Submitted:
31 May 2024
Posted:
03 June 2024
You are already at the latest version
Abstract
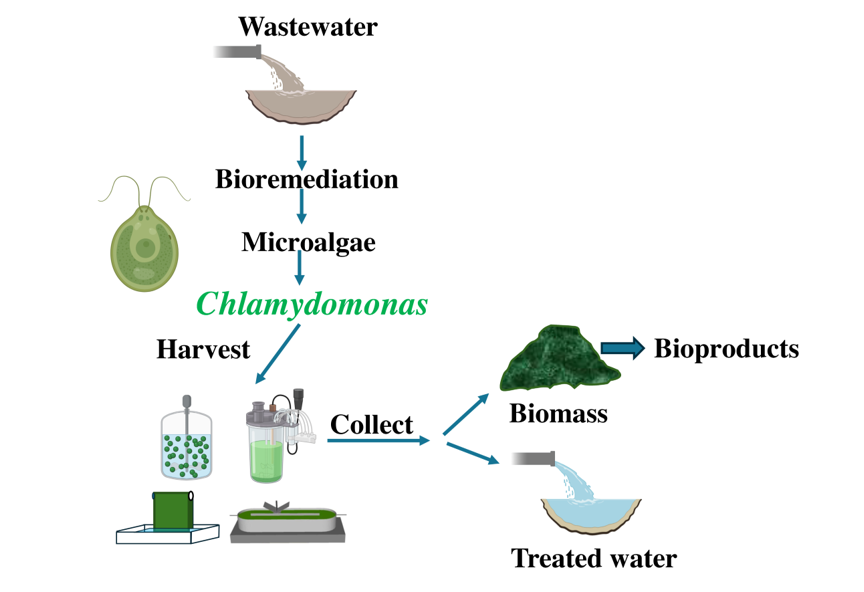
Keywords:
1. Introduction. Why Microalgae and Why Chlamydomonas?
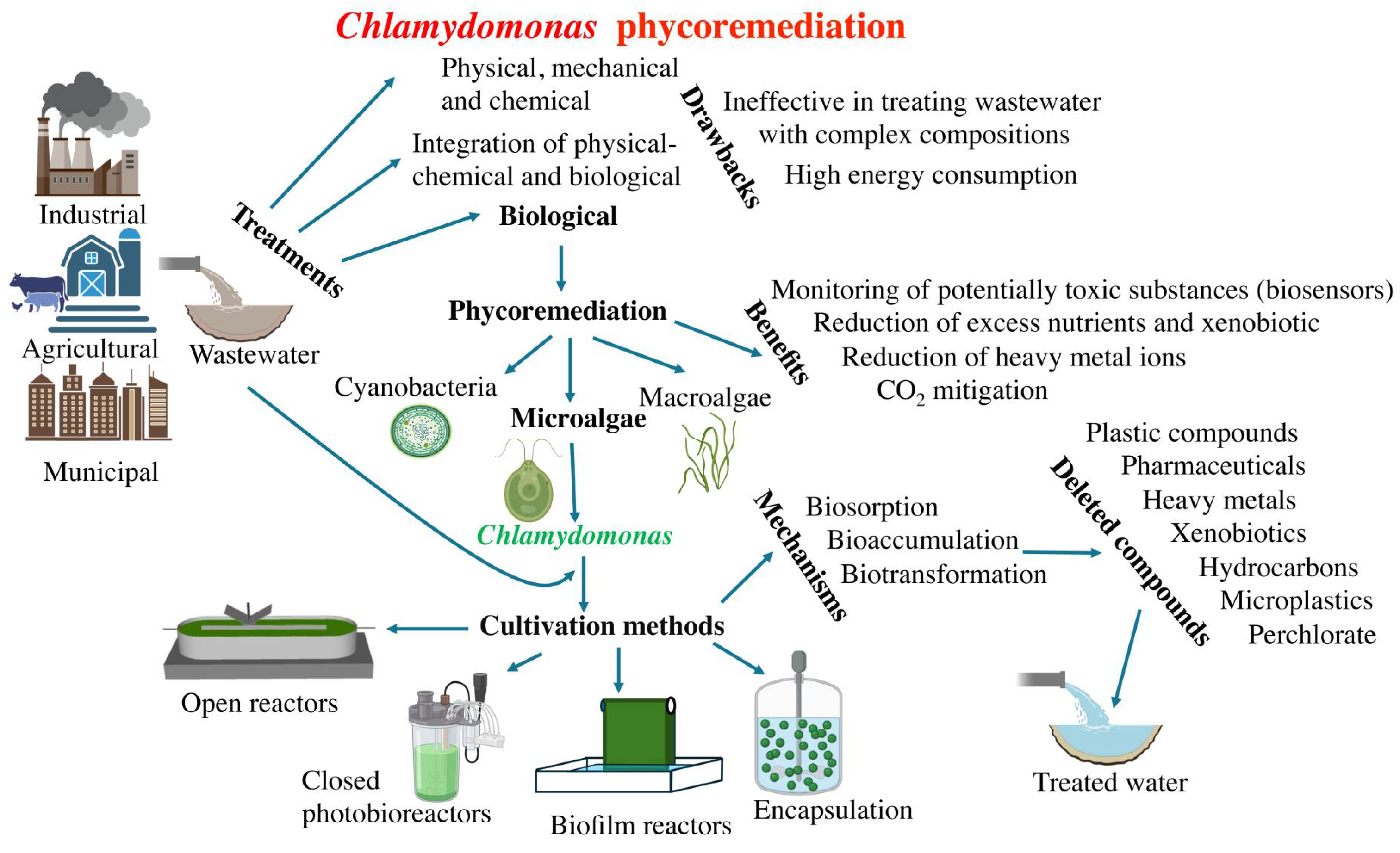
2. Wastewater and Advantages of Using Microalgae for Its Bioremediation
3. Microalgae Cultivation Methods
3.1. Open Reactors
3.2. Close Photoreactors
3.3. Biofilm Reactors
3.4. Encapsulation
4. Chlamydomonas Phycoremediation
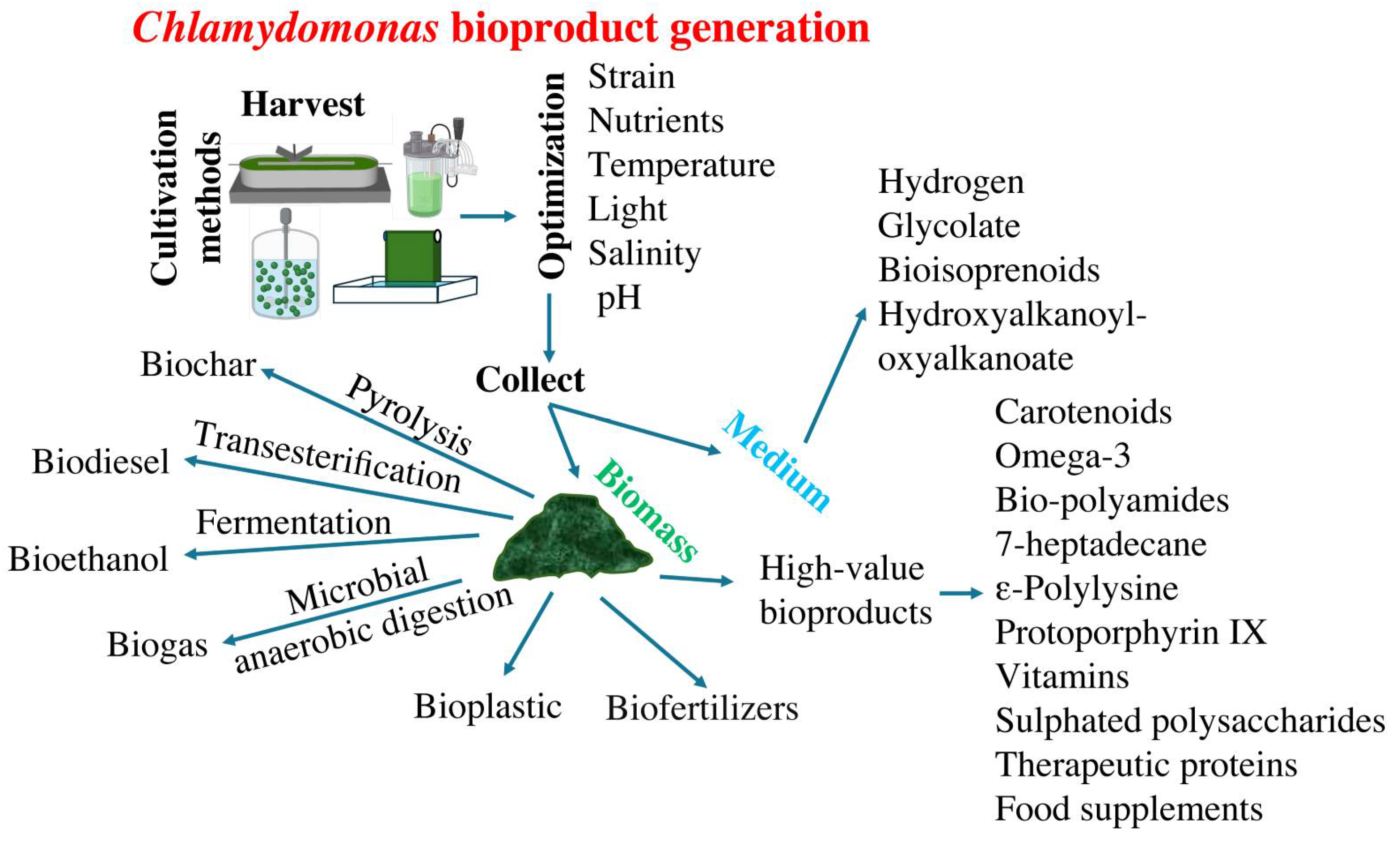
5. Chlamydomonas Bioproduct Generation
5.1. Biomass
5.2. Biochar
5.3. Biofertilizers
5.4. Bioplastic
5.5. Biofuels
5.5.1. Biodiesel
5.5.2. Bioethanol
5.5.3. Biogas
5.5.4. Hydrogen
5.6. High-Value Bioproducts
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rani, S.; Gunjyal, N.; Ojha, C.S.P.; Singh, R.P. Review of Challenges for Algae-Based Wastewater Treatment: Strain Selection, Wastewater Characteristics, Abiotic, and Biotic Factors. J. Hazardous, Toxic, Radioact. Waste 2021, 25, 03120004. [Google Scholar] [CrossRef]
- Falkowski, P.G. The Role of Phytoplankton Photosynthesis in Global Biogeochemical Cycles. Photosynth. Res. 1994, 39, 235–258. [Google Scholar] [CrossRef]
- Ochoa De Alda, J.A.G.; Esteban, R.; Diago, M.L.; Houmard, J. The Plastid Ancestor Originated among One of the Major Cyanobacterial Lineages. Nat. Commun. 2014, 5, 4937. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, S.J.; Archibald, J.M. Genomic Insights into Plastid Evolution. Genome Biol. Evol. 2020, 12, 978–990. [Google Scholar] [CrossRef] [PubMed]
- de Cassia Soares Brandão, B.; Oliveira, C.Y.B.; dos Santos, E.P.; de Abreu, J.L.; Oliveira, D.W.S.; da Silva, S.M.B.C.; Gálvez, A.O. Microalgae-Based Domestic Wastewater Treatment: A Review of Biological Aspects, Bioremediation Potential, and Biomass Production with Biotechnological High-Value. Environ. Monit. Assess. 2023, 195, 1384. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ye, X.; Bi, H.; Shen, Z. Microalgae Biofuels: Illuminating the Path to a Sustainable Future amidst Challenges and Opportunities. Biotechnol. Biofuels Bioprod. 2024, 17, 10. [Google Scholar] [CrossRef]
- Gao, S.; Chen, W.; Cao, S.; Sun, P.; Gao, X. Microalgae as Fishmeal Alternatives in Aquaculture: Current Status, Existing Problems, and Possible Solutions. Environ. Sci. Pollut. Res. 2024, s11356. [Google Scholar] [CrossRef]
- Gupta, A.; Kang, K.; Pathania, R.; Saxton, L.; Saucedo, B.; Malik, A.; Torres-Tiji, Y.; Diaz, C.J.; Dutra Molino, J.V.; Mayfield, S.P. Harnessing Genetic Engineering to Drive Economic Bioproduct Production in Algae. Front. Bioeng. Biotechnol. 2024, 12, 1350722. [Google Scholar] [CrossRef] [PubMed]
- Udaypal; Goswami, R. K.; Mehariya, S.; Verma, P. Advances in Microalgae-Based Carbon Sequestration: Current Status and Future Perspectives. Environ. Res. 2024, 249, 118397. [Google Scholar] [CrossRef]
- Sasso, S.; Stibor, H.; Mittag, M.; Grossman, A.R. From Molecular Manipulation of Domesticated Chlamydomonas Reinhardtii to Survival in Nature. Elife 2018, 7, e39233. [Google Scholar] [CrossRef]
- Salomé, P.A.; Merchant, S.S. A Series of Fortunate Events: Introducing Chlamydomonas as a Reference Organism. Plant Cell 2019, 31, 1682–1707. [Google Scholar] [CrossRef] [PubMed]
- Oz Yasar, C.; Fletcher, L.; Camargo-Valero, M.A. Effect of Macronutrients (Carbon, Nitrogen, and Phosphorus) on the Growth of Chlamydomonas Reinhardtii and Nutrient Recovery under Different Trophic Conditions. Environ. Sci. Pollut. Res. 2023, 30, 111369–111381. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, U. The Chlamydomonas Sourcebook. Volume 1: Introduction to Chlamydomonas and Its Laboratory Use. Elsevier Acad. Press. [CrossRef]
- Calatrava, V.; Tejada-Jimenez, M.; Sanz-Luque, E.; Fernandez, E.; Galvan, A.; Llamas, A. Chlamydomonas Reinhardtii, a Reference Organism to Study Algal–Microbial Interactions: Why Can’t They Be Friends? Plants 2023, 12, 788. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Q.; Zhou, R.; Feng, J.; Zhang, K.; Li, X.; Ma, X.; Dietrich, A.M. Occurrence of Free Amino Acids in the Source Waters of Zhejiang Province, China, and Their Removal and Transformation in Drinking Water Systems. Water (Switzerland) 2020, 12, 73. [Google Scholar] [CrossRef]
- Ahamed, A.; Ge, L.; Zhao, K.; Veksha, A.; Bobacka, J.; Lisak, G. Environmental Footprint of Voltammetric Sensors Based on Screen-Printed Electrodes: An Assessment towards “Green” Sensor Manufacturing. Chemosphere 2021, 278, 130462. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Dutta, D.; Singh, A.; Dubey, R.; Kamboj, D.V. Recent Advances in the Elimination of Persistent Organic Pollutants by Photocatalysis. Front. Environ. Sci. 2022, 10, 872514. [Google Scholar] [CrossRef]
- Wagner, T. V.; Rempe, F.; Hoek, M.; Schuman, E.; Langenhoff, A. Key Constructed Wetland Design Features for Maximized Micropollutant Removal from Treated Municipal Wastewater: A Literature Study Based on 16 Indicator Micropollutants. Water Res. 2023, 244, 120534. [Google Scholar] [CrossRef] [PubMed]
- Morillas-España, A.; Lafarga, T.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; González-López, C. Microalgae Based Wastewater Treatment Coupled to the Production of High Value Agricultural Products: Current Needs and Challenges. Chemosphere 2022, 291, 132968. [Google Scholar] [CrossRef]
- Elangovan, B.; Detchanamurthy, S.; Senthil Kumar, P.; Rajarathinam, R.; Deepa, V.S. Biotreatment of Industrial Wastewater Using Microalgae: A Tool for a Sustainable Bioeconomy. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Talukdar, A.; Kundu, P.; Bhattacharya, S.; Dutta, N. Microplastic Contamination in Wastewater: Sources, Distribution, Detection and Remediation through Physical and Chemical-Biological Methods. Sci. Total Environ. 2024, 916, 170254. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of Emerging Micropollutants Originating from Pharmaceuticals and Personal Care Products (PPCPs) in Water and Wastewater by Advanced Oxidation Processes: A Review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Singh, A.; Pal, D.B.; Mohammad, A.; Alhazmi, A.; Haque, S.; Yoon, T.; Srivastava, N.; Gupta, V.K. Biological Remediation Technologies for Dyes and Heavy Metals in Wastewater Treatment: New Insight. Bioresour. Technol. 2022, 343, 126154. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A. Wastewater Treatment and Reuse for Sustainable Water Resources Management: A Systematic Literature Review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Yadav, G.; Shanmugam, S.; Sivaramakrishnan, R.; Kumar, D.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A.; Rajendran, K. Mechanism and Challenges behind Algae as a Wastewater Treatment Choice for Bioenergy Production and Beyond. Fuel 2021, 285, 119093. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.O.; et al. Wastewater Based Microalgal Biorefinery for Bioenergy Production: Progress and Challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef]
- Razaviarani, V.; Arab, G.; Lerdwanawattana, N.; Gadia, Y. Algal Biomass Dual Roles in Phycoremediation of Wastewater and Production of Bioenergy and Value-Added Products. Int. J. Environ. Sci. Technol. 2023, 20, 8199–8216. [Google Scholar] [CrossRef]
- Dayana Priyadharshini, S.; Suresh Babu, P.; Manikandan, S.; Subbaiya, R.; Govarthanan, M.; Karmegam, N. Phycoremediation of Wastewater for Pollutant Removal: A Green Approach to Environmental Protection and Long-Term Remediation. Environ. Pollut. 2021, 290, 117989. [Google Scholar] [CrossRef]
- Ibrahim, F.G.G.; Alonso Gómez, V.; Muñoz Torre, R.; de Godos Crespo, I. Scale-down of High-Rate Algae Ponds Systems for Urban Wastewater Reuse. J. Water Process Eng. 2023, 56, 104342. [Google Scholar] [CrossRef]
- Jebali, A.; Acién, F.G.; Rodriguez Barradas, E.; Olguín, E.J.; Sayadi, S.; Molina Grima, E. Pilot-Scale Outdoor Production of Scenedesmus Sp. in Raceways Using Flue Gases and Centrate from Anaerobic Digestion as the Sole Culture Medium. Bioresour. Technol. 2018, 262, 1–8. [Google Scholar] [CrossRef]
- Villalba, M.R.; Cervera, R.; Sánchez, J. Green Solutions for Urban Sustainability: Photobioreactors for Algae Cultivation on Façades and Artificial Trees. Buildings 2023, 13, 1541. [Google Scholar] [CrossRef]
- Takache, H.; Christophe, G.; Cornet, J.F.; Pruvost, J. Experimental and Theoretical Assessment of Maximum Productivities for the Microalgae Chlamydomonas Reinhardtii in Two Different Geometries of Photobioreactors. Biotechnol. Prog. 2010, 26, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Solsvik, J.; Wagner, J.L.; Zhang, D.; Hellgardt, K.; Park, C.W. CFD and Kinetic-Based Modeling to Optimize the Sparger Design of a Large-Scale Photobioreactor for Scaling up of Biofuel Production. Biotechnol. Bioeng. 2019, 116, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, L.; Torzillo, G. Hydrogen Production with the Microalga Chlamydomonas Reinhardtii Grown in a Compact Tubular Photobioreactor Immersed in a Scattering Light Nanoparticle Suspension. Int. J. Hydrogen Energy 2012, 37, 16951–16961. [Google Scholar] [CrossRef]
- Martinez-Carvajal, G.D.; Taidi, B.; Jarrahi, M. Towards a Low Energy, Stirless Photobioreactor Using Photosynthetic Motile Microalgae. Algal Res. 2024, 77, 103350. [Google Scholar] [CrossRef]
- Rodríguez-Bolaños, M.; Vargas-Romero, G.; Jaguer-García, G.; Aguilar-Gonzalez, Z.I.; Lagos-Romero, V.; Miranda-Astudillo, H. V. Antares I: A Modular Photobioreactor Suitable for Photosynthesis and Bioenergetics Research. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Moreno Osorio, J.H.; Pollio, A.; Frunzo, L.; Lens, P.N.L.; Esposito, G. A Review of Microalgal Biofilm Technologies: Definition, Applications, Settings and Analysis. Front. Chem. Eng. 2021, 3, 737710. [Google Scholar] [CrossRef]
- Kreis, C.T.; Grangier, A.; Bäumchen, O. In Vivo Adhesion Force Measurements of Chlamydomonas on Model Substrates. Soft Matter 2019, 15, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Catalan, R.E.; Fragkopoulos, A.A.; von Trott, N.; Kelterborn, S.; Baidukova, O.; Hegemann, P.; Bäumchen, O. Light-Regulated Adsorption and Desorption of Chlamydomonas Cells at Surfaces. Soft Matter 2023, 19, 306. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, J.; Waghela, B.; Falcao, B.; Vavilala, S.L. Algal Polysaccharide’s Potential to Combat Respiratory Infections Caused by Klebsiella Pneumoniae and Serratia Marcescens Biofilms. Appl. Biochem. Biotechnol. 2022, 194, 671–693. [Google Scholar] [CrossRef]
- Vieira, M. V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Han, M.; Zhang, C.; Ho, S.H. Immobilized Microalgal System: An Achievable Idea for Upgrading Current Microalgal Wastewater Treatment. Environ. Sci. Ecotechnology 2023, 14, 100227. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Xie, P.; Ren, N.; Ho, S.H. Cytoprotective Alginate Microcapsule Serves as a Shield for Microalgal Encapsulation Defensing Sulfamethoxazole Threats and Safeguarding Nutrient Recovery. J. Hazard. Mater. 2024, 465, 133454. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeong, D.; Im, S.J.; Jang, A. Optimization of Alginate Bead Size Immobilized with Chlorella Vulgaris and Chlamydomonas Reinhardtii for Nutrient Removal. Bioresour. Technol. 2020, 302, 122891. [Google Scholar] [CrossRef] [PubMed]
- Homburg, S.V.; Kruse, O.; Patel, A. V. Growth and Photosynthetic Activity of Chlamydomonas Reinhardtii Entrapped in Lens-Shaped Silica Hydrogels. J. Biotechnol. 2019, 302, 58–66. [Google Scholar] [CrossRef]
- Zhang, B.B.; Wang, L.; Charles, V.; Rooke, J.C.; Su, B.L. Robust and Biocompatible Hybrid Matrix with Controllable Permeability for Microalgae Encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 8939–8946. [Google Scholar] [CrossRef] [PubMed]
- Mandsberg, N.K.; Liao, W.; Yamanouchi, Y.A.; Boisen, A.; Ejima, H. Encapsulation of Chlamydomonas Reinhardtii into a Metal-Phenolic Network. Algal Res. 2022, 61, 102569. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Bioremediation of Heavy Metals Using Microalgae: Recent Advances and Mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Hoyos, B.S.; Hernandez-Tenorio, F.; Miranda, A.M.; Villanueva-Mejía, D.F.; Sáez, A.A. Systematic Analysis of Genes Related to Selenium Bioaccumulation in Microalgae: A Review. Biology (Basel). 2023, 12, 703. [Google Scholar] [CrossRef]
- Touliabah, H.E.S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A Review of Microalgae-and Cyanobacteria-Based Biodegradation of Organic Pollutants. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Arora, N.; Patel, A.; Sartaj, K.; Pruthi, P.A.; Pruthi, V. Bioremediation of Domestic and Industrial Wastewaters Integrated with Enhanced Biodiesel Production Using Novel Oleaginous Microalgae. Env. Sci Pollut Res 2016, 23, 20997–21007. [Google Scholar] [CrossRef]
- Hasan, R. Bioremediation of Swine Wastewater and Biofuel Potential by Using Chlorella Vulgaris, Chlamydomonas Reinhardtii, and Chlamydomonas Debaryana. J. Pet. Environ. Biotechnol. 2014, 05, 3–7. [Google Scholar] [CrossRef]
- Kong, Q.X.; Li, L.; Martinez, B.; Chen, P.; Ruan, R. Culture of Microalgae Chlamydomonas Reinhardtii in Wastewater for Biomass Feedstock Production. Appl. Biochem. Biotechnol. 2010, 160, 9–18. [Google Scholar] [CrossRef]
- Sasi, P.; Viswanathan, A.; Mechery, J.; Thomas, D.; Jacob, J.; Paulose, S. Phycoremediation of Paper and Pulp Mill Effluent Using Planktochlorella Nurekis and Chlamydomonas Reinhardtii-A Comparative Study. J. Environ. Treat. Tech. 2020, 809–817. [Google Scholar]
- Abou-Shanab, R.A.I.; Ji, M.-K.; Kim, H.-C.; Paeng, K.-J.; Jeon, B.-H. Microalgal Species Growing on Piggery Wastewater as a Valuable Candidate for Nutrient Removal and Biodiesel Production. J. Environ. Manage. 2013, 115, 257–264. [Google Scholar] [CrossRef]
- Leong, Y.K.; Huang, C.Y.; Chang, J.S. Pollution Prevention and Waste Phycoremediation by Algal-Based Wastewater Treatment Technologies: The Applications of High-Rate Algal Ponds (HRAPs) and Algal Turf Scrubber (ATS). J. Environ. Manage. 2021, 296, 113193. [Google Scholar] [CrossRef] [PubMed]
- Grönlund, E.; Hanaeus, J.; Johansson, E.; Falk, S. Performance of an Experimental Wastewater Treatment High-Rate Algal Pond in Subarctic Climate. Water Environ. Res. a Res. Publ. Water Environ. Fed. 2010, 82, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Godos, I. de; Blanco, S.; García-Encina, P.A.; Becares, E.; Muñoz, R. Long-Term Operation of High Rate Algal Ponds for the Bioremediation of Piggery Wastewaters at High Loading Rates. Bioresour. Technol. 2009, 100, 4332–4339. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Phan, D.; Spierling, R.E.; Kopachevsky, A.M.; Bouwer, E.J.; Lundquist, T.J.; Betenbaugh, M.J. Production of Lipid-Containing Algal-Bacterial Polyculture in Wastewater and Biomethanation of Lipid Extracted Residues: Enhancing Methane Yield through Hydrothermal Pretreatment and Relieving Solvent Toxicity through Co-Digestion. Sci. Total Environ. 2019, 653, 1377–1394. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, T.; Xie, Y.; Chen, J.; Ho, S.H.; Wang, Y.; Huang, F. Attached Culture of Chlamydomonas Sp. JSC4 for Biofilm Production and TN/TP/Cu(II) Removal. Biochem. Eng. J. 2019, 141, 1–9. [Google Scholar] [CrossRef]
- Schaedig, E.; Cantrell, M.; Urban, C.; Zhao, X.; Greene, D.; Dancer, J.; Gross, M.; Sebesta, J.; Chou, K.J.; Grabowy, J.; et al. Isolation of Phosphorus-Hyperaccumulating Microalgae from Revolving Algal Biofilm (RAB) Wastewater Treatment Systems. Front. Microbiol. 2023, 14, 1219318. [Google Scholar] [CrossRef] [PubMed]
- de-Bashan, L.E.; Bashan, Y. Immobilized Microalgae for Removing Pollutants: Review of Practical Aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef] [PubMed]
- Nazos, T.T.; Ghanotakis, D.F. Biodegradation of Phenol by Alginate Immobilized Chlamydomonas Reinhardtii Cells. Arch. Microbiol. 2021, 203, 5805–5816. [Google Scholar] [CrossRef]
- Saavedra, R.; Muñoz, R.; Taboada, M.E.; Vega, M.; Bolado, S. Comparative Uptake Study of Arsenic, Boron, Copper, Manganese and Zinc from Water by Different Green Microalgae. Bioresour. Technol. 2018, 263, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Han, B.; Kong, F.; You, T.; Bi, R.; Zeng, X.; Wang, S.; Jia, Y. Enhancement of Arsenic Uptake and Accumulation in Green Microalga Chlamydomonas Reinhardtii through Heterologous Expression of the Phosphate Transporter DsPht1. J. Hazard. Mater. 2023, 459, 132130. [Google Scholar] [CrossRef]
- Nam, S.H.; Kwak, J. Il; An, Y.J. Assessing Applicability of the Paper-Disc Method Used in Combination with Flow Cytometry to Evaluate Algal Toxicity. Environ. Pollut. 2018, 234, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Ibuot, A.; Webster, R.E.; Williams, L.E.; Pittman, J.K. Increased Metal Tolerance and Bioaccumulation of Zinc and Cadmium in Chlamydomonas Reinhardtii Expressing a AtHMA4 C-Terminal Domain Protein. Biotechnol. Bioeng. 2020, 117, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Cervera, B.; García-Balboa, C.; Díaz-Alejo, H.M.; Costas, E.; López-Rodas, V. Rapid Colonization of Uranium Mining-Impacted Waters, the Biodiversity of Successful Lineages of Phytoplankton Extremophiles. Microb. Ecol. 2020, 79, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Balzano, S.; Sardo, A.; Blasio, M.; Chahine, T.B.; Dell’Anno, F.; Sansone, C.; Brunet, C. Microalgal Metallothioneins and Phytochelatins and Their Potential Use in Bioremediation. Front. Microbiol. 2020, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tang, Y.; Yu, F.; Peng, Z.; Yao, S.; Deng, X.; Long, H.; Wang, X.; Huang, K. Translatomics and Physiological Analyses of the Detoxification Mechanism of Green Alga Chlamydomonas Reinhardtii to Cadmium Toxicity. J. Hazard. Mater. 2023, 448, 130990. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, B.; Li, Z.; Deng, P.; Deng, X.; Long, H.; Wang, X.; Huang, K. Overexpression of the Sulfate Transporter-Encoding SULTR2 Increases Chromium Accumulation in Chlamydomonas Reinhardtii. Biotechnol. Bioeng. 2023, 120, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, P.; Fu, H.; Chen, J.; Ye, M.; Zhai, S.; Hu, F.; Zhang, C.; Ge, Y.; Fortin, C. A Comparative Study of the Accumulation and Detoxification of Copper and Zinc in Chlamydomonas Reinhardtii: The Role of Extracellular Polymeric Substances. Sci. Total Environ. 2023, 871, 161995. [Google Scholar] [CrossRef] [PubMed]
- Millet, R.T.; Santos, J.P.; Slaveykova, V.I. Exploring the Subcellular Distribution of Mercury in Green Alga Chlamydomonas Reinhardtii and Diatom Cyclotella Meneghiniana : A Comparative Study. Aquat. Toxicol. 2024, 267, 106836. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Jiang, Z.; Yu, H.; Li, Z.; Zhang, C.; Ge, Y. Assessment of Joint Toxicity of Arsenate and Lead by Multiple Endpoints in Chlamydomonas Reinhardtii. Bull. Environ. Contam. Toxicol. 2023, 111, 30. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, Z.; Yan, Z.; Zhou, G.; Zhang, W.; Wang, Y.; Li, X. Defense Pathways of Chlamydomonas Reinhardtii under Silver Nanoparticle Stress: Extracellular Biosorption, Internalization and Antioxidant Genes. Chemosphere 2022, 291, 132764. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.P.; Luo, K.; Zhang, S.; Zheng, Q.; Yang, H. Bioaccumulation and Catabolism of Prometryne in Green Algae. Chemosphere 2012, 87, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Cao, J.; Ma, X.; Ping, J.; Zhang, C.; Ke, T.; Zhang, Y.; Tao, Y.; Chen, L. The Simultaneous Removal of the Combined Pollutants of Hexavalent Chromium and O-Nitrophenol by Chlamydomonas Reinhardtii. Ecotoxicol. Environ. Saf. 2020, 198, 110648. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Kurade, M.B.; Abou-Shanab, R.A.I.; Ji, M.K.; Choi, J.; Kim, J.O.; Jeon, B.H. Biodegradation of Carbamazepine Using Freshwater Microalgae Chlamydomonas Mexicana and Scenedesmus Obliquus and the Determination of Its Metabolic Fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef]
- Wan, L.; Wu, Y.; Ding, H.; Zhang, W. Toxicity, Biodegradation, and Metabolic Fate of Organophosphorus Pesticide Trichlorfon on the Freshwater Algae Chlamydomonas Reinhardtii. J. Agric. Food Chem. 2020, 68, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Deng, J.; Cui, L.; Chang, P.; Dai, X.; Yang, C.; Li, N.; Ren, Z.; Zhang, X. The Potential Assessment of Green Alga Chlamydomonas Reinhardtii CC-503 in the Biodegradation of Benz(a)Anthracene and the Related Mechanism Analysis. Chemosphere 2020, 249, 126097. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Zhang, C.; Zhou, X.; Yin, Z.; Hu, T.; Hu, D.; Liu, C.; Zhu, L. Influence of Polystyrene Microplastics on the Growth, Photosynthetic Efficiency and Aggregation of Freshwater Microalgae Chlamydomonas Reinhardtii. Sci. Total Environ. 2020, 714, 136767. [Google Scholar] [CrossRef]
- Carbó, M.; Chaturvedi, P.; Álvarez, A.; Pineda-Cevallos, D.; Ghatak, A.; González, P.R.; Cañal, M.J.; Weckwerth, W.; Valledor, L. Ferroptosis Is the Key Cellular Process Mediating Bisphenol A Responses in Chlamydomonas and a Promising Target for Enhancing Microalgae-Based Bioremediation. J. Hazard. Mater. 2023, 448, 130997. [Google Scholar] [CrossRef]
- Hena, S.; Gutierrez, L.; Croué, J.P. Removal of Pharmaceutical and Personal Care Products (PPCPs) from Wastewater Using Microalgae: A Review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.Q.; Zheng, H.S.; Li, S.; Du, J.S.; Feng, X.C.; Yin, R.L.; Wu, Q.L.; Ren, N.Q.; Chang, J.S. Removal of Cephalosporin Antibiotics 7-ACA from Wastewater during the Cultivation of Lipid-Accumulating Microalgae. Bioresour. Technol. 2016, 221, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Kurade, M.B.; Jeon, B.H. Ecotoxicological Effects of Enrofloxacin and Its Removal by Monoculture of Microalgal Species and Their Consortium. Environ. Pollut. 2017, 226, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-J.; Ying, G.-G.; Liu, S.; Zhou, L.-J.; Chen, Z.-F.; Peng, F.-Q. Simultaneous Removal of Inorganic and Organic Compounds in Wastewater by Freshwater Green Microalgae. Environ. Sci. Process. Impacts 2014, 16, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, S.; Huang, F.; Lin, L.; Hu, Z.; Zheng, Y. Toxicological Effects of Microplastics and Sulfadiazine on the Microalgae Chlamydomonas Reinhardtii. Front. Microbiol. 2022, 13, 865768. [Google Scholar] [CrossRef] [PubMed]
- Seoane, M.; Conde-Pérez, K.; Esperanza, M.; Cid, Á.; Rioboo, C. Unravelling Joint Cytotoxicity of Ibuprofen and Oxytetracycline on Chlamydomonas Reinhardtii Using a Programmed Cell Death-Related Biomarkers Panel. Aquat. Toxicol. 2023, 257, 106455. [Google Scholar] [CrossRef] [PubMed]
- Hom-Diaz, A.; Jaén-Gil, A.; Rodríguez-Mozaz, S.; Barceló, D.; Vicent, T.; Blánquez, P. Insights into Removal of Antibiotics by Selected Microalgae (Chlamydomonas Reinhardtii, Chlorella Sorokiniana, Dunaliella Tertiolecta and Pseudokirchneriella Subcapitata). Algal Res. 2022, 61, 102560. [Google Scholar] [CrossRef]
- Hom-Diaz, A.; Llorca, M.; Rodríguez-Mozaz, S.; Vicent, T.; Barceló, D.; Blánquez, P. Microalgae Cultivation on Wastewater Digestate: β-Estradiol and 17α-Ethynylestradiol Degradation and Transformation Products Identification. J. Environ. Manage. 2015, 155, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Liakh, I.; Harshkova, D.; Hrouzek, P.; Bišová, K.; Aksmann, A.; Wielgomas, B. Green Alga Chlamydomonas Reinhardtii Can Effectively Remove Diclofenac from the Water Environment – A New Perspective on Biotransformation. J. Hazard. Mater. 2023, 455, 131570. [Google Scholar] [CrossRef] [PubMed]
- Stravs, M.A.; Pomati, F.; Hollender, J. Exploring Micropollutant Biotransformation in Three Freshwater Phytoplankton Species. Environ. Sci. Process. Impacts 2017, 19, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Beuchel, C.; Liers, C.; Reisser, W.; Harms, H.; Schlosser, D. Laccase-like Enzyme Activities from Chlorophycean Green Algae with Potential for Bioconversion of Phenolic Pollutants. FEMS Microbiol. Lett. 2015, 362, fnv072. [Google Scholar] [CrossRef]
- Míguez, L.; Esperanza, M.; Seoane, M.; Cid, Á. Assessment of Cytotoxicity Biomarkers on the Microalga Chlamydomonas Reinhardtii Exposed to Emerging and Priority Pollutants. Ecotoxicol. Environ. Saf. 2021, 208, 111646. [Google Scholar] [CrossRef]
- Yadav, N.; Ahn, H.J.; Kurade, M.B.; Ahn, Y.; Park, Y.K.; Khan, M.A.; Salama, E.S.; Li, X.; Jeon, B.H. Fate of Five Bisphenol Derivatives in Chlamydomonas Mexicana: Toxicity, Removal, Biotransformation and Microalgal Metabolism. J. Hazard. Mater. 2023, 454, 131504. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A Bacterium That Degrades and Assimilates Poly(Ethylene Terephthalate). Science (80-. ). 2016, 351, 6278. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, G.; Taunt, H.N.; Berto, M.; Jackson, H.O.; Piccinini, D.; Carletti, A.; Scurani, G.; Braidi, N.; Purton, S. A PETase Enzyme Synthesised in the Chloroplast of the Microalga Chlamydomonas Reinhardtii Is Active against Post-Consumer Plastics. Sci. Rep. 2023, 13, 10028. [Google Scholar] [CrossRef] [PubMed]
- de Carpentier, F.; Maes, A.; Marchand, C.H.; Chung, C.; Durand, C.; Crozet, P.; Lemaire, S.D.; Danon, A. How Abiotic Stress-Induced Socialization Leads to the Formation of Massive Aggregates in Chlamydomonas. Plant Physiol. 2022, 190, 1927–1940. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Chen, Z.; Gu, P.; Li, X.; Wang, G. Exploring Cell Aggregation as a Defense Strategy against Perchlorate Stress in Chlamydomonas Reinhardtii through Multi-Omics Analysis. Sci. Total Environ. 2023, 905, 167045. [Google Scholar] [CrossRef]
- Nakanishi, A.; Sakihama, Y.; Ozawa, N. Improvement of Growth of Chlamydomonas Reinhardtii in CO2 – Stepwisely Aerating Condition. J. Appl. Biotechnol. Reports 2021, 8, 37–40. [Google Scholar] [CrossRef]
- Hariz, H.B.; Takriff, M.S.; Mohd Yasin, N.H.; Ba-Abbad, M.M.; Mohd Hakimi, N.I.N. Potential of the Microalgae-Based Integrated Wastewater Treatment and CO2 Fixation System to Treat Palm Oil Mill Effluent (POME) by Indigenous Microalgae; Scenedesmus Sp. and Chlorella Sp. J. Water Process Eng. 2019, 32, 100907. [Google Scholar] [CrossRef]
- Choi, H. Il; Hwang, S.W.; Kim, J.; Park, B.; Jin, E.S.; Choi, I.G.; Sim, S.J. Augmented CO2 Tolerance by Expressing a Single H+-Pump Enables Microalgal Valorization of Industrial Flue Gas. Nat. Commun. 2021, 12, 6049. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Tahir, F.; Ashfaq, H.; Akbar, I.; Razzaque, N.; Haider, M.N.; Xu, J.; Zhu, H.; Wang, N.; Shahid, A. Decontamination of Industrial Wastewater Using Microalgae Integrated with Biotransformation of the Biomass to Green Products. Energy Nexus 2022, 6, 100089. [Google Scholar] [CrossRef]
- Ahmad, A.; W. Hassan, S.; Banat, F. An Overview of Microalgae Biomass as a Sustainable Aquaculture Feed Ingredient: Food Security and Circular Economy. Bioengineered 2022, 13, 9521–9547. [Google Scholar] [CrossRef]
- Ma, X.; Mi, Y.; Zhao, C.; Wei, Q. A Comprehensive Review on Carbon Source Effect of Microalgae Lipid Accumulation for Biofuel Production. Sci. Total Environ. 2022, 806. [Google Scholar] [CrossRef]
- Cheng, C.L.; Lo, Y.C.; Huang, K. Lou; Nagarajan, D.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Effect of PH on Biomass Production and Carbohydrate Accumulation of Chlorella Vulgaris JSC-6 under Autotrophic, Mixotrophic, and Photoheterotrophic Cultivation. Bioresour. Technol. 2022, 351, 127021. [Google Scholar] [CrossRef]
- Beigbeder, J.B.; Lavoie, J.M. Effect of Photoperiods and CO2 Concentrations on the Cultivation of Carbohydrate-Rich P. Kessleri Microalgae for the Sustainable Production of Bioethanol. J. CO2 Util. 2022, 58, 101934. [Google Scholar] [CrossRef]
- Salman, J.M.; Grmasha, R.A.; Stenger-Kovács, C.; Lengyel, E.; Al-sareji, O.J.; AL-Cheban, A.M.A.A.; Meiczinger, M. Influence of Magnesium Concentrations on the Biomass and Biochemical Variations in the Freshwater Algae, Chlorella Vulgaris. Heliyon 2023, 9, e13072. [Google Scholar] [CrossRef] [PubMed]
- Fields, F.J.; Ostrand, J.T.; Mayfield, S.P. Fed-Batch Mixotrophic Cultivation of Chlamydomonas Reinhardtii for High-Density Cultures. Algal Res. 2018, 33, 109–117. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Yang, Y.; Heredia, V.; Horn, S.J.; Keremane, S.R.; Jin, M.M.; Mayfield, S.P. Optimized Production of a Bioactive Human Recombinant Protein from the Microalgae Chlamydomonas Reinhardtii Grown at High Density in a Fed-Batch Bioreactor. Algal Res. 2022, 66, 102786. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Microalgae-Based Biochar Production and Applications: A Comprehensive Review. Bioresour. Technol. 2023, 389, 129782. [Google Scholar] [CrossRef]
- Fan, X.; Du, C.; Zhou, L.; Fang, Y.; Zhang, G.; Zou, H.; Yu, G.; Wu, H. Biochar from Phytoremediation Plant Residues: A Review of Its Characteristics and Potential Applications. Environ. Sci. Pollut. Res. 2024, 31, 16188–16205. [Google Scholar] [CrossRef]
- Torri, C.; Samorì, C.; Adamiano, A.; Fabbri, D.; Faraloni, C.; Torzillo, G. Preliminary Investigation on the Production of Fuels and Bio-Char from Chlamydomonas Reinhardtii Biomass Residue after Bio-Hydrogen Production. Bioresour. Technol. 2011, 102, 8707–8713. [Google Scholar] [CrossRef]
- Gan, Y.Y.; Ong, H.C.; Show, P.L.; Ling, T.C.; Chen, W.H.; Yu, K.L.; Abdullah, R. Torrefaction of Microalgal Biochar as Potential Coal Fuel and Application as Bio-Adsorbent. Energy Convers. Manag. 2018, 165, 152–162. [Google Scholar] [CrossRef]
- Zheng, H.; Guo, W.; Li, S.; Chen, Y.; Wu, Q.; Feng, X.; Yin, R.; Ho, S.H.; Ren, N.; Chang, J.S. Adsorption of P-Nitrophenols (PNP) on Microalgal Biochar: Analysis of High Adsorption Capacity and Mechanism. Bioresour. Technol. 2017, 244, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Hernandez-Tenorio, F.; Villalta, F.; Vargas, G.J.; Sáez, A.A. Advances in the Development of Biofertilizers and Biostimulants from Microalgae. Biology (Basel). 2024, 13. [Google Scholar] [CrossRef]
- Sido, M.Y.; Tian, Y.; Wang, X.; Wang, X. Application of Microalgae Chlamydomonas Applanata M9V and Chlorella Vulgaris S3 for Wheat Growth Promotion and as Urea Alternatives. Front. Microbiol. 2022, 13, 1035791. [Google Scholar] [CrossRef] [PubMed]
- Mutale-joan, C.; Redouane, B.; Najib, E.; Yassine, K.; Lyamlouli, K.; Laila, S.; Zeroual, Y.; Hicham, E.A. Screening of Microalgae Liquid Extracts for Their Bio Stimulant Properties on Plant Growth, Nutrient Uptake and Metabolite Profile of Solanum Lycopersicum L. Sci. Rep. 2020, 10, 2820. [Google Scholar] [CrossRef] [PubMed]
- Gitau, M.M.; Farkas, A.; Balla, B.; Ördög, V.; Futó, Z.; Maróti, G. Strain-Specific Biostimulant Effects of Chlorella and Chlamydomonas Green Microalgae on Medicago Truncatula. Plants 2021, 10, 1060. [Google Scholar] [CrossRef]
- Martini, F.; Beghini, G.; Zanin, L.; Varanini, Z.; Zamboni, A.; Ballottari, M. The Potential Use of Chlamydomonas Reinhardtii and Chlorella Sorokiniana as Biostimulants on Maize Plants. Algal Res. 2021, 60, 102515. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Ördög, V.; Staden, J. Van; Jäger, K. Cytokinin-and Auxin-like Activity in Cyanophyta and Microalgae. J. Appl. Phycol. 2002, 14, 215–221. [Google Scholar] [CrossRef]
- Metting, B. Population Dynamics of Chlamydomonas Sajao and Its Influence on Soil Aggregate Stabilization in the Field. Appl. Environ. Microbiol. 1986, 51, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Arora, Y.; Sharma, S.; Sharma, V. Microalgae in Bioplastic Production: A Comprehensive Review. Arab. J. Sci. Eng. 2023, 48, 7225–7241. [Google Scholar] [CrossRef] [PubMed]
- Chaogang, W.; Zhangli, H.; Anping, L.; Baohui, J. Biosynthesis of Poly-3-Hydroxybutyrate (PHB) in the Transgenic Green Alga Chlamydomonas Reinhardtii. J. Phycol. 2010, 46, 396–402. [Google Scholar] [CrossRef]
- Hao, Z.; Songlin, M.; Xiaotan, D.; Ru, C.; Han, L.; Zhanyou, C.; Song, X.; Yonghua, L.-B.; Fantao, K. Harnessing Algal Peroxisomes for Efficient Poly Hydroxybutyrate Production. ACS Sustain. Chem. Eng 2024, 12, 3312–3321. [Google Scholar] [CrossRef]
- Kato, N. Production of Crude Bioplastic-Beads with Microalgae: Proof-of-Concept. Bioresour. Technol. Reports 2019, 5, 326–330. [Google Scholar] [CrossRef]
- Nakanishi, A.; Nemoto, S.; Yamamoto, N.; Iritani, K.; Watanabe, M. Identification of Cell-Attachment Factors Derived from Green Algal Cells Disrupted by Sonication in Fabrication of Cell Plastics. Bioengineering 2023, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Chaos-Hernández, D.; Reynel-Ávila, H.E.; Bonilla-Petriciolet, A.; Villalobos-Delgado, F.J. Extraction Methods of Algae Oils for the Production of Third Generation Biofuels – A Review. Chemosphere 2023, 341, 139856. [Google Scholar] [CrossRef]
- Daneshvar, E.; Sik Ok, Y.; Tavakoli, S.; Sarkar, B.; Shaheen, S.M.; Hong, H.; Luo, Y.; Rinklebe, J.; Song, H.; Bhatnagar, A. Insights into Upstream Processing of Microalgae: A Review. Bioresour. Technol. 2021, 329, 124870. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Suresh, S.; Kanwal, S.; Ramadoss, G.; Ramprakash, B.; Incharoensakdi, A. Microalgal Biorefinery Concepts’ Developments for Biofuel and Bioproducts: Current Perspective and Bottlenecks. Int. J. Mol. Sci. 2022, 23, 2623. [Google Scholar] [CrossRef]
- Chen, C.L.; Huang, C.C.; Ho, K.C.; Hsiao, P.X.; Wu, M.S.; Chang, J.S. Biodiesel Production from Wet Microalgae Feedstock Using Sequential Wet Extraction/Transesterification and Direct Transesterification Processes. Bioresour. Technol. 2015, 194, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.H.; Ng, I.S. CRISPRi Mediated Phosphoenolpyruvate Carboxylase Regulation to Enhance the Production of Lipid in Chlamydomonas Reinhardtii. Bioresour. Technol. 2017, 245, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Rengel, R.; Smith, R.T.; Haslam, R.P.; Sayanova, O.; Vila, M.; León, R. Overexpression of Acetyl-CoA Synthetase (ACS) Enhances the Biosynthesis of Neutral Lipids and Starch in the Green Microalga Chlamydomonas Reinhardtii. Algal Res. 2018, 31, 183–193. [Google Scholar] [CrossRef]
- Kong, F.; Liang, Y.; Légeret, B.; Beyly-Adriano, A.; Blangy, S.; Haslam, R.P.; Napier, J.A.; Beisson, F.; Peltier, G.; Li-Beisson, Y. Chlamydomonas Carries out Fatty Acid β-Oxidation in Ancestral Peroxisomes Using a Bona Fide Acyl-CoA Oxidase. Plant J. 2017, 90, 358–371. [Google Scholar] [CrossRef]
- Shin, Y.S.; Jeong, J.; Nguyen, T.H.T.; Kim, J.Y.H.; Jin, E.S.; Sim, S.J. Targeted Knockout of Phospholipase A2 to Increase Lipid Productivity in Chlamydomonas Reinhardtii for Biodiesel Production. Bioresour. Technol. 2019, 271, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-F.; Lin, J.-Y.; Pan, K.-Y.; Huang, C.-K.; Chu, Y.-K. Overexpressing Ferredoxins in Chlamydomonas Reinhardtii Increase Starch and Oil Yields and Enhance Electric Power Production in a Photo Microbial Fuel Cell. Int. J. Mol. Sci. 2015, 16, 19308–19325. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Tanaka, K.; Imamura, S. Microalgal Target of Rapamycin (TOR): A Central Regulatory Hub for Growth, Stress Response and Biomass Production. Plant Cell Physiol. 2020, 61, 675–684. [Google Scholar] [CrossRef]
- Tan, K.W.M.; Lee, Y.K. Expression of the Heterologous Dunaliella Tertiolecta Fatty Acyl-ACP Thioesterase Leads to Increased Lipid Production in Chlamydomonas Reinhardtii. J. Biotechnol. 2017, 247, 60–67. [Google Scholar] [CrossRef]
- Zhu, Z.; Yuan, G.; Fan, X.; Fan, Y.; Yang, M.; Yin, Y.; Liu, J.; Liu, Y.; Cao, X.; Tian, J.; et al. The Synchronous TAG Production with the Growth by the Expression of Chloroplast Transit Peptide-Fused ScPDAT in Chlamydomonas Reinhardtii. Biotechnol. Biofuels 2018, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Iskandarov, U.; Sitnik, S.; Shtaida, N.; Didi-Cohen, S.; Leu, S.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. Cloning and Characterization of a GPAT-like Gene from the Microalga Lobosphaera Incisa (Trebouxiophyceae): Overexpression in Chlamydomonas Reinhardtii Enhances TAG Production. J. Appl. Phycol. 2016, 28, 907–919. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Hu, G.; Sommerfeld, M.; Hu, Q. Inhibition of Starch Synthesis Results in Overproduction of Lipids in Chlamydomonas Reinhardtii. Biotechnol. Bioeng. 2010, 107, 258–268. [Google Scholar] [CrossRef]
- Kato, Y.; Oyama, T.; Inokuma, K.; Vavricka, C.J.; Matsuda, M.; Hidese, R.; Satoh, K.; Oono, Y.; Chang, J.S.; Hasunuma, T.; et al. Enhancing Carbohydrate Repartitioning into Lipid and Carotenoid by Disruption of Microalgae Starch Debranching Enzyme. Commun. Biol. 2021, 4, 450. [Google Scholar] [CrossRef]
- Khoo, K.S.; Ahmad, I.; Chew, K.W.; Iwamoto, K.; Bhatnagar, A.; Show, P.L. Enhanced Microalgal Lipid Production for Biofuel Using Different Strategies Including Genetic Modification of Microalgae: A Review. Prog. Energy Combust. Sci. 2023, 96, 101071. [Google Scholar] [CrossRef]
- Zheng, S.; Zou, S.; Wang, H.; Feng, T.; Sun, S.; Chen, H.; Wang, Q. Reducing Culture Medium Nitrogen Supply Coupled with Replenishing Carbon Nutrient Simultaneously Enhances the Biomass and Lipid Production of Chlamydomonas Reinhardtii. Front. Microbiol. 2022, 13, 1019806. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, U.; Blaby, I.; Casero, D.; Gallaher, S.D.; Goodson, C.; Johnson, S.; Lee, J.H.; Merchant, S.S.; Pellegrini, M.; Roth, R.; et al. The Path to Triacylglyceride Obesity in the Sta6 Strain of Chlamydomonas Reinhardtii. Eukaryot. Cell 2014, 13, 591–613. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ahn, J.W.; Park, E.J.; Choi, J.L. Overexpression of S-Adenosylmethionine Synthetase in Recombinant Chlamydomonas for Enhanced Lipid Production. J. Microbiol. Biotechnol. 2023, 33, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Kulikovskiy, M.; Maltseva, S. Nitrogen and Phosphorus Stress as a Tool to Induce Lipid Production in Microalgae. Microb. Cell Fact. 2023, 22. [Google Scholar] [CrossRef]
- Gonzalez, D.I.; Ynalvez, R.A. Comparison of the Effects of Nitrogen-, Sulfur- and Combined Nitrogen- and Sulfur-Deprivations on Cell Growth, Lipid Bodies and Gene Expressions in Chlamydomonas Reinhardtii Cc5373-Sta6. BMC Biotechnol. 2023, 23, 35. [Google Scholar] [CrossRef]
- Salama, E.S.; Kim, H.C.; Abou-Shanab, R.A.I.; Ji, M.K.; Oh, Y.K.; Kim, S.H.; Jeon, B.H. Biomass, Lipid Content, and Fatty Acid Composition of Freshwater Chlamydomonas Mexicana and Scenedesmus Obliquus Grown under Salt Stress. Bioprocess Biosyst. Eng. 2013, 36, 827–833. [Google Scholar] [CrossRef] [PubMed]
- James, G.O.; Hocart, C.H.; Hillier, W.; Price, G.D.; Djordjevic, M.A. Temperature Modulation of Fatty Acid Profiles for Biofuel Production in Nitrogen Deprived Chlamydomonas Reinhardtii. Bioresour. Technol. 2013, 127, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ge, Y.; Liu, K.; Yamaoka, Y.; Zhang, D.; Chi, Z.; Akkaya, M.; Kong, F. Overexpression of a MYB1 Transcription Factor Enhances Triacylglycerol and Starch Accumulation and Biomass Production in the Green Microalga Chlamydomonas Reinhardtii. J. Agric. Food Chem. 2023, 71, 17833–17841. [Google Scholar] [CrossRef]
- Singh, P.; Kumari, S.; Guldhe, A.; Misra, R.; Rawat, I.; Bux, F. Trends and Novel Strategies for Enhancing Lipid Accumulation and Quality in Microalgae. Renew. Sustain. Energy Rev. 2016, 55, 1–16. [Google Scholar] [CrossRef]
- Figueroa-Torres, G.M.; Pittman, J.K.; Theodoropoulos, C. Optimisation of Microalgal Cultivation via Nutrient-Enhanced Strategies: The Biorefinery Paradigm. Biotechnol. Biofuels 2021, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Poulhazan, A.; Arnold, A.A.; Mentink-Vigier, F.; Muszyński, A.; Azadi, P.; Halim, A.; Vakhrushev, S.Y.; Joshi, H.J.; Wang, T.; Warschawski, D.E.; et al. Molecular-Level Architecture of Chlamydomonas Reinhardtii’s Glycoprotein-Rich Cell Wall. Nat. Commun. 2024, 15, 986. [Google Scholar] [CrossRef]
- Choi, S.P.; Nguyen, M.T.; Sim, S.J. Enzymatic Pretreatment of Chlamydomonas Reinhardtii Biomass for Ethanol Production. Bioresour. Technol. 2010, 101, 5330–5336. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Choi, S.P.; Lee, J.; Lee, J.H.; Sim, S.J. Hydrothermal Acid Pretreatment of Chlamydomonas Reinhardtii Biomass for Ethanol Production. J. Microbiol. Biotechnol. 2009, 19, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.N.; Zachleder, V.; Vítová, M.; Barbosa, M.J.; Bišová, K. Starch Production in Chlamydomonas Reinhardtii through Supraoptimal Temperature in a Pilot-Scale Photobioreactor. Cells 2021, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- De Marco, M.A.; Curatti, L.; Martínez-Noël, G.M.A. High Auxin Disrupts Expression of Cell-Cycle Genes, Arrests Cell Division and Promotes Accumulation of Starch in Chlamydomonas Reinhardtii. Algal Res. 2024, 78, 103419. [Google Scholar] [CrossRef]
- Qu, W.; Loke Show, P.; Hasunuma, T.; Ho, S.H. Optimizing Real Swine Wastewater Treatment Efficiency and Carbohydrate Productivity of Newly Microalga Chlamydomonas Sp. QWY37 Used for Cell-Displayed Bioethanol Production. Bioresour. Technol. 2020, 305, 123072. [Google Scholar] [CrossRef]
- Kunatsa, T.; Xia, X. A Review on Anaerobic Digestion with Focus on the Role of Biomass Co-Digestion, Modelling and Optimisation on Biogas Production and Enhancement. Bioresour. Technol. 2022, 344, 126311. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Ghosh, S.; Panja, A.; Kumar, V.; Singh, A.K.; Prasad, R. Microalgae and Biogas: A Boon to Energy Sector. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as Substrates for Fermentative Biogas Production in a Combined Biorefinery Concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef]
- Veerabadhran, M.; Gnanasekaran, D.; Wei, J.; Yang, F. Anaerobic Digestion of Microalgal Biomass for Bioenergy Production, Removal of Nutrients and Microcystin: Current Status. J. Appl. Microbiol. 2021, 131, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Klassen, V.; Blifernez-Klassen, O.; Wibberg, D.; Winkler, A.; Kalinowski, J.; Posten, C.; Kruse, O. Highly Efficient Methane Generation from Untreated Microalgae Biomass. Biotechnol. Biofuels 2017, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from Microalgae: Technologies, Challenges and Opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.J.; de la Lama-Calvente, D.; Jiménez-Rodríguez, A.; Borja, R.; Rincón-Llorente, B. Influence of the Cell Wall of Chlamydomonas Reinhardtii on Anaerobic Digestion Yield and on Its Anaerobic Co-Digestion with a Carbon-Rich Substrate. Process Saf. Environ. Prot. 2019, 128, 167–175. [Google Scholar] [CrossRef]
- Barros, R.; Raposo, S.; Morais, E.G.; Rodrigues, B.; Afonso, V.; Gonçalves, P.; Marques, J.; Cerqueira, P.R.; Varela, J.; Teixeira, M.R.; et al. Biogas Production from Microalgal Biomass Produced in the Tertiary Treatment of Urban Wastewater: Assessment of Seasonal Variations. Energies 2022, 15, 5713. [Google Scholar] [CrossRef]
- Klassen, V.; Blifernez-Klassen, O.; Bax, J.; Kruse, O. Wastewater-Borne Microalga Chlamydomonas Sp.: A Robust Chassis for Efficient Biomass and Biomethane Production Applying Low-N Cultivation Strategy. Bioresour. Technol. 2020, 315, 123825. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, N.; Praveen, G.; AmitKumar, S.; SundarRajan, P.S.; Baskaran, A.; Priyadharsini, P.; SanjayKumar, S.P.; Dawn, S.S.; Pavithra, K.G.; Arun, J.; et al. A Review on Biological Biohydrogen Production: Outlook on Genetic Strain Enhancements, Reactor Model and Techno-Economics Analysis. Sci. Total Environ. 2023, 896, 165143. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, Q.; Yu, D. The Future of Hydrogen Energy: Bio-Hydrogen Production Technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- King, S.J.; Jerkovic, A.; Brown, L.J.; Petroll, K.; Willows, R.D. Synthetic Biology for Improved Hydrogen Production in Chlamydomonas Reinhardtii. Microb. Biotechnol. 2022, 15, 1946–1965. [Google Scholar] [CrossRef]
- Frenkel, A.W. Hydrogen Evolution by the Flagellate Green Alga, Chlamydomonas Moewusii. Arch Biochem Biophys 1951, 38, 219–230. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Erbes, D.L.; Riederer-Henderson, M.A.; Peavey, D.G.; Gibbs, M. H2 Metabolism in Photosynthetic Organisms: I. Dark H2 Evolution and Uptake by Algae and Mosses. Plant Physiol 1975, 56, 72–77. [Google Scholar] [CrossRef]
- Melis, A.; Zhang, L.; Forestier, M.; Ghirardi, M.L.; Seibert, M. Sustained Photobiological Hydrogen Gas Production upon Reversible Inactivation of Oxygen Evolution in the Green Alga Chlamydomonas Reinhardtii. Plant Physiol. 2000, 122, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Oller, J.L.; Dubini, A.; Galván, A.; Fernández, E.; González-Ballester, D. Low Oxygen Levels Contribute to Improve Photohydrogen Production in Mixotrophic Non-Stressed Chlamydomonas Cultures. Biotechnol. Biofuels 2015, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Elman, T.; Yacoby, I. A Two-Phase Protocol for Ambient Hydrogen Production Using Chlamydomonas Reinhardtii. STAR Protoc. 2022, 3, 101640. [Google Scholar] [CrossRef] [PubMed]
- Fakhimi, N.; Gonzalez-Ballester, D.; Fernández, E.; Galván, A.; Dubini, A. Algae-Bacteria Consortia as a Strategy to Enhance H2 Production. Cells 2020, 9, 1353. [Google Scholar] [CrossRef]
- Elman, T.; Schweitzer, S.; Shahar, N.; Swartz, J.; Yacoby, I. Engineered Clostridial [FeFe]-Hydrogenase Shows Improved O2 Tolerance in Chlamydomonas Reinhardtii. Int. J. Hydrogen Energy 2020, 45, 30201–30210. [Google Scholar] [CrossRef]
- Nagy, V.; Podmaniczki, A.; Vidal-Meireles, A.; Kuntam, S.; Herman, É.; Kovács, L.; Tóth, D.; Scoma, A.; Tóth, S.Z. Thin Cell Layer Cultures of Chlamydomonas Reinhardtii L159I-N230Y, Pgrl1 and Pgr5 Mutants Perform Enhanced Hydrogen Production at Sunlight Intensity. Bioresour. Technol. 2021, 333, 125217. [Google Scholar] [CrossRef]
- Milrad, Y.; Schweitzer, S.; Feldman, Y.; Yacoby, I. Green Algal Hydrogenase Activity Is Outcompeted by Carbon Fixation before Inactivation by Oxygen Takes Place. Plant Physiol. 2018, 177, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Kosourov, S.; Nagy, V.; Shevela, D.; Jokel, M.; Messinger, J.; Allahverdiyeva, Y. Water Oxidation by Photosystem II Is the Primary Source of Electrons for Sustained H2 Photoproduction in Nutrient-Replete Green Algae. PNAS 2020, 117, 29629–29636. [Google Scholar] [CrossRef]
- Kanygin, A.; Milrad, Y.; Thummala, C.; Reifschneider, K.; Baker, P.; Marco, P.; Yacoby, I.; Redding, K.E. Rewiring Photosynthesis: A Photosystem I-Hydrogenase Chimera That Makes H2: In Vivo. Energy Environ. Sci. 2020, 13, 2903–2914. [Google Scholar] [CrossRef]
- Lakatos, G.; Balogh, D.; Farkas, A.; Ördög, V.; Nagy, P. .; Bíró, T.; Maróti, G. Factors Influencing Algal Photobiohydrogen Production in Algal-Bacterial Co-Cultures. Algal Res 2017, 28, 161–171. [Google Scholar] [CrossRef]
- Masi, A.; Leonelli, F.; Scognamiglio, V.; Gasperuzzo, G.; Antonacci, A. ; Terzidis, chael A. Chlamydomonas Reinhardtii: A Factory of Nutraceutical and Food Supplements for Human Health. Molecules 2023, 28, 1185. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y. Disruption of the Glycolate Dehydrogenase Gene in the High-CO2-Requiring Mutant HCR89 of Chlamydomonas Reinhardtii. Can. J. Bot. 2005, 87, 820–833. [Google Scholar] [CrossRef]
- Wang, Y.; Stessman, D.J.; Spalding, M.H. The CO2 Concentrating Mechanism and Photosynthetic Carbon Assimilation in Limiting CO2: How Chlamydomonas Works against the Gradient. Plant J. 2015, 82, 429–448. [Google Scholar] [CrossRef]
- Yun, E.J.; Zhang, G.-C.; Atkinson, C.; Lane, S.; Liu, J.-J.; Ort, D.R.; Jin, Y.-S. Glycolate Production by a Chlamydomonas Reinhardtii Mutant Lacking Carbon-Concentrating Mechanism. J. Biotechnol. 2021, 335, 39–46. [Google Scholar] [CrossRef]
- Taubert, A.; Jakob, T.; Wilhelm, C. Glycolate from Microalgae: An Efficient Carbon Source for Biotechnological Applications. Plant Biotechnol. J. 2019, 17, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Zabaleta, E.; Wang, Y.; Zhao, L.; Shi, M. Identification and Characterization of Genes Encoding the Hydroxypyruvate Reductases in Chlamydomonas Reveal Their Distinct Roles in Photorespiration. Front. Plant Sci. 2021, 12, 690296. [Google Scholar] [CrossRef] [PubMed]
- Lauersen, K.J. Eukaryotic Microalgae as Hosts for Light-Driven Heterologous Isoprenoid Production. Planta 2019, 249, 155–180. [Google Scholar] [CrossRef]
- Yahya, R.Z.; Wellman, G.B.; Overmans, S.; Lauersen, K.J. Engineered Production of Isoprene from the Model Green Microalga Chlamydomonas Reinhardtii. Metab. Eng. Commun. 2023, 16, e00221. [Google Scholar] [CrossRef] [PubMed]
- Miró-Vinyals, B.; Artigues, M.; Wostrikoff, K.; Monte, E.; Broto-Puig, F.; Leivar, P.; Planas, A. Chloroplast Engineering of the Green Microalgae Chlamydomonas Reinhardtii for the Production of HAA, the Lipid Moiety of Rhamnolipid Biosurfactants. N. Biotechnol. 2023, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moulin, S.L.Y.; Beyly-Adriano, A.; Cuiné, S.; Blangy, S.; Légeret, B.; Floriani, M.; Burlacot, A.; Sorigué, D.; Samire, P.P.; Li-Beisson, Y.; et al. Fatty Acid Photodecarboxylase Is an Ancient Photoenzyme That Forms Hydrocarbons in the Thylakoids of Algae. Plant Physiol. 2021, 186, 1455–1472. [Google Scholar] [CrossRef] [PubMed]
- Salma-Ancane, K.; Sceglovs, A.; Tracuma, E.; Wychowaniec, J.K.; Aunina, K.; Ramata-Stunda, A.; Nikolajeva, V.; Loca, D. Effect of Crosslinking Strategy on the Biological, Antibacterial and Physicochemical Performance of Hyaluronic Acid and ɛ-Polylysine Based Hydrogels. Int. J. Biol. Macromol. 2022, 208, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Suresh, S.; Incharoensakdi, A. Chlamydomonas Sp. as Dynamic Biorefinery Feedstock for the Production of Methyl Ester and ɛ-Polylysine. Bioresour. Technol. 2019, 272, 281–287. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, J.Y.; Ahn, J.H.; Ahn, Y.-J.; Lee, S.Y. Current Advancements in the Bio-Based Production of Polyamides. Trends Chem. 2023, 5, 873–891. [Google Scholar] [CrossRef]
- Freudenberg, R.A.; Baier, T.; Einhaus, A.; Wobbe, L.; Kruse, O. High Cell Density Cultivation Enables Efficient and Sustainable Recombinant Polyamine Production in the Microalga Chlamydomonas Reinhardtii. Bioresour. Technol. 2021, 323, 124542. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, R.A.; Wittemeier, L.; Einhaus, A.; Baier, T.; Kruse, O. Advanced Pathway Engineering for Phototrophic Putrescine Production. Plant Biotechnol. J. 2022, 20, 1968–1982. [Google Scholar] [CrossRef] [PubMed]
- Fields, F.J.; Lejzerowicz, F.; Schroeder, D.; Ngoi, S.M.; Tran, M.; McDonald, D.; Jiang, L.; Chang, J.T.; Knight, R.; Mayfield, S. Effects of the Microalgae Chlamydomonas on Gastrointestinal Health. J. Funct. Foods 2020, 65, 103738. [Google Scholar] [CrossRef]
- Murbach, T.S.; Glávits, R.; Moghadam Maragheh, N.; Endres, J.R.; Hirka, G.; Goodman, R.E.; Lu, G.; Vértesi, A.; Béres, E.; Pasics Szakonyiné, I. Evaluation of the Genotoxic Potential of Protoporphyrin IX and the Safety of a Protoporphyrin IX-Rich Algal Biomass. J. Appl. Toxicol. 2022, 42, 1253–1275. [Google Scholar] [CrossRef]
- Grande, T.; Vornoli, A.; Lubrano, V.; Vizzarri, F.; Raffaelli, A.; Gabriele, M.; Novoa, J.; Sandoval, C.; Longo, V.; Echeverria, M.C.; et al. Chlamydomonas Agloeformis from the Ecuadorian Highlands: Nutrients and Bioactive Compounds Profiling and In Vitro Antioxidant Activity. Foods 2023, 12, 3147. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Gasmi, A.; Lenchyk, L.; Shanaida, M.; Zafar, S.; Mujawdiya, P.K.; Lysiuk, R.; Antonyak, H.; Noor, S.; Akram, M.; et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules 2022, 27, 7167. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Tambat, V.S.; Chen, C.W.; Chauhan, A.S.; Kumar, P.; Vadrale, A.P.; Huang, C.Y.; Dong, C. Di; Singhania, R.R. Recent Advancements in Astaxanthin Production from Microalgae: A Review. Bioresour. Technol. 2022, 364, 128030. [Google Scholar] [CrossRef]
- Perozeni, F.; Cazzaniga, S.; Baier, T.; Zanoni, F.; Zoccatelli, G.; Lauersen, K.J.; Wobbe, L.; Ballottari, M. Turning a Green Alga Red: Engineering Astaxanthin Biosynthesis by Intragenic Pseudogene Revival in Chlamydomonas Reinhardtii. Plant Biotechnol. J. 2020, 18, 2053–2067. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Perozeni, F.; Baier, T.; Ballottari, M. Engineering Astaxanthin Accumulation Reduces Photoinhibition and Increases Biomass Productivity under High Light in Chlamydomonas Reinhardtii. Biotechnol. Biofuels Bioprod. 2022, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.G.; Cho, K.; Kim, U.; Yun, J.H.; Cho, D. hyun; Heo, J.; Park, S. Bin; Kim, J.W.; Lee, Y.J.; Ramanan, R.; et al. Enhancement of Β-Carotene Production by Regulating the Autophagy-Carotenoid Biosynthesis Seesaw in Chlamydomonas Reinhardtii. Bioresour. Technol. 2019, 292, 121937. [Google Scholar] [CrossRef] [PubMed]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging Microalgal Vitamins for Human Health. Microb. Cell Fact. 2020, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Meireles, A.; Neupert, J.; Zsigmond, L.; Rosado-Souza, L.; Kovács, L.; Nagy, V.; Galambos, A.; Fernie, A.R.; Bock, R.; Tóth, S.Z. Regulation of Ascorbate Biosynthesis in Green Algae Has Evolved to Enable Rapid Stress-Induced Response via the VTC2 Gene Encoding GDP-l-Galactose Phosphorylase. New Phytol. 2017, 214, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Darwish, R.; Gedi, M.A.; Akepach, P.; Assaye, H.; Zaky, A.S.; Gray, D.A. Chlamydomonas Reinhardtii Is a Potential Food Supplement with the Capacity to Outperform Chlorella and Spirulina. Appl. Sci. 2020, 10, 6736. [Google Scholar] [CrossRef]
- Kamble, P.; Cheriyamundath, S.; Lopus, M.; Sirisha, V.L. Chemical Characteristics, Antioxidant and Anticancer Potential of Sulfated Polysaccharides from Chlamydomonas Reinhardtii. J. Appl. Phycol. 2018, 30, 1641–1653. [Google Scholar] [CrossRef]
- Choudhary, S.; Save, S.N.; Vavilala, S.L. Unravelling the Inhibitory Activity of Chlamydomonas Reinhardtii Sulfated Polysaccharides against α-Synuclein Fibrillation. Sci. Rep. 2018, 8, 5692. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, J.; Vavilala, S.L. Evaluating the Antibacterial and Antibiofilm Potential of Sulphated Polysaccharides Extracted from Green Algae Chlamydomonas Reinhardtii. J. Appl. Microbiol. 2019, 127, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.D.; Oliveira, C.F.M. de; Molino, J.V.D.; Ferreira-Camargo, L.S.; Matsudo, M.C.; Carvalho, J.C.M. de Production of Recombinant Biopharmaceuticals in Chlamydomonas Reinhardtii. Int. J. Plant Biol. 2023, 14, 39–52. [Google Scholar] [CrossRef]
- Kiefer, A.M.; Niemeyer, J.; Probst, A.; Erkel, G.; Schroda, M. Production and Secretion of Functional SARS-CoV-2 Spike Protein in Chlamydomonas Reinhardtii. Front. Plant Sci. 2022, 13, 988870. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




