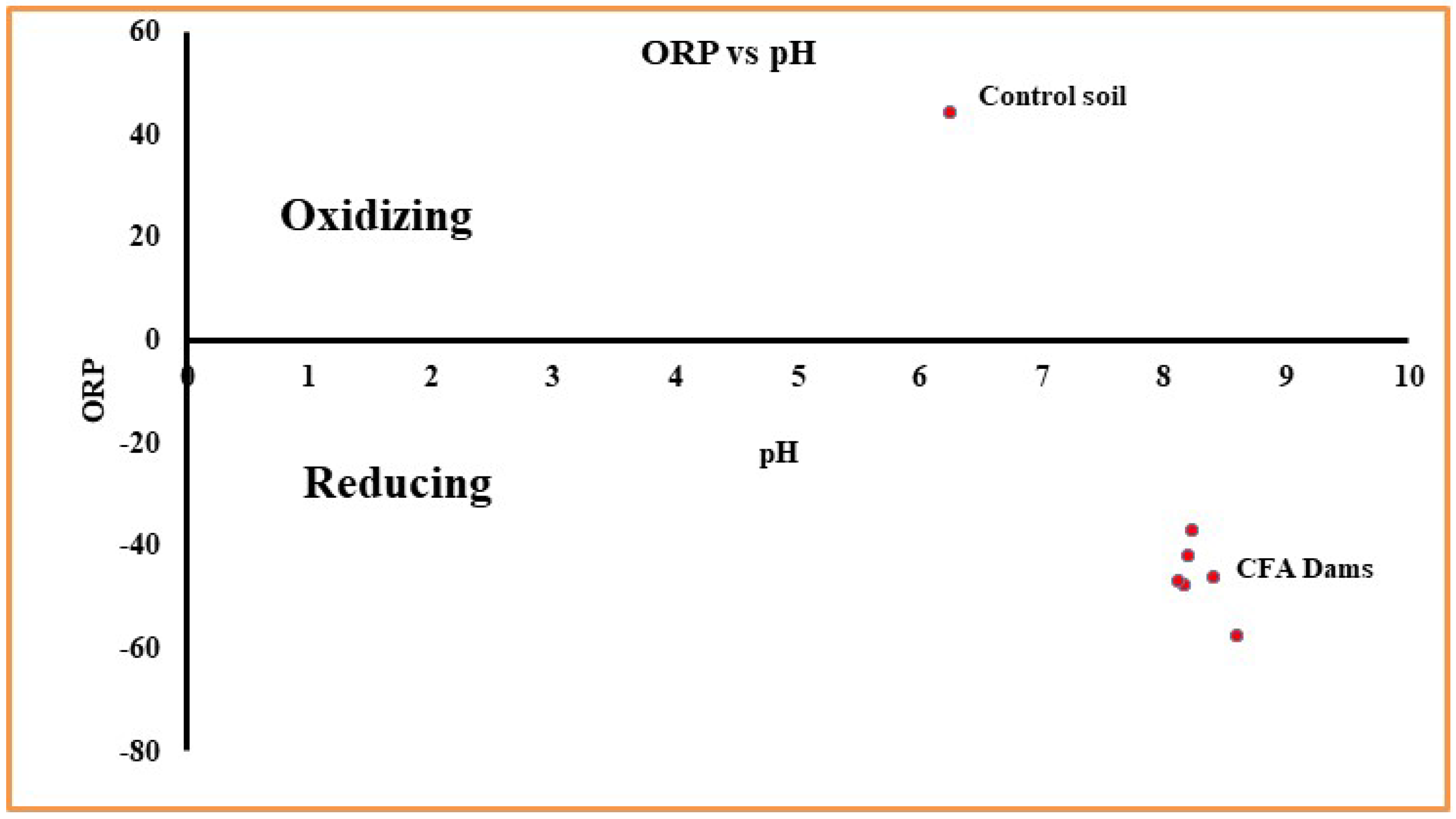
| Sampling sites | pH | ORP (mV) | EC (µS/cm) @ 25ºC | TOC (mg/kg) |
|---|---|---|---|---|
| Dam 3B | 8.17 ± 0.13 | -47.7±2.2 | 1027.30 | 84.37±0.80 |
| Dam 3C | 8.40 ± 0.06 | -45.9±11.3 | 632.70 | 2.68±2.68 |
| Dam 4A | 8.60 ± 0.03 | -57.5±1.6 | 301.70 | 83.74± |
| Dam 4B | 8.11 ± 0.07 | -46.9±2.5 | 757.70 | 2.22±0.98 |
| Dam 5A | 8.20 ± 0.16 | -41.8±3.9 | 1159.00 | 85.02±1.42 |
| Dam 5C | 8.23 ± 0.07 | -37.1±4.1 | 1515.00 | 2.46±0.28 |
| Control Soil | 6.24 ± 0.13 | 44.3±15.8 | 103.10 | 85.82±0.60 |
| Un-spiked CRM | - | - | - | 2.50±0.54 |
| Spiked CRM | - | - | - | 27.14±0.31 |
| Sample ID | Ag | Al | B | Ba | Mn | Cr | Pb | Fe | Ni | Mg | Ca | Co | Cu | Zn | Na | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFA 3B | 1.15±0.07 | 493.92±0.01 | 3.07±0.00 | 12.27±0.01 | 5.17±0.01 | 2.04±0.01 | 0.05±0.28 | 375.37±0.00 | 0.30±0.00 | 158.50±0.00 | 432.80±0.00 | 0.06±0.01 | 0.41±0.00 | 1.21±0.01 | 18.47±0.01 | 71.14±0.01 |
| CFA 3C | 0.97±0.12 | 347.92±0.00 | 3.34±0.01 | 13.37±0.01 | 4.62±0.00 | 1.58±0.01 | 0.13±0.07 | 285.98±0.01 | 0.25±0.01 | 13.40±0.00 | 543.14±0.00 | 0.08±0.02 | 0.45±0.00 | 1.31±0.01 | 0.51±0.16 | 70.38±0.02 |
| CFA 4B | 2.56±0.24 | 1178.59±0.00 | 4.94±0.00 | 16.85±0.00 | 8.45±0.00 | 4.93±0.00 | 0.20±0.03 | 939.81±0.00 | 0.95±0.00 | 211.91±0.00 | 599.76±0.00 | 0.03±0.00 | 0.01±0.00 | ND | 0.17±0.00 | 346.02±0.00 |
| CFA 5A | 1.25±0.34 | 481.23±0.01 | 1.77±0.00 | 9.62±0.03 | 5.30±0.01 | 2.20±0.00 | 0.04±0.16 | 376.25±0.00 | 0.40±0.03 | 137.90±0.00 | 362.62±0.00 | 0.10±0.02 | 0.40±0.00 | 0.46±0.00 | 8.73±0.00 | 234.30±0.00 |
| CFA 5C | 1.20±0.27 | 359.47±0.00 | 3.12±0.00 | 12.77±0.01 | 5.27±0.00 | 1.82±0.00 | 0.03±0.40 | 354.81±0.00 | 0.33±0.01 | 162.37±0.00 | 476.02±0.00 | 0.08±0.01 | 0.40±0.00 | 1.36±0.00 | 20.63±0.00 | 242.97±0.01 |
| CRM | 1.95±0.12 | 500.47±0.01 | 10.83±0.01 | 6.94±0.00 | 4.46±0.01 | 2.98±0.00 | 0.62±0.05 | 856.50±0.01 | 0.61±0.00 | 13.35±0.00 | 384.33±0.00 | 0.20±0.02 | 1.12±0.00 | 0.31±0.00 | 0.16±0.00 | 118.90±0.00 |
| SOIL | 2.70±0.21 | 407.04±0.00 | 2.90±0.17 | 2.12±0.01 | 10.48±0.01 | 6.20±0.00 | 0.12±0.16 | 952.82±0.00 | 0.74±0.01 | 175.65±0.00 | 486.88±0.00 | 0.24±0.00 | 0.86±0.01 | 1.12±0.00 | 2.85±0.00 | 233.36±0.00 |
| Species | Molality | Activity | Molality | Activity | Gamma |
|---|---|---|---|---|---|
| Al 7.648e-03 | |||||
| Al(OH)4- | 7.622e-03 | 4.112e-03 | -2.118 | -2.386 | -0.268 |
| Al(OH)3 | 2.467e-05 | 2.467e-05 | -4.608 | -4.608 | 0.000 |
| Al(OH)2+ | 1.593e-06 | 9.337e-07 | -5.798 | -6.030 | -0.232 |
| AlOH+2 | 7.521e-09 | 8.878e-10 | -8.124 | -9.052 | -0.928 |
| Al+3 | 2.570e-17 | 6.705e-13 | -16.590 | -12.174 | 4.417 |
| Ca 2.772e+00 | |||||
| Ca+2 | 2.761e+00 | 2.535e+02 | 0.441 | 2.404 | 1.963 |
| CaOH+ | 1.093e-02 | 6.696e-03 | -1.961 | -2.174 | -0.213 |
| CaH2BO3+ | 1.261e-04 | 4.914e-05 | -3.899 | -4.309 | -0.409 |
| Co(2) 4.717e-07 | |||||
| Co+2 | 4.717e-07 | 7.392e-12 | -6.326 | -11.131 | -4.805 |
| CoOH+ | 3.104e-12 | 1.953e-13 | -11.508 | -12.709 | -1.201 |
| Co(OH)2 | 2.054e-14 | 2.054e-14 | -13.687 | -13.687 | 0.000 |
| Co2OH+3 | 4.682e-15 | 7.235e-26 | -14.330 | -25.141 | -10.811 |
| Co(OH)3- | 8.624e-18 | 5.426e-19 | -17.064 | -18.265 | -1.201 |
| CoOOH- | 2.271e-18 | 1.429e-19 | -17.644 | -18.845 | -1.201 |
| Co(OH)4-2 | 7.266e-21 | 1.139e-25 | -20.139 | -24.944 | -4.805 |
| Co4(OH)4+4 | 4.811e-24 | 0.000e+00 | -23.318 | -42.537 | -19.219 |
| Co(3) 1.263e-32 | |||||
| CoOH+2 | 1.263e-32 | 1.980e-37 | -31.898 | -36.703 | -4.805 |
| Co+3 | 0.000e+00 | 0.000e+00 | -47.948 | -43.531 | 4.417 |
| Cr(2) 3.996e-18 | |||||
| Cr+2 | 3.996e-18 | 6.263e-23 | -17.398 | -22.203 | -4.805 |
| Cr(3) 1.747e-05 | |||||
| Cr+3 | 1.696e-05 | 2.622e-16 | -4.770 | -15.581 | -10.811 |
| Cr(OH)+2 | 4.846e-07 | 7.595e-12 | -6.315 | -11.119 | -4.805 |
| Cr(OH)2+ | 1.945e-08 | 1.224e-09 | -7.711 | -8.912 | -1.201 |
| CrO2- | 6.656e-10 | 4.188e-11 | -9.177 | -10.378 | -1.201 |
| Cr(OH)3 | 6.086e-10 | 6.086e-10 | -9.216 | -9.216 | 0.000 |
| Cr(OH)4- | 5.099e-10 | 3.208e-11 | -9.293 | -10.494 | -1.201 |
| Cr(6) 1.695e-28 | |||||
| HCrO4- | 1.204e-28 | 7.576e-30 | -27.919 | -29.121 | -1.201 |
| NaCrO4- | 2.824e-29 | 1.777e-30 | -28.549 | -29.750 | -1.201 |
| KCrO4- | 1.737e-29 | 1.093e-30 | -28.760 | -29.961 | -1.201 |
| CrO4-2 | 3.520e-30 | 3.232e-28 | -29.453 | -27.491 | 1.963 |
| H2CrO4 | 4.449e-38 | 4.449e-38 | -37.352 | -37.352 | 0.000 |
| Cr2O7-2 | 0.000e+00 | 0.000e+00 | -51.875 | -56.680 | -4.805 |
| Cu(1) 2.893e-06 | |||||
| Cu+ | 2.893e-06 | 1.820e-07 | -5.539 | -6.740 | -1.201 |
| Cu(2) 1.126e-08 | |||||
| Cu2(OH)2+2 | 3.882e-09 | 6.084e-14 | -8.411 | -13.216 | -4.805 |
| CuOH+ | 3.058e-09 | 1.556e-09 | -8.515 | -8.808 | -0.293 |
| Cu(OH)2 | 4.111e-10 | 4.111e-10 | -9.386 | -9.386 | 0.000 |
| Cu(OH)3- | 1.775e-11 | 1.117e-12 | -10.751 | -11.952 | -1.201 |
| Cu+2 | 4.048e-12 | 3.717e-10 | -11.393 | -9.430 | 1.963 |
| Cu(OH)4-2 | 7.425e-13 | 1.164e-17 | -12.129 | -16.934 | -4.805 |
| Fe(2) 2.951e-03 | |||||
| Fe+2 | 2.951e-03 | 4.625e-08 | -2.530 | -7.335 | -4.805 |
| FeOH+ | 4.303e-09 | 2.438e-09 | -8.366 | -8.613 | -0.247 |
| Fe(OH)2 | 2.564e-12 | 2.564e-12 | -11.591 | -11.591 | 0.000 |
| Fe(OH)3- | 1.895e-12 | 1.074e-12 | -11.722 | -11.969 | -0.247 |
| Fe(3) 3.933e-05 | |||||
| Fe3(OH)4+5 | 1.310e-05 | 1.222e-35 | -4.883 | -34.913 | -30.030 |
| Fe2(OH)2+4 | 7.405e-09 | 4.467e-28 | -8.130 | -27.350 | -19.219 |
| Fe(OH)2+ | 3.228e-09 | 1.892e-09 | -8.491 | -8.723 | -0.232 |
| Fe(OH)3 | 2.691e-09 | 2.691e-09 | -8.570 | -8.570 | 0.000 |
| Fe(OH)4- | 5.659e-10 | 3.317e-10 | -9.247 | -9.479 | -0.232 |
| FeOH+2 | 3.564e-14 | 3.673e-15 | -13.448 | -14.435 | -0.987 |
| Fe+3 | 1.647e-25 | 4.296e-21 | -24.783 | -20.367 | 4.417 |
| Mg 1.931e-03 | |||||
| Mg+2 | 1.794e-03 | 1.647e-01 | -2.746 | -0.783 | 1.963 |
| MgOH+ | 1.373e-04 | 8.680e-05 | -3.862 | -4.061 | -0.199 |
| MgH2BO3+ | 4.938e-08 | 1.924e-08 | -7.306 | -7.716 | -0.409 |
| Ni 2.217e-06 | |||||
| NiOH+ | 2.115e-06 | 1.331e-07 | -5.675 | -6.876 | -1.201 |
| Ni+2 | 8.697e-08 | 7.984e-06 | -7.061 | -5.098 | 1.963 |
| Ni(OH)2 | 1.400e-08 | 1.400e-08 | -7.854 | -7.854 | 0.000 |
| Ni(OH)3- | 2.946e-10 | 1.853e-11 | -9.531 | -10.732 | -1.201 |
| Pb 1.127e-07 | |||||
| Pb2OH+3 | 3.969e-08 | 6.134e-19 | -7.401 | -18.212 | -10.811 |
| Pb4(OH)4+4 | 6.898e-09 | 4.162e-28 | -8.161 | -27.381 | -19.219 |
| PbOH+ | 5.702e-09 | 3.588e-10 | -8.244 | -9.445 | -1.201 |
| Pb(OH)2 | 1.502e-11 | 1.502e-11 | -10.823 | -10.823 | 0.000 |
| Pb+2 | 1.175e-12 | 1.079e-10 | -11.930 | -9.967 | 1.963 |
| Pb(OH)4-2 | 4.115e-13 | 6.450e-18 | -12.386 | -17.190 | -4.805 |
| Pb(OH)3- | 3.161e-13 | 1.989e-14 | -12.500 | -13.701 | -1.201 |
| Pb3(OH)4+2 | 3.099e-17 | 4.857e-22 | -16.509 | -21.314 | -4.805 |
| Zn 8.230e-06 | |||||
| ZnOH+ | 8.068e-06 | 5.076e-07 | -5.093 | -6.294 | -1.201 |
| Zn(OH)2 | 1.065e-07 | 1.065e-07 | -6.973 | -6.973 | 0.000 |
| Zn+2 | 4.176e-08 | 3.834e-06 | -7.379 | -5.416 | 1.963 |
| Zn(OH)3- | 1.124e-08 | 7.069e-10 | -7.949 | -9.151 | -1.201 |
| Zn(OH)4-2 | 2.378e-09 | 3.726e-14 | -8.624 | -13.429 | -4.805 |
| Phase | I** | log IAP | log K | (298 K,1 atm) |
|---|---|---|---|---|
| Al(OH)3(am) | 1.38 | 12.18 | 10.80 | Al(OH)3 |
| Al2O3 | 4.78 | 24.43 | 19.65 | Al2O3 |
| Boehmite | 3.63 | 12.20 | 8.58 | AlOOH |
| CoFe2O4 | 16.70 | 13.17 | -3.53 | CoFe2O4 |
| Cupricferrite | 8.88 | 14.87 | 5.99 | CuFe2O4 |
| Cuprite | 4.19 | 2.78 | -1.41 | Cu2O |
| Cuprousferrite | 14.33 | 5.41 | -8.92 | CuFeO2 |
| Diaspore | 5.33 | 12.20 | 6.87 | AlOOH |
| FeCr2O4 | 0.20 | 7.40 | 7.20 | FeCr2O4 |
| Ferrihydrite | 0.80 | 3.99 | 3.19 | Fe(OH)3 |
| Gibbsite | 3.89 | 12.18 | 8.29 | Al(OH)3 |
| Goethite | 3.52 | 4.01 | 0.49 | FeOOH |
| Hematite | 9.46 | 8.04 | -1.42 | Fe2O3 |
| Maghemite | 1.66 | 8.04 | 6.39 | Fe2O3 |
| Magnesioferrite | 6.66 | 23.52 | 16.86 | Fe2MgO4 |
| Magnetite | 13.56 | 16.97 | 3.40 | Fe3O4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




