Submitted:
31 May 2024
Posted:
06 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
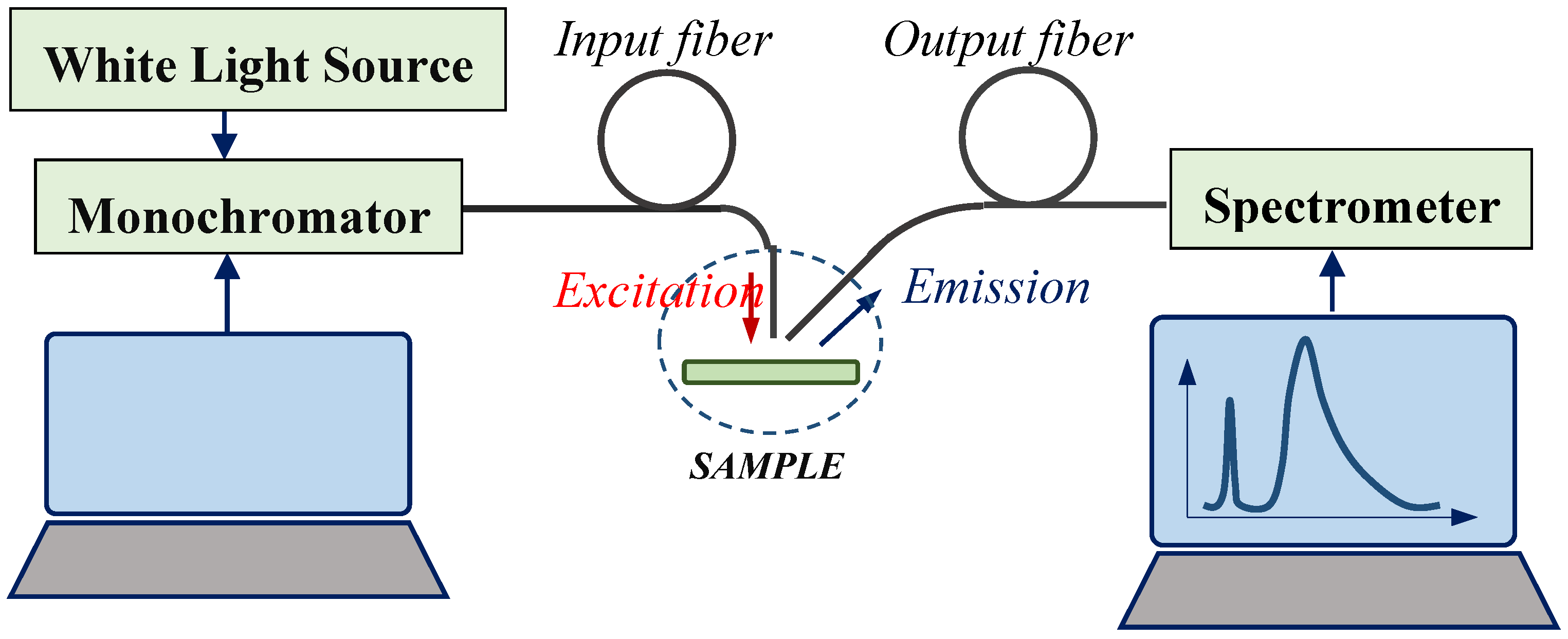
2.2. Sample Analysis
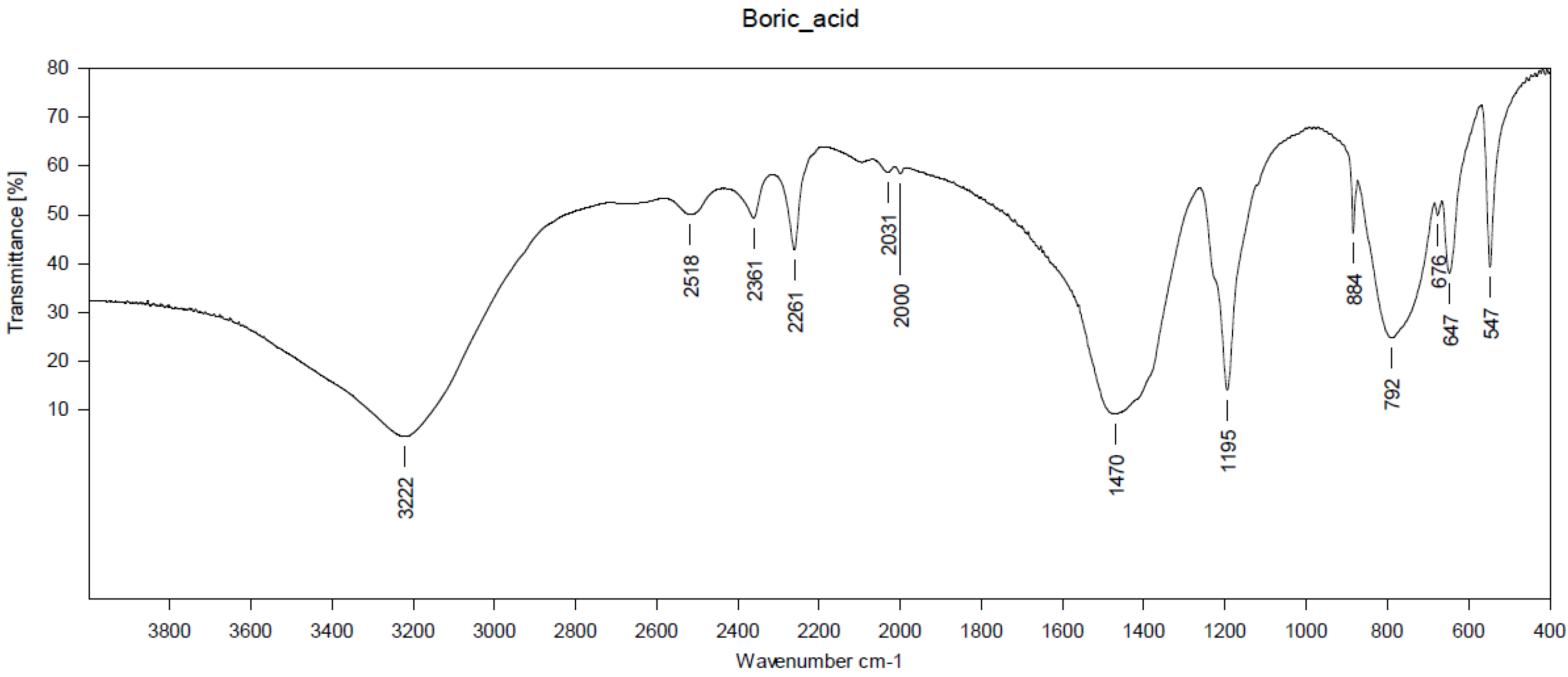
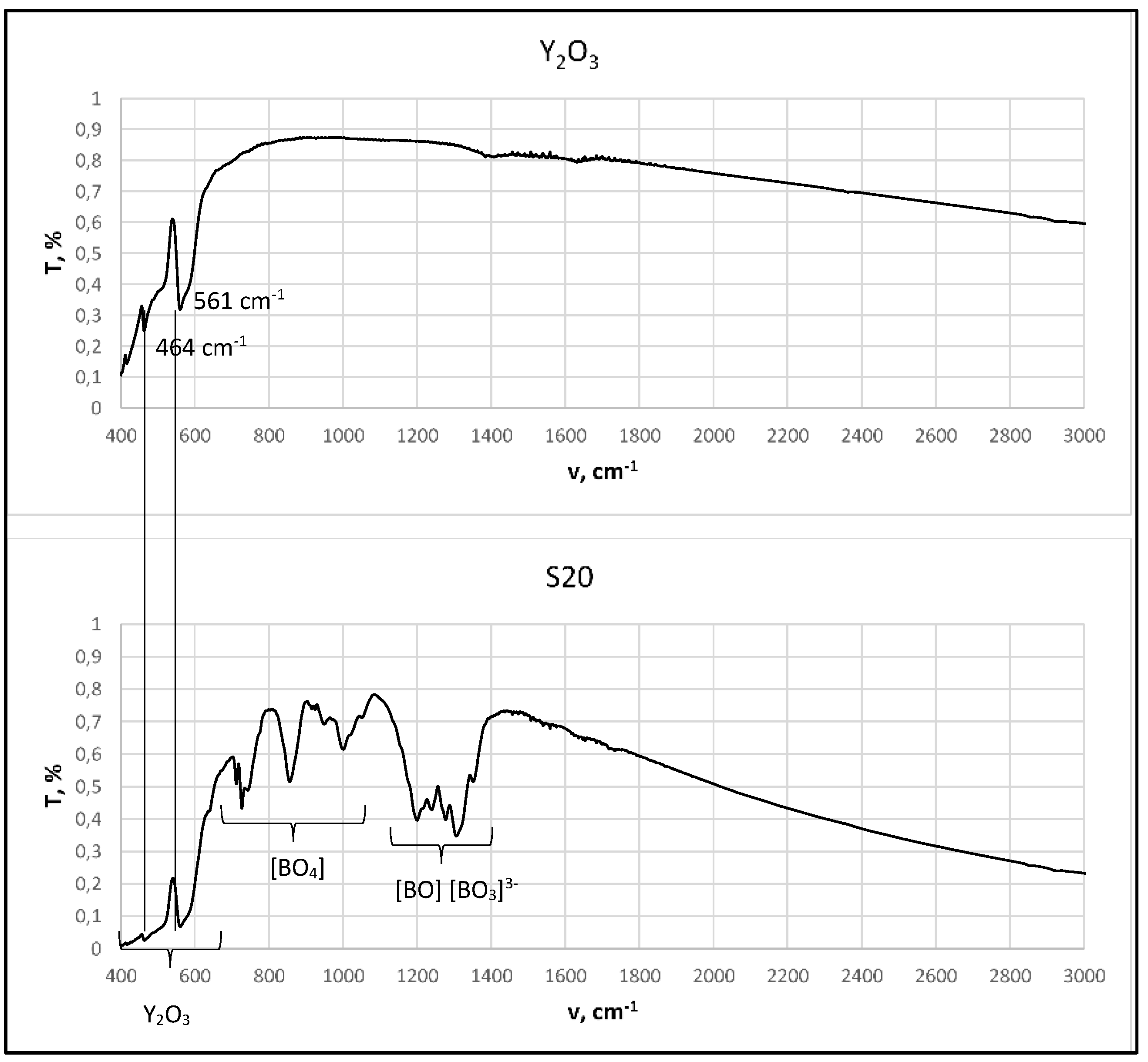
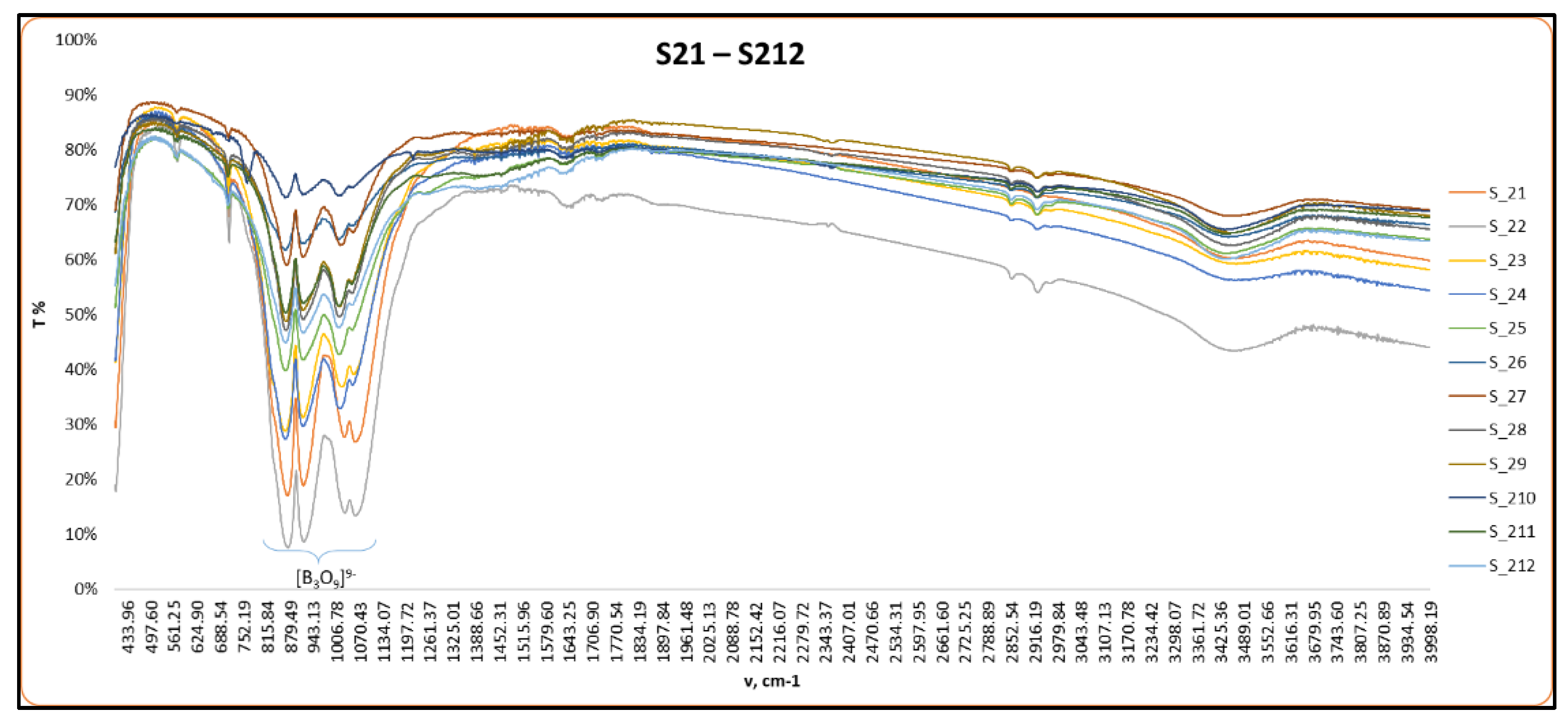
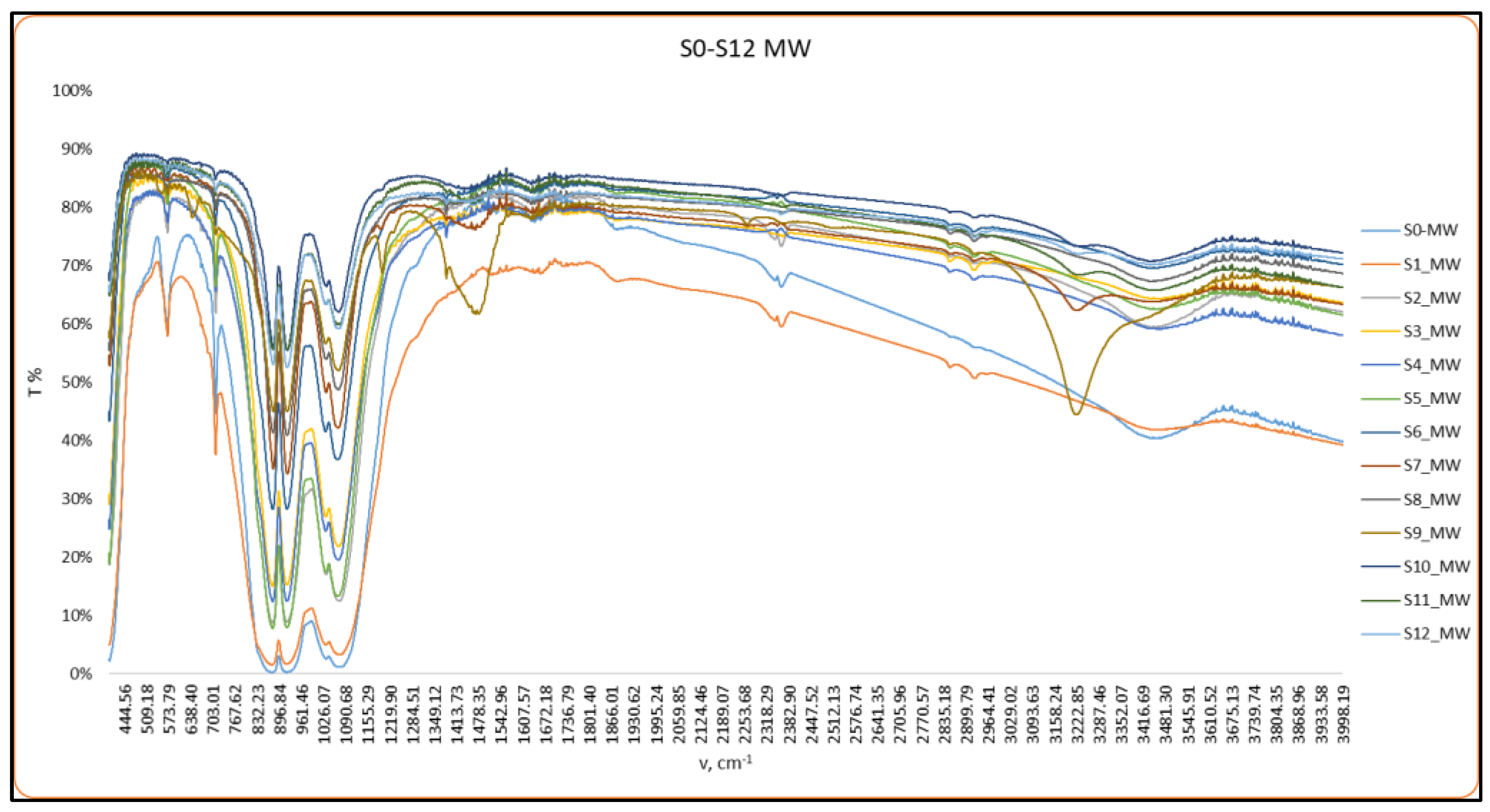
2.2.1. FT-IR Analysis
2.2.2. Photoluminescent Analysis
3. Results
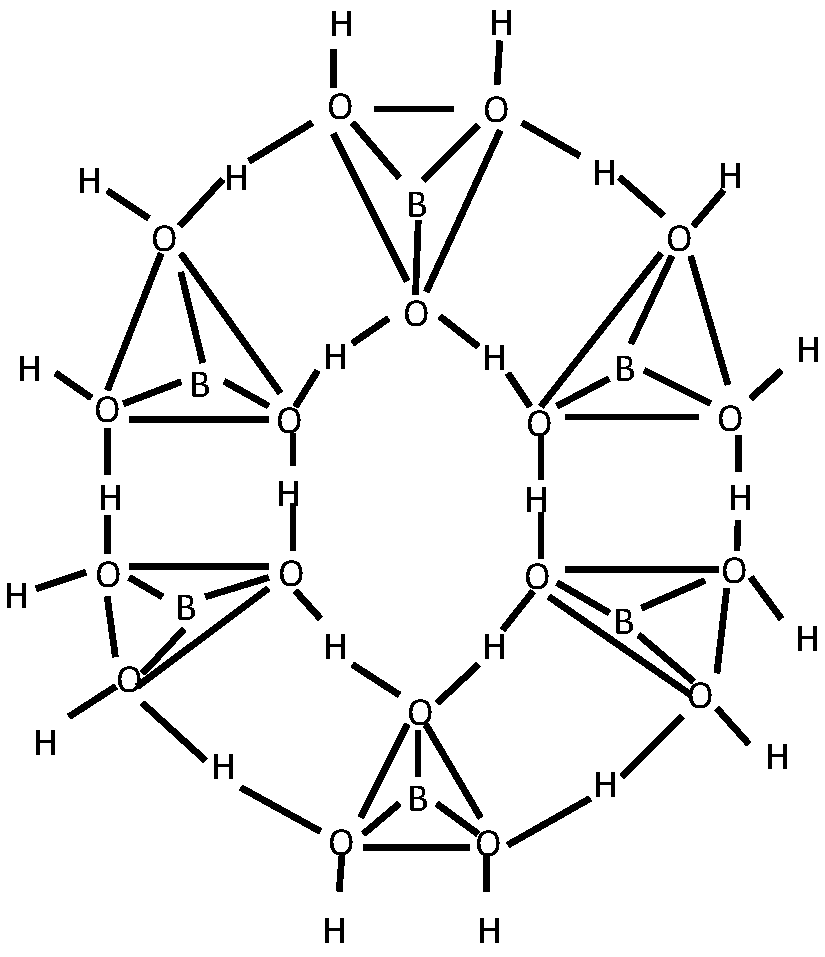
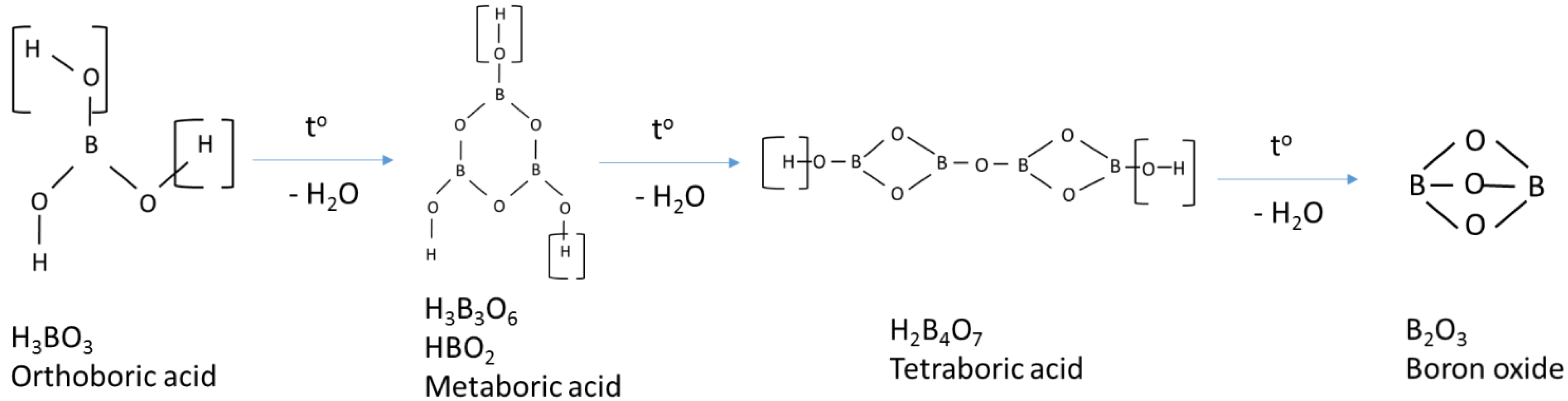
3.1. Structural Characterization
3.2. Photoluminescent Properties
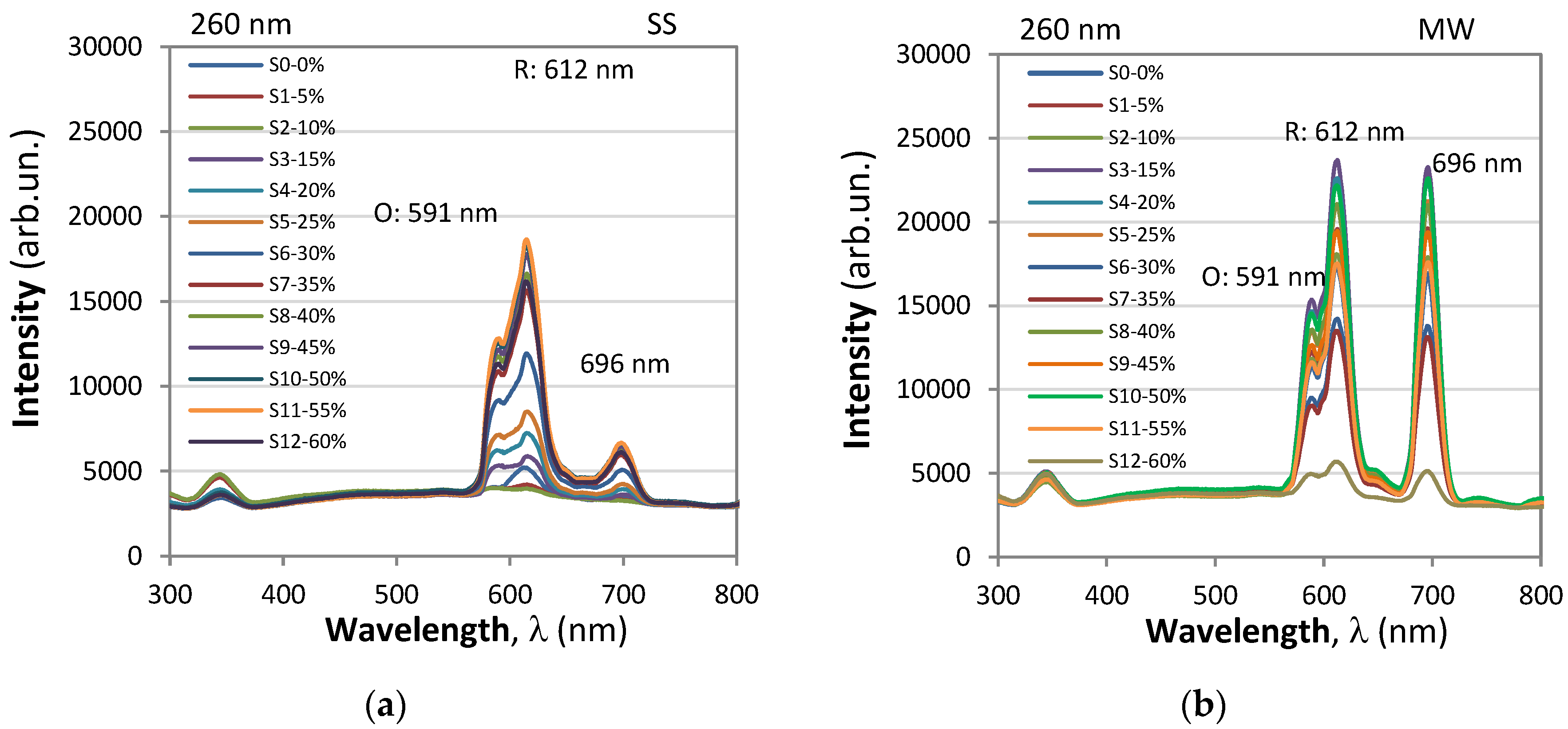
- Orange emission (5D₀→7F₁): A peak at 591 nm was identified, corresponding to the 5D₀→7F₁ transition, resulting in orange emission.
- Red emission (5D₀→7F₂): Peaks at 612 nm and 620 nm were observed, corresponding to the 5D₀→7F₂ transition, resulting in red emission.
-
Transition intensity and matrix influence:
- When Eu³⁺ ions occupy inversion center sites, the 5D₀→7F₁ transitions were expected to be relatively strong, while the 5D₀→7F₂ transitions were relatively weak.
- The transition 5D₀→7F₁ due to magnetic-dipole is independent of the host matrix, whereas the electric-dipole allowed 5D₀→7F₂ transition is strongly influenced by the local structure.
-
Emission intensity ratio (R/O Ratio):
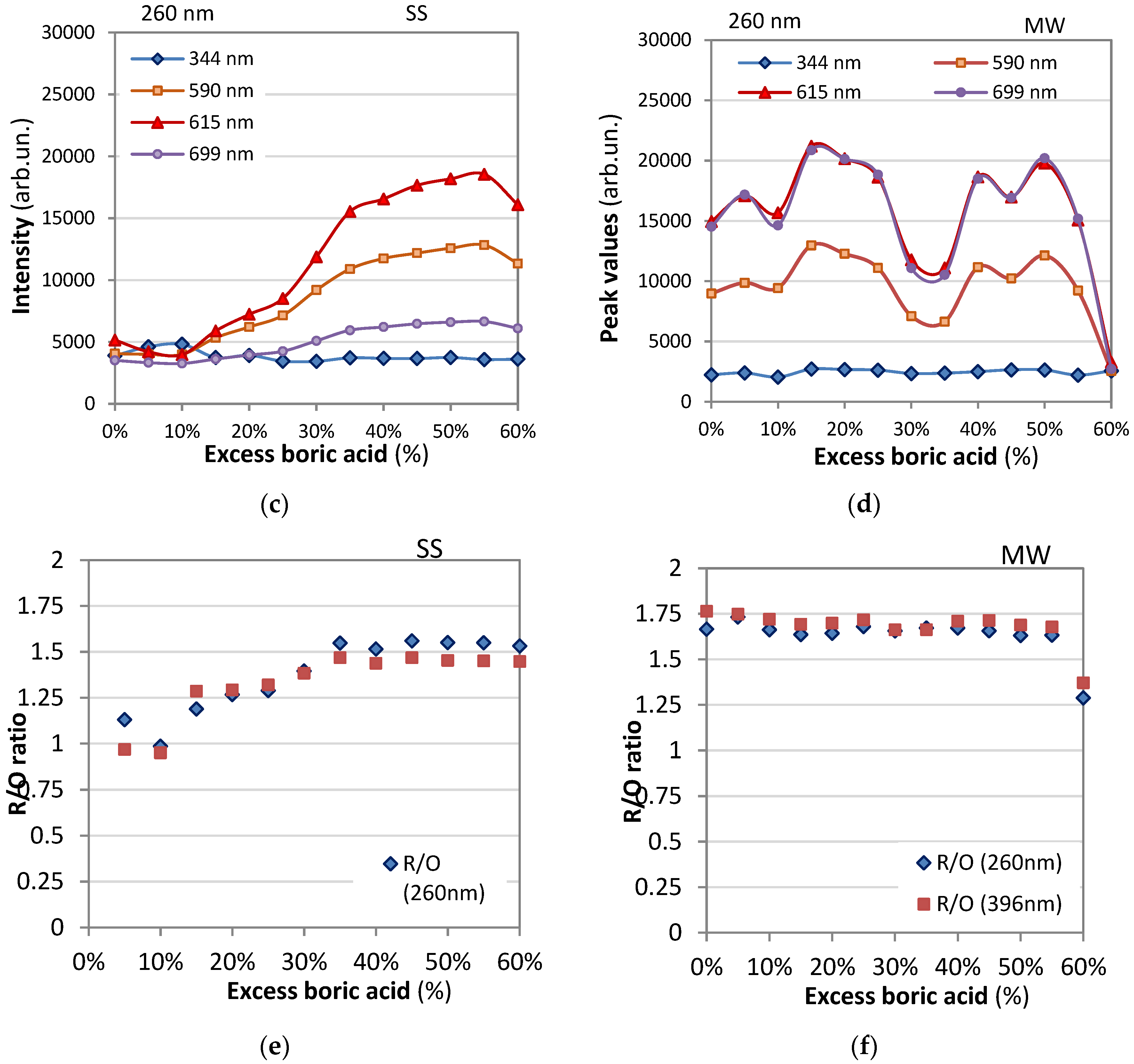
- The emission intensity ratio between red and orange color transitions, denoted as R/O (I(5D₀→7F₂)/I(5D₀→7F₁)), was calculated by considering the sum of integral intensity of red emission peak observed at 612 nm for contribution of 5D0/7F2 transition. The intensities of the different 5D0 - 7FJ transitions and the splitting of these emission peaks depend on the local symmetry of the crystal field of the Eu3+ ion. If the Eu3+ ion occupies a centrosymmetric site in the crystal lattice, the magnetic dipole transition 5D0 − 7F 1 (orange) is the dominant transition; otherwise, the electric dipole transition 5D0 − 7F2 (red) becomes dominant.
- This ratio, also known as the asymmetric ratio, color purity, or red-to-orange emission ratio, provides insights into the relative strengths of the red and orange emissions. YBO3:Eu3+ has a hexagonal structure of the vaterite type, with Eu 3+ ions occupying the Y3+ site, which has point symmetry S 6. As a result, the orange emission at 592 nm from the 5 D0 − 7F1 transition is dominant, leading to a lower value of the intensity ratio (R/O) between red and orange emission. Good color purity requires a high R/O value, and thus many studies aim to improve this ratio.
- Emission at 696 nm whose intensity is comparable to that of the 612 nm peak for 396 nm excitation and considerably lower for 260 nm excitation.
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Plewa, J.; Jüstel, T. ; Phase transition of YBO3. Journal of Thermal Analysis and Calorimetry 2007, 88, 531–535. [Google Scholar] [CrossRef]
- Fuchs, B.; Schröder, F.; Heymann, G.; Siegel, R.; Senker, J.; Jüstel, T.; and Huppertz, H. ; Crystal structure re-determination, spectroscopy, and photoluminescence of π-YBO3:Eu3+. Z. anorg. allg. Chem. 2021, 647, 2035–2046. [Google Scholar] [CrossRef]
- Sevim, F.; Demir, F.; Bilen, M.; Okur, H. Kinetic analysis of thermal decomposition of boric acid from thermogravimetric data. Korean J Chem Eng. 2006, 23, 736–740. [Google Scholar] [CrossRef]
- Harabor, A.; Rotaru, P.; Scorei, R.I.; Harabor, N.A. Non-conventional hexagonal structure for boric acid. J Therm Anal Calorim. 2014, 118, 1375–1384. [Google Scholar] [CrossRef]
- Huber, C.; Jahromy, S.S.; Birkelbach, F.; et al. The multistep decomposition of boric acid. Energy Sci Eng. 2020, 8, 1650–1666. [Google Scholar] [CrossRef]
- Ben Smida, Y.; Marzouki, R.; Kaya, S.; Erkan, S.; Faouzi Zid, M.; Hichem Hamzaoui, A. Synthesis Methods in Solid-State Chemistry. Synthesis Methods and Crystallization, Editor Riadh Marzouki, InTech Open, 2020, 1-13. [CrossRef]
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia-Ruiz, N.; Carassiti, L.; Harrison, A.; Gregory, D.H. Modern Microwave Methods in Solid-State Inorganic Materials Chemistry: From Fundamentals to Manufacturing. Chemical Reviews 2023, 114, 1170–1206. [Google Scholar] [CrossRef]
- Liu, F.W.; Hsu, C.H.; Chen, F.S.; Lu, C.H. Microwave-assisted solvothermal preparation and photoluminescence properties of Y2O3:Eu3+ phosphors. Ceramics International 2012, 38, 1577–1584. [Google Scholar] [CrossRef]
- Boyer, D.; Bertrand-Chadeyron, G.; Mahiou, R.; Brioude, A.; Mugnier, J. Synthesis and characterization of sol–gel derived Y3BO6:Eu3+ powders and films. Optical Materials 2003, 24, 35–41. [Google Scholar] [CrossRef]
- Maia, L.J.Q.; Mastelaro, V.R.; Pairis, S.; Hernandes, A.C.; Ibanez, A. A sol–gel route for the development of rare-earth aluminum borate nanopowders and transparent thin films. Journal of Solid State Chemistry 2007, 180, 611–618. [Google Scholar] [CrossRef]
- Gangwar, A.K.; Nagpal, K.; Kumar, P.; Singh, N.; Gupta, B.K. New insight into printable europium-doped yttrium borate luminescent pigment for security ink applications. Journal of Applied Physics 2019, 125, 074903. [Google Scholar] [CrossRef]
- Medvedev, E.F.; Komarevskaya, A.S. IR spectroscopic study of the phase composition of boric acid as a component of glass batch. Glass and Ceramics 2007, 64, 42–46. [Google Scholar] [CrossRef]
- Vlasov, A. G. and Florinskaya, V. A. (eds.), Structure and Physicochemical Properties of Inorganic Glasses [in Russian], Khimiya, Leningrad 1974.
- Kazuo N., Infrared and Raman Spectra of Inorganic and Coordination Compounds: Theory and Applications in Inorganic Chemistry, 6th ed., Wiley, New York 1997.
- Stefanovskii, S.V.; Ivanov, I.A.; Gulin, A.N. IR and EPR spectra of aluminoborosilicate and aluminophosphate glasses simulating vitrified radioactive wastes. Fiz. Khim. Stekla 1991, 17, 120–125. [Google Scholar]
- Beregi, E.; Watterich, A.; Kovács, L.; Madarász, J. Solid-state reactions in Y2O3:3Al2O3:4B2O3 system studied by FTIR spectroscopy and X-ray diffraction. Vibrational Spectroscopy 2000, 22, 169–173. [Google Scholar] [CrossRef]
- Onani, M.O.; Okil, J.O.; Dejene, F.B. Solution–combustion synthesis and photoluminescence properties of YBO3:Tb3+ phosphor powders. Physica B: Condensed Matter 2014, 439, 133–136. [Google Scholar] [CrossRef]
- Zhang, F.; Bian, G.; Ding, H.; Tang, J.; Li, X.; Zhang, C.; Li, S.; Jia, G. Controllable synthesis and tunable luminescence of yttrium orthoborate microcrystals with multiform morphologies and dimensions. J. of Luminescence 2020, 219, 116890. [Google Scholar] [CrossRef]
- Hristova, K.; Kostova, I.P.; Eftimov, T.A.; Brabant, D.; Fouzar, S. Rare-Earth-Ion (RE3+)-Doped Aluminum and Lanthanum Borates for Mobile-Phone-Interrogated Luminescent Markers. Photonics 2024, 11, 434. [Google Scholar] [CrossRef]
| Sample code, series SS | Sample code, series MW | H3BO3, g (% excess) |
|---|---|---|
| S20 SS | S20 MW | 0.3537 (0 %) |
| S21 SS | S21 MW | 0.3713 (5%) |
| S22 SS | S22 MW | 0.3891 (10 %) |
| S23 SS | S23 MW | 0.4067 (15 %) |
| S24 SS | S24 MW | 0.4244 (20%) |
| S25 SS | S25 MW | 0.4421 (25 %) |
| S26 SS | S26 MW | 0.4598 (30 %) |
| S27 SS | S27 MW | 0.4772 (35 %) |
| S28 SS | S28 MW | 0.4949 (40 %) |
| S29 SS | S29 MW | 0.5126 (45 %) |
| S210 SS | S210 MW | 0.5303 (50 %) |
| S211 SS | S211 MW | 0.5479 (55 %) |
| S212 SS | S212 MW | 0.5656 (60 %) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





