Submitted:
04 June 2024
Posted:
04 June 2024
You are already at the latest version
Abstract
Keywords:
Introduction
An Actual Rise in the Prevalence of ASD?
“What is the reason for the high [1/645] prevalence rates? We doubt that differences in diagnostic criteria and survey methods account for these high rates, although such factors may explain the large differences between the studies done between 1966 and 1980 and the studies done since 1983. In fact, the earlier studies were carefully performed using good survey methods. Although in some studies the diagnostic criteria may have been different, the diagnostic criteria were similar in the study by Hoshino et al (reference) and in our study, and both studies were done in Japan in similar cultural, ethnic, and socioeconomic regions. However our prevalence is three times that of Hoshino et al. Could the real prevalence of autism be increasing in the world?”
“…it was not possible to account entirely for the effect of the diagnostic criteria on the prevalence estimates as the ICD-10 and DSM-IV diagnostic schema leave some scope for variation in their interpretation and application.”
“If MMR and thimerosal [factors related to vaccination] aren’t responsible, what’s the causative factor for autism? The simple answer is that it is not known.”
Spectrums of Drug Induced Developmental Injury
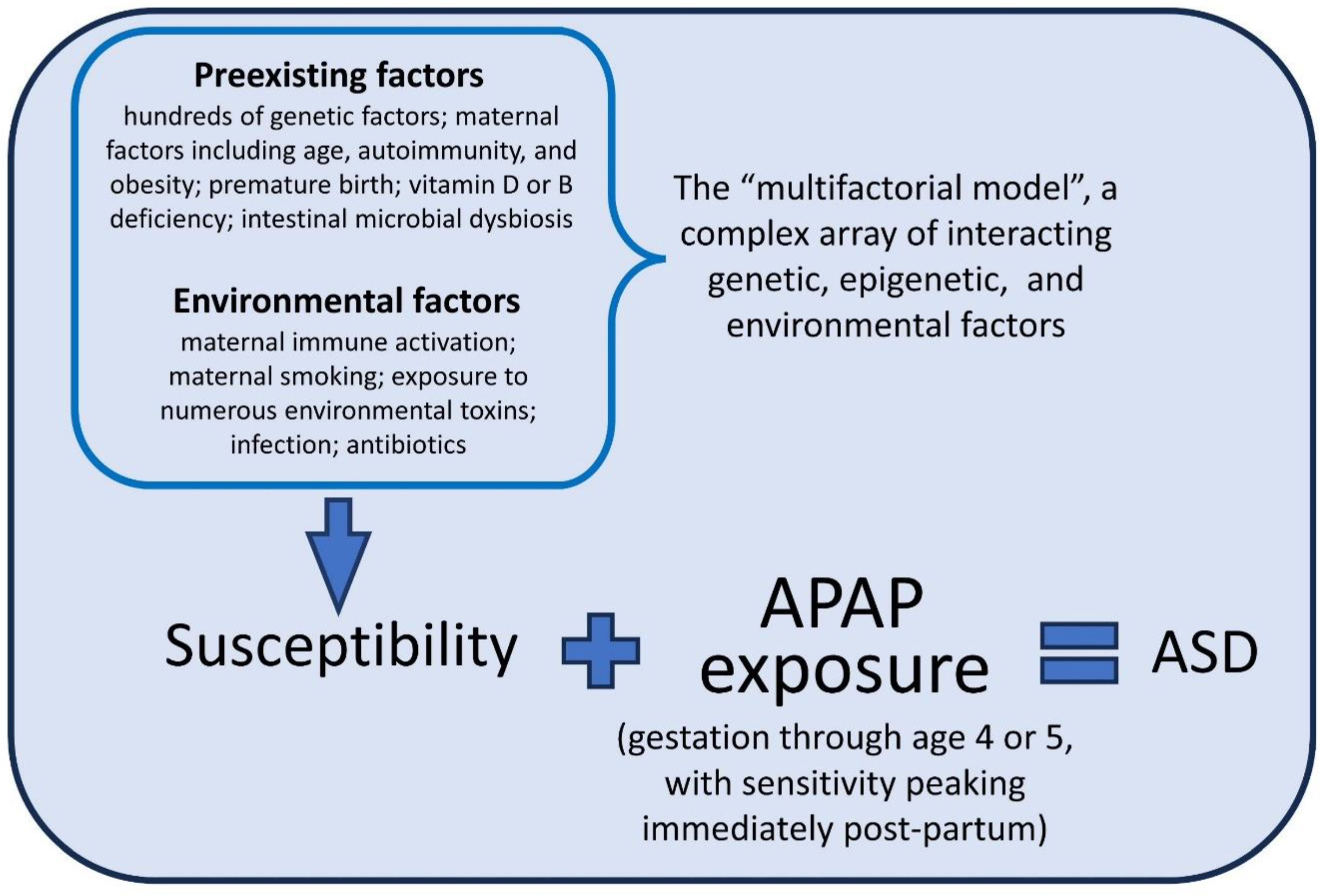
The Metabolism of Acetaminophen and the Etiology of ASD
The Weight of Evidence and the Role of Acetaminophen in the Etiology of ASD
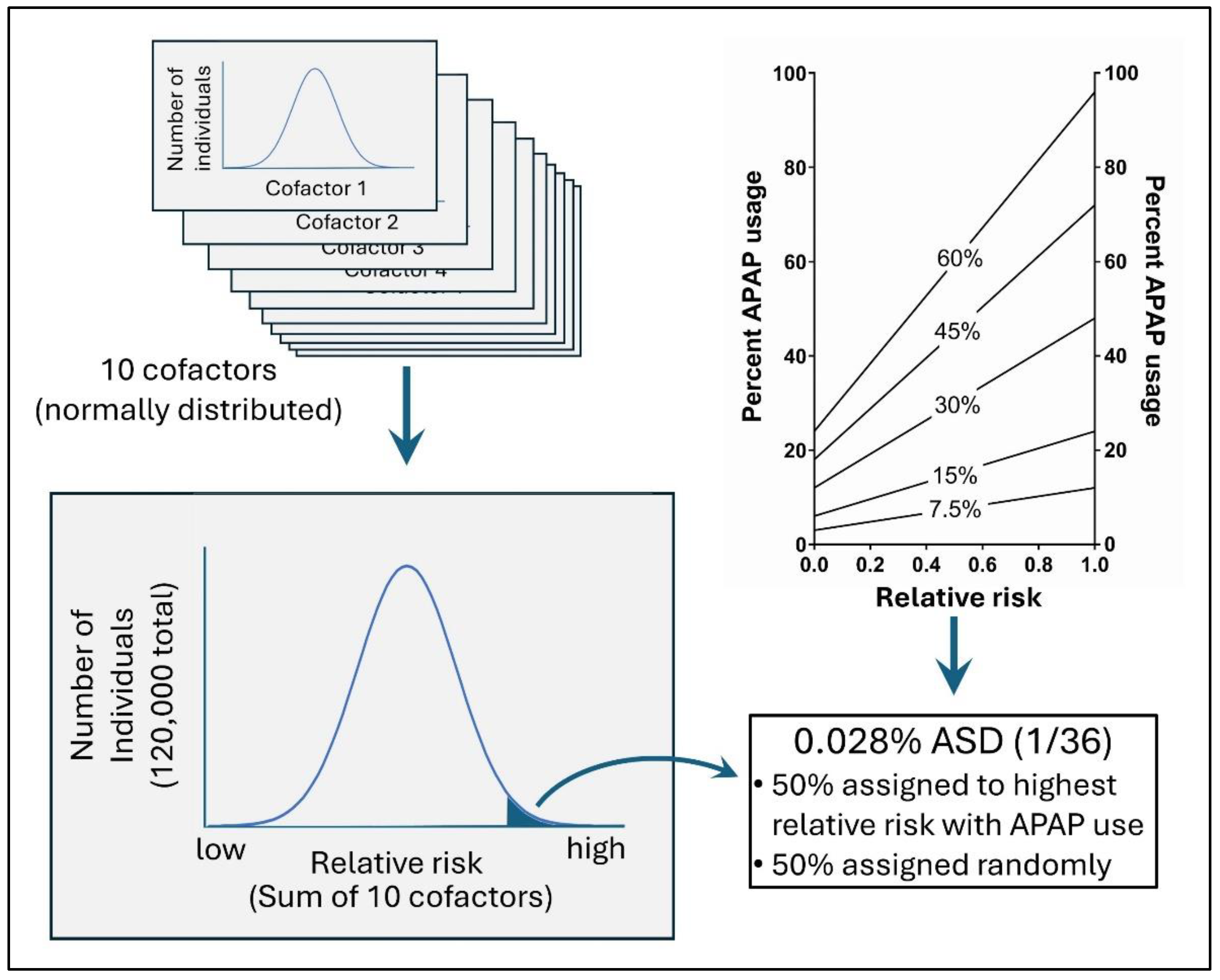
Multivariate Analysis of Cohort Data: Assessment of Methods by an In Silico Study
Methods:
In Silico Evaluation of the Cox Regression Model
Results
The Effect of Treating Cofactors (Predisposing Factors) as Confounding Factors in a Cox Regression Analysis
The Effects of Under-Reported Acetaminophen Use during Pregnancy in a Cox Regression Analysis
Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Patel E, Jones Iii JP, 3rd, Bono-Lunn D, Kuchibhatla M, Palkar A, Cendejas Hernandez J, et al. The safety of pediatric use of paracetamol (acetaminophen): a narrative review of direct and indirect evidence. Minerva pediatrics. 2022, 74, 774–788. [CrossRef] [PubMed]
- Zhao L, Jones J, Anderson L, Konsoula Z, Nevison C, Reissner K, et al. Acetaminophen causes neurodevelopmental injury in susceptible babies and children: no valid rationale for controversy. Clinical and experimental pediatrics 2023. [CrossRef] [PubMed]
- Parker W, Anderson LG, Jones JP, Anderson R, Williamson L, Bono-Lunn D, et al. The Dangers of Acetaminophen for Neurodevelopment Outweigh Scant Evidence for Long-Term Benefits. Children. 2024, 11, 44. [CrossRef]
- Schultz, S.T.; Klonoff-Cohen, H.S.; Wingard, D.L.; Akshoomoff, N.A.; Macera, C.A.; Ji, M. Acetaminophen (paracetamol) use, measles-mumps-rubella vaccination, and autistic disorder. The results of a parent survey. Autism. 2008, 12, 293–307. [Google Scholar] [CrossRef]
- Freed, G.L.; Clark, S.J.; Butchart, A.T.; Singer, D.C.; Davis, M.M. Parental vaccine safety concerns in 2009. Pediatrics. 2010, 125, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, A.; Zeldin, A.; Schuster, E.; Barrett, C.; Lehrer, D. Vaccine-related beliefs and practices of parents of children with autism spectrum disorders. American journal on intellectual and developmental disabilities. 2012, 117, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Ji Y, Azuine RE, Zhang Y, Hou W, Hong X, Wang G, et al. Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure With Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry. 2020, 77, 180–189. [CrossRef] [PubMed] [PubMed Central]
- Frisch, M.; Simonsen, J. Ritual circumcision and risk of autism spectrum disorder in 0- to 9-year-old boys: national cohort study in Denmark. J R Soc Med. 2015, 108, 266–279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Durso, G.R.O.; Luttrell, A.; Way, B.M. Over-the-Counter Relief From Pains and Pleasures Alike: Acetaminophen Blunts Evaluation Sensitivity to Both Negative and Positive Stimuli. Psychol Sci. 2015, 26, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Dewall CN, Macdonald G, Webster GD, Masten CL, Baumeister RF, Powell C, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010, 21, 931–937. [CrossRef] [PubMed]
- Roberts, I.D.; Krajbich, I.; Way, B.M. Acetaminophen influences social and economic trust. Scientific Reports. 2019, 9, 4060. [Google Scholar] [CrossRef] [PubMed]
- Cendejas-Hernandez J, Sarafian J, Lawton V, Palkar A, Anderson L, Lariviere V, et al. Paracetamol (Acetaminophen) Use in Infants and Children was Never Shown to be Safe for Neurodevelopment: A Systematic Review with Citation Tracking. Eur J Pediatr. 2022, 181, 1835–1857. [CrossRef] [PubMed]
- Hinz, B.; Cheremina, O.; Brune, K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008, 22, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Hogestatt ED, Jonsson BA, Ermund A, Andersson DA, Bjork H, Alexander JP, et al. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005, 280, 31405–31412. [CrossRef] [PubMed]
- Ottani, A.; Leone, S.; Sandrini, M.; Ferrari, A.; Bertolini, A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur J Pharmacol. 2006, 531, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Viberg, H.; Eriksson, P.; Gordh, T.; Fredriksson, A. Paracetamol (Acetaminophen) Administration During Neonatal Brain Development Affects Cognitive Function and Alters Its Analgesic and Anxiolytic Response in Adult Male Mice. Toxicol Sci. 2013, 138, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Herrington, J.A.; Guss Darwich, J.; Harshaw, C.; Brigande, A.M.; Leif, E.B.; Currie, P.J. Elevated ghrelin alters the behavioral effects of perinatal acetaminophen exposure in rats. Dev Psychobiol. 2022, 64, e22252. [Google Scholar] [CrossRef] [PubMed]
- Harshaw, C.; Warner, A.G. Interleukin-1β-induced inflammation and acetaminophen during infancy: Distinct and interactive effects on social-emotional and repetitive behavior in C57BL/6J mice. Pharmacol Biochem Behav. 2022, 220, 173463. [Google Scholar] [CrossRef]
- Philippot, G.; Gordh, T.; Fredriksson, A.; Viberg, H. Adult neurobehavioral alterations in male and female mice following developmental exposure to paracetamol (acetaminophen): characterization of a critical period. J Appl Toxicol. 2017, 37, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Suda N, Hernandez JC, Poulton J, Jones JP, Konsoula Z, Smith C, et al. Therapeutic doses of paracetamol with co-administration of cysteine and mannitol during early development result in long term behavioral changes in laboratory rats. PLoS One. 2020, 16, e0253543. [CrossRef]
- Blecharz-Klin K, Wawer A, Jawna-Zboińska K, Pyrzanowska J, Piechal A, Mirowska-Guzel D, et al. Early paracetamol exposure decreases brain-derived neurotrophic factor (BDNF) in striatum and affects social behaviour and exploration in rats. Pharmacol Biochem Behav. 2018, 168, 25–32. [CrossRef] [PubMed]
- Dean, S.L.; Knutson, J.F.; Krebs-Kraft, D.L.; McCarthy, M.M. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur J Neurosci. 2012, 35, 1218–1229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Posadas, I.; Santos, P.; Blanco, A.; Muñoz-Fernández, M.; Ceña, V. Acetaminophen induces apoptosis in rat cortical neurons. PLoS One. 2010, 5, e15360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sher, D.A.; Gibson, J.L. Pioneering, prodigious and perspicacious: Grunya Efimovna Sukhareva's life and contribution to conceptualising autism and schizophrenia. European child & adolescent psychiatry. 2023, 32, 475–490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanner, L. Autistic disturbances of affective contact. Nervous child. 1943, 2, 217–250. [Google Scholar]
- Asperger, H. Die „Autistischen Psychopathen” im Kindesalter. Archiv für Psychiatrie und Nervenkrankheiten. 1944, 117, 76–136. [Google Scholar] [CrossRef]
- Baron-Cohen, S. Leo Kanner, Hans Asperger, and the discovery of autism. The Lancet. 2015, 386, 1329–1330. [Google Scholar] [CrossRef]
- Easton T, Herrera S. J&J's dirty little secret. Forbes 1998, 42–44.
- ProPublica. Use Only As Directed2013 June 28, 2018 [cited 2013 September 20, 2013]. Available from: https://www.propublica.org/article/tylenol-mcneil-fda-use-only-as-directed.
- Matsuishi, T.; Shiotsuki, Y.; Yoshimura, K.; Shoji, H.; Imuta, F.; Yamashita, F. High prevalence of infantile autism in Kurume City, Japan. J Child Neurol. 1987, 2, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Coury, D.L.; Nash, P.L. Epidemiology and etiology of autistic spectrum disorders difficult to determine. Pediatr Ann. 2003, 32, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Nyhan, W.L. Toxicity of drugs in the neonatal period. J Pediatr. 1961, 59, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lotter, V. Childhood autism in africa. Journal of child psychology and psychiatry, and allied disciplines. 1978, 19, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Neph, A. A Comparison of Healthcare between Nicaragua and the United States and the Feasibility of Naturopathic Medicine. Honors Projects. 2011, 73. [Google Scholar]
- Vargas-Palacios, E.; Pineda, R.; Galán-Rodas, E. The politicised and crumbling Nicaraguan health system. Lancet. 2018, 392, 2694–2695. [Google Scholar] [CrossRef] [PubMed]
- Spinazzi NA, Santoro JD, Pawlowski K, Anzueto G, Howe YJ, Patel LR, et al. Co-occurring conditions in children with Down syndrome and autism: a retrospective study. Journal of neurodevelopmental disorders. 2023, 15, 9. [CrossRef] [PubMed] [PubMed Central]
- Maenner MJ, Warren Z, Williams AR, Amoakohene E, Bakian AV, Bilder DA, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). 2023, 72, 1–14. [CrossRef] [PubMed] [PubMed Central]
- Decoteau, C.L. The "Western disease": Autism and Somali parents' embodied health movements. Soc Sci Med. 2017, 177, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Sterwald, C.; Baker, J. Frosted Intellectuals: How Dr. Leo Kanner Constructed the Autistic Family. Perspect Biol Med. 2019, 62, 690–709. [Google Scholar] [CrossRef] [PubMed]
- Reid, N.; Shanley, D.C.; Logan, J.; White, C.; Liu, W.; Hawkins, E. International Survey of Specialist Fetal Alcohol Spectrum Disorder Diagnostic Clinics: Comparison of Diagnostic Approach and Considerations Regarding the Potential for Unification. International journal of environmental research and public health 2022, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hus, Y.; Segal, O. Challenges Surrounding the Diagnosis of Autism in Children. Neuropsychiatric disease and treatment. 2021, 17, 3509–3529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hen-Herbst, L.; Jirikowic, T.; Hsu, L.Y.; McCoy, S.W. Motor performance and sensory processing behaviors among children with fetal alcohol spectrum disorders compared to children with developmental coordination disorders. Res Dev Disabil. 2020, 103, 103680. [Google Scholar] [CrossRef] [PubMed]
- Coll, S.-M.; Foster, N.E.V.; Meilleur, A.; Brambati, S.M.; Hyde, K.L. Sensorimotor skills in autism spectrum disorder: A meta-analysis. Research in Autism Spectrum Disorders. 2020, 76, 101570. [Google Scholar] [CrossRef]
- Antshel, K.M.; Russo, N. Autism Spectrum Disorders and ADHD: Overlapping Phenomenology, Diagnostic Issues, and Treatment Considerations. Current psychiatry reports. 2019, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.; Benz, J.; Pei, J.; Andrew, G.; Schuller, G.; Abele-Webster, L.; et al. The impact of an ADHD co-morbidity on the diagnosis of, F.A.S.D. The Canadian journal of clinical pharmacology = Journal canadien de pharmacologie clinique. 2010, 17, e165–e176. [Google Scholar] [PubMed]
- Lai MC, Kassee C, Besney R, Bonato S, Hull L, Mandy W, et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. The lancet Psychiatry. 2019, 6, 819–829. [CrossRef] [PubMed]
- Weyrauch, D.; Schwartz, M.; Hart, B.; Klug, M.G.; Burd, L. Comorbid Mental Disorders in Fetal Alcohol Spectrum Disorders: A Systematic Review. J Dev Behav Pediatr. 2017, 38, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Matson, J.L.; Shoemaker, M. Intellectual disability and its relationship to autism spectrum disorders. Res Dev Disabil. 2009, 30, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Chokroborty-Hoque, A.; Alberry, B.; Singh, S.M. Exploring the complexity of intellectual disability in fetal alcohol spectrum disorders. Frontiers in pediatrics. 2014, 2, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bell SH, Stade B, Reynolds JN, Rasmussen C, Andrew G, Hwang PA, et al. The remarkably high prevalence of epilepsy and seizure history in fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010, 34, 1084–1089. [CrossRef] [PubMed]
- Hanlon-Dearman, A.; Chen, M.L.; Olson, H.C. Understanding and managing sleep disruption in children with fetal alcohol spectrum disorder. Biochem Cell Biol. 2018, 96, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Amos-Kroohs RM, Fink BA, Smith CJ, Chin L, Van Calcar SC, Wozniak JR, et al. Abnormal Eating Behaviors Are Common in Children with Fetal Alcohol Spectrum Disorder. J Pediatr. 2016, 169, 194–200. [CrossRef] [PubMed] [PubMed Central]
- Reid, N.; Moritz, K.M.; Akison, L.K. Adverse health outcomes associated with fetal alcohol exposure: A systematic review focused on immune-related outcomes. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2019, 30, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Al-Beltagi, M. Autism medical comorbidities. World journal of clinical pediatrics. 2021, 10, 15–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, H.; Zhu, Y.; Wang, T.; Zhang, X.; Zhang, K.; Zhang, Z. Genetic risk factors for autism-spectrum disorders: a systematic review based on systematic reviews and meta-analysis. Journal of neural transmission (Vienna, Austria : 1996). 2021, 128, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Sambo, D.; Goldman, D. Genetic Influences on Fetal Alcohol Spectrum Disorder. Genes 2023, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Molecular autism. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- May, P.A.; Gossage, J.P. Maternal risk factors for fetal alcohol spectrum disorders: not as simple as it might seem. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2011, 34, 15–26. [Google Scholar] [PubMed] [PubMed Central]
- Daniolou, S.; Pandis, N.; Znoj, H. The Efficacy of Early Interventions for Children with Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. J Clin Med. 2022, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reid N, Dawe S, Shelton D, Harnett P, Warner J, Armstrong E, et al. Systematic Review of Fetal Alcohol Spectrum Disorder Interventions Across the Life Span. Alcohol Clin Exp Res. 2015, 39, 2283–2295. [CrossRef] [PubMed]
- Thompson, T.; Oram, C.; Correll, C.U.; Tsermentseli, S.; Stubbs, B. Analgesic Effects of Alcohol: A Systematic Review and Meta-Analysis of Controlled Experimental Studies in Healthy Participants. The journal of pain. 2017, 18, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, N.; Kohno, T. Analgesic Effect of Acetaminophen: A Review of Known and Novel Mechanisms of Action. Front Pharmacol. 2020, 11, 580289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, G.; Huang, L.; Zhang, Z. The molecular mechanisms of acetaminophen-induced hepatotoxicity and its potential therapeutic targets. Exp Biol Med (Maywood). 2023, 248, 412–424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zakhari, S. Overview: how is alcohol metabolized by the body? Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2006, 29, 245–254. [Google Scholar] [PubMed] [PubMed Central]
- McGill, M.R.; Jaeschke, H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharmaceutical research. 2013, 30, 2174–2187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alberti, A.; Pirrone, P.; Elia, M.; Waring, R.H.; Romano, C. Sulphation deficit in "low-functioning" autistic children: a pilot study. Biol Psychiatry. 1999, 46, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Pagan C, Benabou M, Leblond C, Cliquet F, Mathieu A, Lemière N, et al. Decreased phenol sulfotransferase activities associated with hyperserotonemia in autism spectrum disorders. Translational Psychiatry. 2021, 11, 23. [CrossRef] [PubMed]
- Geier, D.A.; Kern, J.K.; Garver, C.R.; Adams, J.B.; Audhya, T.; Geier, M.R. A prospective study of transsulfuration biomarkers in autistic disorders. Neurochem Res. 2009, 34, 386–393. [Google Scholar] [CrossRef] [PubMed]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004, 80, 1611–1617. [CrossRef] [PubMed]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Feldon, J.; Dammann, O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011, 69, 26R–33R. [Google Scholar] [CrossRef] [PubMed]
- Stancioiu, F.; Bogdan, R.; Dumitrescu, R. Neuron-Specific Enolase (NSE) as a Biomarker for Autistic Spectrum Disease (ASD). Life (Basel, Switzerland) 2023, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, D.; Zielke, H.R.; Yeh, D.; Yang, P. Cellular stress and apoptosis contribute to the pathogenesis of autism spectrum disorder. Autism research : official journal of the International Society for Autism Research. 2018, 11, 1076–1090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frye, R.E.; Sequeira, J.M.; Quadros, E.V.; James, S.J.; Rossignol, D.A. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol Psychiatry. 2013, 18, 369–381. [Google Scholar] [CrossRef] [PubMed]
- James, S.J. Autism and Folate-dependent One-carbon Metabolism: Serendipity and Critical Branch-point Decisions in Science. Global advances in health and medicine. 2013, 2, 48–51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parker W, Hornik CD, Bilbo S, Holzknecht ZE, Gentry L, Rao R, et al. The role of oxidative stress, inflammation and acetaminophen exposure from birth to early childhood in the induction of autism. J Int Med Res. 2017, 45, 407–438. [CrossRef] [PubMed]
- McFadden, J. Razor sharp: The role of Occam's razor in science. Ann N Y Acad Sci. 2023, 1530, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ahlqvist VH, Sjöqvist H, Dalman C, Karlsson H, Stephansson O, Johansson S, et al. Acetaminophen Use During Pregnancy and Children's Risk of Autism, ADHD, and Intellectual Disability. Jama. 2024, 331, 1205–1214, Neobiomics AB, a startup company located at the Karolinska Campus that works with niche food supplement solutions for infants. Dr Gardner reported receiving grants from Swedish Research Council during the conduct of the study. Dr Lee reported receiving personal fees from Beasley Allen Law Firm, Patterson Belknap Webb & Tyler LLP, and AlphaSights and grants from NIH (1R01NS107607) during the conduct of the study and grants from NIH (1P50HD11142-01, 3 P50HD111142-02S1, 1 R01 NS131433-01), Pennsylvania Department of Human Services, US Department of Defense, and Pennsylvania Department of Health CURE SAP (# 410008574)7 outside the submitted work. No other disclosures were reported. [CrossRef] [PubMed] [PubMed Central]
- Vatcheva, K.P.; Lee, M.; McCormick, J.B.; Rahbar, M.H. The Effect of Ignoring Statistical Interactions in Regression Analyses Conducted in Epidemiologic Studies: An Example with Survival Analysis Using Cox Proportional Hazards Regression Model. Epidemiology (Sunnyvale, Calif) 2015, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, C.; Frewer, V.; Cox, G.; Williams, K.; Ure, A. Prevalence and Age of Onset of Regression in Children with Autism Spectrum Disorder: A Systematic Review and Meta-analytical Update. Autism research : official journal of the International Society for Autism Research. 2021, 14, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Bandoli, G.; Palmsten, K.; Chambers, C. Acetaminophen use in pregnancy: Examining prevalence, timing, and indication of use in a prospective birth cohort. Paediatr Perinat Epidemiol. 2020, 34, 237–246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woodbury, M.L.; Cintora, P.; Ng, S.; Hadley, P.A.; Schantz, S.L. Examining the relationship of acetaminophen use during pregnancy with early language development in children. Pediatr Res. 2023. [CrossRef]
- Bornehag CG, Reichenberg A, Hallerback MU, Wikstrom S, Koch HM, Jonsson BA, et al. Prenatal exposure to acetaminophen and children's language development at 30 months. Eur Psychiatry. 2018, 51, 98–103. [CrossRef] [PubMed]
- Westerlund, T.; Barzi, S.; Bernsten, C. Consumer views on safety of over-the-counter drugs, preferred retailers and information sources in Sweden: after re-regulation of the pharmacy market. Pharmacy practice. 2017, 15, 894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Case 1, 22-md-03043-DLC Document 1381, Page 111. Filed 12/18/23. US Federal Court; 2024.
- Alemany S, Avella-García C, Liew Z, García-Esteban R, Inoue K, Cadman T, et al. Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: Meta-analysis in six European population-based cohorts. Eur J Epidemiol. 2021, 36, 993–1004. [CrossRef] [PubMed]
- Ertmann, R.K.; Møller, J.J.; Waldorff, F.B.; Siersma, V.; Reventlow, S.; Söderström, M. The majority of sick children receive paracetamol during the winter. Danish medical journal. 2012, 59, A4555. [Google Scholar] [PubMed]
- Lewerissa, E.I.; Nadif Kasri, N.; Linda, K. Epigenetic regulation of autophagy-related genes: Implications for neurodevelopmental disorders. Autophagy. 2024, 20, 15–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miners, J.O.; Robson, R.A.; Birkett, D.J. Paracetamol metabolism in pregnancy. Br J Clin Pharmacol. 1986, 22, 359–362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O'Hara, K.; Wright, I.M.; Schneider, J.J.; Jones, A.L.; Martin, J.H. Pharmacokinetics in neonatal prescribing: evidence base, paradigms and the future. Br J Clin Pharmacol. 2015, 80, 1281–1288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: the immune system feels the heat. Nature reviews Immunology. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Sullivan, J.E.; Farrar, H.C. Fever and Antipyretic Use in Children. Pediatrics. 2011, 127, 580–587. [Google Scholar] [CrossRef]
- El-Radhi, A.S.M. Fever management: Evidence vs current practice. Clin Pediatr. 2012, 1, 29–33. [Google Scholar]
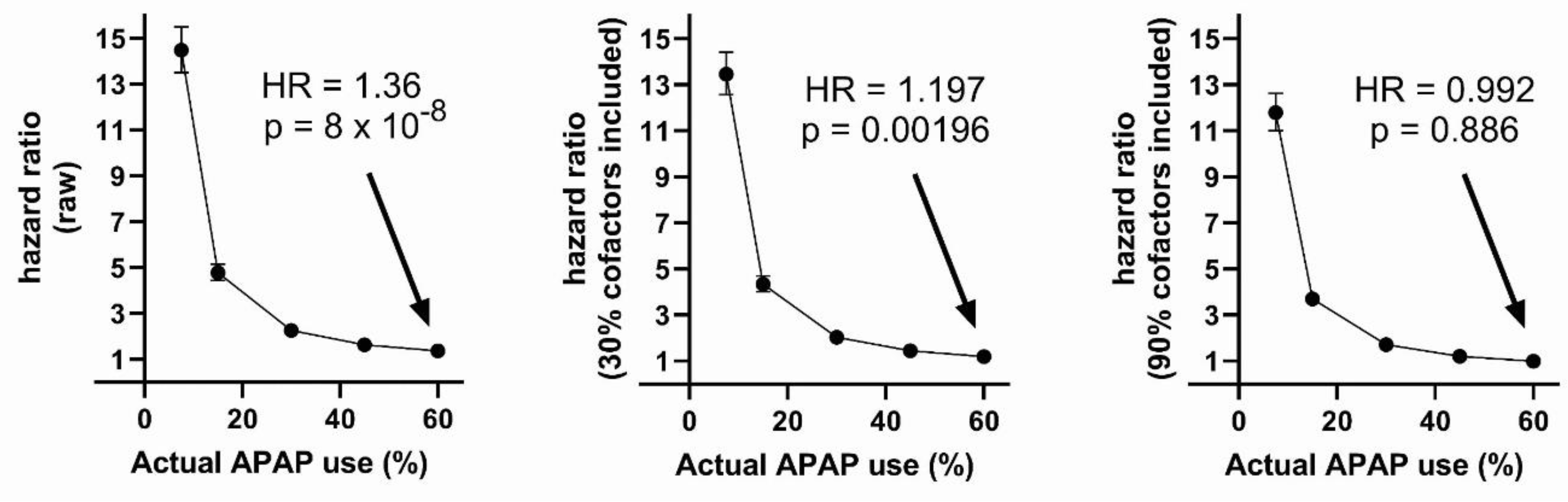
| Number cofactors included | Variable | HR (p-value) | Lower-upper 95% CI |
|---|---|---|---|
| 0 | APAP | 2.461 (2e-16) | 2.675- 2.265 |
| 3 | Cofactor 1 | 1.219 (2e-16) | 1.241 - 1.198 |
| 3 | Cofactor 2 | 1.197 (2e-16) | 1.217 - 1.176 |
| 3 | Cofactor 3 | 1.186 (2e-16) | 1.207 - 1.166 |
| 3 | APAP | 1.844 (2e-16) | 2.007 - 1.694 |
| 9 | Cofactor 1 | 1.250 (2e-16) | 1.275 - 1.231 |
| 9 | Cofactor 2 | 1.210 (2e-16) | 1.233 - 1.192 |
| 9 | Cofactor 3 | 1.203 (2e-16) | 1.227 - 1.185 |
| 9 | Cofactor 4 | 1.221 (2e-16) | 1.245 - 1.203 |
| 9 | Cofactor 5 | 1.215 (2e-16) | 1.238 - 1.197 |
| 9 | Cofactor 6 | 1.235 (2e-16) | 1.260 - 1.217 |
| 9 | Cofactor 7 | 1.216 (2e-16) | 1.240 - 1.199 |
| 9 | Cofactor 8 | 1.227 (2e-16) | 1.251 - 1.208 |
| 9 | Cofactor 9 | 1.215 (2e-16) | 1.238 - 1.197 |
| 9 | APAP | 0.905 (0.0264) | 0.828 - 0.988 |
| Number cofactors included | Variable | HR (p-value) | Lower-upper 95% CI |
|---|---|---|---|
| 0 | APAP | 1.364 (8.13e-08) | 1.218 - 1.528 |
| 3 | Cofactor 1 | 1.238 (2e-16) | 1.217 - 1.260 |
| 3 | Cofactor 2 | 1.216 (2e-16) | 1.196 - 1.237 |
| 3 | Cofactor 3 | 1.205 (2e-16) | 1.185 - 1.226 |
| 3 | APAP | 1.197 (0.00196) | 1.068 - 1.341 |
| 9 | Cofactor 1 | 1.250 (2e-16) | 1.229 - 1.272 |
| 9 | Cofactor 2 | 1.210 (2e-16) | 1.190 - 1.230 |
| 9 | Cofactor 3 | 1.203 (2e-16) | 1.183 - 1.224 |
| 9 | Cofactor 4 | 1.221 (2e-16) | 1.200 - 1.242 |
| 9 | Cofactor 5 | 1.215 (2e-16) | 1.194 - 1.236 |
| 9 | Cofactor 6 | 1.235 (2e-16) | 1.214 - 1.256 |
| 9 | Cofactor 7 | 1.216 (2e-16) | 1.196 - 1.237 |
| 9 | Cofactor 8 | 1.227 (2e-16) | 1.206 - 1.248 |
| 9 | Cofactor 9 | 1.215 (2e-16) | 1.194 - 1.235 |
| 9 | APAP | 0.992 (0.886) | 0.885 - 1.111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





