Submitted:
03 June 2024
Posted:
05 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Substrate and Microorganism
2.3. Biological Treatment of Grape Pomace by Trametes Versicolor
2.3.1. Laboratory Jars
2.3.2. Tray Bioreactor
2.4. Enzyme Activity Measurement
2.5. Determination of Biomass Concentration
2.6. Analysis of Chemical Composition of Grape Pomace
2.7. Statistical Analysis
3. Results and Discussion
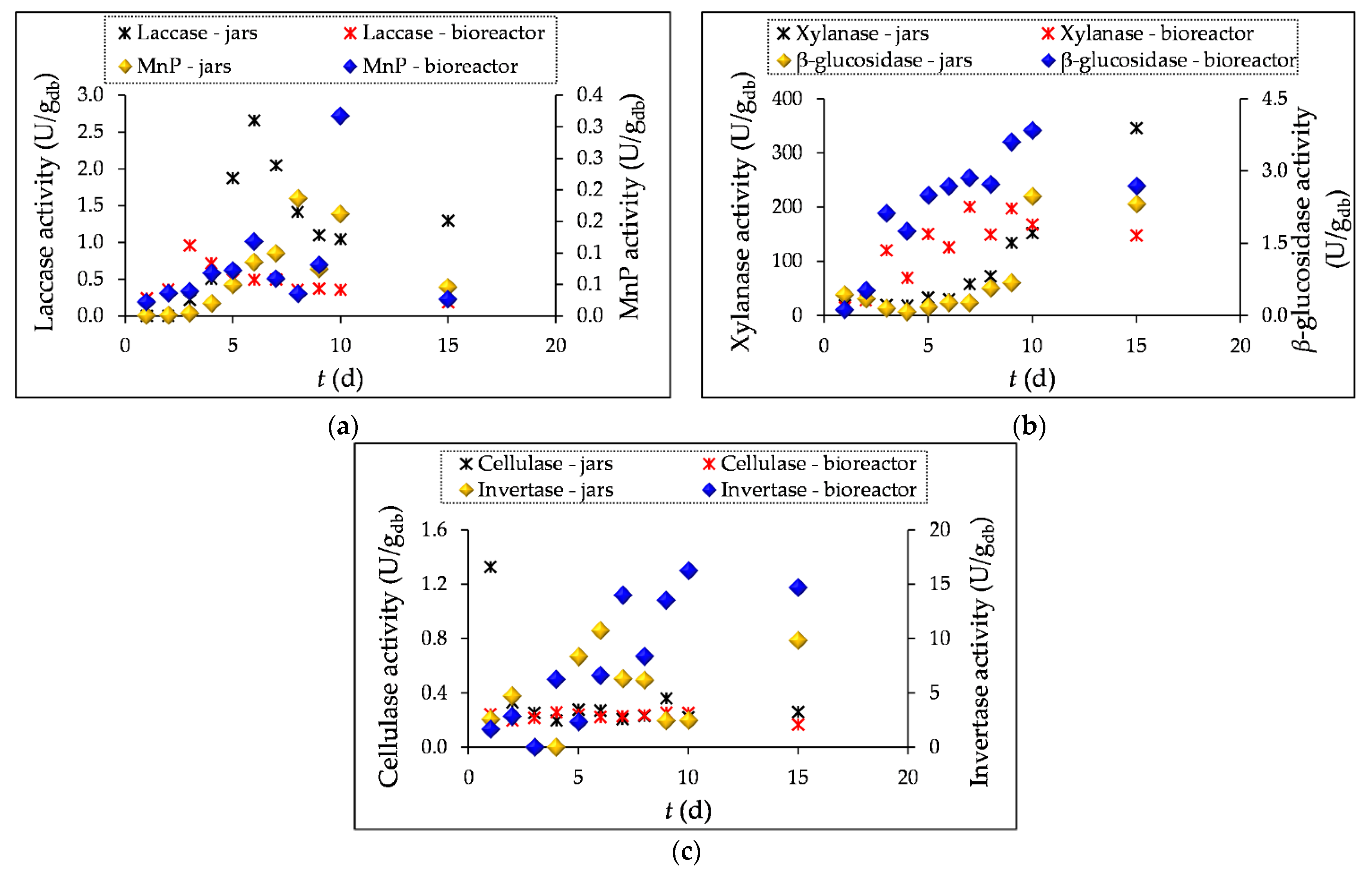
3.1. Enzyme Activity Measurement
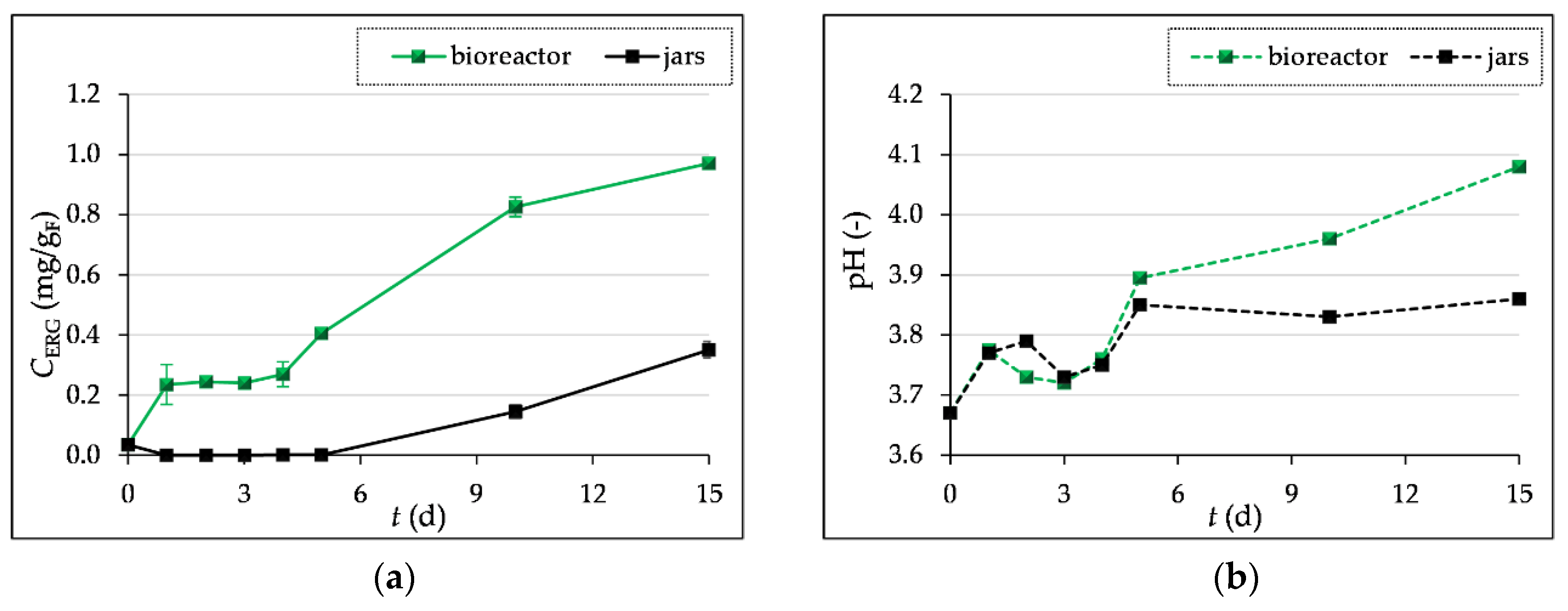
3.2. Determination of Biomass Concentration and pH Measurement
3.3. Chemical Composition of Grape Pomace
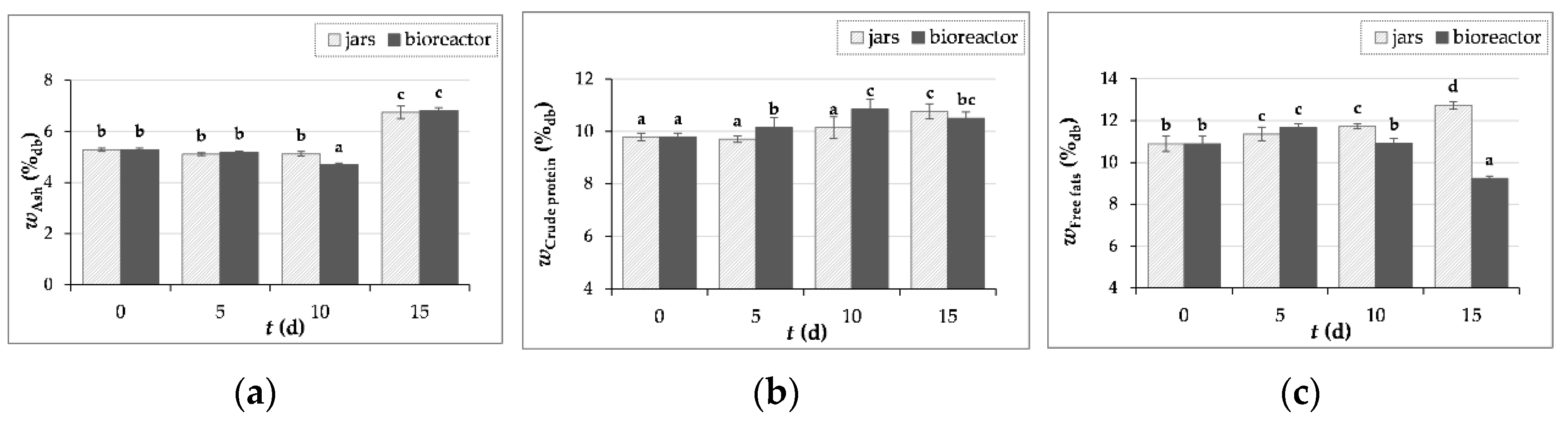
3.3.1. Ash, Crude Proteins and Free Fats Content Determination
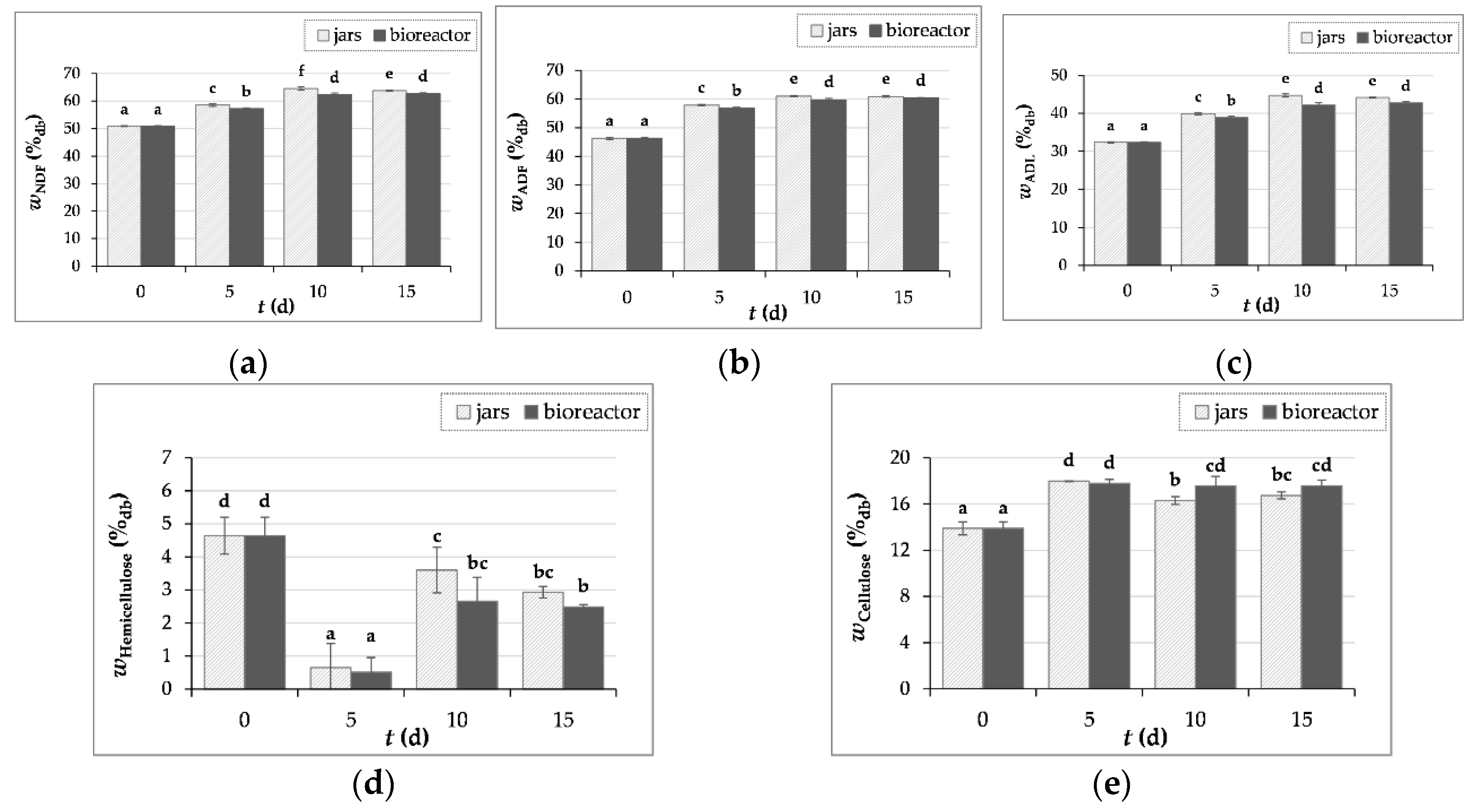
3.3.2. Crude Fiber Content Determination
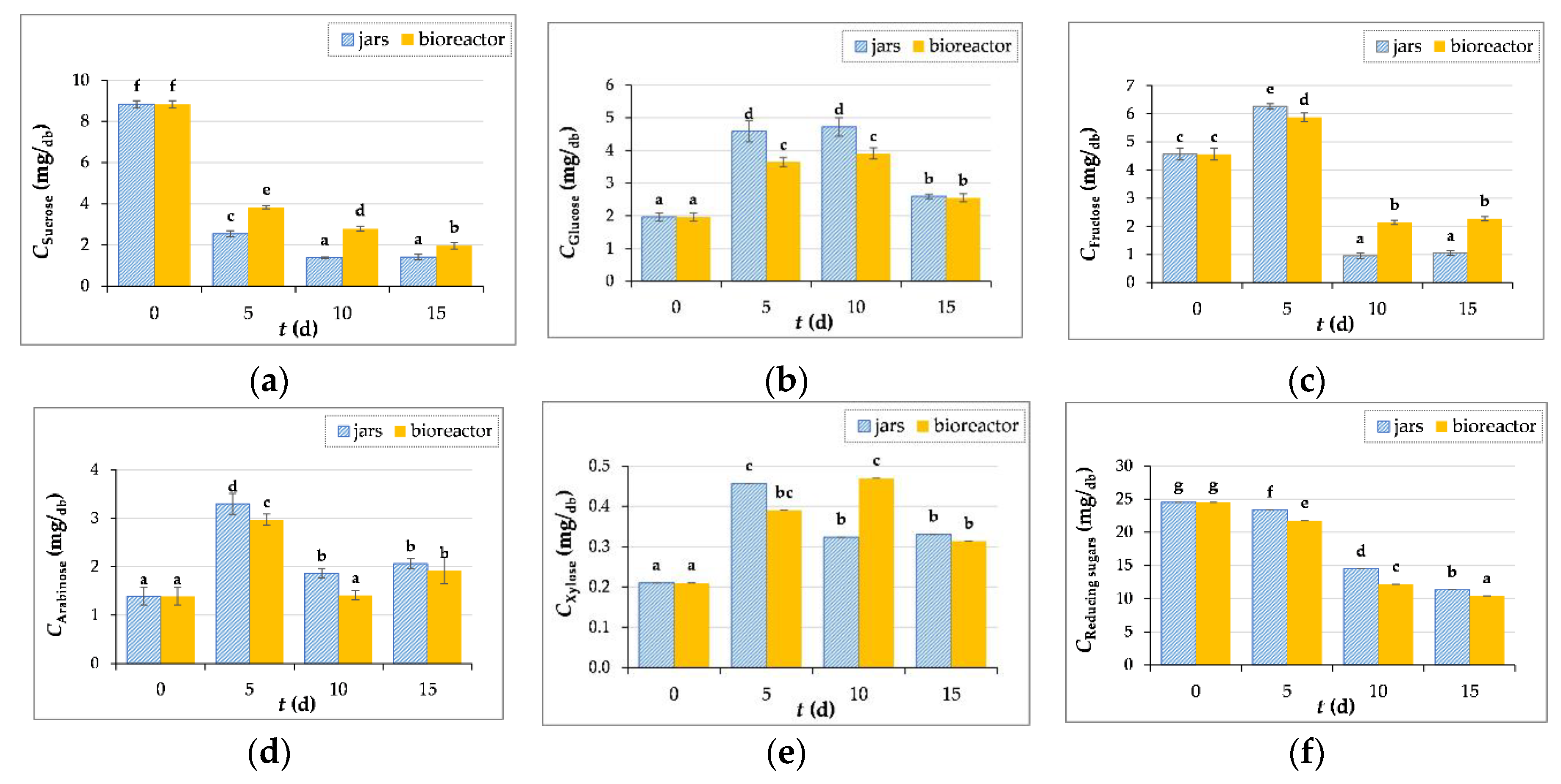
3.3.3. Measurement of Sugar Concentration
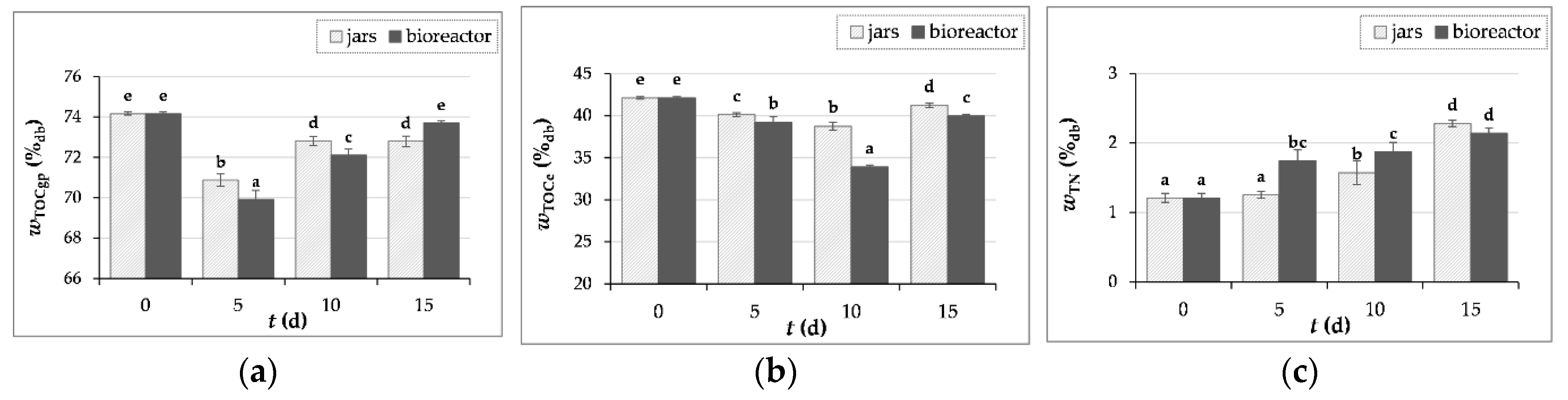
3.3.4. Measurement of Carbon and Nitrogen Content
3.4. Phenolic Compound and Antioxidant Activity Measurement
3.4.1. Determination of Total Phenolic Compound and Antioxidant Activity
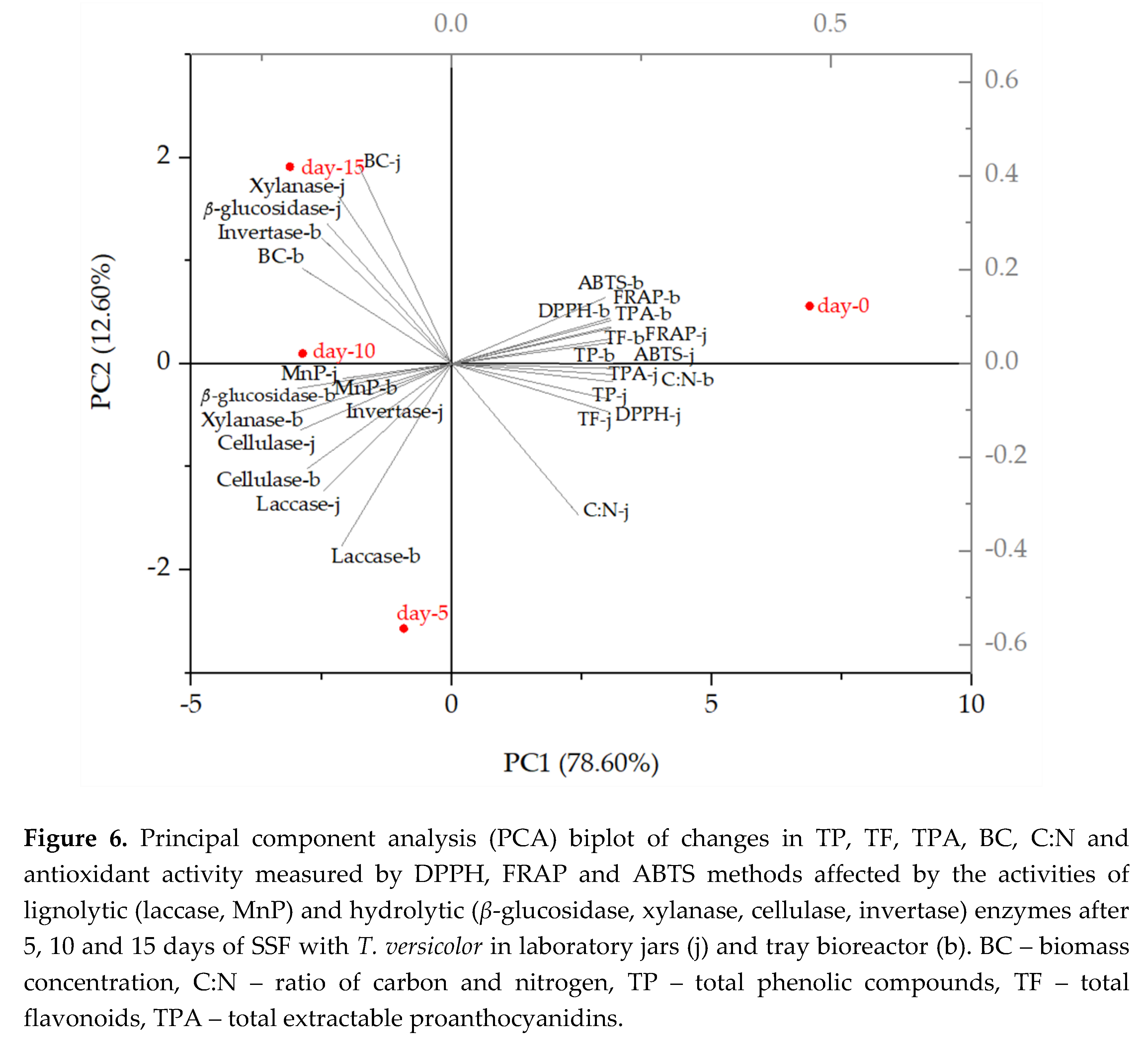
3.4.2. Principal Components Analysis
3.4.3. Determination of Individual Phenolic Compound Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 May 2022).
- Cui, W.; Wang, Y.; Sun, Z.; Cui, C.; Li, H.; Luo, K.; Cheng, A. Effects of Steam Explosion on Phenolic Compounds and Dietary Fiber of Grape Pomace. LWT 2023, 173, 114350. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Tišma, M.; Šelo, G.; Grgić, J.; Perković, G.; Planinić, M. Winery Production Residues as Feedstocks within the Biorefinery Concept. Engineering Power 2022, 17, 11–17. [Google Scholar]
- Siller-Sánchez, A.; Luna-Sánchez, K.A.; Bautista-Hernández, I.; Chávez-González, M.L. Use of Grape Pomace from the Wine Industry for the Extraction of Valuable Compounds with Potential Use in the Food Industry. Curr Food Sci Tech Rep 2024, 2, 7–16. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of Phenolic Compounds from Grape Pomace Using Ohmic Heating: Chemical Composition, Bioactivity and Bioaccessibility. Food Chemistry 2024, 436, 137780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, D.; Zhang, J.; Shen, J.; Cao, J.; Gu, H.; Cui, M.; He, L.; Chen, G.; Liu, S.; et al. Improving Soluble Phenolic Profile and Antioxidant Activity of Grape Pomace Seeds through Fungal Solid-State Fermentation. Foods 2024, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, V.; Igrejas, G.; Aires, A.; Falco, V.; Valentão, P.; Poeta, P. Phenolic Compounds Classification and Their Distribution in Winemaking By-Products. Eur Food Res Technol 2023, 249, 207–239. [Google Scholar] [CrossRef]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. Physicochemical Characterization and Evaluation of Gastrointestinal In Vitro Behavior of Alginate-Based Microbeads with Encapsulated Grape Pomace Extracts. Pharmaceutics 2023, 15, 980. [Google Scholar] [CrossRef] [PubMed]
- Šelo, G.; Planinić, M.; Tišma, M.; Martinović, J.; Perković, G.; Bucić-Kojić, A. Bioconversion of Grape Pomace with Rhizopus Oryzae under Solid-State Conditions: Changes in the Chemical Composition and Profile of Phenolic Compounds. Microorganisms 2023, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability 2023, 15, 9075. [Google Scholar] [CrossRef]
- Abid, K.; Boudagga, S.; Abid, O.; Najar, T.; Jaouani, A. Bioconversion of Grape Pomace Waste into Suitable Alternative Feed for Ruminants with Pleurotus Cornucopiae and Ganoderma Resinaceum via Solid-State Fermentation Bioprocess. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Gerardi, C.; D’Amico, L.; Durante, M.; Tufariello, M.; Giovinazzo, G. Whole Grape Pomace Flour as Nutritive Ingredient for Enriched Durum Wheat Pasta with Bioactive Potential. Foods 2023, 12, 2593. [Google Scholar] [CrossRef] [PubMed]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. In Vitro Bioaccessibility Assessment of Phenolic Compounds from Encapsulated Grape Pomace Extract by Ionic Gelation. Molecules 2023, 28, 5285. [Google Scholar] [CrossRef] [PubMed]
- Perković, G.; Martinović, J.; Šelo, G.; Bucić-Kojić, A.; Planinić, M.; Ambrus, R. Characterization of Grape Pomace Extract Microcapsules: The Influence of Carbohydrate Co-Coating on the Stabilization of Goat Whey Protein as a Primary Coating. Foods 2024, 13, 1346. [Google Scholar] [CrossRef] [PubMed]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda-Torre, L.; Torres-León, C.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Aguilar, C.N. Solid-State Fermentation for the Recovery of Phenolic Compounds from Agro-Wastes. Resources 2023, 12, 36. [Google Scholar] [CrossRef]
- Tišma, M.; Žnidaršič-Plazl, P.; Šelo, G.; Tolj, I.; Šperanda, M.; Bucić-Kojić, A.; Planinić, M. Trametes Versicolor in Lignocellulose-Based Bioeconomy: State of the Art, Challenges and Opportunities. Bioresource Technology 2021, 124997. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, A.; Beccaccioli, M.; Verni, M.; Cecchetti, V.; Minisci, A.; Reverberi, M.; Pontonio, E.; Rizzello, C.G. Combined Use of Trametes Versicolor Extract and Sourdough Fermentation to Extend the Microbiological Shelf-Life of Baked Goods. LWT 2023, 189, 115467. [Google Scholar] [CrossRef]
- Planinić, M.; Zelić, B.; Čubel, I.; Bucić-Kojić, A.; Tišma, M. Corn Forage Biological Pretreatment by Trametes versicolor in a Tray Bioreactor. Waste Manag Res 2016, 34, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory Testing of Methods for Assay of Xylanase Activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of Cellulase Activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Karpe, A.V.; Dhamale, V.V.; Morrison, P.D.; Beale, D.J.; Harding, I.H.; Palombo, E.A. Winery Biomass Waste Degradation by Sequential Sonication and Mixed Fungal Enzyme Treatments. Fungal Genet. Biol. 2017, 102, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Margetić, A.; Vujčić, Z. Comparative Study of Stability of Soluble and Cell Wall Invertase from Saccharomyces Cerevisiae. Prep. Biochem. Biotechnol. 2017, 47, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Lueangjaroenkit, P.; Kunitake, E.; Sakka, M.; Kimura, T.; Teerapatsakul, C.; Sakka, K.; Chitradon, L. Light Regulation of Two New Manganese Peroxidase-Encoding Genes in Trametes polyzona KU-RNW027. Microorganisms 2020, 8, 852. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; Wiley: Wrolstad, 2001. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, Proanthocyanidins, Flavones and Flavonols in Some Plant Materials and Their Antioxidant Activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of Solvent and Temperature on Extraction of Phenolic Compounds from Grape Seed, Antioxidant Activity and Colour of Extract. International Journal of Food Science & Technology 2009, 44, 2394–2401. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Grgić, J.; Perković, G.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comparative Study of the Influence of Various Fungal-Based Pretreatments of Grape Pomace on Phenolic Compounds Recovery. Foods 2022, 11, 1665. [Google Scholar] [CrossRef]
- Alarcón, E.; Hernández, C.; García, G.; Ziarelli, F.; Gutiérrez-Rivera, B.; Musule, R.; Vázquez-Marrufo, G.; Gardner, T.G. Changes in Chemical and Structural Composition of Sugarcane Bagasse Caused by Alkaline Pretreatments [Ca(OH)2 and NaOH] Modify the Amount of Endoglucanase and β-Glucosidase Produced by Aspergillus niger in Solid-State Fermentation. Chem. Eng. Commun. 2021, 209, 594–606. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- AOAC. AOAC. AOAC Official Method 942.05. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications). In Agriculture Handbook; No. 379; Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1970; pp. 1–24. [Google Scholar]
- AOAC. AOAC. AOAC Official Method 2001.11. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. AOAC. AOAC Official Method 945.16. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Mostafa, Y.S.; Širić, I.; Alamri, S.A.M.; Alrumman, S.A.; Kumar, P.; Abou Fayssal, S.; Zjalić, S.; Singh, R.; Eid, E.M. Assessment of Metal Elements and Biochemical Constituents of Wild Turkey Tail (Trametes versicolor) Mushrooms Collected from the Shivalik Foothills of the Himalayas, India. Forests 2023, 14, 2247. [Google Scholar] [CrossRef]
- Fabros, J.A.; Lazo, M.K.M.; Magpantay, J.E.S.; Abon, M.D.; Dulay, R.M.R.; Kalaw, S.P.; Reyes, R.G. The Effect of Nutritional and Physical Factors on the Growth of Trametes versicolor (L.) Lloyd and Its Mycochemical and Cytotoxic Properties. Studies in Fungi 2023, 8. [Google Scholar] [CrossRef]
- Kumar, D. Fungal Lipase Production by Solid State Fermentation-An Overview. Journal of Analytical & Bioanalytical Techniques 2015, 06. [Google Scholar] [CrossRef]
- Athanasiou, P.E.; Gkountela, C.I.; Patila, M.; Fotiadou, R.; Chatzikonstantinou, A.V.; Vouyiouka, S.N.; Stamatis, H. Laccase-Mediated Oxidation of Phenolic Compounds from Wine Lees Extract towards the Synthesis of Polymers with Potential Applications in Food Packaging. Biomolecules 2024, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- Erskine, E.; Ozkan, G.; Lu, B.; Capanoglu, E. Effects of Fermentation Process on the Antioxidant Capacity of Fruit Byproducts. ACS Omega 2023, 8, 4543–4553. [Google Scholar] [CrossRef] [PubMed]
- Botella, C.; Ory, I. de; Webb, C.; Cantero, D.; Blandino, A. Hydrolytic Enzyme Production by Aspergillus awamori on Grape Pomace. Biochemical Engineering Journal 2005, 26, 100–106. [Google Scholar] [CrossRef]
- De Bellis, P.; Maggiolino, A.; Albano, C.; De Palo, P.; Blando, F. Ensiling Grape Pomace With and Without Addition of a Lactiplantibacillus plantarum Strain: Effect on Polyphenols and Microbiological Characteristics, in Vitro Nutrient Apparent Digestibility, and Gas Emission. Front. Vet. Sci. 2022, 9, 808293. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Ahmed, I.A.; Özcan, M.M.; Al Juhaimi, F.; Babiker, E.F.E.; Ghafoor, K.; Banjanin, T.; Osman, M.A.; Gassem, M.A.; Alqah, H.A.S. Chemical Composition, Bioactive Compounds, Mineral Contents, and Fatty Acid Composition of Pomace Powder of Different Grape Varieties. Journal of Food Processing and Preservation 2020, 44, e14539. [Google Scholar] [CrossRef]
- Altop, A.; Güngör, E.; Erener, G. Aspergillus Niger May Improve Nutritional Quality of Grape Seed and Its Usability in Animal Nutrition through Solid-State Fermentation. International Advanced Researches and Engineering Journal 2018, 2, 273–277. [Google Scholar]
- Gungor, E.; Altop, A.; Erener, G. Effect of Raw and Fermented Grape Pomace on the Growth Performance, Antioxidant Status, Intestinal Morphology, and Selected Bacterial Species in Broiler Chicks. Animals 2021, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J. Fungal Cell Wall: Structure, Synthesis, and Assembly; 1st ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 5–18. [Google Scholar]
- Sukma, A.; Jos, B.; Sumardiono, S. Kinetic of Biomass Growth and Protein Formation on Rice Bran Fermentation Using Rhizopus oryzae. MATEC Web Conf. 2018, 156, 01023. [Google Scholar] [CrossRef]
- Machado, A.R.; Voss, G.B.; Machado, M.; Paiva, J.A.; Nunes, J.; Pintado, M. Chemical Characterization of the Cultivar ‘Vinhão’ (Vitis vinifera L.) Grape Pomace Towards Its Circular Valorisation. SSRN - Elsevier. [CrossRef]
- Zheng, Y.; Lee, C.; Yu, C.; Cheng, Y.-S.; Simmons, C.W.; Zhang, R.; Jenkins, B.M.; VanderGheynst, J.S. Ensilage and Bioconversion of Grape Pomace into Fuel Ethanol. J. Agric. Food Chem. 2012, 60, 11128–11134. [Google Scholar] [CrossRef] [PubMed]
- Manara, P.; Zabaniotou, A.; Vanderghem, C.; Richel, A. Lignin Extraction from Mediterranean Agro-Wastes: Impact of Pretreatment Conditions on Lignin Chemical Structure and Thermal Degradation Behavior. Catal. Today 2014, 223, 25–34. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Oliveira, R.A.; Bon, E.P.S.; Terzi, S.C.; Souza, E.F.; Gottschalk, L.M.F.; Tonon, R.V. Use of Grape Pomace for the Production of Hydrolytic Enzymes by Solid-State Fermentation and Recovery of Its Bioactive Compounds. Food Research International 2019, 120, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dilokpimol, A.; Kabel, M.A.; de Vries, R.P. Fungal Xylanolytic Enzymes: Diversity and Applications. Bioresource Technology 2022, 344, 126290. [Google Scholar] [CrossRef] [PubMed]
- Zamora Zamora, H.D.; Silva, T.A.L.; Varão, L.H.R.; Baffi, M.A.; Pasquini, D. Simultaneous Production of Cellulases, Hemicellulases, and Reducing Sugars by Pleurotus Ostreatus Growth in One-Pot Solid State Fermentation Using Alstroemeria Sp. Waste. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Yu, H.; Xie, B.; Khan, R.; Dong, J.; Shen, G. The Changes in Macronutrients and Microbial Community Structure during the Co-Composting of White Wine Distillers’ Grains and Potassium Silicate. J. Clean. Prod. 2021, 319, 128681. [Google Scholar] [CrossRef]
- Zambrano, C.; Kotogán, A.; Bencsik, O.; Papp, T.; Vágvölgyi, C.; Mondal, K.C.; Krisch, J.; Takó, M. Mobilization of Phenolic Antioxidants from Grape, Apple and Pitahaya Residues via Solid State Fungal Fermentation and Carbohydrase Treatment. LWT - Food Sci. Technol. 2018, 89, 457–465. [Google Scholar] [CrossRef]
- Chen, Q.; Su, J.; Zhang, Y.; Li, C.; Zhu, S. Phytochemical Profile and Bioactivity of Bound Polyphenols Released from Rosa Roxburghii Fruit Pomace Dietary Fiber by Solid-State Fermentation with Aspergillus Niger. Molecules 2024, 29, 1689. [Google Scholar] [CrossRef]
- Tian, Z.-X.; Li, Y.-F.; Long, M.-X.; Liang, Q.; Chen, X.; Huang, D.-M.; Ran, Y.-Q. Effects of Six Different Microbial Strains on Polyphenol Profiles, Antioxidant Activity, and Bioaccessibility of Blueberry Pomace with Solid-State Fermentation. Front Nutr 2023, 10, 1282438. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, M.; Çoklar, H.; Bulut, A.N.; Hosseini, S.R. Evaluation of Black Grape Pomace, a Fruit Juice by-Product, in Shalgam Juice Production: Effect on Phenolic Compounds, Anthocyanins, Resveratrol, Tannin, and in Vitro Antioxidant Activity. Food Science & Nutrition n/a. [CrossRef]
- Fuchs, G.; Boll, M.; Heider, J. Microbial Degradation of Aromatic Compounds — from One Strategy to Four. Nat Rev Microbiol 2011, 9, 803–816. [Google Scholar] [CrossRef] [PubMed]
| Lignolytic enzymes | Buffer solution | pH |
| Laccase | 50 mM sodium malonate buffer | 4.5 |
| Manganese peroxidase (MnP) | 50 mM sodium malonate buffer | 4.5 |
| Hydrolyticenzymes | Buffer solution | pH |
| Xylanase | 50 mM sodium citrate buffer | 5.3 |
| Cellulase | 50 mM sodium citrate buffer | 4.8 |
| β-glucosidase | 100 mM sodium acetate buffer | 5.0 |
| Invertase | 100 mM sodium acetate buffer | 4.5 |
| Liquid extract preparation | Analysis |
|
|
|
|
|
|
| Solid samples of GP | Analysis |
|
| day “0” | day 1 | day 2 | day 3 | day 4 | day 5 | day 10 | day 15 | |
|---|---|---|---|---|---|---|---|---|
| Weight loss - jars (%db) | - | 9.0 | 11.7 | 14.8 | 15.6 | 16.4 | 25.2 | 38.5 |
| Moisture content- jars (%db) | 71.8 | 71.7 | 70.5 | 68.5 | 65.9 | 68.4 | 60.6 | 51.1 |
| Moisture content- bioreactor (%db) | 66.9 | 58.7 | 57.1 | 57.2 | 57.1 | 49.4 | 45.4 | 29.7 |
| Tbioreactor (°C) | - | 27.3 | 27.0 | 27.5 | 27.5 | 27.6 | 27.2 | 27.5 |
| TGP in bioreactor (°C) | - | 26.7 | 26.6 | 27.6 | 27.7 | 27.6 | 27.4 | 27.3 |
| Compound | GP | SSF in laboratory jars | SSF in a tray bioreactor | ||||
|---|---|---|---|---|---|---|---|
| day “0” | day 5 | day 10 | day 15 | day 5 | day 10 | day 15 | |
| TP (mg/gdb) | 50.08±0.08 m | 23.68±0.11 h | 14.79±0.28 e | 12.43±0.07 b | 14.31±0.04 d | 12.64±0.06 b | 11.62±0.07 a |
| TF (mg/gdb) | 25.14±0.06 n | 13.91±0.03 j | 8.23±0.06 g | 5.99±0.03 d | 6.91±0.04 f | 4.52±0.03 b | 4.22±0.07 a |
| TPA (mg/gdb) | 8.55±0.04 n | 3.32±0.04 i | 1.80±0.03 f | 1.44±0.03 d | 1.55±0.03 e | 1.32±0.02 c | 1.09±0.04 a |
| DPPH (mgT/gdb) | 57.50±0.00 g | 27.50±0.00 c | 18.50±0.00 b | 10.50±0.00 a | 11.50±0.00 a | 10.50±0.00 a | 10.0±0.00 a |
| ABTS (mgT/gdb) | 314.00±0.00 k | 114.00±0.01 f | 85.50±0.00 d | 57.50±0.00 a | 72.00±0.00 b | 102.50±0.00 e | 88.50±0.00 d |
| FRAP (mgT/gdb) | 212.50±0.00 h | 67.50±0.01 d | 58.50±0.01 c | 48.50±0.00 b | 37.50±0.00 a | 35.00±0.00 a | 34.00±0.00 a |
| Phenolic compound | day “0” | SSF in laboratory jars | SSF in tray bioreactor | ||||
|---|---|---|---|---|---|---|---|
| Co (µg/gdb)* | Ci,max. (µg/gdb)* | p** | tSSF (d) | Ci,max. (µg/gdb)* | p** | tSSF (d) | |
| GA | 267.77 ± 11.78 | 275.59 ± 11.90 | 0.6249 | 1. | 248.40 ± 4.41 | 0.0450 | 1. |
| EA | 34.65 ± 3.66 | 129.11 ± 14.82 | 0.0125 | 1. | 136.02 ± 3.84 | 0.0000 | 1. |
| p-HBA | 5.05 ± 2.15 | 9.33 ± 0.02 | 0.0007 | 10. | 10.96 ± 0.43 | 0.0040 | 15. |
| SA | 86.37 ± 2.15 | 95.79 ± 3.22 | 0.0933 | 10. | 86.64 ± 2.86 | 0.5661 | 3. |
| 3,4-DHBA | 138.61 ± 9.87 | 237.46 ± 5.73 | 0.0082 | 10. | 338.03 ± 2.19 | 0.0005 | 3. |
| o-CoA | 4.43 ± 0.11 | 7.74 ± 0.33 | 0.0082 | 10. | 6.83 ± 0.58 | 0.0263 | 4. |
| EPG | 166.69 ± 8.42 | 246.27 ± 7.32 | 0.0128 | 2. | 373.05 ± 4.25 | 0.0001 | 2. |
| GCG | 291.57 ± 2.35 | 408.79 ± 15.58 | 0.0042 | 2. | 480.89 ± 4.18 | 0.0000 | 2. |
| QU | 173.32 ± 16.54 | 504.31 ± 16.98 | 0.0034 | 1. | 507.29 ± 31.17 | 0.0067 | 1. |
| KA | 10.22 ± 1.06 | 33.78 ± 0.65 | 0.0017 | 1. | 35.75 ± 1.09 | 0.0024 | 1. |
| PB1 | 304.27 ± 0.37 | 460.39 ± 12.31 | 0.0019 | 2. | 510.34 ± 18.72 | 0.0028 | 1. |
| RES | 46.07 ± 3.48 | 56.65 ± 3.54 | 0.1204 | 1. | 54.46 ± 0.67 | 0.0353 | 2. |
| VIN | 17.52 ± 1.64 | 44.53 ± 1.12 | 0.0035 | 1. | 46.55 ± 1.30 | 0.0034 | 1. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





