1. Introduction
A wide range of medical applications use autologous platelet-rich plasma (PRP) formulations and plasma protein-based biological preparations for non-surgical tissue repair, regeneration, and wound healing applications [
1,
2]. The employment of PRP and protein-based technologies have generated promising patient outcomes, as these complex biological preparations and processes involve a multitude of local and systemic cellular and molecular activities in a sequential manner, aimed at restoring the integrity and function of damaged tissues.
PRP preparations employ centrifuges and gravitational cellular density separation protocols to separate a unit of fresh whole blood into a platelet-poor plasma (PPP) fraction, a platelet-buffy coat stratum, with or without leukocytes, and a red blood cell (RBC) fraction [
3]. The PRP induced tissue repair, regeneration, and wound healing potential is based on the release of a plethora of platelet constituents from alpha, dense, and lysosomal granules, as well as the activities of many platelet adhesion molecules. Furthermore, the absence, or presence, of particular leukocytes has an effect on platelet cellular functions, like angiogenesis, immunomodulation, cell signaling and metabolism, and ultimately tissue repair and functional tissue restoration [
1,
4].
Regretfully, there is no consensus on a comprehensive PRP classification system or on autologous biological preparations, resulting in an oversaturation of PRP devices with numerous preparation methods [
5,
6]. Therefore, PRP functional characteristics, such as centrifugation protocols, platelet dosing, leukocyte and RBC content, and cellular delivery methods to treat specific pathological tissue conditions, differ substantially [
7,
8].
There are three main variables in a simplified classification system, namely platelets, leukocytes, and plasma proteins [
9]. In terms of these biological variables, PRP can be classified into four general categories [
10]. A number of variables differentiate PRP devices, including the volume of processed whole blood, the device platelet capture rate which influences the platelet concentration, and the prepared PRP volume. In addition, the presence and concentration of leukocytes is an underestimated factor in PRP treatment outcomes. Accordingly, the literature denotes to poor patient outcomes when PRP devices with low platelet numbers and inconsistent other cellular content were used [
11,
12,
13,
14].
Following PRP preparation, the platelet concentrate is removed from the device for clinical application, while the PPP fraction, with its own molecular and cellular components, is usually discarded as it is not considered therapeutically beneficial. In spite of this, certain growth factors, such as insulin-like growth factor-1 (IGF-1) and hepatocyte growth factor HGF), are predominantly found in the PPP fraction, outside of platelets [
15,
16]. The PPP fractions also contain exosomes and other macrovesicular structures that are essential for cell-to-cell communication and for cell signaling [
17,
18].
The advancement of technology has led to the development of new concepts for biological preparations, such as the concentration of PPP by modified ultrafiltration in order to increase plasma levels of plasma proteins, such as fibrinogen, alpha 2 macroglobulin (A2M), IGF-1, HGF, cytokines, and other biomolecules [
17,
19]. Modern low volume ultrafiltration devices, with a small membrane pore size create a protein rich product that can be used as a standalone product or as a combination with high definition PRP formulations to form a consolidated multicellular protein rich platelet concentrate (PRPC) preparation. In comparison to PRP alone, PRPC contains a greater amount of diverse growth factors and other important non-platelet derivates [
17], with several studies reporting on synergistic effects between platelet-derived growth factors and non-platelet-derived growth factors [
20,
21]. PRPCs function as a biological matrix once activated, retaining and facilitating interactions between the matrix cellular and molecular constituents [
19]. Moreover, the versatility of PRPC technology facilitates the creation of patient-specific formulations for non-surgical as well as surgical applications.
Our objective in this review is to provide a comprehensive overview of a multipurpose biological platform comprised of high-definition PRP and viable protein-rich plasma, as well as their typical biological contributions in tissue repair, regeneration, and wound healing.
2. The Impact of PRP in PRPC
In an era in which traumatic and degenerative diseases are becoming more prevalent, regenerative medicine therapies aims to develop strategies to repair and regenerate damaged tissues to restore normal function [
22].
Regenerative medicine strategies are primarily focused on non-surgical and interventional approaches, treating conditions where conventional therapies are ineffective or insufficient, using autologous biological preparations (ABP) that are capable of tissue repair and regeneration. In biosurgical procedures, regenerative medicine technologies are used to support in post-surgical wound healing to minimize complications and improve clinical outcomes through the release of many biologically active components present in numerous autologous preparations, like several PRP formulations, bone marrow concentrates, and a variety of adipose tissue preparations [
23,
24]. In this review, we focus merely on PRP bioformulation and their rationale in PRPC.
2.1. Rationale for PRP Therapies
Physiological pathways can be stimulated following the precise delivery of concentrated platelets into degenerated and injured tissues to initiate tissue repair through the release of many platelet-derived growth factors, cytokines, molecules, and adhesive proteins. Additionally, local tissue regulators act through cell signaling, paracrine, autocrine, and intracrine mechanisms after PRP administration to facilitate tissue repair.
2.2. PRP Classification
PRP is a centrifugated and processed liquid fraction of harvested fresh peripheral blood, obtained by gravitational density separation, and is characterized by a heterogeneous and complex composition of multi-cellular components and a significant increase in platelet concentration compared to baseline values (
Figure 1). Currently, the recommended dose of platelets in PRP is > 1billion platelets per ml [
3,
25].
Due to the lack of consensus regarding standardization and preparation methods, PRP devices may be categorized as pure PRP (P-PRP), leukocyte-poor PRP (LP-PRP), leukocyte-rich PRP (LR-PRP), and varying compositions of plasma based preparations, such as platelet-rich fibrin (PRF) [
10]. However, PRP and plasma-based PRP preparations differ significantly in composition, as demonstrated by a proteomic study [
26]. Encouraging results following PRP applications have been reported [
27]. Nevertheless, poor and even negative patient outcomes have also been reported, with PRP platelet dosing and cellular characterization being the most prevalent variables involved, largely due to differences in PRP device architecture and preparation techniques [
28].
2.3. Platelet Structures and Biological Content
Platelets are anucleate and small cells, pinched off from megakaryocytes in the red bone marrow before they are released on a continuous basis into the peripheral circulation, on a continuous basis [
29]. The platelet cell membrane is made of phospholipids and glycoproteins that interact with various ligands, like collagen and thrombin, and mediate platelet adhesion and aggregation [
30]. Furthermore, glycoproteins facilitate cellular signaling to interact with extra cellular molecules and cells [
31].
Platelets contain three major types of secretory granules, including α-granules, dense granules, and lysosomes, containing unique cellular and molecular composites [
32]. Proteomic based analysis provided detailed information on platelet granular content following platelet activation [
33]. Dense granules and α-granules are the most well-studied and the most physiologically important as they are involved in numerous cellular activities related to tissue repair and regeneration [
3]. On overview of platelet structures, their main content, and other platelet elements are shown in table 1.
2.3.1. α-Granules
α-granules are unique to platelets, and they are they are most abundantly present, with approximately 50-80 granules per platelet, accounting for 10% of its volume [
34].
Platelet proteomic studies have acknowledged that there are more than 300 soluble proteins in platelets [
35]. Platelet α- granules contain a variety of proteins, including numerous platelet growth factors (PGFs) with their typical isoforms, like vascular endothelium growth factor (VEGF), platelet-derived growth factor (PDGF) with their various isoforms, transforming growth factor-beta (TGF-β), fibroblast growth factor (FGF), epidermal growth factor (EGF), and to a lesser extend hepatocyte growth factor (HGF), and insulin-like growth factor-1 (IGF-1) [
36]. Furthermore, a variety of angiogenetic protein regulators, coagulation factors, and immunomodulatory molecules are stored in the alpha granules, as well many chemokines and cytokines [
35]. They serve as pro-inflammatory and immune modulating factors when they are secreted, among others Regulated by T-Cell activation and probably secreted by T-Cells (RANTES), and platelet factor 4, also known as chemokine ligand 4 (CXCL4) [
37,
38]. Additionally, α-granules contain numerous platelet membrane bound proteins receptors, like P-selectin and integrins, that are expressed on the platelet surface [
39].
2.3.1.1. Platelet-Derived Exosomes
Activated PRP will lead to the secretion of platelet granules as well as the release of extracellular vesicles (EVs), including exosomes, originating from intracytoplasmic multivescicular bodies (MVBs) [
40,
41]. In recent years, exosomes received a lot of attention in regenerative medicine applications as active biological carriers in tissue repair and regeneration. Exosomes are secreted by many different metabolically active cells, like immune cells and mesenchymal stem cells (MSCs), and they are found in blood, bone marrow, synovial fluid, and urine [
42,
43,
44,
45]. Exosomes are small, differently sized EVs, 30–150 nm in diameter, and act as carriers of, among others, many biologically active proteins, mRNA, miRNA, and other bioactive substances [
46,
47].
Exosomes have a significant impact on many cellular functions, including immune response, and cell signaling [
48,
49], thereby playing crucial roles in intercellular communication [
50] and platelet-cell communication [
51]], transporting their cargo between cells and organs during normal biological processes.
Platelet-derived exosomes (PLT-Exos) are stored in platelet alpha granules and MVBs [
52]. The concentration of exosomes in plasma has reported to range from 0.9-1.3 x 10
9/mL [
53], with PLT-Exos constituting approximately 75% of total plasma exosomes [
54].
Torreggiani et al. isolated exosomes from PRP-platelets (PRP-Exos) and demonstrated their potential effects on the proliferation and differentiation of bone marrow MSCs [
55], advocating their roles in tissue regeneration. It has been suggested that PRP-Exos may deliver a nano-delivery system, contributing the healing mechanisms of PRP [
56]. Interestingly, several released PGF can be encapsulated into exosomes in high concentrations and transported across the extra cellular space to target tissues capable of promoting angiogenesis and fibroblast proliferation [
55,
57,
58].
The potential for exosomes and other EVs in tissue repair, regeneration, and wound healing is extensive. However, a better understanding and optimization of PRP-Exos preparation methods, and their function in PGF transportation need to be further explored and positioned against commercial purification processes.
2.3.2. Dense Granules
The second most prevalent intra-platelet structure are the dense granules. These storage organelles contain small molecules such as histamine, ADP, polyphosphates, serotonin (5-hydroxytryptamine, 5-HT), and epinephrine [
59]. Their key function is converting platelets into an active state, initiating thrombus formation with the subsequent release of α-granular constituents.
It is also noteworthy that several secretory molecules are capable of immunomodulation [
60]. Particularly, platelet ADP is connected to dendritic cellular activity, potentially resulting in increased antigen endocytosis, and steer an immune response by linking the innate and adaptive immune systems [
61]. Importantly, 5-HT is stored at high concentrations in dense granules and has been recognized as an important neurotransmitter, involved in neuropsychological processes. Furthermore, 5-HT contributes to the regulation of several biological functions, including pain, inflammation, and immunomodulation [
62,
63,
64].
2.3.3. Lysosomes
Platelet lysosomal functions have not been well studied. Few studies mention the in vivo release of platelet lysosomal content, which contain an array of acid glycohydrolases, as reflected in table 1. These enzymes can destroy glycoproteins and glycosaminoglycans [
65] and they act as contributors to the cell digestive system, destroying materials taken up from outside the cell and to digest archaic cytosolic components [
66]. Furthermore, Heijnen and van der Sluis mentioned lysosomal activities in extracellular functions, such as fibrinolysis, supportive in vasculature remodeling, and the degradation of extracellular matrix components [
67].
On this matter, lysosome proteases have been implicated in tendon homeostasis mechanisms due to their ability to cleave the tendon ECM [
68]. Interestingly, since lysosomes contain proteases and cationic proteins with bactericidal activities, they interact as well with macrophages in phagocytosis [
69].
2.4. Variability in Leukocyte Presence in PRP and PRPC
Throughout the years, practitioners and scientists have defined various formulations of PRP, including P-PRP and LP-PRP preparations which result in minimal leukocyte contamination. Others refer to PRP as a biological preparation containing increased levels of platelets, leukocytes, or LR-PRP [
10,
36,
70]. Furthermore, another classification of PRP is platelet-rich fibrin (PRF) or platelet-leukocyte rich fibrin (L-PRF), containing greater levels of fibrin and bioactive proteins [
9]. Specifically, with regard to the various PRP formulations, neutrophils, lymphocytes, monocytes, and macrophages have a significant role in the intrinsic biology of classical wound healing cascades and have an impact on chronic tissue pathologies as a result of their ability to interact with platelets, immune cells, and host defense mechanisms [
36,
71]
Currently available PRP devices and technologies produce a broad range of bioformulations with regards to platelet dosage, leukocyte populations, and concentrations, potentially resulting in varying effects on inflammation, immunomodulation, angiogenesis, nociception, and ultimately tissue repair, regeneration, and wound healing [
28,
72,
73].
The role of leukocytes in PRP preparations, as well as their impact on tissue regeneration and repair, is still widely underestimated. Preferably, PRP bioformulations should be substantiated based on the cellular properties of pathological tissue structures.
2.5. Tissue Repair and Regeneration Provoked by PRP
Tissue regeneration and repair utilizing PRP technology following degenerative processes or trauma is a complex biological process that involves numerous biological components and mechanisms. One of the key aspects in this process is the role of platelets secreting various substances to incite immunomodulation, angiogenesis, and modulate pain to promote tissue repair and restore tissue functionality.
2.5.1. Immunomodulation
The innate immune system identifies imposing microbes and fragments from tissue damage fragments when surface-expressed pattern recognition receptors, like toll-like receptors (TLRs) [
74], bind to damaged and pathogen-associated molecular patterns. Because of TLR activation, nuclear factor kappa B (NF-
KB) is activated, which regulates innate and adaptive immune responses. An adaptive immune system’s function follows the identification of pathogens or tissue damage by employing antigen-specific receptors to eliminate the pathogens.
Platelets are essential cells of both the innate and adaptive immune systems. They are among the first cells to identify microbial pathogens and are proficient in identifying endothelial injury [
75]. In compromised tissues, non-activated platelets become rapidly activated and undergo exocytosis, releasing their granular and molecular content, and express platelet chemokine receptors, immediately followed by platelet aggregation and the subsequent initiation of inflammatory pathways [
76].
When PRP, consisting of a high concentration of platelets, is delivered to pathological tissues, a similar cascade of events is elicited with platelets interacting with local immune cells that express several surface immunomodulatory receptor molecules, such as platelet-specific TLRs, P-selectin, and other cytokines to regulate and optimize tissue repair, wound healing, and halt degeneration [
77,
78]. As opposed to LP-PRP formulations, LR-PRP contains leukocytic cells, which may initiate early-phase immune responses in response to platelet activation [
79]. Specifically, TLR-4 induces various interactions between platelets and neutrophils [
80], including neutrophil degranulation with release of reactive oxygen species and creation of neutrophil-extracellular traps (NETs) that are capable of trapping and destroying pathogens through the process of NETosis [
81]
Similarly, as a result of platelet activation, monocyte and macrophage effector functions are modulated, contributing to tissue inflammation, the differentiation of immune cells, and they initiate T- and B cell responses via dendritic cells [
82,
83]. Furthermore, monocytes migrate into degenerative and diseased tissues and are able to alter chemotaxis and modify proteolysis by adhering to and secreting inflammatory mediators [
84].
2.5.2. Angiogenesis
During wound healing, tissue repair and regeneration, optimization of angiogenetic pathways is required. Angiogenesis in pathological microenvironments involves the sprouting and organization of micro vessels from preexisting blood vessels [
85]. It requires the restoration of numerous angiogenic mechanisms to overcome low oxygen tensions, low pH levels, and high lactate levels [
28].
As a result of PRP therapy, PGFs and platelet pro- and anti-angiogenic factors mediate cell–cell communication and cell–matrix interactions, contributing to the formation of functional blood vessels [
86,
87]. PRP formulations containing a high platelet dose (> 1.5 x 10
6 platelets/µL) and therefore containing a high concentration of proangiogenic VEGF were found to be most effective in stimulating angiogenesis [
88]. Moreover, it has been demonstrated that the synergy between PDGF-BB and VEGF enhances the formation of a mature vascular network [
89].
Activated leukocytes in LR-PRP are vigorously involved in angiogenesis [
86]. Neutrophils, monocytes, and lymphocytes produce MMP-2 and MMP-9, contributing to the proliferation and migration of endothelial cells (ECs) [
90]. Interestingly, matrix metalloproteinases (MMPs) are also released by platelets on a platelet dose-dependent basis, increasing the total concentration of MMPs [
89]. Additionally, monocyte-derived macrophages secrete significant amounts of VEGF-A, important in tissue repair induced angiogenesis [
91]
According to Yuan et al., LR-PRP generates greater angiogenesis than LP-PRP, and the blood supply is more abundant when LR-PRP is applied [
92]. The restoration of blood flow is essential during tissue repair processes as new blood vessels can deliver oxygen and nutrients while simultaneously removing catabolic byproducts [
93].
2.5.3. Analgesic Effects
Platelet dosing and the PRP bioformulations have been identified as important features in PRP preparations to contribute to analgesic effects [
94,
95]. Obviously, patients who experience less pain tend to have better outcomes and more effective post-operative rehabilitation results.
Different mechanisms may be involved in the induction of painkilling mechanisms by PRP, both direct and indirect. According to Mohammadi et al., the physiological aspects of wound pain in post-surgical wound care patients were related to vascular injury and skin tissues hypoxia [
93]. However, the application of PRP facilitated the reinstitution of neo-angiogenesis, resulting in improved tissue oxygenation and nutrient delivery, and patients experience the benefit of reduced pain as a result.
Similar painkilling observations were reported in a systematic review regarding PRP utilization in orthopedic surgical and non-surgical spine procedures [
95,
96]. In another study, higher platelet concentrations were significantly associated with greater analgesic responses and higher post treatment outcome satisfaction [
97]. The impact of platelet concentration on painkilling was also demonstrated in a small animal study. PRP containing a platelet concentration of 1 x 10
6/µL completely relieved pain, while a reduction in platelet concentration by 50% induced significantly less pain relief [
98].
In 2008, a mechanistic role for platelet-derived 5-HT was implied by Everts et al. [
24]. They hypothesized that 5-HT, a critical neurotransmitter, plays a variety of well-defined analgesic roles in the central nervous system, with specific functions for the many 5-HT receptors [
99]. Further, they hypothesized that administered PRP, containing huge amounts of 5-HT, stored in platelet dense granules, may contribute to analgesic effects, as Sprott et al. reported significant improvements in pain scores associated with a significant reduction in platelet 5-HT levels and a concomitant increase in serum serotonin levels [
100]. It is noteworthy that Kuffler found that several PRP preparation aspects had varying effects on chronic pain relief, with a significant role for the analgesic effects of platelet 5-HT [
101].
3. The Contribution of PPP in PRPC
Blood plasma is a complex liquid base, protein rich, biological matrix in which platelets, leukocyte, and red blood cells are suspended [
102]. Based on a quantitative proteomic analysis, we found that PPP contains plasma-based growth factors, a substantial amount of proteins, and cytokines [
33].
3.1. Platelet-Poor Plasma Composition
PPP comprises of biomolecules, plasma-based growth factors like IGF-1 and HGF [
103]. When compared to PRP, they are more prevalent in PPP and are transported by several plasma proteins with active roles in several tissue repair and regeneration processes [
104,
105].
According to Anderson et al., blood plasma contains more than 1,500 different proteins at varying concentrations [
106], with 21 proteins accounting for approximately 99% of the total plasma protein composition [
107]. As the plasma proteome is in direct contact with most cells in the body, it contains a broad variety of proteins from a wide range of intracellular locations and membrane proteins [
107]. Plasma proteins can be coarsely classified into three groups: proteins present in high concentrations, “leakage” proteins which originate from pathological tissues [
106], and several cytokines involved in immune disorders and inflammation [
108].
The total plasma protein concentration has been determined to range from 60 to 80 mg/mL, with albumin (~60%), globulins (~35%), and fibrinogen (~4%) as 3 major protein groups. The remaining 1% of blood proteins represent several thousands of low-abundant proteins [
102,
109].
Table 2 outlines the most abundant human proteins, as well as their respective plasma concentrations and molecular weights.
The utilization of specific, concentrated, plasma proteins have gained interest in biotechnology, regenerative medicine, and orthobiological applications [
19]. More specifically, the use of albumin in bioengineering, the employment of alpha-2-macroglobulins (A2Ms) to function as a protease inhibitor in osteoarthritis, and the employment of fibrin as a scaffold consolidated with other biologics, with emphasis on sustained cell release and tissue repair.
3.1.1. Hepatocyte Growth Factor
HGF is a multifunctional protein, primarily synthesized by hepatocytes and circulates abundantly in plasma upon cell migration [
110]. HGF has a relative molecular mass of 82kDa and is a heterodimer molecule, made up of an alpha unit and beta unit [
111]. HGF, secreted by fibroblasts and expressed in MSCs, affects epithelial cell proliferation, morphology, epithelial cell motility [
112], and can induce epithelial tub formation [
110,
113].
HGF possesses mitogenic, morphogenic, and motogenic, activities by interacting with HGF/c-Met axis [
111,
114]. Additionally, HGF is a potent proliferative critical factor for angiogenesis [
115]. Furthermore, HGF stimulates the proliferation and migration of progenitor cells and plays an important role in tissue regeneration [
111,
112], including skeletal muscle repair following injury [
116].
Synergistic effects between HGF and VEGF have been reported [
117], contributing to an increase in neovascularization with amplification of the angiogenic response as HGF stimulates the release of VEGF-A [
28,
40,
118].
3.1.2. Insulin-like Growth Factor-1
The IGF-1 is a small peptide (7.6 kDa), with a molecular structure similar to insulin, displaying both anabolic and catabolic effects in many cells and tissues [
105]. About 98% of the circulating IGF-1 in humans is bound to IGF binding proteins (IGFBPs) with over 90% bound to IGFBP-3 [
119], with a molecular weight range of 39 to 53 kDa. IGFBPs can regulate the activities of IGF-1 by reducing the free IGF-1 fractions and by prolonging its half-life time [
120]. Importantly, IGF-1 bound to its specific binding proteins prevents passing through the hollow fiber pores of the ultrafiltration device, keeping IGF-1 in the final protein-rich PPP
IGF-1 is responsible for cell growth and differentiation, as well as cell survival, protein synthesis, cell motility and proliferation, DNA and matrix synthesis, particularly collagen-I [
121].
IGF-1 has a high binding affinity for specific IGF-1 receptors, and to a lesser extend for insulin receptors [
122,
123]. IGF-1 receptor binding activates PI3 kinase/Akt [
124], the Ras-MAPK [
125] and the PLC pathways [
126] which are important in cell survival and cell signaling. Particularly in tendons, Ras-MAPK pathways regulate tenocyte proliferation and collagen synthesis [
127]. Other IGF-1 receptor pathways are key mediators for several PGF, such as EGF, FGF, and PDGF [
128].
There are 3 IGF-1 isoforms described: IGF-1Ea, IGF-1Eb, and IGF-1Ec [
129]. The later isoform is a highly force-sensitive mechano-growth factor [
130] and incites bone marrow MSC migration [
131], stimulating tendon repair due to potent cell proliferative mechanisms. Potent IGF-1 anabolic effects have been demonstrated in chondrocyte regeneration in a dose dependent manner [
132]. Further, IGF-1 acts synergistically with platelet anabolic TGF-β1 growth factors [
133].
Besides the IGF-1 anabolic effects, it is noticed that the biological IGF-1 activities lead to decreased catabolic events like, inhibiting degradative ECM effects by downgrading MMP-1, MMP-3, MMP-8, and MMP-13, a significantly reduction in GAG release, blocking collagen from being released from the ECM, and inhibition of apoptosis [
134,
135]. Additional IGF-1 anabolic and catabolic mechanisms were mentioned in skeletal muscle pathways [
136].
Importantly, endocrine, autocrine, and paracrine IGF-1 mechanisms stimulate angiogenesis by regulating some of VEGF’s activities by promoting vascular EC migration and tube formation [
137,
138].
IGF-1 is not found in significant quantities in platelet granules, as demonstrated in human and equine studies [
139,
140]. Therefore, mixing IGF-1 prepared from PPP, with PRP-based TGF-β makes a strong case as a novel therapeutic biological preparation to treat OA, cartilage, tendon, and muscle pathologies. Hence, IGF-1 combined with PRP constituents might be considered a new effective biological product which can create both, anabolic and catabolic tissue environments and potentially optimize inhibitory degradative pathways, induce MSC migration, stimulate chondrogenesis, and regulate apoptosis [
141,
142].
3.1.3. Human Albumin
Human plasma albumin, produced in the liver, makes up about 60% of the total protein content in plasma with a plasma concentration of approximately 40g/L and molecular mass of 66kDa [
143]. Its main function is to maintain the colloid osmotic pressure of plasma and it can bind and transport fatty acids, hormones, and other molecules in the circulation [
144]. Albumin is omnipresent in regenerative medicine research and bioengineering applications because of its structure and protein properties [
145]. The multifaceted physiological roles of albumin include the recruitment by endogenous stem cells promoting bone growth, anti-bacterial properties, and albumin can act as a free radical scavenger [
146,
147]. These typical albumin properties resulted in an omnipresence in regenerative medicine research and bioengineering applications in tissue matrix remodeling [
148].
3.1.4. Alpha-2-Мacroglobulin
A2M is one of the largest plasma proteins in the human body, produced in the liver and released into the bloodstream [
149]. The A2M glycoprotein is a homo-tetrameric glycoprotein containing four identical subunits, each with a molecular mass of 190kD and an average plasma concentration of 1.4 g/L [
150], whereas healthy synovial fluid contains approximately one tenth of the plasma concentration [
151].
A2M proteins inhibit proteolytic proteases that are known to be harmful to cartilage. In their mechanism of action, they utilize a tetrameric cage that physically surrounds a broad range of catalytic proteinases, a mechanism known as the protease snap-trap mechanism [
152,
153]. When large substrate molecules are trapped in the cage, they trigger A2M activation (a-A2M), which results in conformational protein changes [
154]. Furthermore, in addition to capturing proteases, a-A2M exposes a reactive thioester which forms covalent a-A2M/protease complexes with small primary amines [
155,
156].
The presence of A2M in synovial fluid has been postulated to inhibit various types of proteinases harmful to cartilage, possibly reducing OA symptoms and encouraging cartilage formation [
157]. Platelet growth factors TGF-β1, FGF-2, macrophage activation factors, and TNF-α have a high binding affinity for aA2M [
158,
159,
160,
161]. Additionally, A2M captures and inhibits activated MMPs by the creation of A2M/MMP complexes and traps IL1β, thus reducing chondrocyte collagenase upregulation [
162,
163,
164]. In a similar manner, A2M inhibits the activity of disintegrin and metalloproteinases with thrombospondin motifs (ADAMTS)-1,4,5,7, and 12, which contribute to the degradation of the ECM and OA [
165].
The CORE™ ultrafiltration device (EmCyte Corporation® Fort Myers FL, USA) utilizes hollow fibers with a molecular weight cutoff of 20kDa. Data from a case study revealed that the device efficiently concentrates plasma proteins, including A2M, when an average of 23 ml PPP yielded 6.6 ml of protein concentrate (Personal communications Dr. N. Stephens, director Biofyl Scientific Research, Fort Myers, FL). Furthermore, an enzyme linked immunoassay test determined that A2M concentrations ranged from 3,800 to 6,700 µg/mL, with a capture rate of 89.7%, which was approximately three times higher than the A2M concentration in the non-concentrated PPP volume. Early clinical case studies indicate that concentrated A2M supplementation may be beneficial in a number of MSK disorders, in particular in OA [
166,
167]. Further clinical studies are warranted and should be directed towards a better understanding of concentrated A2M dosing and immunomodulatory capacities, related to chronic nociceptive inflammatory cartilage pathologies.
3.1.5. Fibrinogen
Fibrinogen is a complex glycoprotein abundantly present in plasma at high concentrations, ranging from 1.5-4 g/L, with varying chain molecular masses, see
Table 2 [
168]. Fibrinogen consists of 3 pairs of non-identical polypeptide chains (α, β, γ) that are jointly connected by disulfide bridges to form two symmetrical half molecules [
169]. The 6 fibrinogen polypeptides are arranged with N-termini in a central E-Domain, and two outer D-domains via C-termini with both domains connected by coiled-coil regions [
170].
Table 2.
Overview of plasma proteins.
Table 2.
Overview of plasma proteins.
| Plasma Protein Chains |
Concentration (g/L) |
Molecular Weight (kDa) |
| Albumin |
40 |
66 |
| IgG y-chain |
12 |
50 |
| Transferrin |
2.3 |
25 |
| IgA α-chain |
2 |
60 |
| Apolipoprotein A1 |
1.4 |
28 |
| α2-macroglobulin |
1.4 |
190 |
| α-1antitripsin |
1.1 |
52 |
| Fibrinogen α-chain |
0.95 |
95 |
| IgM µ chains |
0.75 |
75 |
| Hemopexin |
0.75 |
60 |
| Apolipoprotein B |
0.72 |
250 |
| α1-acid glycoprotein |
0.61 |
41 |
| Fibrinogen β-chain |
0.56 |
56 |
| Apolipoprotein AII |
0.3 |
110 |
| Fibrinogen y-chain |
0.5 |
50 |
| Complement C3 β-chain |
0.39 |
75 |
| Antithrombin III |
0.32 |
58 |
| Apolipoprotein AII |
0.3 |
17 |
| Haptoglobin α-chain |
0.29 |
40 |
| Pre-albumin |
0.26 |
16 |
| Ceruloplasmin |
0.21 |
132 |
| Haptoglobin β-chain |
0.14 |
20 |
| Fibronectin |
0.11 |
230 |
| Plasminogen α-chain |
0.099 |
60 |
| Complement C4 α-chain |
0.082 |
98 |
| Complement C4 β-chain |
0.061 |
73 |
| Plasminogen β-chain |
0.041 |
25 |
| Complement C4 y-chain |
0.028 |
33 |
| Other |
0.038 |
N/A |
Fibrinogen is a soluble acute-phase protein macromolecule, and acts as a precursor for fibrin formation when thrombin cleavages fibrinogen into an insoluble three-dimensional fibrin network, which is then cross-linked to form fibrin clots [
171]. Furthermore, thrombin-mediated proteolytic cleavage activates platelets, and supports invading macrophages, fibroblasts, ECs, and other molecules by entrapping them in the fibrin network [
168,
172]. Therefore, fibrinogen plays fundamental roles in hemostatic and homeostasis physiological processes to control bleeding, to promote tissue repair, and to enhance wound healing [
173,
174]. Additionally, fibrin(ogen) matrices are implicated in antimicrobial host defense mechanisms as they prevent microbial invasion by entrapping bacterial invaders and the recruitment of leukocytes [
175], undertaking immunoregulatory functions through cell receptors of macrophage, neutrophil, and ECs [
176,
177,
178].
4. PRPC Characteristics
PRPC is a liquid autologous product derived from a unit of whole blood following PRP processing. Typically, the PPP fractions are discarded after PRP processing, however, when preparing PRPC, the PPP fraction is processed with a concentrating device. A new generation biocompatible ultrafiltration device has been developed to concentrate the PPP proteins by processing this volume through hollow fiber semipermeable ultrafiltration [
179]. By reducing plasma water in a controlled manner, a volume of PPP plasma proteins with a molecular weight, larger than the ultrafiltrate membrane pore size, are concentrated, along with several important extra-platelet growth factors, to a low volume viscous protein-rich plasma product [
180].
The highly concentrated PRP fraction and other cells are re-suspended in a low volume of plasma and extracted from the PRP device,
Figure 2.
There are many commercial PRP devices utilizing either one-step, or two-step centrifugation and preparation protocols to produce platelet concentrates with many different bioformulations reported [
5,
6]. PRP device and preparation variable factors include the whole blood PRP processing volume, the prepared PRP volume, presence of leukocyte, the device platelet capture rate, affecting the PRP platelet concentration and platelet dose, as indicated in
Table 3. In particular, when low concentration PRP devices are used to create PRPCs, the cell concentration and the number of released matrices bound cells per time unit will be even lower, hence limiting the regenerative potential of the matrix.
The data are extrapolated and calculated from relevant published studies to demonstrate the variances in platelet and leukocyte concentrations, and platelet dosages of four different PRP formulations. Abbreviations: PRPv: platelet rich plasma volume; PLTc: platelet concentration; PLTd: platelet dose; WBCc: white blood cell concentration; MONc: monocyte concentration; NEUc: neutrophil concentration; RBCc: red blood cell concentration; R: references; P-PRP: pure platelet-rich plasma; PRF: platelet-rich fibrin; LP-PRP: leukocyte-poor PRP; LR-PRP: leukocyte-rich PRP.
A PRPC comprises of a small volume of highly concentrated plasma proteins compounded with a low volume of highly conccentrated PRP. To prevent preparations with low platelet concentrations embedded in the final PRPC matrix, ideally, PRP preparations with high platelet concentrations and platelet dose should be considered for PRPC preparations. Since higher platelet and leukocyte concentrations initiate significantly greater cell proliferative, signaling, and angiogenetic activities during tissue repair and regeneration, as compared to PRP with low cell concentrations [
25,
28,
36]. Dissimilarities in embedded matrix cellular platelet and leukocyte concentrations, as well as the total available platelet numbers for LP-PRP, LR-PRP, and PRF matrices are indicated in
Table 4 and visualized
Figure 3.
Three different matrix formulations are displayed, demonstrating the differences among them regarding platelet concentration, total available platelets, and leukocyte cell concentrations. The PRF matrix data are composed from the data in
Table 3. The hematological data for PRF, and both LP and LR-PRPC formulations originate from a same-donor experimental model. Both PRPC matrix formulations consisted of a mixture of 3ml of PRP with 3ml of protein-rich plasma, to a consolidated volume of 6ml. Abbreviations: PRF: platelet-rich fibrin; LP-PRP: leukocyte-poor PRP; LR-PRP: leukocyte-rich PRP PLTs: platelets; PLTc: platelet concentration; MONc: monocyte concentration; NEUc: neutrophil concentration; RBCc: red blood cell concentration.
4.1. PRPC Matrix Formation
During tissue repair, regeneration, and wound healing, fibrin and platelet clot formation are integral parts of the natural healing process, as well as angiogenetic cascades [
190] through intrinsic, extrinsic, and common pathways [
191].
The formation of an engineered PRPC clot is very similar to ex vivo fibrin clot formation protocols, which involves moving cuvettes around their vertical axis at 37 °C to form a fibrin clot [
192]. After the preparation of PRP, the PPP volume is processed in the ultrafiltration device, removing water from the PPP fraction, significantly reducing the PPP volume, while simultaneously concentrating plasma proteins, plasma-based growth factors, and other molecules. The low volume-concentrated PPP fraction is then gently mixed and agitated with a low volume-high concentration PRP volume. The in one syringe consolidated soluble PRP and concentrated PPP fractions are labelled PRPC. A calculated volume of CaCl 10% (Ca
++) is added to the PRPC volume, resulting in the conversion of prothrombin to thrombin. A complex multistep clotting process is then instigated, mediated by thrombin. Fibrin formation is initiated by the serine protease thrombin, which cleaves fibrinopeptides A and B (FpA, FpB) from fibrinogen [
170]. The cleavage of FpA occurs first, inducing polymerization of soluble fibrin monomers into protofibrils of half-staggered overlapping fibrin units. Thereafter, the released FpB is interrelated with lateral aggregation of protofibrils, contributing to the clot tensile strength and fiber thickness.
In the next phase, as thrombin and Ca
++ ions cleave the activation peptide from FXIII-a, a cross-linked three-dimensional insoluble fibrin network is formed, initiated by FXIII [
193]. During the fibrin polymerization process, covalent bonds are formed between fibrin γ-γ chains of the D domains, the γ and α-chains of the E-domain, and other plasma molecules [
194]. Furthermore, at pathological tissue sites the delivered PRPC will be exposed to factor III, also known as tissue factor (TF), a membrane-bound protein that will trigger thrombin generation and ultimately fibrin polymerization [
195].
After fibrin polymerization by FXIII, the compact provisional three-dimensional PRPC matrix includes high concentrations of platelets trapped within the fibrin scaffold. Leukocytes and RBCs may also be embedded in the matrix depending on the formulation of the prepared PRP, see
Figure 4.
4.2. PRPC Matrix Fibrinolysis
Fibrinolysis is an enduring physiological process of biodegradation of fibrin clots and is characterized by a well-regulated balance between fibrin formation and breakdown [
196]. This complex and dynamic process is mediated by plasmin, a serine fibrinolytic protease, which is obtained through the activation of the inactive glycoprotein plasminogen [
197].
The cross-linked fibrin matrix promotes the rapid conversion of plasminogen, by tissue-type plasminogen (t-PA) or urokinase-type plasminogen (u-PA) surface activators, to the catalytically active protease plasmin [
198]. tPA is the most abundant protease and acts primarily on fibrinolysis while u-PA primarily targets neutrophils and macrophages’ cell surfaces for purposes such as ECM remodeling and monocyte migration [
199]. Subsequently, plasmin initiates plasma hydrolysis of the matrix fibrin polymers [
200].
In response to the proteolytic plasmin generation, plasma protein α2-antiplasmin and plasminogen activator inhibitor-1(PAI-1) are two foremost inhibitors of fibrinolysis acting as a covalent inhibitors of plasmin to prevent plasminogen to bind to fibrin and to regulate the dissolution of fibrin polymers into soluble fragments, such as fibrin degradation products (FDP) and D-dimers [
201,
202].
In addition, after PPP ultrafiltration, high fibrinogen concentrations provide a denser fibrin matrix structure. Denser fibrin matrices have smaller pores, and hence are less susceptible to fibrinolysis. A lower vulnerability can be attributed to the fact that smaller matrix pores inhibit plasminogen from binding to tPA surface activators [
203,
204]. Therefore, PRPC matrix biological components are released more slowly than in thin, non-concentrated fiber matrices with larger pores, leading to longer cell signaling and extended tissue repair processes [
205].
4.3. Sustained Cellular Matrix Release
Upon application to pathological tissue and injury sites, soluble PRPC will become a fibrin-based matrix with typical mechanical properties, and will exhibit several non-fibrin-based biological properties due to the embedded platelet and plasma-based growth factors, ECM proteins (fibronectin, vitronectin), and enzymes (plasminogen, tissue plasminogen activator) [
168]. Furthermore, the provisional three dimensional PRPC matrix serves as an environment for resident cells, homing MSCs, cytokines, and other cells that are involved in tissue remodeling, cell signaling, migration, and proliferation, and ultimately tissue repair [
206,
207,
208].
As a result of the dense fibrin architecture of the PRPC matrix, the embedded PRP cellular content, the ECM, and local resident tissue cells interact in a multidirectional and synergistic manner, allowing platelets to release their granular content, inflammatory cells, and leukocytes in a slow and progressive manner, steering the complex mechanisms involved in angiogenesis, immunomodulation, and tissue repair.
Interestingly, following fibrinolysis, the generation of FDP employ pro-inflammatory and anti-inflammatory properties [
1,
2]. Pro-inflammatory D-dimers and fibrin fragment E stimulate the production of IL-6, IL-8, TNF-α, IL-1β, and chemokines, activating neutrophils and monocytes [
209]. Whereas fibrin fragment Bβ15-42 express anti-inflammatory effects [
210].
In studies using PRFs and PRF membranes, the provisional fibrin matrix has been shown to allow for the sustained release of platelets and molecules [
208,
211]. However, PRPC matrices, consisting of LP or LR-PRP possess a much greater biological profile than PRF-like and test tube, preparations, given that PRPCs contain higher platelet concentrations, as specified in
Table 4. Furthermore, following PPP ultrafiltration, the PRPC matrix fibrinogen concentration is significantly increased. Consequently, this results in fibrinolysis resistance facilitating a prolonged sustained cellular and molecular release period [
212], ultimately contributes to an increased level of cellular activity and angiogenetic stimulation [
213,
214]. A positive effect of a prolonged sustained release period of matrix embedded PGFs was confirmed in an animal model, demonstrating enhanced angiogenesis [
215].
Notably, the preservation of PGFs in fibrin scaffolds and the sustained release of matrix cells over a prolonged period of time resulted in significantly greater cumulative amounts of growth factors as compared to the initial burst release of PRP [
216].
5. PRPC Matrix Biological Properties
PRPC, with fibrinogen as its main component, functions as temporary three-dimensional scaffold. Fibrinogen will be converted into fibrin and the PRPC cellular components will be embedded in the matrix. Following PRPC administration, the activated matrix demonstrates two main functions. First, the matrix displays mechanical properties, mimicking the ECM, able to fill partial and full-thickness tissue tears, therefore preventing the leakage of reparative cells in the affected area. Second, the matrix provides a molecular link for local tissue resident cells and ECs to connect and invade the matrix microenvironment, mediating cellular interactions and signaling pathways [
217] (
Figure 5).
5.1. Fibrin, Fibrinogen, and Macrophage Responses
PRPC and other fibrin-based matrices, are not only capable of providing scaffolds into which cells can be embedded or cells can be infiltrated, but they are also capable of direct molecular cell signaling as these biological matrixes contain multiple molecular binding sites for growth factors, integrins, and other ECM molecules [
219].
The provisional fibrin extracellular matrix provides a structural scaffold for cell infiltration and matrix anchoring at tissue site, with a dynamic role for monocytes and macrophages in advancing and resolving inflammation in response to cues in their microenvironment [
220].
Delgado et al. investigated the mechanical properties and cell behavior of three different fibrin-based matrix formulations, including a matrix with a high fibrinogen content, similar to the concentrated PPP following ultrafiltration [
221]. Interestingly, a fibrinogen-concentration-dependent cell adhesion was observed, which was confirmed by others [
222,
223]. It was concluded that high fibrinogen concentrations improved the matrix mechanical properties with better cushioning properties, and cell adhesion capacity without impeding the cell viability due to a longer sustained release period. Further, the improved cushioning effects contributed to better chondrogenic properties, and it was suggested that high density matrices might be suitable for the treatment of chondral defects [
224].
Importantly, the utilization of high fibrinogen content matrices, did not alter cell properties or viability due to swelling, and a normal nutrient diffusion capacity was observed. Additionally, no decrease, or inhibition, of cell migration and proliferation were seen [
225].
There is extensive knowledge about how soluble factors such as cytokines and chemokines regulate macrophage polarization [
226,
227]. However, the effects of the insoluble fibrin matrix on macrophage behavior are not well understood. However, is has been demonstrated that fibrin(ogen) can modulate the activities of monocytes and macrophages, and play an important role in the transition from inflammation to tissue repair [
228].
Hsieh et al. used cultured macrophages and activated fibrinogen with thrombin to produce a fibrin gel [
229]. They concluded that cultured macrophages on fibrin gels secrete the anti-inflammatory cytokine IL-10, suggesting that fibrin can promote an anti-inflammatory response in macrophages, even when stimulated by IFN-γ.
5.2. Fibrin to Support in Immune Responses and Inflammation
Immune cells contain an arsenal of fibrinolytic regulators and plasmin, and participate in host immune responses, providing access for immune cells to migrate to the dissolving matrix and FDP. In turn, the fibrinolytic proteins have diverse roles in immunoregulation [
230].
Macrophage-1 antigen, a surface receptor on monocytes, macrophages, and neutrophils contributes to the adherence to ECM proteins, regulates adhesion dependent processes in leukocytes, and functions as well as an important fibrinogen receptor [
231]. Porous fibrinogen 3D scaffolds led to resolving local inflammatory responses in a bone fracture model, measured by decreased pro-inflammatory cytokines, like IL-8, IL-6, IL-17, IL-1b, and TNF-
α. It was concluded that the fibrinogen scaffolds provided a temporary support matrix to promoting a pro-regenerative local environment [
232]. Almeida et al. investigated the role of fibrinogen in immunomodulation and concluded that fibrinogen instigated immune cell responses, with increased recruitment of MSCs and downregulation of pro-inflammatory molecules [
233].
More specific for PRPC matrix formulations, activated platelets shed platelet micro particles (PMPs) that exercise anti-inflammatory and immunosuppressive effects and down regulate macrophage and dendritic cell activation to control inflammation and immune responses [
234]. Additionally, PMPs can support in resolving tissue inflammation by inhibiting IL-17 production by regulatory T cells [
235], as well PMPs can alter the monocyte and macrophage polarization to less reactive phenotypes [
236].
Noteworthy, Kim et al demonstrated that three-dimensional fibrin-based scaffolds improve the paracrine effects of bone marrow derived MSCs, resulting in elevated level of immunomodulatory factors and function [
237]. PRPC clinical application potential
The use of autologously prepared protein-rich fibrin matrices is widespread in clinical procedures and biomolecular engineering applications in which they have been recognized as a versatile carrier for many cell types and biomaterials [
238]. They can be used in non-surgical tissue repair and regeneration procedures as a standalone injectable preparation, or as a cellular remodellable tissue adhesive [
239,
240]. Furthermore, fibrin matrices can be safely and effectively used as fibrin sealants in biosurgical procedures to improve patient outcomes [
241,
242].
Table 5 illustrates the multiple roles and clinical potential of fibrin matrixes utilization.
5.3. Endothelial Cell Interactions with Fibrin Matrix
Fibrin matrices are portraited as an excellent matrix for the invasion and adhesion of ECs followed by the formation of new capillary-like structures [
243]. Fibrin stabilizes the expression of vitronectin receptor avβ3-integrin on ECs, promoting their migration to the matrix proteins [
244] and several integrins and other receptors, like ICAM-1, and VE-Cadherin 1, facilitate the binding of ECs to the fibrin(ogen) and leukocytes [
245].
After PRPC delivery and the subsequent fibrin, protein, activation, leukocytes infiltrate into the developed matrix followed by ECs from adjacent tissues [
246]. Matrix embedded ECs associate with angiogenesis and tissue repair processes as they realign with new vascular structures [
219]. Furthermore, encapsulated ECs produce and secrete t-PA and u-PA, converting plasminogen into plasmin, initiating matrix fibrinolysis [
247]. The ensuing proteolytic matrix components are found to be essential in prompting an early angiogenetic response [
248]. FDP increase the release of endothelial cell-derived growth factors [
249] and D-dimers induces the secretion of IL-1 from macrophages, stimulating the expression of PAI-1 in ECs [
250].
5.4. Role of Fibrin Matrix in Angiogenesis
Fibrin-based matrices and angiogenesis are closely connected physiological processes. Restoring the microcirculation to its former state can be supported by PRP utilization and is determined by the release of PGFs, pro- and anti-angiogenic factors, and EC responses [
28]. However, fibrin matrix-based angiogenesis is driven by biochemical mechanisms, the matrix milieu and mass, and the attachment of ECs to the matrix [
251]. Similarly, fibrin induces the expression of
αv
β3 integrin, an ECM cell-surface receptor, which allows ECs to bind to fibrin itself, and ECM proteins fibronectin and vitronectin, promoting angiogenesis and wound repair [
244].
As opposed to non-protein concentrated fibrin matrixes with a low platelet concentration, PRPC matrix formulations contribute significantly to angiogenic mechanisms and tissue repair. As part of the PRPC matrix formulation, high definition PRP is an integral component, embedding high levels of PGFs, angiogenetic factors, and other molecules in a dense three-dimensional matrix structure. Furthermore, angiomorphogenic effects and capillary morphogenesis are dependent on the availability of PRP growth factor concentrations, and the matrix rigidity and structure [
252].
5.5. Antimicrobial Activities of Fibrin(ogen)
In addition to its role in hemostasis, fibrinogen plays an important role in host defense against microorganisms. In response to pathogens and their environment, the fibrin matrix may trigger a protective immune response with antimicrobial properties mediated by fibrin(ogen) through two distinct mechanisms [
175]. Firstly, the fibrin matrix acts as a protective barrier which physically entraps bacteria or encapsulates foci of bacteria to limit their growth and dissemination. Secondly, fibrin can stimulate immune cell recruitment and activation, which results in elimination of pathogenic microbes.
The non-exhaustive
Table 5 displays a variety of biological characteristics and therapeutic goals of fibrin(ogen) and compound PRPC matrixes. Fibrin-based matrices have distinct therapeutic objectives based on their biological characteristics. In addition to sharing many biological functions and application objectives with fibrin-based matrices, PRPC matrixes provide the additional benefit of embedding the cellularity of PRP formulations directly within the matrix, thereby allowing a broader range of therapeutic applications and treatment objectives.
It has been demonstrated in several studies that multiple bacterial species are susceptible to host fibrinogen molecules, including neutrophils and macrophages, when exposed to fibrin deposits [
253,
254]. Conversely, pathogen clearance in mice lacking fibrinogen was significantly compromised, suggesting a failure in innate immune cell function by fibrinogen integrin Mac-1 [
255].
5.6. Matrix Similarities and Differences
Based on their respective cellular components, fibrin(ogen) and PRPC matrixes display similar biological and therapeutic characteristics, along with distinct differences, as indicated in
Table 5. Autologous PRPC and fibrin matrices, such as PRF, PRFM, and Vivostat™ technology [
256,
257,
258], are prepared from patient’s whole blood and are highly regarded for their safety and versatility. One of the key properties of autologous PRPC and fibrin matrices is their rapid polymerization, enabling them to be utilized as a fibrin tissue sealant or adhesive, depending on specific therapeutic goals. Both PRPC treatment modalities possess the same biological functions as the fibrin(ogen)-based approaches, albeit with a few notable differences. A key difference between PRPC matrix and fibrin matrix is the presence of concentrated fibrinogen in PRPC matrix. This biocomponent contributes to a denser sealant and matrix, making it more resistant to fibrinolysis. Additionally, it promotes a prolonged sustained cellular release phenomenon. The second important difference between the two matrices is the use of high PRP concentrations in the PRPC matrix. Whether with or without specific leukocytes, these high platelet concentrations guarantee a significant amount of additional physiological and biological functions that are unique for platelets and leukocytes, allowing for a surplus of repair and regenerative, synergistically, functioning pathways. By leveraging these pathways, the sealant and matrix PRPC modalities enhance the overall regenerative potential of the treatment, while offering a broader range of effective treatment options for patients.
6. Conclusions
In our opinion, fibrin-based matrices have emerged as promising biological and multipurpose platform, due to their high cellular adhesion capacity.
Protein rich autologous platelet concentrates, and their matrixes are complex multidimensional autologous biological products that are a combination of a high concentration of platelets and a high concentration of proteins.
High volume 2-spin PRP devices employing variable biological preparation methods are well-suited for preparing different PRPC formulations. These devices effectively capture high platelet numbers, accommodate variations in bioformulations, and produce sufficient volumes of PPP to concentrate plasma proteins, in particular A2M, and fibrinogen.
After activation, the PRPC protein network, in particular fibrinogen, is enzymatically converted into fibrin and the liquid PRPC will transform into an insoluble and elastic three-dimensional porous matrix. The concentrated fibrin(ogen) provides the matrix with additional strength, shape, and stability, when compared to non-concentrated fibrinogen matrices.
The PRPC matrix is embedded with platelets and other cells, creating a favorable environment for cellular interactions, and cell signaling. Furthermore, matrix cell receptors connect and bind with invading MSCs, macrophages, fibroblasts, and ECM proteins. As a result of naturally occurring biologically fibrinolysis, matrix ingrained platelets and other biomolecules are released over time, ensuring a progressive delivery cells and biomolecules to the local, pathological, microenvironment. Synergistic matrix properties are initiated in the presence of high concentrations of reparative matrix cells, which enhance cell signaling and promote reparative activities, including cell migration and proliferation, immunomodulation, angiogenesis, and inflammation control, thereby supporting and initiating reparative and regenerative effects.
In our PRPC preparations, we obtained 52 ml of autologous blood to produce 3 ml of high concentration PRP and 3 ml of concentrated protein rich PPP, creating a 6ml biological active matrix for tissue repair, regeneration, and wound healing. To determine the biological impact of PRPC matrix formulations on patient outcomes, more studies are required comparing PRPC matrices, non-concentrated fibrinogen matrix formulations, and non-matrix based biological preparations.
References
- Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. IJMS [Internet]. 2020 Oct 21 [cited 2022 Feb 1];21(20):7794. Available from: https://www.mdpi.com/1422-0067/21/20/7794.
- Andia I, Maffulli N. New biotechnologies for musculoskeletal injuries. The Surgeon [Internet]. 2019 Aug [cited 2019 Dec 11];17(4):244–55. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1479666X18300969.
- Everts PA, Mazzola T, Mautner K, Randelli PS, Podesta L. Modifying Orthobiological PRP Therapies Are Imperative for the Advancement of Treatment Outcomes in Musculoskeletal Pathologies. Biomedicines [Internet]. 2022 Nov 15 [cited 2024 Mar 21];10(11):2933. Available from: https://www.mdpi.com/2227-9059/10/11/2933.
- Everts PA, Lana JF, Onishi K, Buford D, Peng J, Mahmood A, et al. Angiogenesis and Tissue Repair Depend on Platelet Dosing and Bioformulation Strategies Following Orthobiological Platelet-Rich Plasma Procedures: A Narrative Review. 2023.
- Fadadu PP, Mazzola AJ, Hunter CW, Davis TT. Review of concentration yields in commercially available platelet-rich plasma (PRP) systems: a call for PRP standardization. Reg Anesth Pain Med [Internet]. 2019 Jun [cited 2020 Jun 25];44(6):652–9. Available from: http://rapm.bmj.com/lookup/doi/10.1136/rapm-2018-100356.
- Magalon J, Brandin T, Francois P, Degioanni C, De Maria L, Grimaud F, et al. Technical and biological review of authorized medical devices for platelets-rich plasma preparation in the field of regenerative medicine. Platelets [Internet]. 2021 Feb 17 [cited 2021 Nov 21];32(2):200–8. Available from: https://www.tandfonline.com/doi/full/10.1080/09537104.2020.1832653.
- Chahla J, Cinque ME, Piuzzi NS, Mannava S, Geeslin AG, Murray IR, et al. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting: A Systematic Review of the Clinical Orthopaedic Literature. The Journal of Bone and Joint Surgery [Internet]. 2017 Oct [cited 2020 Jan 26];99(20):1769–79. Available from: http://Insights.ovid.com/crossref?an=00004623-201710180-00009.
- Murray IR, Robinson PG, West CC, Goudie EB, Yong LY, White TO, et al. Reporting Standards in Clinical Studies Evaluating Bone Marrow Aspirate Concentrate: A Systematic Review. Arthroscopy: The Journal of Arthroscopic & Related Surgery [Internet]. 2018 Apr [cited 2019 Oct 4];34(4):1366–75. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0749806317314822.
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends in Biotechnology [Internet]. 2009 Mar [cited 2020 Jul 13];27(3):158–67. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167779909000158.
- M. Dohan Ehrenfest D, Bielecki T, Mishra A, Borzini P, Inchingolo F, Sammartino G, et al. In Search of a Consensus Terminology in the Field of Platelet Concentrates for Surgical Use: Platelet-Rich Plasma (PRP), Platelet-Rich Fibrin (PRF), Fibrin Gel Polymerization and Leukocytes. CPB [Internet]. 2012 May 1 [cited 2019 Oct 6];13(7):1131–7. Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1389-2010&volume=13&issue=7&spage=1131.
- Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The Effect of Platelet-Rich Fibrin Matrix on Rotator Cuff Tendon Healing: A Prospective, Randomized Clinical Study. Am J Sports Med [Internet]. 2012 Jun [cited 2022 Aug 23];40(6):1234–41. Available from: http://journals.sagepub.com/doi/10.1177/0363546512442924.
- Longo UG, Castricini R, De Benedetto M, Panfoli N, Pirani P, Zini R, et al. Paper # 117: Platelet-Rich Fibrin Matrix Augmentation for Arthroscopic Rotator Cuff Repair: A Randomized Controlled Trial. Arthroscopy: The Journal of Arthroscopic & Related Surgery [Internet]. 2011 Oct [cited 2023 May 31];27(10):e145–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0749806311008279.
- Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, et al. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA [Internet]. 2021 Nov 23 [cited 2022 Mar 21];326(20):2021. Available from: https://jamanetwork.com/journals/jama/fullarticle/2786501.
- Atiyeh B, Oneisi A, Ghieh F. Platelet-Rich Plasma Facial Rejuvenation: Myth or Reality? Aesth Plast Surg [Internet]. 2021 May 17 [cited 2021 Nov 21]; Available from: https://link.springer.com/10.1007/s00266-021-02300-9.
- Beitia M, Delgado D, Mercader J, Sánchez P, López De Dicastillo L, Sánchez M. Action of Platelet-Rich Plasma on In Vitro Cellular Bioactivity: More than Platelets. IJMS [Internet]. 2023 Mar 10 [cited 2023 May 11];24(6):5367. Available from: https://www.mdpi.com/1422-0067/24/6/5367.
- Acebes-Huerta A, Arias-Fernández T, Bernardo Á, Muñoz-Turrillas MC, Fernández-Fuertes J, Seghatchian J, et al. Platelet-derived bio-products: Classification update, applications, concerns and new perspectives. Transfusion and Apheresis Science [Internet]. 2020 Feb [cited 2020 Jul 13];59(1):102716. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1473050219302897.
- Mercader Ruiz J, Beitia M, Delgado D, Sánchez P, Guadilla J, Pérez De Arrilucea C, et al. Method Based on Ultrafiltration to Obtain a Plasma Rich in Platelet and Plasma Growth Factors. JCM [Internet]. 2023 Sep 13 [cited 2024 Mar 21];12(18):5941. Available from: https://www.mdpi.com/2077-0383/12/18/5941.
- Delgado D, Garate A, Sánchez P, Bilbao AM, Garcia Del Cano G, Salles J, et al. Biological and structural effects after intraosseous infiltrations of age-dependent platelet-rich plasma: an in vivo study. Journal of Orthopaedic Research®. 2020;38(9):1931–41.
- Muir SM, Reisbig N, Baria M, Kaeding C, Bertone AL. The Concentration of Plasma Provides Additional Bioactive Proteins in Platelet and Autologous Protein Solutions. Am J Sports Med [Internet]. 2019 Jul [cited 2024 Mar 9];47(8):1955–63. Available from: http://journals.sagepub.com/doi/10.1177/0363546519849671.
- Zhang Z, Li L, Yang W, Cao Y, Shi Y, Li X, et al. The effects of different doses of IGF-1 on cartilage and subchondral bone during the repair of full-thickness articular cartilage defects in rabbits. Osteoarthritis and cartilage. 2017;25(2):309–20.
- Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, et al. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-β signaling. Journal of Bone and Mineral Research. 2006;21(4):626–36.
- Yamada S, Behfar A, Terzic A. Regenerative medicine clinical readiness. Regenerative Medicine. 2021;16(03):309–22.
- Everts PAM, Devilee RJJ, Oosterbos CJM, Mahoney CB, Schattenkerk ME, Knape JTA, et al. Autologous platelet gel and fibrin sealant enhance the efficacy of total knee arthroplasty: improved range of motion, decreased length of stay and a reduced incidence of arthrofibrosis. Knee Surg Sports Traumatol Arthr [Internet]. 2007 Jul [cited 2019 Oct 4];15(7):888–94. Available from: http://link.springer.com/10.1007/s00167-007-0296-x.
- Everts PA, Devilee RJJ, Brown Mahoney C, van Erp A, Oosterbos CJM, Stellenboom M, et al. Exogenous Application of Platelet-Leukocyte Gel during Open Subacromial Decompression Contributes to Improved Patient Outcome. Eur Surg Res [Internet]. 2008 [cited 2019 Oct 6];40(2):203–10. Available from: https://www.karger.com/Article/FullText/110862.
- Gentile P, Garcovich S. Systematic Review—The Potential Implications of Different Platelet-Rich Plasma (PRP) Concentrations in Regenerative Medicine for Tissue Repair. IJMS [Internet]. 2020 Aug 9 [cited 2020 Oct 21];21(16):5702. Available from: https://www.mdpi.com/1422-0067/21/16/5702.
- Miroshnychenko O, Chalkley RJ, Leib RD, Everts PA, Dragoo JL. Proteomic analysis of platelet-rich and platelet-poor plasma. Regenerative Therapy [Internet]. 2020 Dec [cited 2023 May 11];15:226–35. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2352320420300730.
- Wu J, Piao Y, Liu Q, Yang X. Platelet-rich plasma-derived extracellular vesicles: A superior alternative in regenerative medicine? Cell Proliferation [Internet]. 2021 Dec [cited 2024 Apr 1];54(12):e13123. Available from: https://onlinelibrary.wiley.com/doi/10.1111/cpr.13123.
- Everts PA, Lana JF, Onishi K, Buford D, Peng J, Mahmood A, et al. Angiogenesis and Tissue Repair Depend on Platelet Dosing and Bioformulation Strategies Following Orthobiological Platelet-Rich Plasma Procedures: A Narrative Review. 2023.
- Andia I, Maffulli N. A contemporary view of platelet-rich plasma therapies: moving toward refined clinical protocols and precise indications. Regenerative Medicine [Internet]. 2018 Sep [cited 2020 Jun 24];13(6):717–28. Available from: https://www.futuremedicine.com/doi/10.2217/rme-2018-0042.
- Kunicki TJ. Platelet membrane glycoproteins and their function: an overview. Blut. 1989;59:30–4.
- Gauthier NP. Types of Glycoprotein Receptors and Signal Transduction Pathways.
- Polasek J. Platelet secretory granules or secretory lysosomes? Platelets. 2005;16(8):500–1.
- Miroshnychenko O. Proteomic analysis of platelet-rich and platelet-poor plasma. Regenerative Therapy. 2020;10.
- Sharda A, Flaumenhaft R. The life cycle of platelet granules. F1000Res [Internet]. 2018 Feb 28 [cited 2023 Sep 14];7:236. Available from: https://f1000research.com/articles/7-236/v1.
- Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood reviews [Internet]. 2009 Jul;23(4):177—189. Available from: https://europepmc.org/articles/PMC2720568.
- Everts P, Onishi K, Jayaram P, Lana JF, Mautner and K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020 [Internet]. MEDICINE & PHARMACOLOGY; 2020 Oct [cited 2020 Oct 21]. Available from: https://www.preprints.org/manuscript/202010.0069/v1.
- Blair P. Platelet α–granules: Basic biology and clinical correlates. 2010;29.
- Gleissner CA, Von Hundelshausen P, Ley K. Platelet chemokines in vascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(11):1920–7.
- Maynard DM, Heijnen HFG, Horne MK, White JG, Gahl WA. Proteomic analysis of platelet α-granules using mass spectrometry. Journal of Thrombosis and Haemostasis [Internet]. 2007 Sep [cited 2024 Apr 3];5(9):1945–55. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1538783622103156.
- Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics [Internet]. 2017 [cited 2024 Apr 4];7(1):81–96. Available from: http://www.thno.org/v07p0081.htm.
- Rui S, Yuan Y, Du C, Song P, Chen Y, Wang H, et al. Comparison and Investigation of Exosomes Derived from Platelet-Rich Plasma Activated by Different Agonists. Cell Transplant [Internet]. 2021 Jan 1 [cited 2024 Apr 4];30:096368972110178. Available from: http://journals.sagepub.com/doi/10.1177/09636897211017833.
- Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral diseases. 2010;16(1):34–8.
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature reviews immunology. 2009;9(8):581–93.
- Zhao Y, Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. International Orthopaedics. 2018;42:2865–72.
- Burke J, Kolhe R, Hunter M, Isales C, Hamrick M, Fulzele S. Stem cell-derived exosomes: a potential alternative therapeutic agent in orthopaedics. Stem Cells International. 2016;2016.
- Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem cell research. 2010;4(3):214–22.
- Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9(21):4997–5000.
- Li J, Zhang Y, Dong PY, Yang GM, Gurunathan S. A comprehensive review on the composition, biogenesis, purification, and multifunctional role of exosome as delivery vehicles for cancer therapy. Biomedicine & Pharmacotherapy [Internet]. 2023 Sep [cited 2024 Apr 1];165:115087. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0753332223008788.
- Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. In Elsevier; 2015. p. 72–81.
- Beit-Yannai E, Tabak S, Stamer WD. Physical exosome: exosome interactions. Journal of cellular and molecular medicine. 2018;22(3):2001–6.
- Huber HJ, Holvoet P. Exosomes: emerging roles in communication between blood cells and vascular tissues during atherosclerosis. Current opinion in lipidology. 2015;26(5):412–9.
- Heijnen HFG, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived From Exocytosis of Multivesicular Bodies and ␣-Granules.
- Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC genomics. 2013;14:1–14.
- Arraud N, Linares R, Tan S, Gounou C, Pasquet J -M., Mornet S, et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. Journal of Thrombosis and Haemostasis [Internet]. 2014 May [cited 2024 Apr 5];12(5):614–27. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1538783622039411.
- Torreggiani E, Perut F, Roncuzzi L, Zini N, Baglìo SR, Baldini N. Exosomes: novel effectors of human platelet lysate activity. Eur Cell Mater. 2014;28:137–51.
- Burgess, DJ. Vesicle vehicles of genetic information. Nature Reviews Genetics. 2014;15(8):514–514.
- Mistry DS, Chen Y, Sen GL. Progenitor function in self-renewing human epidermis is maintained by the exosome. Cell stem cell. 2012;11(1):127–35.
- Ruan G, Kazlauskas A. Axl is essential for VEGF-A-dependent activation of PI3K/Akt. The EMBO journal. 2012;31(7):1692–703.
- Iberg CA, Hawiger D. Natural and Induced Tolerogenic Dendritic Cells. JI [Internet]. 2020 Feb 15 [cited 2020 Sep 3];204(4):733–44. Available from: http://www.jimmunol.org/lookup/doi/10.4049/jimmunol.1901121.
- Ganor Y, Besser M, Ben-Zakay N, Unger T, Levite M. Human T cells express a functional ionotropic glutamate receptor GluR3, and glutamate by itself triggers integrin-mediated adhesion to laminin and fibronectin and chemotactic migration. Journal of immunology (Baltimore, Md : 1950) [Internet]. 2003 Apr;170(8):4362—4372. [CrossRef]
- Łukasik ZM, Makowski M, Makowska JS. From blood coagulation to innate and adaptive immunity: the role of platelets in the physiology and pathology of autoimmune disorders. Rheumatology international. 2018;38(6):959–74.
- Herr N, Bode C, Duerschmied D. The Effects of Serotonin in Immune Cells. Front Cardiovasc Med [Internet]. 2017 Jul 20 [cited 2020 Sep 7];4:48. Available from: http://journal.frontiersin.org/article/10.3389/fcvm.2017.00048/full.
- Cloez-Tayarani I. Differential effect of serotonin on cytokine production in lipopolysaccharide-stimulated human peripheral blood mononuclear cells: involvement of 5-hydroxytryptamine2A receptors. International Immunology [Internet]. 2003 Feb 1 [cited 2020 Sep 7];15(2):233–40. Available from: https://academic.oup.com/intimm/article-lookup/doi/10.1093/intimm/dxg027.
- Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velázquez MA, Garcés-Alvarez ME, Hurtado-Alvarado G, et al. Immunomodulatory Effects Mediated by Serotonin. Journal of Immunology Research [Internet]. 2015 [cited 2020 Sep 7];2015:1–21. Available from: http://www.hindawi.com/journals/jir/2015/354957/.
- Ciferri S, Emiliani C, Guglielmini G, Orlacchio A, Nenci GG, Gresele P. Platelets release their lysosomal content in vivo in humans upon activation. Thrombosis and haemostasis. 2000;83(01):157–64.
- Cooper GM, Hausman R. A molecular approach. The Cell 2nd ed Sunderland, MA: Sinauer Associates. 2000.
- Heijnen H, van der Sluijs P. Platelet secretory behaviour: as diverse as the granules … or not? J Thromb Haemost [Internet]. 2015 Dec [cited 2022 Sep 16];13(12):2141–51. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jth.13147.
- Wunderli SL, Blache U, Beretta Piccoli A, Niederöst B, Holenstein CN, Passini FS, et al. Tendon response to matrix unloading is determined by the patho-physiological niche. Matrix Biology [Internet]. 2020 Jul [cited 2022 Sep 16];89:11–26. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0945053X19304019.
- Rendu F, Brohard-Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets [Internet]. 2001 Jan [cited 2022 Sep 16];12(5):261–73. Available from: http://www.tandfonline.com/doi/full/10.1080/09537100120068170.
- Lana JFSD, Purita J, Paulus C, Huber SC, Rodrigues BL, Rodrigues AA, et al. Contributions for classification of platelet rich plasma – proposal of a new classification: MARSPILL. Regenerative Medicine [Internet]. 2017 Jul [cited 2020 Jul 9];12(5):565–74. Available from: https://www.futuremedicine.com/doi/10.2217/rme-2017-0042.
- Everts PA, Flanagan G, Podesta L. Autologous Orthobiologics. In: Mostoufi SA, George TK, Tria Jr. AJ, editors. Clinical Guide to Musculoskeletal Medicine: A Multidisciplinary Approach [Internet]. Cham: Springer International Publishing; 2022. p. 651–79. [CrossRef]
- Bielecki T, M. Dohan Ehrenfest D, A. Everts P, Wiczkowski A. The Role of Leukocytes from L-PRP/L-PRF in Wound Healing and Immune Defense: New Perspectives. CPB [Internet]. 2012 May 1 [cited 2019 Oct 6];13(7):1153–62. Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1389-2010&volume=13&issue=7&spage=1153.
- Lana JF. Leukocyte-rich PRP versus leukocyte-poor PRP - The role of monocyte/macrophage function in the healing cascade. Journal of Clinical Orthopaedics and Trauma. 2019;6.
- Newton K, Dixit VM. Signaling in Innate Immunity and Inflammation. Cold Spring Harbor Perspectives in Biology [Internet]. 2012 Mar 1 [cited 2020 Sep 6];4(3):a006049–a006049. Available from: http://cshperspectives.cshlp.org/lookup/doi/10.1101/cshperspect.a006049.
- Clemetson K, Clemetson J, Proudfoot A, Power C, Baggiolini M, Wells T. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood [Internet]. 2000 Dec;96(13):4046—4054. Available from: http://www.bloodjournal.org/cgi/content/full/96/13/4046.
- Woodell-May JE, Sommerfeld SD. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J Orthop Res [Internet]. 2020 Feb [cited 2020 Sep 6];38(2):253–7. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/jor.24457.
- Dib PRB, Quirino-Teixeira AC, Merij LB, Pinheiro MBM, Rozini SV, Andrade FB, et al. Innate immune receptors in platelets and platelet-leukocyte interactions. Journal of Leucocyte Biology. 2020;108(4):1157–82.
- Li C, Li J, Li Y, Lang S, Yougbare I, Zhu G, et al. Crosstalk between Platelets and the Immune System: Old Systems with New Discoveries. Advances in Hematology [Internet]. 2012 [cited 2020 Sep 6];2012:1–14. Available from: http://www.hindawi.com/journals/ah/2012/384685/.
- Scopelliti F, Cattani C, Dimartino V, Mirisola C, Cavani A. Platelet Derivatives and the Immunomodulation of Wound Healing. IJMS [Internet]. 2022 Jul 28 [cited 2022 Aug 22];23(15):8370. Available from: https://www.mdpi.com/1422-0067/23/15/8370.
- Rayes J, Bourne JH, Brill A, Watson SP. The dual role of platelet-innate immune cell interactions in thrombo-inflammation. Research and practice in thrombosis and haemostasis. 2020;4(1):23–35.
- Chen T, Li Y, Sun R, Hu H, Liu Y, Herrmann M, et al. Receptor-mediated NETosis on neutrophils. Frontiers in Immunology. 2021;12:775267.
- Patel AA, Ginhoux F, Yona S. Monocytes, macrophages, dendritic cells and neutrophils: an update on lifespan kinetics in health and disease. Immunology. 2021;163(3):250–61.
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nature reviews immunology. 2006;6(3):173–82.
- Ribeiro LS, Migliari Branco L, Franklin BS. Regulation of innate immune responses by platelets. Frontiers in immunology. 2019;10:460217.
- Ribatti D, Crivellato E. “Sprouting angiogenesis”, a reappraisal. Developmental biology. 2012;372(2):157–65.
- Brill, A. Differential role of platelet granular mediators in angiogenesis. Cardiovascular Research [Internet]. 2004 Aug [cited 2020 Jul 19];63(2):226–35. Available from: https://academic.oup.com/cardiovascres/article-lookup/doi/10.1016/j.cardiores.2004.04.012.
- Bir SC, Esaki J, Marui A, Sakaguchi H, Kevil CG, Ikeda T, et al. Therapeutic Treatment with Sustained-Release Platelet-Rich Plasma Restores Blood Perfusion by Augmenting Ischemia-Induced Angiogenesis and Arteriogenesis in Diabetic Mice. J Vasc Res [Internet]. 2011 [cited 2023 Jun 30];48(3):195–205. Available from: https://www.karger.com/Article/FullText/318779.
- Giusti I, Rughetti A, D’Ascenzo S, Millimaggi D, Pavan A, Dell’Orso L, et al. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion [Internet]. 2009 Apr [cited 2020 Sep 10];49(4):771–8. Available from: http://doi.wiley.com/10.1111/j.1537-2995.2008.02033.x.
- Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol [Internet]. 2001 Nov [cited 2020 Jul 19];19(11):1029–34. Available from: http://www.nature.com/articles/nbt1101-1029.
- Łanocha-Arendarczyk N, Baranowska-Bosiacka I, Gutowska I, Kolasa-Wołosiuk A, Kot K, Łanocha A, et al. The activity of matrix metalloproteinases (MMP-2, MMP-9) and their tissue inhibitors (TIMP-1, TIMP-3) in the cerebral cortex and hippocampus in experimental acanthamoebiasis. International Journal of Molecular Sciences. 2018;19(12):4128.
- Jaipersad AS, Lip GYH, Silverman S, Shantsila E. The Role of Monocytes in Angiogenesis and Atherosclerosis. Journal of the American College of Cardiology [Internet]. 2014 Jan [cited 2023 May 12];63(1):1–11. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109713054521.
- Yuan Z, Wang Y, Li Y, Lin C, Wang S, Wang J, et al. Comparison of Leukocyte-Rich and Leukocyte-Poor Platelet-Rich Plasma on Pressure Ulcer in a Rat Model. Journal of Burn Care & Research [Internet]. 2023 Jan 2 [cited 2023 May 11];irac191. Available from: https://academic.oup.com/jbcr/advance-article/doi/10.1093/jbcr/irac191/6967031.
- Mohammadi S, Nasiri S, Mohammadi MH, Malek Mohammadi A, Nikbakht M, Zahed Panah M, et al. Evaluation of platelet-rich plasma gel potential in acceleration of wound healing duration in patients underwent pilonidal sinus surgery: A randomized controlled parallel clinical trial. Transfusion and Apheresis Science [Internet]. 2017 Apr [cited 2020 Jul 19];56(2):226–32. Available from: https://linkinghub.elsevier.com/retrieve/pii/S147305021630218X.
- Bansal H, Leon J, Pont JL, Wilson DA, St SM, Bansal A, et al. Clinical Efficacy of a Single-dose of Platelet Rich Plasma in the Management of Early Knee Osteoarthritis: A Randomized Controlled Study With Mri Assessment and Evaluation of Optimal Dose [Internet]. In Review; 2020 Jun [cited 2020 Jul 7]. Available from: https://www.researchsquare.com/article/rs-33058/v1.
- Jain D, Goyal T, Verma N, Paswan AK, Dubey RK. Intradiscal Platelet-Rich Plasma Injection for Discogenic Low Back Pain and Correlation with Platelet Concentration: A Prospective Clinical Trial. Pain Medicine [Internet]. 2020 Nov 1 [cited 2022 Feb 1];21(11):2719–25. Available from: https://academic.oup.com/painmedicine/article/21/11/2719/5899837.
- Johal H, Khan M, Yung S hang P, Dhillon MS, Fu FH, Bedi A, et al. Impact of Platelet-Rich Plasma Use on Pain in Orthopaedic Surgery: A Systematic Review and Meta-analysis. Sports Health [Internet]. 2019 Jul [cited 2020 Jun 24];11(4):355–66. Available from: http://journals.sagepub.com/doi/10.1177/1941738119834972.
- Lutz C, Cheng J, Prysak M, Zukofsky T, Rothman R, Lutz G. Clinical outcomes following intradiscal injections of higher-concentration platelet-rich plasma in patients with chronic lumbar discogenic pain. International Orthopaedics (SICOT) [Internet]. 2022 Mar 28 [cited 2022 Apr 26]; Available from: https://link.springer.com/10.1007/s00264-022-05389-y.
- Yoshida M, Funasaki H, Marumo K. Efficacy of autologous leukocyte-reduced platelet-rich plasma therapy for patellar tendinopathy in a rat treadmill model. Muscles, ligaments and tendons journal [Internet]. 2016;6(2):205—215. Available from: https://europepmc.org/articles/PMC5115252.
- Berger M, Gray JA, Roth BL. The Expanded Biology of Serotonin. Annu Rev Med [Internet]. 2009 Feb [cited 2020 Sep 7];60(1):355–66. Available from: http://www.annualreviews.org/doi/10.1146/annurev.med.60.042307.110802.
- Sprott H, Franke S, Kluge H, Hein G. Pain treatment of fibromyalgia by acupuncture. Rheumatology International [Internet]. 1998 Jun [cited 2020 Jul 21];18(1):35–6. Available from: http://link.springer.com/10.1007/s002960050051.
- Kuffler D. Variables affecting the potential efficacy of PRP in providing chronic pain relief. JPR [Internet]. 2018 Dec [cited 2020 Jun 24];Volume 12:109–16. Available from: https://www.dovepress.com/variables-affecting-the-potential-efficacy-of-prp-in-providing-chronic-peer-reviewed-article-JPR.
- Leeman M, Choi J, Hansson S, Storm MU, Nilsson L. Proteins and antibodies in serum, plasma, and whole blood—size characterization using asymmetrical flow field-flow fractionation (AF4). Anal Bioanal Chem [Internet]. 2018 Aug [cited 2024 Mar 21];410(20):4867–73. Available from: http://link.springer.com/10.1007/s00216-018-1127-2.
- Hayashi S, Aso H, Watanabe K, Nara H, Rose MT, Ohwada S, et al. Sequence of IGF-I, IGF-II, and HGF expression in regenerating skeletal muscle. Histochemistry and cell biology. 2000;122:427–34.
- Mungunsukh O, McCart E, Day R. Hepatocyte Growth Factor Isoforms in Tissue Repair, Cancer, and Fibrotic Remodeling. Biomedicines [Internet]. 2014 Nov 5 [cited 2024 Apr 16];2(4):301–26. Available from: http://www.mdpi.com/2227-9059/2/4/301.
- Garoufalia Z, Papadopetraki A, Karatza E, Vardakostas D, Philippou A, Kouraklis G, et al. Insulin-like growth factor-I and wound healing, a potential answer to non-healing wounds: A systematic review of the literature and future perspectives. Biomed Rep [Internet]. 2021 Jun 8 [cited 2024 Apr 16];15(2):66. Available from: http://www.spandidos-publications.com/10.3892/br.2021.1442.
- Anderson NL, Anderson NG. The Human Plasma Proteome: History, Character, and Diagnostic Prospects. Molecular & Cellular Proteomics [Internet]. 2003 Jan [cited 2024 Apr 11];2(1):50. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1535947620342420.
- Gerszten RE, Accurso F, Bernard GR, Caprioli RM, Klee EW, Klee GG, et al. Challenges in translating plasma proteomics from bench to bedside: update from the NHLBI Clinical Proteomics Programs. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008;295(1):L16–22.
- Monastero RN, Pentyala S. Cytokines as biomarkers and their respective clinical cutoff levels. International journal of inflammation. 2017;2017.
- Gonzalez-Quintela A, Alende R, Gude F al, Campos J, Rey J, Meijide L, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clinical & Experimental Immunology. 2008;151(1):42–50.
- Matsumoto K, Nakamura T. Hepatocyte growth factor: molecular structure, roles in liver regeneration, and other biological functions. Critical reviews in oncogenesis. 1992;3(1–2):27–54.
- Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proceedings of the Japan Academy, Series B. 2010;86(6):588–610.
- Huh CG, Factor VM, Sánchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proceedings of the National Academy of Sciences. 2004;101(13):4477–82.
- Qi Y, Li M, Xu L, Chang Z, Shu X, Zhou L. Therapeutic role of human hepatocyte growth factor (HGF) in treating hair loss. PeerJ. 2016; 4: e2624.
- Choi W, Lee J, Lee J, Lee SH, Kim S. Hepatocyte Growth Factor Regulates Macrophage Transition to the M2 Phenotype and Promotes Murine Skeletal Muscle Regeneration. Front Physiol [Internet]. 2019 Jul 25 [cited 2024 Apr 22];10:914. Available from: https://www.frontiersin.org/article/10.3389/fphys.2019.00914/full.
- Yasuda S, Goto Y, Sumida H, Noguchi T, Baba T, Miyazaki S, et al. Angiotensin-converting enzyme inhibition restores hepatocyte growth factor production in patients with congestive heart failure. Hypertension. 1999;33(6):1374–8.
- Sisson TH, Nguyen MH, Yu B, Novak ML, Simon RH, Koh TJ. Urokinase-type plasminogen activator increases hepatocyte growth factor activity required for skeletal muscle regeneration. Blood, The Journal of the American Society of Hematology. 2009;114(24):5052–61.
- Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, et al. Hepatocyte Growth Factor Enhances Vascular Endothelial Growth Factor-Induced Angiogenesis in Vitro and in Vivo. The American Journal of Pathology [Internet]. 2001 Mar [cited 2023 May 11];158(3):1111–20. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002944010640588.
- Zhang Z, Li Y, Zhang T, Shi M, Song X, Yang S, et al. Hepatocyte Growth Factor-Induced Tendon Stem Cell Conditioned Medium Promotes Healing of Injured Achilles Tendon. Front Cell Dev Biol [Internet]. 2021 Apr 7 [cited 2023 May 11];9:654084. Available from: https://www.frontiersin.org/articles/10.3389/fcell.2021.654084/full.
- Bailes J, Soloviev M. Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules [Internet]. 2021 Feb 4 [cited 2024 May 14];11(2):217. Available from: https://www.mdpi.com/2218-273X/11/2/217.
- Baxter, RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. American Journal of Physiology-Endocrinology and Metabolism. 2000;278(6):E967–76.
- Miescher I, Rieber J, Calcagni M, Buschmann J. In Vitro and In Vivo Effects of IGF-1 Delivery Strategies on Tendon Healing: A Review. IJMS [Internet]. 2023 Jan 25 [cited 2024 Apr 1];24(3):2370. Available from: https://www.mdpi.com/1422-0067/24/3/2370.
- Werner H, Weinstein D, Bentov I. Similarities and differences between insulin and IGF-I: structures, receptors, and signalling pathways. Archives of physiology and biochemistry. 2008;114(1):17–22.
- Philippou A, Halapas A, Maridaki M, Koutsilieris M. Type I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophy. J Musculoskelet Neuronal Interact. 2007;7(3):208–18.
- Wang T, Thien C, Wang C, Ni M, Gao J, Wang A, et al. 3D uniaxial mechanical stimulation induces tenogenic differentiation of tendon-derived stem cells through a PI3K/AKT signaling pathway. The FASEB Journal. 2018;32(9):4804–14.
- Guo Y, Pan W, Liu S, Shen Z, Xu Y, Hu L. ERK/MAPK signalling pathway and tumorigenesis. Experimental and therapeutic medicine. 2020;19(3):1997–2007.
- Pinto MCX, Kihara AH, Goulart VA, Tonelli FM, Gomes KN, Ulrich H, et al. Calcium signaling and cell proliferation. Cellular signalling. 2015;27(11):2139–49.
- Disser NP, Sugg KB, Talarek JR, Sarver DC, Rourke BJ, Mendias CL. Insulin-like growth factor 1 signaling in tenocytes is required for adult tendon growth. The FASEB Journal. 2019;33(11):12680.
- Lavoie H, Therrien M. Regulation of RAF protein kinases in ERK signalling. Nature reviews Molecular cell biology. 2015;16(5):281–98.
- Ascenzi F, Barberi L, Dobrowolny G, Villa Nova Bacurau A, Nicoletti C, Rizzuto E, et al. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging cell. 2019;18(3):e12954.
- Dai Z, Wu F, Yeung EW, Li Y. IGF-IEc expression, regulation and biological function in different tissues. Growth Hormone & IGF Research. 2010;20(4):275–81.
- Luo Q, Wu K, Zhang B, Song G. Mechano growth factor E peptide promotes rat bone marrow-derived mesenchymal stem cell migration through CXCR4-ERK1/2. Growth Factors. 2015;33(3):210–9.
- Ashraf S, Cha BH, Kim JS, Ahn J, Han I, Park H, et al. Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthritis and cartilage. 2016;24(2):196–205.
- Asparuhova MB, Riedwyl D, Aizawa R, Raabe C, Couso-Queiruga E, Chappuis V. Local concentrations of TGF-β1 and IGF-1 appear determinant in regulating bone regeneration in human postextraction tooth sockets. International journal of molecular sciences. 2023;24(9):8239.
- Lo MY, Kim HT. Chondrocyte apoptosis induced by collagen degradation: inhibition by caspase inhibitors and IGF-1. Journal of orthopaedic research. 2004;22(1):140–4.
- Hui W, Rowan A, Cawston T. Insulin-like growth factor 1 blocks collagen release and down regulates matrix metalloproteinase-1,-3,-8, and-13 mRNA expression in bovine nasal cartilage stimulated with oncostatin M in combination with interleukin 1α. Annals of the rheumatic diseases. 2001;60(3):254–61.
- Yoshida T, Delafontaine P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells. 2020;9(9):1970.
- Nakao-Hayashi J, Ito H, Kanayasu T, Morita I, Murota S itsu. Stimulatory effects of insulin and insulin-like growth factor I on migration and tube formation by vascular endothelial cells. Atherosclerosis. 1992;92(2–3):141–9.
- Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nature medicine. 1999;5(12):1390–5.
- Wasterlain AS, Braun HJ, Harris AHS, Kim HJ, Dragoo JL. The Systemic Effects of Platelet-Rich Plasma Injection. Am J Sports Med [Internet]. 2013 Jan [cited 2021 Nov 29];41(1):186–93. Available from: http://journals.sagepub.com/doi/10.1177/0363546512466383.
- Ionita CR, Troillet AR, Vahlenkamp TW, Winter K, Brehm W, Ionita JC. Comparison of humoral insulin-like growth factor-1, platelet-derived growth factor-BB, transforming growth factor-β1, and interleukin-1 receptor antagonist concentrations among equine autologous blood-derived preparations. American journal of veterinary research. 2016;77(8):898–905.
- Gugjoo MB, Abdelbaset-Ismail A, Aithal HP, Kinjavdekar P, Pawde AM, Kumar GS, et al. Mesenchymal stem cells with IGF-1 and TGF-β1 in laminin gel for osteochondral defects in rabbits. Biomedicine & Pharmacotherapy. 2017;93:1165–74.
- Seifarth C, Csaki C, Shakibaei M. Anabolic actions of IGF-I and TGF-B1 on Interleukin-1B-treated human articular chondrocytes: Evaluation in two and three dimensional cultures. Histology and histopathology. 2009.
- van de Wouw J, Joles JA. Albumin is an interface between blood plasma and cell membrane, and not just a sponge. Clinical Kidney Journal. 2022;15(4):624–34.
- Lee EH, Hui JHP. The potential of stem cells in orthopaedic surgery. THE JOURNAL OF BONE AND JOINT SURGERY. 2006;88(7):11.
- Horváthy DB, Simon M, Schwarz CM, Masteling M, Vácz G, Hornyák I, et al. Serum albumin as a local therapeutic agent in cell therapy and tissue engineering. BioFactors. 2017;43(3):315–30.
- Herrmann M, Vaudaux PE, Pittet D, Auckenthaler R, Lew PD, Perdreau FS, et al. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. Journal of Infectious Diseases. 1988;158(4):693–701.
- Ishida K, Sawada N, Yamaguchi M. Expression of albumin in bone tissues and osteoblastic cells: involvement of hormonal regulation. International journal of molecular medicine. 2004;14(5):891–5.
- Mijiritsky E, Assaf HD, Peleg O, Shacham M, Cerroni L, Mangani L. Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation—A Narrative Review. Biology [Internet]. 2021 Apr 10 [cited 2021 Nov 14];10(4):317. Available from: https://www.mdpi.com/2079-7737/10/4/317.
- Vandooren J, Itoh Y. Alpha-2-Macroglobulin in Inflammation, Immunity and Infections. Front Immunol [Internet]. 2021 Dec 14 [cited 2023 Aug 2];12:803244. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2021.803244/full.
- Perrin A. Identification and characterisation of host-pathogen protein-protein interactions in the blood stages of malaria.
- Wang S, Wei X, Zhou J, Zhang J, Li K, Chen Q, et al. Identification of α 2 -Macroglobulin as a Master Inhibitor of Cartilage-Degrading Factors That Attenuates the Progression of Posttraumatic Osteoarthritis: α 2 M Attentuates Posttraumatic OA Progression. Arthritis & Rheumatology [Internet]. 2014 Jul [cited 2023 Aug 2];66(7):1843–53. Available from: https://onlinelibrary.wiley.com/doi/10.1002/art.38576.
- Armstrong PB, Quigley JP. α2-macroglobulin: an evolutionarily conserved arm of the innate immune system. Developmental & Comparative Immunology. 1999;23(4–5):375–90.
- Marrero A, Duquerroy S, Trapani S, Goulas T, Guevara T, Andersen GR, et al. The crystal structure of human α2-macroglobulin reveals a unique molecular cage. Angewandte Chemie International Edition. 2012;51(14):3340–4.
- Kristensen T, Moestrup SK, Gliemann J, Bendtsen L, Sand O, Sottrup-Jensen L. Evidence that the newly cloned low-density-lipoprotein receptor related protein (LRP) is the α2-macroglobulin receptor. Febs Letters. 1990;276(1–2):151–5.
- Salvesen GS, Barrett AJ. Covalent binding of proteinases in their reaction with α2-macroglobulin. Biochemical Journal. 1980;187(3):695–701.
- Goulas T, Garcia-Ferrer I, Marrero A, Marino-Puertas L, Duquerroy S, Gomis-Rüth FX. Structural and functional insight into pan-endopeptidase inhibition by α2-macroglobulins. Biological Chemistry. 2017;398(9):975–94.
- Zhu M, Zhao B, Wei L, Wang S. Alpha-2-macroglobulin, a native and powerful proteinase inhibitor, prevents cartilage degeneration disease by inhibiting majority of catabolic enzymes and cytokines. Current Molecular Biology Reports. 2021;7:1–7.
- McDaniel MC, Laudico R, Papermaster BW. Association of macrophage-activation factor from a human cultured lymphoid cell line with albumin and α2-macroglobulin. Clinical Immunology and Immunopathology. 1976;5(1):91–104.
- Dennis P, Saksela O, Harpel P, Rifkin D. α2-Macroglobulin is a binding protein for basic fibroblast growth factor. Journal of Biological Chemistry. 1989;264(13):7210–6.
- Huang SS, O’Grady P, Huang J. Human transforming growth factor beta. alpha 2-macroglobulin complex is a latent form of transforming growth factor beta. Journal of Biological Chemistry. 1988;263(3):1535–41.
- Danielpour D, Sporn MB. Differential inhibition of transforming growth factor beta 1 and beta 2 activity by alpha 2-macroglobulin. Journal of Biological Chemistry. 1990;265(12):6973–7.
- Tchetverikov I. Matrix metalloproteinases-3, -8, -9 as markers of disease activity and joint damage progression in early rheumatoid arthritis. Annals of the Rheumatic Diseases [Internet]. 2003 Nov 1 [cited 2023 Aug 2];62(11):1094–9. Available from: https://ard.bmj.com/lookup/doi/10.1136/ard.62.11.1094.
- Wan R, Hu J, Zhou Q, Wang J, Liu P, Wei Y. Application of co-expressed genes to articular cartilage: new hope for the treatment of osteoarthritis. Molecular medicine reports. 2012;6(1):16–8.
- Cuellar JM. Intradiscal Injection of an Autologous Alpha-2-Macroglobulin (A2M) Concentrate Alleviates Back Pain in FAC-Positive Patients. OROAJ [Internet]. 2017 Jan 3 [cited 2023 Aug 2];4(2). Available from: https://juniperpublishers.com/oroaj/OROAJ.MS.ID.555634.php.
- Luan Y, Kong L, Howell DR, Ilalov K, Fajardo M, Bai XH, et al. Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthritis and Cartilage [Internet]. 2008 Nov [cited 2023 Aug 2];16(11):1413–20. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1063458408000940.
- Eliasberg CD, Rodeo SA. Cytokines, Chemokines, Alpha-2-Macroglobulin, Growth Factors. Orthobiologics: Injectable Therapies for the Musculoskeletal System. 2022;123–32.
- Everts PA, Podesta L, Lana JF, Poovendran G, Santos GS, Huber SC. Alpha-2-Macroglobulin Concentrate as Orthobiologic in Osteoarthritis. In: Musculoskeletal Injections Manual: Basics, Techniques and Injectable Agents. Springer; 2024. p. 133–40.
- Weisel JW, Litvinov RI. Fibrin Formation, Structure and Properties. In: Parry DAD, Squire JM, editors. Fibrous Proteins: Structures and Mechanisms [Internet]. Cham: Springer International Publishing; 2017 [cited 2024 Mar 21]. p. 405–56. (Subcellular Biochemistry; vol. 82). Available from: http://link.springer.com/10.1007/978-3-319-49674-0_13.
- Doolittle RF. A detailed consideration of a principal domain of vertebrate fibrinogen and its relatives. Protein Science [Internet]. 1992 Dec [cited 2024 Apr 16];1(12):1563–77. Available from: https://onlinelibrary.wiley.com/doi/10.1002/pro.5560011204.
- Wolberg AS. Fibrinogen and fibrin: synthesis, structure, and function in health and disease. Journal of Thrombosis and Haemostasis [Internet]. 2023 Nov [cited 2024 Apr 29];21(11):3005–15. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1538783623006426.
- Kattula S, Byrnes JR, Wolberg AS. Fibrinogen and Fibrin in Hemostasis and Thrombosis. ATVB [Internet]. 2017 Mar [cited 2024 Apr 16];37(3). Available from: https://www.ahajournals.org/doi/10.1161/ATVBAHA.117.308564.
- Hoppe B. Fibrinogen and factor XIII at the intersection of coagulation, fibrinolysis and inflammation. Thrombosis and haemostasis. 2014;112(10):649–58.
- Pieters M, Wolberg AS. Fibrinogen and fibrin: An illustrated review. Research and Practice in Thrombosis and Haemostasis [Internet]. 2019 Apr [cited 2024 Apr 16];3(2):161–72. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2475037922015394.
- Vilar R, Fish RJ, Casini A, Neerman-Arbez M. Fibrin(ogen) in human disease: both friend and foe. Haematologica [Internet]. 2020 Feb [cited 2024 Apr 16];105(2):284–96. Available from: http://www.haematologica.org/lookup/doi/10.3324/haematol.2019.236901.
- Ko YP, Flick MJ. Fibrinogen is at the interface of host defense and pathogen virulence in Staphylococcus aureus infection. In Thieme Medical Publishers; 2016. p. 408–21.
- Rubel C, Fernández GC, Rosa FA, Gómez S, Bompadre MB, Coso OA, et al. Soluble fibrinogen modulates neutrophil functionality through the activation of an extracellular signal-regulated kinase-dependent pathway. The Journal of Immunology. 2002;168(7):3527–35.
- Forsyth CB, Solovjov DA, Ugarova TP, Plow EF. Integrin αMβ2-mediated cell migration to fibrinogen and its recognition peptides. The Journal of experimental medicine. 2001;193(10):1123–34.
- Bach TL, Barsigian C, Chalupowicz DG, Busler D, Yaen CH, Grant DS, et al. VE-cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels1. Experimental cell research. 1998;238(2):324–34.
- Ji H, Li Y, Su B, Zhao W, Kizhakkedathu JN, Zhao C. Advances in enhancing hemocompatibility of hemodialysis hollow-fiber membranes. Advanced fiber materials. 2023;5(4):1198–240.
- Toma CM, Imre S, Vari CE, Muntean DL, Tero-Vescan A. Ultrafiltration method for plasma protein binding studies and its limitations. Processes. 2021;9(2):382.
- Fitzpatrick J, Bulsara MK, McCrory PR, Richardson MD, Zheng MH. Analysis of Platelet-Rich Plasma Extraction: Variations in Platelet and Blood Components Between 4 Common Commercial Kits. Orthopaedic Journal of Sports Medicine [Internet]. 2017 Jan [cited 2020 Feb 11];5(1):232596711667527. Available from: http://journals.sagepub.com/doi/10.1177/2325967116675272.
- Magalon J, Bausset O, Serratrice N, Giraudo L, Aboudou H, Veran J, et al. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2014;30(5):629–38.
- Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of Growth Factor and Platelet Concentration From Commercial Platelet-Rich Plasma Separation Systems. Am J Sports Med [Internet]. 2011 Feb [cited 2024 May 9];39(2):266–71. Available from: http://journals.sagepub.com/doi/10.1177/0363546510387517.
- Miron RJ, Chai J, Zhang P, Li Y, Wang Y, Mourão CF de AB, et al. A novel method for harvesting concentrated platelet-rich fibrin (C-PRF) with a 10-fold increase in platelet and leukocyte yields. Clin Oral Invest [Internet]. 2020 Aug [cited 2022 Mar 21];24(8):2819–28. Available from: http://link.springer.com/10.1007/s00784-019-03147-w.
- Fernández-Medina T, Vaquette C, Ivanovski S. Systematic Comparison of the Effect of Four Clinical-Grade Platelet Rich Hemoderivatives on Osteoblast Behaviour. IJMS [Internet]. 2019 Dec 11 [cited 2021 Dec 1];20(24):6243. Available from: https://www.mdpi.com/1422-0067/20/24/6243.
- Prysak MH, Lutz CG, Zukofsky TA, Katz JM, Everts PA, Lutz GE. Optimizing the safety of intradiscal platelet-rich plasma: an in vitro study with Cutibacterium acnes. Regenerative Medicine [Internet]. 2019 Oct [cited 2020 Sep 10];14(10):955–67. Available from: https://www.futuremedicine.com/doi/10.2217/rme-2019-0098.
- Everts PA. A Single, Same-Day Procedure Using Multiple PRP Formulations to Treat Complex Knee Pathologies. A Case Report.
- Degen RM, Bernard JA, Oliver KS, Dines JS. Commercial Separation Systems Designed for Preparation of Platelet-Rich Plasma Yield Differences in Cellular Composition. HSS Jrnl [Internet]. 2017 Feb [cited 2020 Jan 26];13(1):75–80. Available from: http://link.springer.com/10.1007/s11420-016-9519-3.
- Jayaram P, Mitchell PJT, Shybut TB, Moseley BJ, Lee B. Leukocyte-Rich Platelet-Rich Plasma Is Predominantly Anti-inflammatory Compared With Leukocyte-Poor Platelet-Rich Plasma in Patients With Mild-Moderate Knee Osteoarthritis: A Prospective, Descriptive Laboratory Study. Am J Sports Med [Internet]. 2023 Jul [cited 2024 May 9];51(8):2133–40. Available from: http://journals.sagepub.com/doi/10.1177/03635465231170394.
- Yesudasan S, Averett RD. Recent advances in computational modeling of fibrin clot formation: A review. Computational biology and chemistry. 2019;83:107148.
- Smith SA, Travers RJ, Morrissey JH. How it all starts: Initiation of the clotting cascade. Critical reviews in biochemistry and molecular biology. 2015;50(4):326–36.
- Sproul EP, Hannan RT, Brown AC. Controlling fibrin network morphology, polymerization, and degradation dynamics in fibrin gels for promoting tissue repair. Biomaterials for Tissue Engineering: Methods and Protocols. 2018;85–99.
- Singh S, Dodt J, Volkers P, Hethershaw E, Philippou H, Ivaskevicius V, et al. Structure functional insights into calcium binding during the activation of coagulation factor XIII A. Scientific reports. 2019;9(1):11324.
- Mosesson MW, Siebenlist KR, Meh DA. The Structure and Biological Features of Fibrinogen and Fibrin. Annals of the New York Academy of Sciences [Internet]. 2006 Jan 25 [cited 2020 Jun 29];936(1):11–30. Available from: http://doi.wiley.com/10.1111/j.1749-6632.2001.tb03491.
- Cimmino G, Ciccarelli G, Golino P. Role of tissue factor in the coagulation network. In Thieme Medical Publishers; 2015. p. 708–17.
- Larsen JB, Hvas AM. Fibrin Clot Formation and Lysis in Plasma. MPs [Internet]. 2020 Sep 25 [cited 2024 May 1];3(4):67. Available from: https://www.mdpi.com/2409-9279/3/4/67.
- Anitua E, Nurden P, Prado R, Nurden AT, Padilla S. Autologous fibrin scaffolds: When platelet- and plasma-derived biomolecules meet fibrin. Biomaterials [Internet]. 2019 Feb [cited 2022 Apr 26];192:440–60. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0142961218308123.
- Hassan MdM, Sharmin S, Kim HJ, Hong ST. Identification and Characterization of Plasmin-Independent Thrombolytic Enzymes. Circulation Research [Internet]. 2021 Feb 5 [cited 2024 Mar 9];128(3):386–400. Available from: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.120.317245.
- Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thrombosis and haemostasis. 2005;93(04):647–54.
- Weisel JW, Litvinov RI. Fibrin Formation, Structure and Properties. In: Parry DAD, Squire JM, editors. Fibrous Proteins: Structures and Mechanisms [Internet]. Cham: Springer International Publishing; 2017 [cited 2024 Mar 9]. p. 405–56. (Subcellular Biochemistry; vol. 82). Available from: http://link.springer.com/10.1007/978-3-319-49674-0_13.
- Schaller J, Gerber SS. The plasmin–antiplasmin system: structural and functional aspects. Cellular and molecular life sciences. 2011;68:785–801.
- Zakrzewski M, Zakrzewska E, Kiciński P, Przybylska-Kuć S, Dybała A, Myśliński W, et al. Evaluation of fibrinolytic inhibitors: alpha-2-antiplasmin and plasminogen activator inhibitor 1 in patients with obstructive sleep apnoea. PloS one. 2016;11(11):e0166725.
- Collet J, Park D, Lesty C, Soria J, Soria C, Montalescot G, et al. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arteriosclerosis, thrombosis, and vascular biology. 2000;20(5):1354–61.
- Gabriel DA, Muga K, Boothroyd EM. The effect of fibrin structure on fibrinolysis. Journal of Biological Chemistry. 1992;267(34):24259–63.
- Risman RA, Paynter B, Percoco V, Shroff M, Bannish BE, Tutwiler V. Internal fibrinolysis of fibrin clots is driven by pore expansion. Sci Rep [Internet]. 2024 Feb 1 [cited 2024 Apr 30];14(1):2623. Available from: https://www.nature.com/articles/s41598-024-52844-4.
- Anitua E, Nurden P, Prado R, Nurden AT, Padilla S. Autologous fibrin scaffolds: When platelet- and plasma-derived biomolecules meet fibrin. Biomaterials [Internet]. 2019 Feb [cited 2021 Nov 30];192:440–60. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0142961218308123.
- Wahl EA, Fierro FA, Peavy TR, Hopfner U, Dye JF, Machens HG, et al. In vitro evaluation of scaffolds for the delivery of mesenchymal stem cells to wounds. BioMed Research International. 2015;2015.
- M. Dohan Ehrenfest D, Bielecki T, Jimbo R, Barbe G, Del Corso M, Inchingolo F, et al. Do the Fibrin Architecture and Leukocyte Content Influence the Growth Factor Release of Platelet Concentrates? An Evidence-based Answer Comparing a Pure Platelet-Rich Plasma (P-PRP) Gel and a Leukocyte- and Platelet-Rich Fibrin (L-PRF). CPB [Internet]. 2012 May 1 [cited 2024 Mar 21];13(7):1145–52. Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1389-2010&volume=13&issue=7&spage=1145.
- Robson S, Shephard E, Kirsch R. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1β, IL-6 and plasminogen activator inhibitors from monocytes in vitro. British journal of haematology. 1994;86(2):322–6.
- Petzelbauer P, Zacharowski PA, Miyazaki Y, Friedl P, Wickenhauser G, Castellino FJ, et al. The fibrin-derived peptide Bβ15–42 protects the myocardium against ischemia-reperfusion injury. Nature medicine. 2005;11(3):298–304.
- Yu M, Wang X, Liu Y, Qiao J. Cytokine release kinetics of concentrated growth factors in different scaffolds. Clinical Oral Investigations. 2019;23:1663–71.
- Alzahrani SH, Hess K, Price JF, Strachan M, Baxter PD, Cubbon R, et al. Gender-specific alterations in fibrin structure function in type 2 diabetes: associations with cardiometabolic and vascular markers. The Journal of Clinical Endocrinology & Metabolism. 2012;97(12):E2282–7.
- Crisci A, Crescenzo UD, Crisci M. Platelet-rich concentrates (L-PRF, PRP) in tissue regeneration: Control of apoptosis and interactions with regenerative cells. 2018;1:5.
- Ratajczak J, Vangansewinkel T, Gervois P, Merckx G, Hilkens P, Quirynen M, et al. Angiogenic Properties of ‘Leukocyte- and Platelet-Rich Fibrin.’ Sci Rep [Internet]. 2018 Oct 2 [cited 2023 May 11];8(1):14632. Available from: https://www.nature.com/articles/s41598-018-32936-8.
- Yang HS, Shin J, Bhang SH, Shin JY, Park J, Im GI, et al. Enhanced skin wound healing by a sustained release of growth factors contained in platelet-rich plasma. Exp Mol Med [Internet]. 2011 [cited 2024 Mar 9];43(11):622. Available from: https://www.nature.com/doifinder/10.3858/emm.2011.43.11.070.
- Nakanishi Y, Matsushita T, Nagai K, Araki D, Hoshino Y, Kuroda R. Fibrin clot and Leukocyte-rich platelet-rich fibrin show similar release kinetics and amount of growth factors: a pilot study. J Orthop Surg Res [Internet]. 2023 Mar 25 [cited 2024 Apr 30];18(1):238. Available from: https://josr-online.biomedcentral.com/articles/10.1186/s13018-023-03709-5.
- Lana JF, Purita J, Everts PA, De Mendonça Neto PAT, De Moraes Ferreira Jorge D, Mosaner T, et al. Platelet-Rich Plasma Power-Mix Gel (ppm)—An Orthobiologic Optimization Protocol Rich in Growth Factors and Fibrin. Gels [Internet]. 2023 Jul 7 [cited 2023 Sep 14];9(7):553. Available from: https://www.mdpi.com/2310-2861/9/7/553.
- Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophysical chemistry. 2004;112(2–3):267–76.
- Laurens N, Koolwijk P, De Maat MPM. Fibrin structure and wound healing. J Thromb Haemost [Internet]. 2006 May [cited 2021 Mar 15];4(5):932–9. Available from: http://doi.wiley.com/10.1111/j.1538-7836.2006.01861.
- Ciano P, Colvin R, Dvorak A, McDonagh J, Dvorak H. Macrophage migration in fibrin gel matrices. Laboratory investigation; a journal of technical methods and pathology. 1986;54(1):62–70.
- Delgado D, Beitia M, Mercader Ruiz J, Sánchez P, Montoya-Alzola M, Fiz N, et al. A Novel Fibrin Matrix Derived from Platelet-Rich Plasma: Protocol and Characterization. IJMS [Internet]. 2024 Apr 6 [cited 2024 May 15];25(7):4069. Available from: https://www.mdpi.com/1422-0067/25/7/4069.
- Dickneite G, Metzner H, Pfeifer T, Kroez M, Witzke G. A comparison of fibrin sealants in relation to their in vitro and in vivo properties. Thrombosis research. 2003;112(1–2):73–82.
- Zhang Z, Zhong W, Li J, Luo J. Mechanical Properties and Cushioning Effectiveness of FPUF-EPS Combination Materials. Materials. 2023;16(21):6886.
- Padilla S, Sánchez M, Orive G, Anitua E. Human-based biological and biomimetic autologous therapies for musculoskeletal tissue regeneration. Trends in biotechnology. 2017;35(3):192–202.
- Balagholi S, Rezaei Kanavi M, Alizadeh S, Dabbaghi R, Karami S, Kheiri B, et al. Effects of fibrin glue as a three-dimensional scaffold in cultivated adult human retinal pigment epithelial cells. Journal of Biomaterials Applications. 2018;33(4):514–26.
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25(12):677–86.
- Lee, KY. M1 and M2 polarization of macrophages: a mini-review. Med Biol Sci Eng. 2019;2(1):1–5.
- Flick MJ, Du X, Degen JL. Fibrin (ogen)-αMβ2 interactions regulate leukocyte function and innate immunity in vivo. Experimental biology and medicine. 2004;229(11):1105–10.
- Hsieh JY, Smith TD, Meli VS, Tran TN, Botvinick EL, Liu WF. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomaterialia [Internet]. 2017 Jan [cited 2024 May 2];47:14–24. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1742706116304974.
- Whyte CS. All tangled up: interactions of the fibrinolytic and innate immune systems. Front Med [Internet]. 2023 Jun 2 [cited 2024 May 5];10:1212201. Available from: https://www.frontiersin.org/articles/10.3389/fmed.2023.1212201/full.
- Tang T, Rosenkranz A, Assmann KJ, Goodman MJ, Gutierrez-Ramos JC, Carroll MC, et al. A role for Mac-1 (CDIIb/CD18) in immune complex–stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcγ receptor–dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. The Journal of experimental medicine. 1997;186(11):1853–63.
- Vasconcelos DM, Gonçalves RM, Almeida CR, Pereira IO, Oliveira MI, Neves N, et al. Fibrinogen scaffolds with immunomodulatory properties promote in vivo bone regeneration. Biomaterials [Internet]. 2016 Dec [cited 2024 May 5];111:163–78. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0142961216305415.
- Almeida CR, Vasconcelos DP, Gonçalves RM, Barbosa MA. Enhanced mesenchymal stromal cell recruitment via natural killer cells by incorporation of inflammatory signals in biomaterials. Journal of The Royal Society Interface. 2012;9(67):261–71.
- Eken C, Sadallah S, Martin PJ, Treves S, Schifferli JA. Ectosomes of polymorphonuclear neutrophils activate multiple signaling pathways in macrophages. Immunobiology. 2013;218(3):382–92.
- Dinkla S, Van Cranenbroek B, Van Der Heijden WA, He X, Wallbrecher R, Dumitriu IE, et al. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood, The Journal of the American Society of Hematology. 2016;127(16):1976–86.
- Laffont B, Corduan A, Rousseau M, Duchez AC, Lee CHC, Boilard E, et al. Platelet microparticles reprogram macrophage gene expression and function. Thrombosis and haemostasis. 2016;115(02):311–23.
- Kim OH, Park JH, Son JI, Yoon OJ, Lee HJ. Bone marrow mesenchymal stromal cells on silk fibroin scaffolds to attenuate polymicrobial sepsis induced by cecal ligation and puncture. Polymers. 2021;13(9):1433.
- Heher P, Mühleder S, Mittermayr R, Redl H, Slezak P. Fibrin-based delivery strategies for acute and chronic wound healing. Advanced drug delivery reviews. 2018;129:134–47.
- Roberts IV, Bukhary D, Valdivieso CYL, Tirelli N. Fibrin Matrices as (Injectable) Biomaterials: Formation, Clinical Use, and Molecular Engineering. Macromolecular Bioscience [Internet]. 2020 Jan [cited 2024 May 2];20(1):1900283. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mabi.201900283.
- O’Connell SM, Impeduglia T, Hessler K, Wang X, Carroll RJ, Dardik H. Autologous platelet-rich fibrin matrix as cell therapy in the healing of chronic lower-extremity ulcers. Wound Repair Regeneration [Internet]. 2008 Nov [cited 2024 Mar 21];16(6):749–56. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1524-475X.2008.00426.
- Everts PAM, Devilee RJJ, Brown Mahoney C, Eeftinck-Schattenkerk M, Box HAM, Knape JTA, et al. Platelet gel and fibrin sealant reduce allogeneic blood transfusions in total knee arthroplasty. Acta Anaesthesiol Scand [Internet]. 2006 May [cited 2019 Oct 4];50(5):593–9. Available from: http://doi.wiley.com/10.1111/j.1399-6576.2006.001005.
- Micovic S, Everts P, Calija B, Strugarevic E, Grubor N, Boricic M, et al. Novel autologous, high concentrated fibrin as advanced hemostatic agent for coronary surgery. Transfusion and Apheresis Science [Internet]. 2021 Aug [cited 2023 May 11];60(4):103171. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1473050221001373.
- Koolwijk P, Van Erck M, De Vree W, Vermeer MA, Weich HA, Hanemaaijer R, et al. Cooperative effect of TNFalpha, bFGF, and VEGF on the formation of tubular structures of human microvascular endothelial cells in a fibrin matrix. Role of urokinase activity. The Journal of cell biology. 1996;132(6):1177–88.
- Feng X, Clark RA, Galanakis D, Tonnesen MG. Fibrin and collagen differentially regulate human dermal microvascular endothelial cell integrins: stabilization of αv/β3 mRNA by fibrin. Journal of investigative dermatology. 1999;113(6):913–9.
- Yakovlev S, Medved L. Interaction of fibrin (ogen) with the endothelial cell receptor VE-cadherin: localization of the fibrin-binding site within the third extracellular VE-cadherin domain. Biochemistry. 2009;48(23):5171–9.
- Gorodetsky R, Vexler A, Levdansky L, Marx G. Fibrin microbeads (FMB) as biodegradable carriers for culturing cells and for accelerating wound healing. Biopolymer Methods in Tissue Engineering. 2004;11–24.
- Oliver JJ, Webb DJ, Newby DE. Stimulated Tissue Plasminogen Activator Release as a Marker of Endothelial Function in Humans. ATVB [Internet]. 2005 Dec [cited 2024 May 6];25(12):2470–9. Available from: https://www.ahajournals.org/doi/10.1161/01.ATV.0000189309.05924.88.
- FUKAO H, UESHIMA S, OKADA K, MATSUO O. The Role of the Pericellular Fibrinolytic System in Angiogenesis. The Japanese Journal of Physiology. 1997;47(2):161–71.
- Harley SL, Sturge J, Powell JT. Regulation by fibrinogen and its products of intercellular adhesion molecule-1 expression in human saphenous vein endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2000;20(3):652–8.
- Hermans P, Hazelzet JA. Plasminogen activator inhibitor type 1 gene polymorphism and sepsis. Clinical Infectious Diseases. 2005;41(Supplement_7):S453–8.
- van HINSBERGH VW, Collen A, Koolwijk P. Role of fibrin matrix in angiogenesis. Annals of the New York Academy of Sciences. 2001;936(1):426–37.
- Nehls V, Herrmann R. The configuration of fibrin clots determines capillary morphogenesis and endothelial cell migration. Microvascular research. 1996;51(3):347–64.
- Sun H, Wang X, Degen JL, Ginsburg D. Reduced thrombin generation increases host susceptibility to group A streptococcal infection. Blood, The Journal of the American Society of Hematology. 2009;113(6):1358–64.
- Degen J, Bugge T, Goguen J. Fibrin and fibrinolysis in infection and host defense. Journal of Thrombosis and Haemostasis. 2007;5:24–31.
- Prasad JM, Gorkun OV, Raghu H, Thornton S, Mullins ES, Palumbo JS, et al. Mice expressing a mutant form of fibrinogen that cannot support fibrin formation exhibit compromised antimicrobial host defense. Blood, The Journal of the American Society of Hematology. 2015;126(17):2047–58.
- Bayer A, Höntsch G, Kaschwich M, Dell A, Siggelkow M, Berndt R, et al. Vivostat Platelet-Rich Fibrin® for complicated or chronic wounds—a pilot study. Biomedicines. 2020;8(8):276.
- Pereira VBS, Lago CAP, Almeida R de AC, Barbirato D da S, Vasconcelos BC do E. Biological and Cellular Properties of Advanced Platelet-Rich Fibrin (A-PRF) Compared to Other Platelet Concentrates: Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2023;25(1):482.
- Hassan S, Dhadse P, Bajaj P, Sethiya K, Subhadarsanee C, Oza R. Comparative evaluation of platelet rich fibrin matrix (PRFM) membrane and platelet rich fibrin (PRF) membrane using the vestibular incision subperiosteal tunnel access (VISTA) approach technique for the treatment of multiple gingival recession in humans: A double-blind, parallel-group, randomized controlled clinical trial. F1000Research. 2023;12:872.
Figure 1.
Portrayal of a 2-spin centrifugation method to produce autologous PRP. PRP preparation involves the collection of a predetermined volume of peripheral blood in collection syringes containing an anticoagulant, like calcium citrate 3.8%. The predonated whole blood is gently loaded in a PRP device for gravitational cellular density separation, using a two-spin centrifugation protocol. After the first spin cycle, the whole blood components are separated into three basic layers: the PPP suspension, the buffy coat demarcation layer, and the RBC layer. During the second centrifugation cycle, the platelet and other cells in the PPP fraction and RBC layer are further separated, resulting in a multicellular buffy coat stratum containing high concentrations of platelets and eventually leukocytes. A calculated portion of the PPP fraction is removed, leaving the platelet concentrate within a small volume of plasma for platelet resuspension. Thereafter, the PRP is extracted from the device. In this graphic, LR-PRP has been prepared, and the multicellular fractions consist of a high concentration of platelets, monocytes, lymphocytes, neutrophils, and some red blood cells. Abbreviations: PPP: platelet-poor plasma; RBC: red blood cells; LR-PRP: leukocyte-rich PRP; PRP: platelet-rich plasma.
Figure 1.
Portrayal of a 2-spin centrifugation method to produce autologous PRP. PRP preparation involves the collection of a predetermined volume of peripheral blood in collection syringes containing an anticoagulant, like calcium citrate 3.8%. The predonated whole blood is gently loaded in a PRP device for gravitational cellular density separation, using a two-spin centrifugation protocol. After the first spin cycle, the whole blood components are separated into three basic layers: the PPP suspension, the buffy coat demarcation layer, and the RBC layer. During the second centrifugation cycle, the platelet and other cells in the PPP fraction and RBC layer are further separated, resulting in a multicellular buffy coat stratum containing high concentrations of platelets and eventually leukocytes. A calculated portion of the PPP fraction is removed, leaving the platelet concentrate within a small volume of plasma for platelet resuspension. Thereafter, the PRP is extracted from the device. In this graphic, LR-PRP has been prepared, and the multicellular fractions consist of a high concentration of platelets, monocytes, lymphocytes, neutrophils, and some red blood cells. Abbreviations: PPP: platelet-poor plasma; RBC: red blood cells; LR-PRP: leukocyte-rich PRP; PRP: platelet-rich plasma.
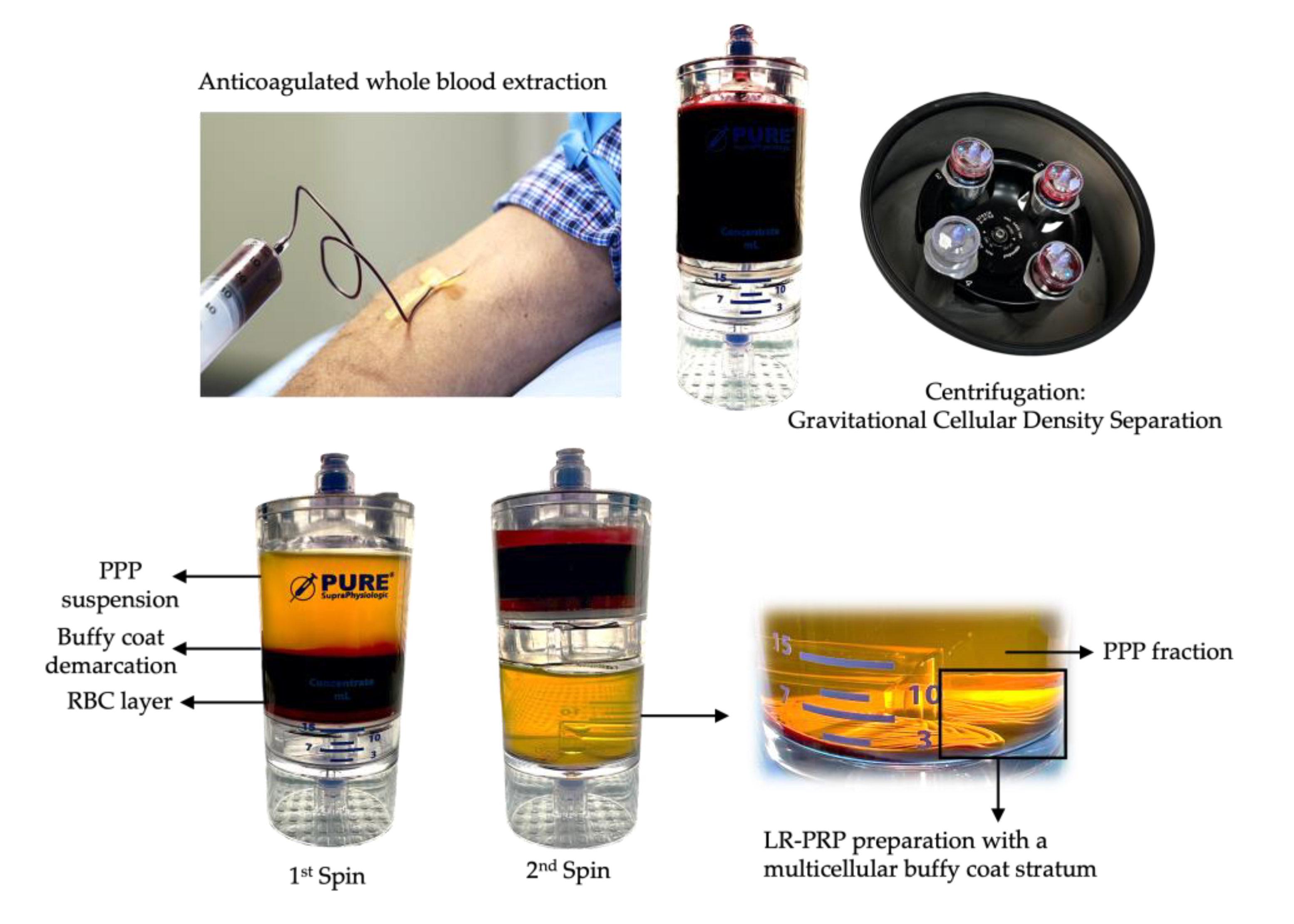
Figure 2.
PRPC preparation. After the second spin, a calculated portion of the PPP fraction is removed with a syringe and attached with an empty syringe to the ultrafiltration device, as well as an effluent collection syringe to collect the eliminated plasma water. The PPP syringes are manually pushed through the device and plasma water, proteins smaller than 20kDA, and cytokines are removed through the hollow fiber pores. After a series of passes, the PPP volume is significantly reduced, leading to a small and viscous volume of protein-rich plasma. The remaining PPP volume in the PRP device is used to resuspend the highly concentrated multicellular LR-PRP fraction and capture it from the PRP device. The concentrated protein-rich PPP and LR-PRP are consolidated into one syringe and gently mixed creating PRPC. Abbreviations: LR-PRP: leukocyte-rich PRP; PPP: platelet-poor plasma; PRP: platelet rich plasma; PRPC: protein-rich platelet concentrate. (The ultrafiltration device shown is the CORE™ Ultrafiltration System, developed by EmCyte Corporation®, Fort Myers FL, USA).
Figure 2.
PRPC preparation. After the second spin, a calculated portion of the PPP fraction is removed with a syringe and attached with an empty syringe to the ultrafiltration device, as well as an effluent collection syringe to collect the eliminated plasma water. The PPP syringes are manually pushed through the device and plasma water, proteins smaller than 20kDA, and cytokines are removed through the hollow fiber pores. After a series of passes, the PPP volume is significantly reduced, leading to a small and viscous volume of protein-rich plasma. The remaining PPP volume in the PRP device is used to resuspend the highly concentrated multicellular LR-PRP fraction and capture it from the PRP device. The concentrated protein-rich PPP and LR-PRP are consolidated into one syringe and gently mixed creating PRPC. Abbreviations: LR-PRP: leukocyte-rich PRP; PPP: platelet-poor plasma; PRP: platelet rich plasma; PRPC: protein-rich platelet concentrate. (The ultrafiltration device shown is the CORE™ Ultrafiltration System, developed by EmCyte Corporation®, Fort Myers FL, USA).
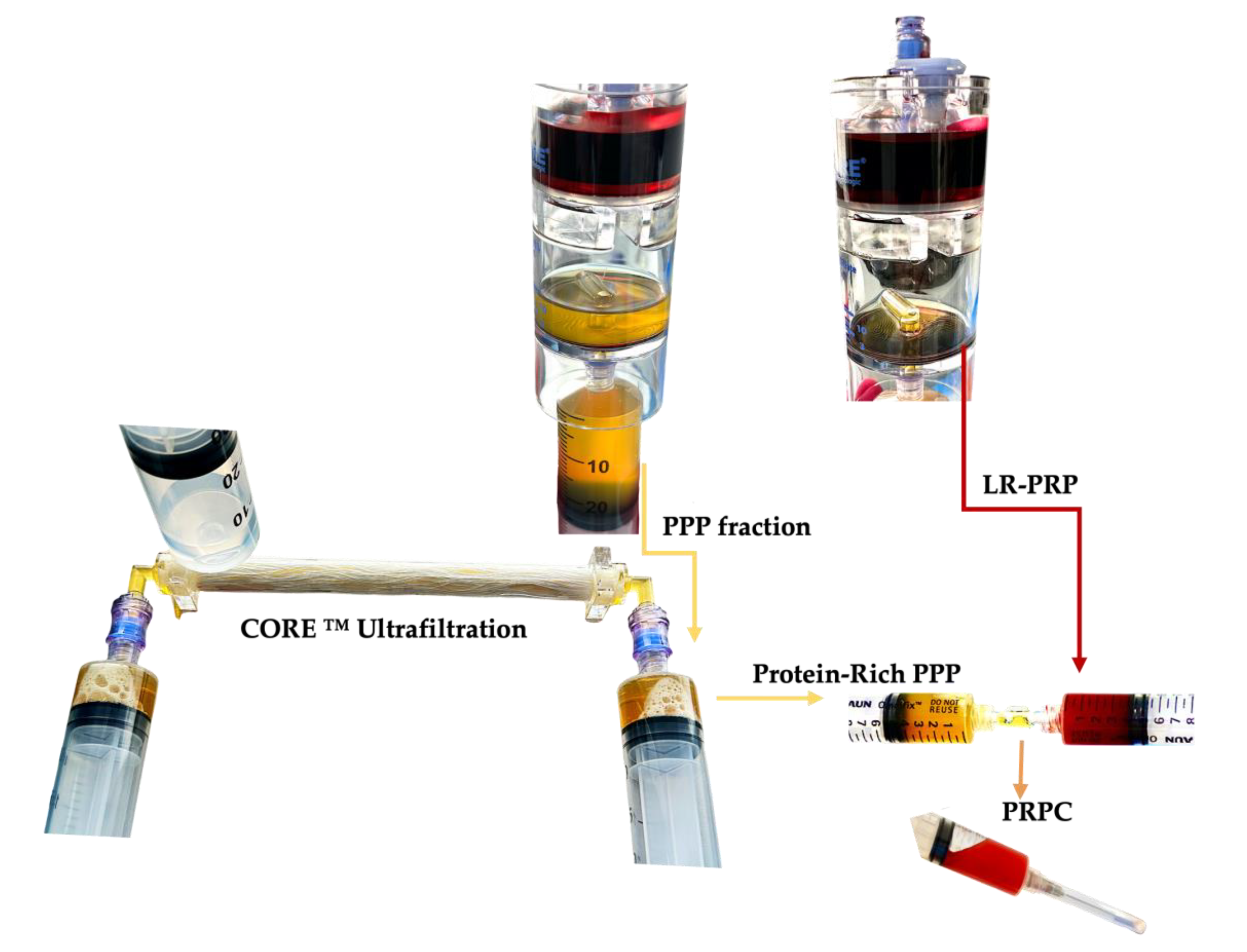
Figure 3.
Visualization of PRF, LP-PRPC, and LR-PRP PRPC matrices. The PRF matrix, leukocyte poor and leukocyte rich PRPC matrices, are exhibited. In a same patient experiment, 130 ml of anticoagulated whole blood was extracted to prepare 10 ml of PRF, LP-PPR and LR-PRP, using two 60 ml 2-spin PRP devices (PurePRP®SP, EmCyte Corporation® Fort Myers FL, USA). After the first spin, the PRF clot was removed from the test tube. After the 2nd spin of the LP and LR-PRP preparations, the PPP fraction was removed for ultrafiltration to concentrate the plasma to produce protein-rich PPP resuspended with the concentrated PRP fractions, described in detail in
Figure 2. Exactly 3ml of both PRP formulations were consolidated with 3ml of protein-rich PPP. To create the PRPC matrices, normal baseline clotting parameters were restored by adding 0.35 ml of NaCl 10% to the 6 ml PRPC volume. Thereafter, 150 IU of bovine thrombin was added to the re-calcified PRPC volume, mimicking the effect of TF, for matrix formation.
Figure 3.
Visualization of PRF, LP-PRPC, and LR-PRP PRPC matrices. The PRF matrix, leukocyte poor and leukocyte rich PRPC matrices, are exhibited. In a same patient experiment, 130 ml of anticoagulated whole blood was extracted to prepare 10 ml of PRF, LP-PPR and LR-PRP, using two 60 ml 2-spin PRP devices (PurePRP®SP, EmCyte Corporation® Fort Myers FL, USA). After the first spin, the PRF clot was removed from the test tube. After the 2nd spin of the LP and LR-PRP preparations, the PPP fraction was removed for ultrafiltration to concentrate the plasma to produce protein-rich PPP resuspended with the concentrated PRP fractions, described in detail in
Figure 2. Exactly 3ml of both PRP formulations were consolidated with 3ml of protein-rich PPP. To create the PRPC matrices, normal baseline clotting parameters were restored by adding 0.35 ml of NaCl 10% to the 6 ml PRPC volume. Thereafter, 150 IU of bovine thrombin was added to the re-calcified PRPC volume, mimicking the effect of TF, for matrix formation.

Figure 4.
Schematic overview of the multistep physiological process of PRPC matrix creation and the sustained release of matrix biological components. The ultrafiltration device concentrates non-activated plasma proteins, including fibrinogen, consisting of two D-domains, composed of α, β, γ chains, and plasma growth factors. After consolidating a small volume of high concentration PRP with the concentrated protein suspension, fibrinogen undergoes a structural change in the presence of thrombin and calcium ions, leading to the cleavage of fibrinogen into FpA and FpB to form stable complex, soluble fibrin monomers. The soluble monomer polymerizes to form half-staggered protofibrils. Several enzymes, including FXIIIa, and in presence of Ca++ ions, protofibrils are converted to the cross-linked fibrin, embedded with highly concentrated PRP cells. Fibrinolysis is initiated when t-PA converts plasminogen into plasmin, whereas PAI-1 inhibits t-PA, preventing the activation of plasminogen, and thus fibrinolysis. Hence, FDP, D-dimers, platelets, and other cells are continuously released from the PRPC matrix, whereas α2 antiplasmin acts by blocking plasmin activity, reducing the proteolytic fibrinolytic break down.
Figure 4.
Schematic overview of the multistep physiological process of PRPC matrix creation and the sustained release of matrix biological components. The ultrafiltration device concentrates non-activated plasma proteins, including fibrinogen, consisting of two D-domains, composed of α, β, γ chains, and plasma growth factors. After consolidating a small volume of high concentration PRP with the concentrated protein suspension, fibrinogen undergoes a structural change in the presence of thrombin and calcium ions, leading to the cleavage of fibrinogen into FpA and FpB to form stable complex, soluble fibrin monomers. The soluble monomer polymerizes to form half-staggered protofibrils. Several enzymes, including FXIIIa, and in presence of Ca++ ions, protofibrils are converted to the cross-linked fibrin, embedded with highly concentrated PRP cells. Fibrinolysis is initiated when t-PA converts plasminogen into plasmin, whereas PAI-1 inhibits t-PA, preventing the activation of plasminogen, and thus fibrinolysis. Hence, FDP, D-dimers, platelets, and other cells are continuously released from the PRPC matrix, whereas α2 antiplasmin acts by blocking plasmin activity, reducing the proteolytic fibrinolytic break down.
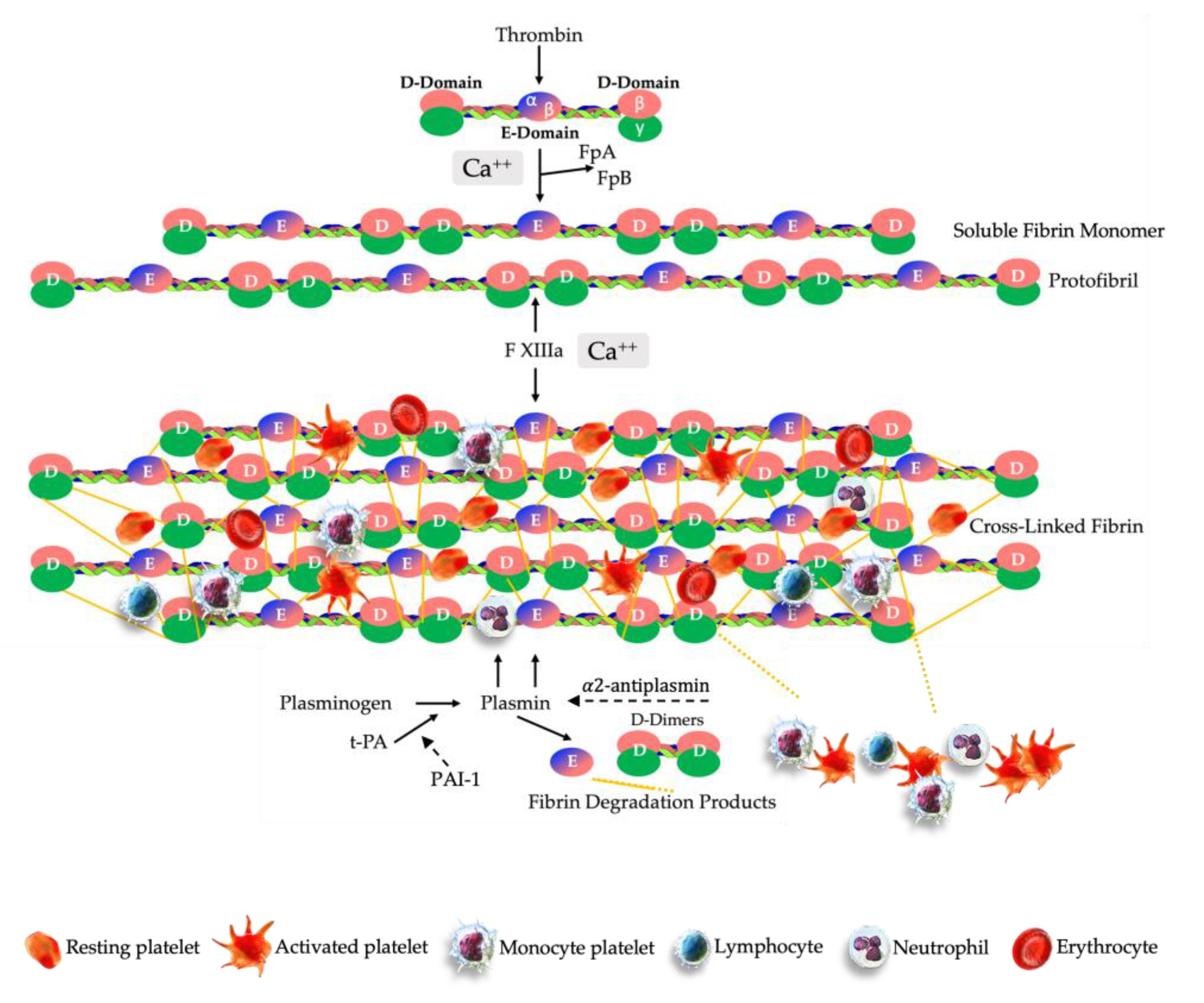
Figure 5.
Illustration of the biological features of a PRPC matrix. The beneficial effects of an accurately prepared and applied PRPC matrix in pathological microenvironments, are depicted by several biologically induced processes that are crucial in the repair and regeneration of diseased tissues. The PRPC matrix, which is composed of concentrated and activated fibrinogen as its main component, serves as a temporary dense insoluble three-dimensional scaffold with high concentrations of PRP cellular content embedded. Importantly, the matrix enhances the viability and functionality of the PRP cellular content. The PRPC matrix is instrumental in providing a molecular link for invading local tissue resident cells, such as MSCs, macrophages, fibroblasts, and ECs. Inside the matrix microenvironment, a multitude of cells and molecules engage in many cellular interactions. Upon fibrinolytic breakdown of the PRPC matrix, an abundance of activated platelets, their PGFs and other biological active molecules and cells are released to the local microenvironment. Ultimately, the sustained release of matrix substances leads to an increase in cellular activity and signaling, angiogenetic and immunomodulatory processes, and antimicrobial activities, contributing to the overall tissue repair and regenerative process. Moreover, as a result of the breaking down of fibrin strands, they provide structural support for the development of new tissues and facilitate the adhesion and migration of cells [
28,
174,
175,
176,
177,
178,
218] .
Figure 5.
Illustration of the biological features of a PRPC matrix. The beneficial effects of an accurately prepared and applied PRPC matrix in pathological microenvironments, are depicted by several biologically induced processes that are crucial in the repair and regeneration of diseased tissues. The PRPC matrix, which is composed of concentrated and activated fibrinogen as its main component, serves as a temporary dense insoluble three-dimensional scaffold with high concentrations of PRP cellular content embedded. Importantly, the matrix enhances the viability and functionality of the PRP cellular content. The PRPC matrix is instrumental in providing a molecular link for invading local tissue resident cells, such as MSCs, macrophages, fibroblasts, and ECs. Inside the matrix microenvironment, a multitude of cells and molecules engage in many cellular interactions. Upon fibrinolytic breakdown of the PRPC matrix, an abundance of activated platelets, their PGFs and other biological active molecules and cells are released to the local microenvironment. Ultimately, the sustained release of matrix substances leads to an increase in cellular activity and signaling, angiogenetic and immunomodulatory processes, and antimicrobial activities, contributing to the overall tissue repair and regenerative process. Moreover, as a result of the breaking down of fibrin strands, they provide structural support for the development of new tissues and facilitate the adhesion and migration of cells [
28,
174,
175,
176,
177,
178,
218] .
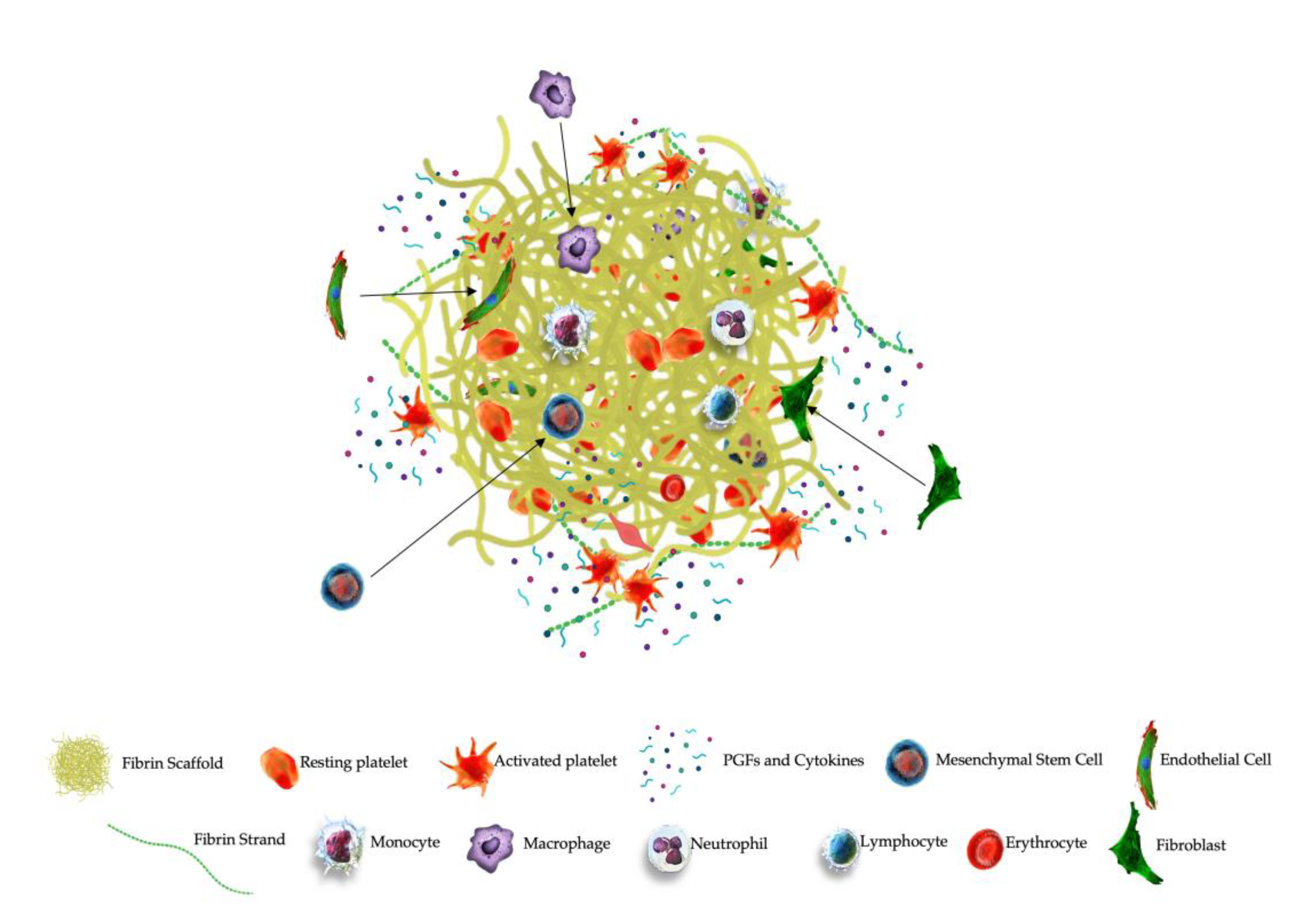
Table 1.
Partial list of PRPC components, structures, and molecular content of platelets, plasma.
Table 1.
Partial list of PRPC components, structures, and molecular content of platelets, plasma.
| PRPC Component |
Structure |
Key Content |
Main Functions |
| Platelet |
α-granules |
Growth Factors:
PDGF (AA-BB-AB-CC), VEGF,
TGF (α-β), FGF (a-b), EGF, CTGF |
Growth factor-based regulation of tissue repair via cell proliferation, differentiation, mitogenesis, chemotaxis, epithelial repair.
|
Adhesive Proteins:
Fibronectin, vitronectin,
fibrinogen, vWF, P-selectin,
integrins αIIbβ, Phosphatidylserine |
Platelet aggregation, platelet-endothelial cell interaction, thrombus formation.
|
Coagulation Factors:
Factors IV, XI, XIII, plasminogen, plasmin, antithrombin, Tissue Factor |
Hemostasis, thrombus formation.
|
Angiogenic Regulators:
IL8, Thrombospondin, Angiostatin, PF-4, TIMP-1,4, MMP-1,2,9, Angiopoietin, Endostatin,
SDF-1, PMP
|
Angiogenesis cascades and re-establishing vasculature. |
Cytokines:
IL1, IL4, IL6, TFNα, SDF-1
|
Chemotaxis, inflammatory response modulation, antimicrobial activity.
|
Chemokines:
RANTES, CXCL4, CXCL7, CCL2, CCL3, CCL5, β-TG |
Inflammation, antimicrobial, bactericidal activity. |
Complement Proteins:
C3, C4 |
Phagocytosis, chemotaxis, platelet activation. |
Exosomes:
mRNA, miRNA, CXCL4, CXCL7
|
Cell adhesion, paracrine communication, regulation of cell fate, modulation of inflammatory response.
|
| Dense granules |
ADP, ATP, TFNα calcium,
serotonin, epinephrine,
pyrophosphates
|
Platelet activation, vasoconstriction.
|
| Lysosomes |
Collagenase, elastase, Cathepsin,
α-arabinoside, β-galactosidase |
Matrix degradation, antimicrobial activity.
|
| Multivescicular Bodies |
Exosomes
Extracellular vesicles |
Cell proliferation, PGF transportation, platelet-cell communication. |
| PPP |
Plasma Proteins (> 300) |
Albumen, Fibrinogen, Alpha-2-macroglobulin, |
Blood clotting, maintain blood pressure, carrier functions, Immunity, pH regulation |
| Coagulation factors |
Tissue Factor, Factor I, II, IV, V, VII, vWF |
Intrinsic and extrinsic coagulation pathways, Clot formation |
| Growth Factors |
IGF-1, HGF |
Bone growth, glucose transport in fat and muscle, muscle production, mitogenesis, cell growth, cell proliferation |
Table 3.
Hematology data from different PRP preparations.
Table 3.
Hematology data from different PRP preparations.
Device
Category |
PRPv
ml |
PLTc
x 103/µL |
PLTd
x 106
|
WBCc
x 103/µL |
MONc
x 103/µL |
NEUc
x 103/µL |
RBCc
x 109/µL |
References |
| P-PRP |
4.8 |
170 |
887 |
0.3 |
0 |
0 |
0 |
[181,182,183] |
| PRF |
5 |
205 |
1,025 |
0.1 |
0 |
0 |
0 |
[5,184,185] |
| LP-PRP |
4.6 |
1,280 |
5,686 |
11.5 |
3.1 |
0.8 |
0.2 |
[97,186,187] |
| LR-PRP |
6.3 |
1,603 |
9,212 |
24.7 |
4.7 |
5.4 |
1.6 |
[186,188,189] |
Table 4.
Indicative matrix cellular differences and total available platelet for 3 different PRP preparations.
Table 4.
Indicative matrix cellular differences and total available platelet for 3 different PRP preparations.
Matrix
Formulation |
PLTc
x 106/µL |
PLTs available
in matrix, x 109
|
MONc
x 103/µL |
NEUc
x 103/µL |
RBCc
x 109/µL |
| PRF |
0,275 |
1,375 |
0 |
0 |
0 |
| LP-PRPC |
2,3 |
6,9 |
4.5 |
6.1 |
0.3 |
| LR-PRPC |
3,9 |
11,8 |
8.2 |
13.5 |
2.4 |
Table 5.
A variety of biological and therapeutic hallmarks of autologous fibrin(ogen) matrices and compounded PRPC matrices.
Table 5.
A variety of biological and therapeutic hallmarks of autologous fibrin(ogen) matrices and compounded PRPC matrices.
| Treatment Modality and Biocomponent |
Biological Function of Matrix |
Therapeutic Goals |
Sealant/Glue
Fibrin(ogen)
Regenerative Glue
Fibrin(ogen) and PRP
|
Post-surgical hemostasis control
Oozing tissues sealing
Controllable biodegradation
+
ECM cellular support
Anti-inflammatory
Active platelet mediated angiogenesis
|
Avoiding seromatous wound leakage
Avoid blood loss
Decrease surgical adhesions
Periodontal membrane
Support in surgical tissue healing
Infection prevention
Chronic wound & bone healing
Scar reduction |
Fibrin Matrix
Fibrin(ogen)
PRPC matrix
Fibrin(ogen) and PRP |
Temporary matrix for invading platelets, leukocytes
EC migration
Cell adhesion
Promotor angiogenesis process
Collagen production
Growth factor carrier
Promotor of immunomodulation
Fibrinolytic sustained release
+
Osteoblast stimulation
Temporary matrix with embedded concentrated platelets and leukocytes
Platelet growth factor reservoir
Plasma growth factor reservoir
Tissue ingrowth stimulation
Instigator immunomodulation
Cell proliferation-differentiation
Organizer angiogenesis process
MSC & HF stem cell paracrine effects
Antimicrobial pathways
Nociception
A2M enzymatic processes |
Tissue repair
Tissue regeneration
Tissue engineering heart valves, patches
Hydrogel transport
Intra-osseous infiltration
Nerve injuries
Myogenesis
Bone defect augmentation
Epithelialization chronic wounds
Tissue infection management
Tissue repair, including nerves
Tissue regeneration
Skin grafting
Wound healing
Partial-Full thickness tendon/ligament biofiller
Immunomodulation
Osteoarthritis
Joint protease inhibitor
Joint cushioning
Hair growth |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).










