Submitted:
29 May 2024
Posted:
30 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
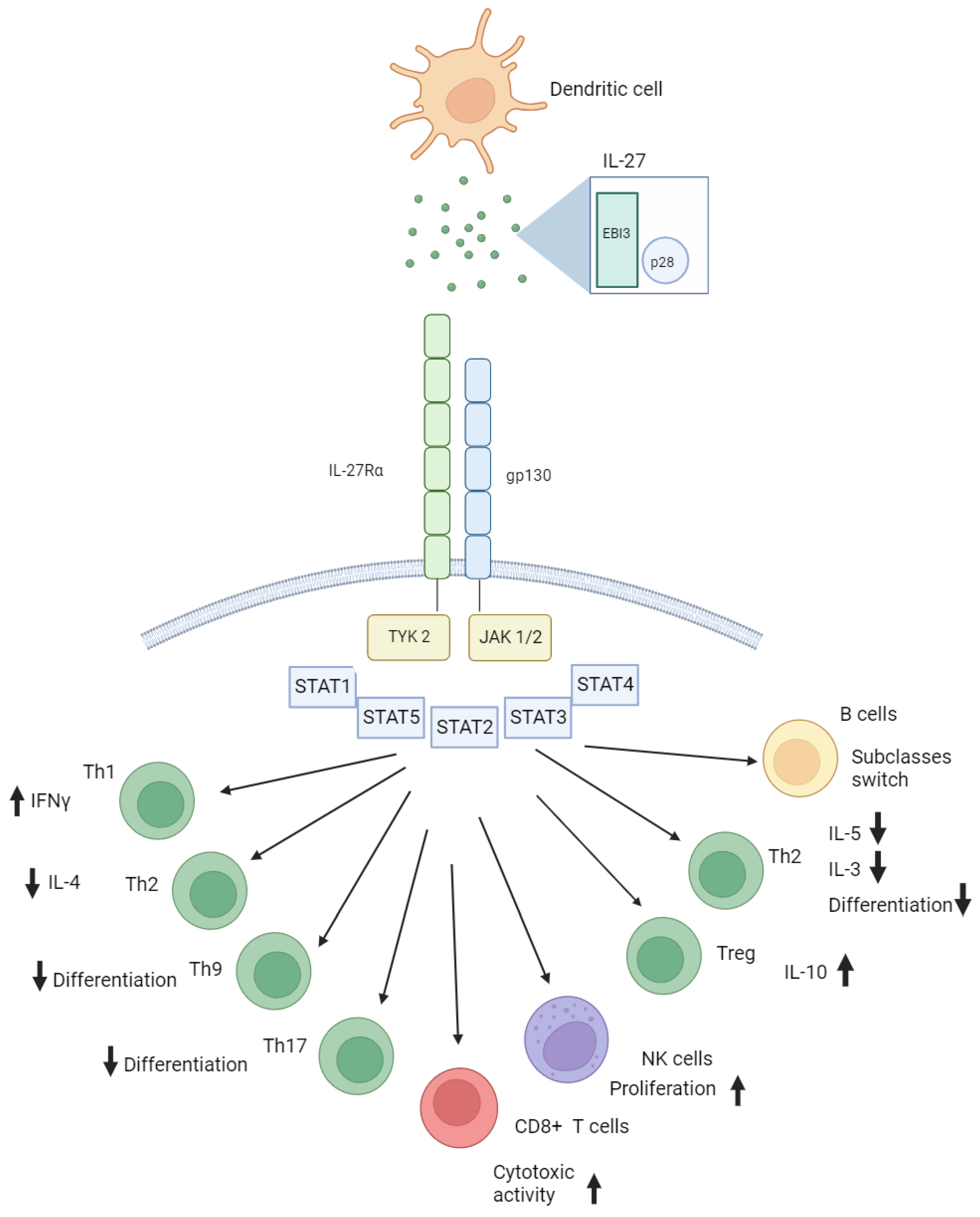
2. Interleukin 27 Conformation and Its Biological Function in Immunity
3. Interleukin 27 in Infectious Pathology
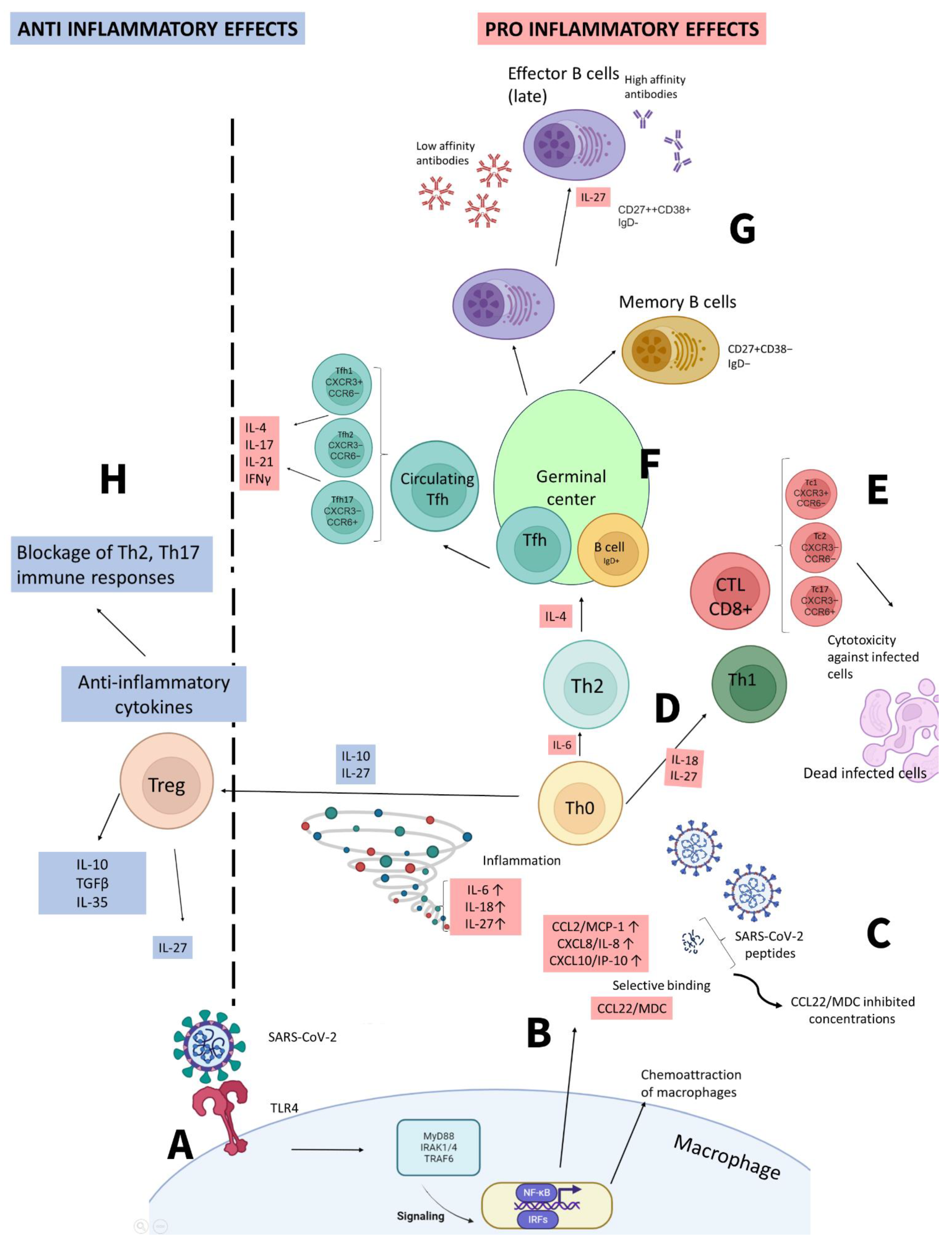
4. Interleukin 27 in COVID-19
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filip, Roxana, Roxana Gheorghita Puscaselu, Liliana Anchidin-Norocel, Mihai Dimian, and Wesley K. Savage. 2022. “Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems.” Journal of Personalized Medicine 12 (8): 1295. [CrossRef]
- Salamanna, Francesca, Melania Maglio, Maria Paola Landini, and Milena Fini. 2020. “Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2.” Frontiers in Medicine 7 (December). [CrossRef]
- Boechat, José Laerte, Inês Chora, António Morais, and Luís Delgado. 2021. “The Immune Response to SARS-CoV-2 and COVID-19 Immunopathology – Current Perspectives.” Pulmonology 27 (5). [CrossRef]
- Lu, Ligong, Hui Zhang, Meixiao Zhan, Jun Jiang, Hua Yin, Danielle J Dauphars, Shiyou Li, Yong Li, and You-Wen He. 2020. “Preventing Mortality in COVID-19 Patients: Which Cytokine to Target in a Raging Storm?” Frontiers in Cell and Developmental Biology 8 (July). [CrossRef]
- Gibson, Peter G, Ling Qin, and Ser Hon Puah. 2020. “COVID -19 Acute Respiratory Distress Syndrome ( ARDS ): Clinical Features and Differences from Typical Pre- COVID -19 ARDS.” Medical Journal of Australia 213 (2). [CrossRef]
- Kastelein, Robert A., Christopher A. Hunter, and Daniel J. Cua. 2007. “Discovery and Biology of IL-23 and IL-27: Related but Functionally Distinct Regulators of Inflammation.” Annual Review of Immunology 25 (1): 221–42. [CrossRef]
- Devergne, O, M Hummel, H Koeppen, M M Le Beau, E C Nathanson, E Kieff, and M Birkenbach. 1996. “A Novel Interleukin-12 P40-Related Protein Induced by Latent Epstein-Barr Virus Infection in B Lymphocytes.” Journal of Virology 70 (2): 1143–53. [CrossRef]
- Pflanz, Stefan, Jackie C Timans, Jeanne Cheung, Rency Rosales, Holger Kanzler, Jonathan Gilbert, Linda Hibbert, et al. 2002. “IL-27, a Heterodimeric Cytokine Composed of EBI3 and P28 Protein, Induces Proliferation of Naive CD4+ T Cells.” Immunity 16 (6): 779–90. [CrossRef]
- Mei, Youwen, Zi Lv, Liling Xiong, Hanwen Zhang, Nanlin Yin, and Hongbo Qi. 2021. “The Dual Role of IL-27 in CD4+T Cells.” Molecular Immunology 138 (October): 172–80. [CrossRef]
- Min, Booki, Dongkyun Kim, and Matthias J Feige. 2021. “IL-30† (IL-27A): A Familiar Stranger in Immunity, Inflammation, and Cancer.” Experimental & Molecular Medicine 53 (5): 823–34. [CrossRef]
- Kishi, Yasuhiro, Takaaki Kondo, Sheng Xiao, Nir Yosef, Jellert Gaublomme, Chuan Wu, Chao Wang, et al. 2016. “Protein c Receptor (PROCR) Is a Negative Regulator of Th17 Pathogenicity.” Journal of Experimental Medicine 213 (11): 2489–2501. [CrossRef]
- Marques, Hanna Santos, Breno Bittencourt de Brito, Filipe Antônio França da Silva, Maria Luísa Cordeiro Santos, Júlio César Braga de Souza, Thiago Macêdo Lopes Correia, Luana Weber Lopes, et al. 2021. “Relationship between Th17 Immune Response and Cancer.” World Journal of Clinical Oncology 12 (10): 845–67. [CrossRef]
- Mchedlidze, T, M Kindermann, A T Neves, D Voehringer, M F Neurath, and S Wirtz. 2016. “IL-27 Suppresses Type 2 Immune Responses in Vivo via Direct Effects on Group 2 Innate Lymphoid Cells.” Mucosal Immunology 9 (6): 1384–94. [CrossRef]
- Do, Jeongsu, Dongkyun Kim, Sohee Kim, Alice Valentin-Torres, Nina Dvorina, Eunjung Jang, Vivekananthan Nagarajavel, et al. 2017. “Treg-Specific IL-27Rα Deletion Uncovers a Key Role for IL-27 in Treg Function to Control Autoimmunity.” Proceedings of the National Academy of Sciences of the United States of America 114 (38): 10190–95. [CrossRef]
- Pyle, Chloe J., Faith I. Uwadiae, David P. Swieboda, and James A. Harker. 2017. “Early IL-6 Signalling Promotes IL-27 Dependent Maturation of Regulatory T Cells in the Lungs and Resolution of Viral Immunopathology.” Edited by Carolina B. Lopez. PLOS Pathogens 13 (9): e1006640. [CrossRef]
- Kourko, Olena, Kyle Seaver, Natalya Odoardi, Sameh Basta, and Katrina Gee. 2019. “IL-27, IL-30, and IL-35: A Cytokine Triumvirate in Cancer.” Frontiers in Oncology 9 (October). [CrossRef]
- Morita, Yugo, Elysia A. Masters, Edward M. Schwarz, and Gowrishankar Muthukrishnan. 2021. “Interleukin-27 and Its Diverse Effects on Bacterial Infections.” Frontiers in Immunology 12 (May). [CrossRef]
- Shimizu, Jun, Fumio Kaneko, and Noboru Suzuki. 2013. “Skewed Helper T-Cell Responses to IL-12 Family Cytokines Produced by Antigen-Presenting Cells and the Genetic Background in Behcet’s Disease.” Genetics Research International 2013 (December): 1–11. [CrossRef]
- Zwirner, Norberto Walter, and Andrea Ziblat. 2017. “Regulation of NK Cell Activation and Effector Functions by the IL-12 Family of Cytokines: The Case of IL-27.” Frontiers in Immunology 8 (January). [CrossRef]
- Yoshimoto, Takayuki, Yukino Chiba, Jun-Ichi Furusawa, Mingli Xu, Ren Tsunoda, Kaname Higuchi, and Izuru Mizoguchi. 2015. “Potential Clinical Application of Interleukin-27 as an Antitumor Agent.” Cancer Science 106 (9): 1103–10. [CrossRef]
- Kourko, Olena, Kyle Seaver, Natalya Odoardi, Sameh Basta, and Katrina Gee. 2019. “IL-27, IL-30, and IL-35: A Cytokine Triumvirate in Cancer.” Frontiers in Oncology 9 (October). [CrossRef]
- Jia, Haiyan, Paula Dilger, Chris Bird, and Meenu Wadhwa. 2016. “IL-27 Promotes Proliferation of Human Leukemic Cell Lines through the MAPK/ERK Signaling Pathway and Suppresses Sensitivity to Chemotherapeutic Drugs.” Journal of Interferon & Cytokine Research 36 (5): 302–16. [CrossRef]
- Sekar, Divya, Christina Hahn, Bernhard Brüne, Edward Roberts, and Andreas Weigert. 2012. “Apoptotic Tumor Cells Induce IL-27 Release from Human DCS to Activate TReg Cells That Express CD69 and Attenuate Cytotoxicity.” European Journal of Immunology 42 (6): 1585–98. [CrossRef]
- Batten, Marcel, Ji Li, Sothy Yi, Noelyn M. Kljavin, Dimitry M. Danilenko, Sophie Lucas, James Lee, Frederic J. de Sauvage, and Nico Ghilardi. 2006. “Interleukin 27 Limits Autoimmune Encephalomyelitis by Suppressing the Development of Interleukin 17–Producing T Cells.” Nature Immunology 7 (9): 929–36. [CrossRef]
- Morishima, Naohiko, Itaru Mizoguchi, Masae Okumura, Yukino Chiba, Mingli Xu, Motomu Shimizu, Masanao Matsui, Junichiro Mizuguchi, and Takayuki Yoshimoto. 2010. “A Pivotal Role for Interleukin-27 in CD8+T Cell Functions and Generation of Cytotoxic T Lymphocytes.” Journal of Biomedicine and Biotechnology 2010 (January): 1–10. [CrossRef]
- Pflanz, Stefan, Jackie C Timans, Jeanne Cheung, Rency Rosales, Holger Kanzler, Jonathan Gilbert, Linda Hibbert, et al. 2002. “IL-27, a Heterodimeric Cytokine Composed of EBI3 and P28 Protein, Induces Proliferation of Naive CD4+ T Cells.” Immunity 16 (6): 779–90. [CrossRef]
- Lucas, Sophie, Nico Ghilardi, Ji Li, and Frédéric J. de Sauvage. 2003. “IL-27 Regulates IL-12 Responsiveness of Naïve CD4+ T Cells through Stat1-Dependent and -Independent Mechanisms.” Proceedings of the National Academy of Sciences of the United States of America 100 (25): 15047–52. [CrossRef]
- Yoshimoto, Tomohiro, Takayuki Yoshimoto, Koubun Yasuda, Junichiro Mizuguchi, and Kenji Nakanishi. 2007. “IL-27 Suppresses Th2 Cell Development and Th2 Cytokines Production from Polarized Th2 Cells: A Novel Therapeutic Way for Th2-Mediated Allergic Inflammation.” The Journal of Immunology 179 (7): 4415–23. [CrossRef]
- Schneider, Raphael, Teodora Yaneva, Diane Beauseigle, Lama El-Khoury, and Nathalie Arbour. 2010. “IL-27 Increases the Proliferation and Effector Functions of Human Naïve CD8+ T Lymphocytes and Promotes Their Development into Tc1 Cells.” European Journal of Immunology 41 (1): 47–59. [CrossRef]
- Harker, James A., Aleksandr Dolgoter, and Elina I. Zuniga. 2013. “Cell-Intrinsic IL-27 and Gp130 Cytokine Receptor Signaling Regulates Virus-Specific CD4+ T Cell Responses and Viral Control during Chronic Infection.” Immunity 39 (3): 548–59. [CrossRef]
- Meka, Rakeshchandra R., Shivaprasad H. Venkatesha, Steven Dudics, Bodhraj Acharya, and Kamal D. Moudgil. 2015. “IL-27-Induced Modulation of Autoimmunity and Its Therapeutic Potential.” Autoimmunity Reviews 14 (12): 1131–41. [CrossRef]
- Do, Jeong-su, Anabelle Visperas, Yibayiri Osee Sanogo, Jennifer J Bechtel, Nina Dvorina, Sohee Kim, Eunjung Jang, et al. 2016. “An IL-27/Lag3 Axis Enhances Foxp3+ Regulatory T Cell–Suppressive Function and Therapeutic Efficacy.” Mucosal Immunology 9 (1): 137–45. [CrossRef]
- Wong, Hector R., Christopher J. Lindsell, Patrick Lahni, Kimberly W. Hart, and Sebastien Gibot. 2013. “Interleukin 27 as a Sepsis Diagnostic Biomarker in Critically Ill Adults.” Shock 40 (5): 382–86. [CrossRef]
- Skouras, Vasileios S, Sophia F Magkouta, Ioannis Psallidas, Irene Tsilioni, Panagiotis Maragozidis, Konstantinos I Gourgoulianis, and Ioannis Kalomenidis. 2015. “Interleukin-27 Improves the Ability of Adenosine Deaminase to Rule out Tuberculous Pleural Effusion regardless of Pleural Tuberculosis Prevalence.” Infectious Diseases 47 (7): 477–83. [CrossRef]
- Gollnick, Hailey, Jamie Barber, Robert J Wilkinson, Sandra Newton, and Ankita Garg. 2023. “IL-27 Inhibits Anti- Mycobacterium Tuberculosis Innate Immune Activity of Primary Human Macrophages.” Tuberculosis 139 (March): 102326–26. [CrossRef]
- Zeng, Jiajia, Shuaini Yang, Yuqing Tuo, Xiaoyu Zha, Ruoyuan Sun, Tingsha Lu, Hong Zhang, Lu Tan, Sai Qiao, and Hong Bai. 2023. “IL-27 Signaling Promotes Th1 Response by Downregulating IL-10 Production in DCs during Chlamydial Respiratory Infection.” Microorganisms 11 (3): 604–4. [CrossRef]
- Liu, Francesca Diane M., Elisabeth E. Kenngott, Micha F. Schröter, Anja Kühl, Silke Jennrich, Ralf Watzlawick, Ute Hoffmann, et al. 2014. “Timed Action of IL-27 Protects from Immunopathology While Preserving Defense in Influenza.” PLoS Pathogens 10 (5): e1004110. [CrossRef]
- Montes de Oca, Marcela, Fabian de Labastida Rivera, Clay Winterford, Teija C. M. Frame, Susanna S. Ng, Fiona H. Amante, Chelsea L. Edwards, et al. 2020. “IL-27 Signalling Regulates Glycolysis in Th1 Cells to Limit Immunopathology during Infection.” Edited by Phillip Scott. PLOS Pathogens 16 (10): e1008994. [CrossRef]
- Ansari, Nasim Akhtar, Rajiv Kumar, Shalini Gautam, Susanne Nylén, Om Prakash Singh, Shyam Sundar, and David Sacks. 2011. “IL-27 and IL-21 Are Associated with T Cell IL-10 Responses in Human Visceral Leishmaniasis.” The Journal of Immunology 186 (7): 3977–85. [CrossRef]
- Arsentieva, N A, N E Liubimova, O K Batsunov, Z R Korobova, O V Stanevich, A A Lebedeva, E A Vorobyov, et al. 2021. “Plasma Cytokines in Patients with COVID-19 during Acute Phase of the Disease and Following Complete Recovery.” Медицинская иммунoлoгия 23 (2): 311–26. [CrossRef]
- Zamani, Batool, Maedeh Najafizadeh, Hossein Motedayyen, and Reza Arefnezhad. 2022. “Predicting Roles of IL-27 and IL-32 in Determining the Severity and Outcome of COVID-19.” International Journal of Immunopathology and Pharmacology 36 (January): 039463202211458-039463202211458. [CrossRef]
- Klingler, Jéromine, Gregory Lambert, Juan C Bandres, Rozita Emami-Gorizi, Arthur Nádas, Kasopefoluwa Y Oguntuyo, Fatima Amanat, et al. 2022. “Immune Profiles to Distinguish Hospitalized versus Ambulatory COVID-19 Cases in Older Patients” 25 (12): 105608–8. [CrossRef]
- Laatifi, Mariam, Samira Douzi, Hind Ezzine, Chadia El Asry, Abdellah Naya, Abdelaziz Bouklouze, Younes Zaid, and Mariam Naciri. 2023. “Explanatory Predictive Model for COVID-19 Severity Risk Employing Machine Learning, Shapley Addition, and LIME.” Scientific Reports 13 (1). [CrossRef]
- Juan Felipe Valdés-López, and Silvio Urcuqui-Inchima. 2023. “Antiviral Response and Immunopathogenesis of Interleukin 27 in COVID-19.” Archives of Virology 168 (7). [CrossRef]
- Valdés-López, J.F., Urcuqui-Inchima, S. Antiviral response and immunopathogenesis of Interleukin 27 in COVID-19. Arch Virol 168, 178 (2023). [CrossRef]
- Arsentieva, Natalia A, Natalia E Liubimova, Oleg K Batsunov, Zoia R Korobova, Raisa N Kuznetsova, Artem A Rubinstein, Oksana V Stanevich, et al. 2023. “Predictive Value of Specific Cytokines for Lethal COVID-19 Outcome.” Infekciâ I Immunitet 12 (5): 859–68. [CrossRef]
- Xu, Fang, Qiong Liu, Shihui Lin, Na Shen, Yibing Yin, and Ju Cao. 2013. “IL-27 Is Elevated in Acute Lung Injury and Mediates Inflammation.” Journal of Clinical Immunology 33 (7): 1257–68. [CrossRef]
- Xu, Zheng, Xin-Ming Wang, Peng Cao, Chen Zhang, Chun-Mei Feng, Ling Zheng, De-Xiang Xu, Lin Fu, and Hui Zhao. 2022. “Serum IL-27 Predicts the Severity and Prognosis in Patients with Community-Acquired Pneumonia: A Prospective Cohort Study.” International Journal of Medical Sciences 19 (1): 74–81. [CrossRef]
- Korobova, Zoia R., Natalia A. Arsentieva, Natalia E. Liubimova, Oleg K. Batsunov, Vladimir G. Dedkov, Anna S. Gladkikh, Alena A. Sharova, et al. 2022. “Cytokine Profiling in Different SARS-CoV-2 Genetic Variants.” International Journal of Molecular Sciences 23 (22): 14146. [CrossRef]
- Kudryavtsev, Igor V., Natalia A. Arsentieva, Zoia R. Korobova, Dmitry V. Isakov, Artem A. Rubinstein, Oleg K. Batsunov, Irina V. Khamitova, Raisa N. Kuznetsova, Tikhon V. Savin, Tatiana V. Akisheva, and et al. 2022. "Heterogenous CD8+ T Cell Maturation and ‘Polarization’ in Acute and Convalescent COVID-19 Patients" Viruses 14, no. 9: 1906. [CrossRef]
- Schneider, Raphael, Teodora Yaneva, Diane Beauseigle, Lama El-Khoury, and Nathalie Arbour. 2010. “IL-27 Increases the Proliferation and Effector Functions of Human Naïve CD8+ T Lymphocytes and Promotes Their Development into Tc1 Cells.” European Journal of Immunology 41 (1): 47–59. [CrossRef]
- Harker, James A., Aleksandr Dolgoter, and Elina I. Zuniga. 2013. “Cell-Intrinsic IL-27 and Gp130 Cytokine Receptor Signaling Regulates Virus-Specific CD4+ T Cell Responses and Viral Control during Chronic Infection.” Immunity 39 (3): 548–59. [CrossRef]
| Year of publication | Authors | Findings | Reference |
|---|---|---|---|
| 2021 | Arsentieva N.A. et al. | Increase in IL-27 levels in acute COVID-19 patients vs. healthy donors (p<0.0001); Decrease of IL-27 concentrations in convalescents when compared to healthy donors (p=0.0015). | [41] |
| 2022 | Arsentieva N.A. et al. | IL-27 showed statistically significant increase in concentrations in COVID-19 acute patients when compared to healthy donors (p<0.001 for non-survivors and p<0.05 for survivors). | [46] |
| 2022 | Korobova Z.R. et al. | IL-27 was one of the four biological markers showing statistically significant changes in concentrations in blood plasma of patients infected with different variants of SARS-CoV-2 (p<0.001). | [49] |
| 2022 | Kudryavtsev I.V. et al. | The correlation between Tc17 cells of central memory and TEMRA cells and serum IL-27 levels was negative in patients with acute COVID-19, a tendency not observed in convalescent or healthy donors. | [50] |
| 2022 | Zamani B. et al. | IL-27, along with IL-32 and neutrophil-to-lymphocyte ratio (NLR) was highlighted as one of the markers of severe COVID-19 and lethal outcomes. | [42] |
| 2022 | Klingler J et al. | In patients with higher demographic risk factors (i.e., male, black/Hispanic descent and median age over 63 years old), IL-27 was suggested as one of the factors to prove the need for hospitalization | [43] |
| 2023 | Laatifi M. et al. | COVID-19 prognosis was based on machine learning and included several cytokines, and IL-27, along with IL-9, IL-12p40, and MCP-3, to play the role of markers for non-severe COVID-19 | [44] |
| 2023 | Valdés-López and Urcuqui-Inchima | IL-27 triggers a strong pro-inflammatory and antiviral reaction that relies on STAT1, without requiring IFN, in COVID-19-derived PBMCs and monocytes, which is linked to a severe clinical outcome of COVID-19. This effect is also seen in macrophages that have been stimulated by S protein. | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





