Submitted:
21 May 2024
Posted:
23 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
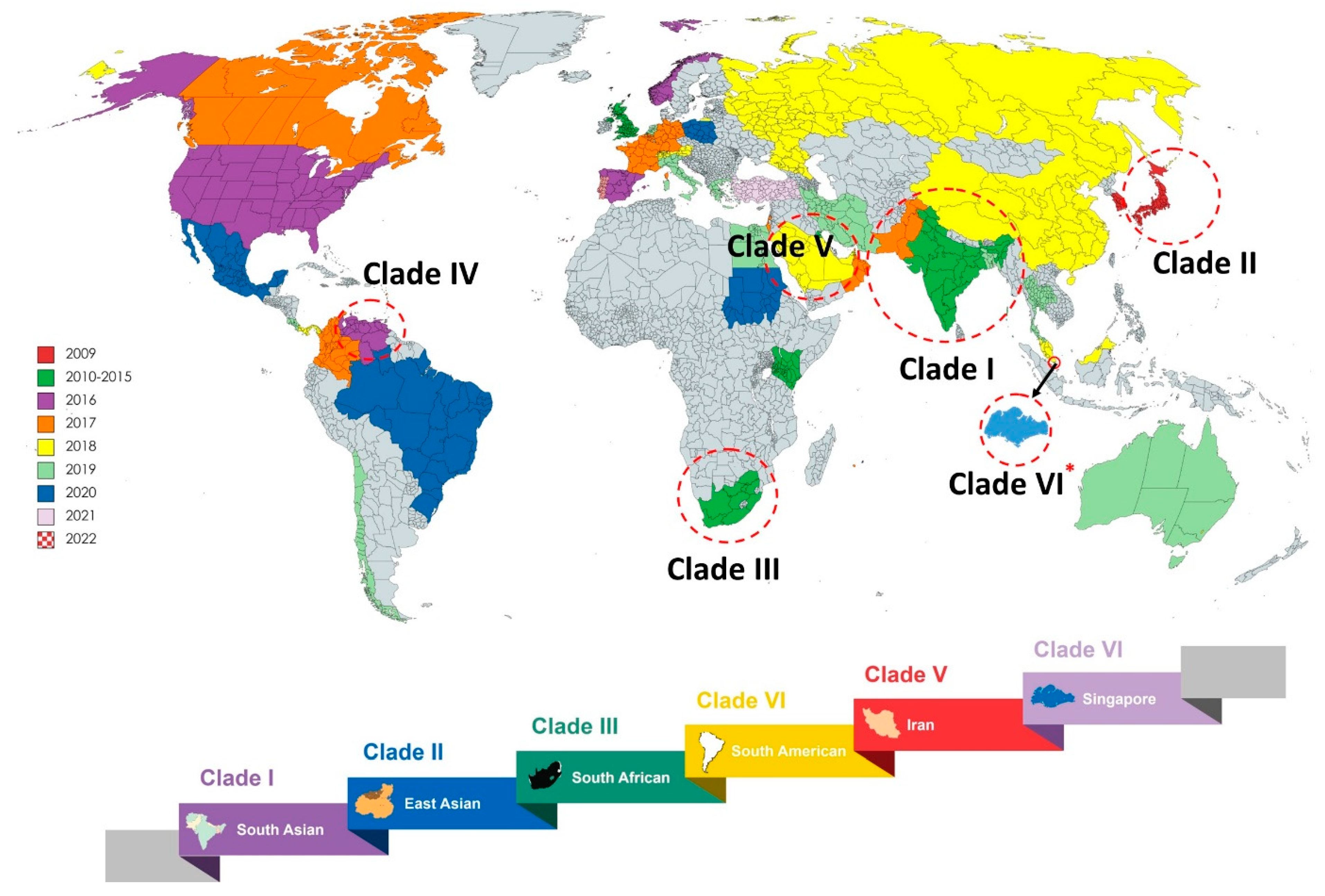
1.1. Candidiasis, Outbreak, and Epidemiology
1.2. Drug Resistance: Molecular Bases
2. Therapeutic Options
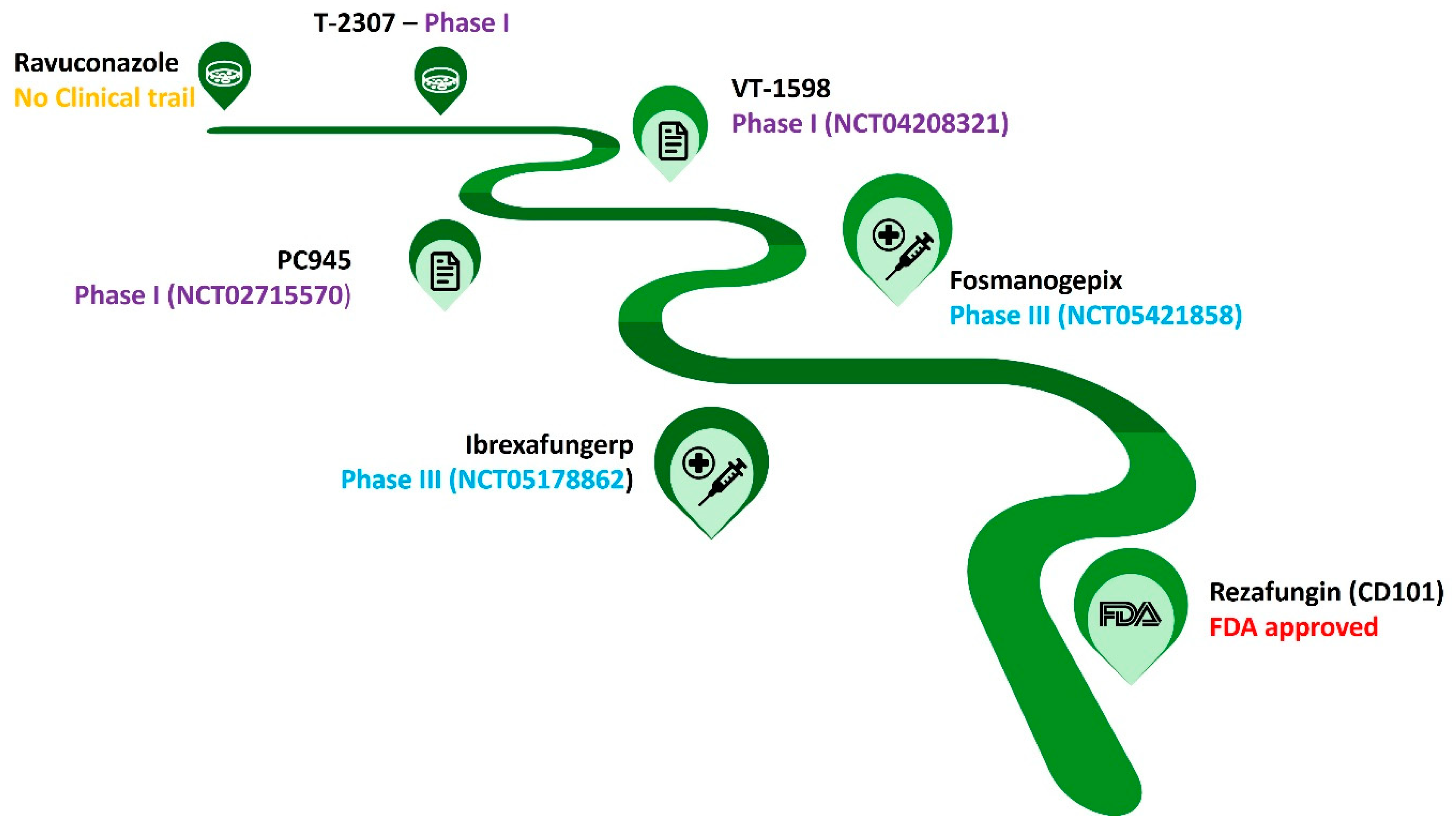
2.1. Current Approved/Considered Antifungal Therapy
2.2. Chemicals as an Emerging Weapon Against C. auris
2.3. Essential Oils Are the Potential Sources of Novel Antifungal Skeletons
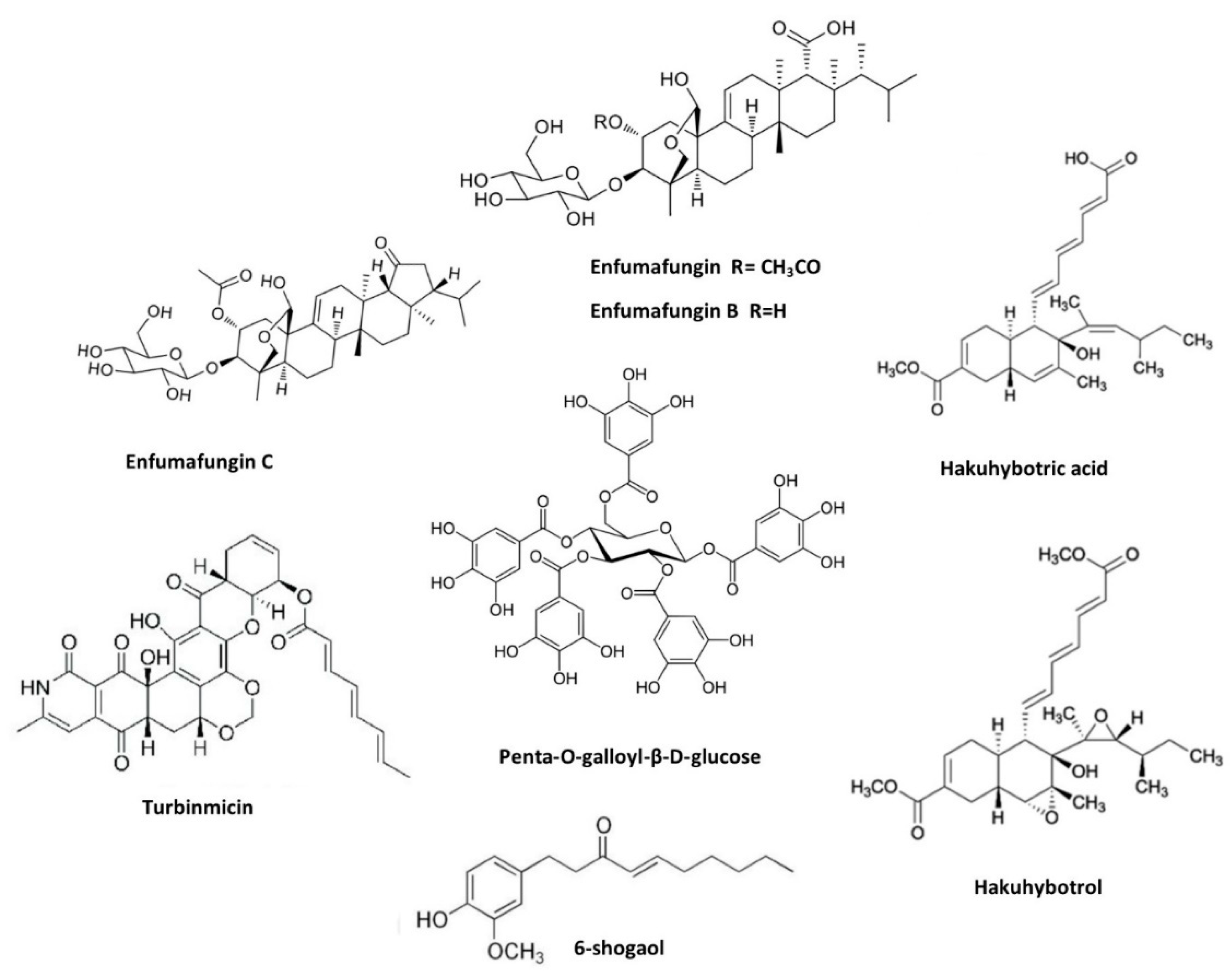
2.4. Natural Products against Candida auris
2.5. Peptide-Based Strategies for Eradicating C. auris
2.6. Antifungal Immune Therapy against C. auris
2.7. Photosensitizers Based Antimicrobial Photodynamic Therapy (APDT)
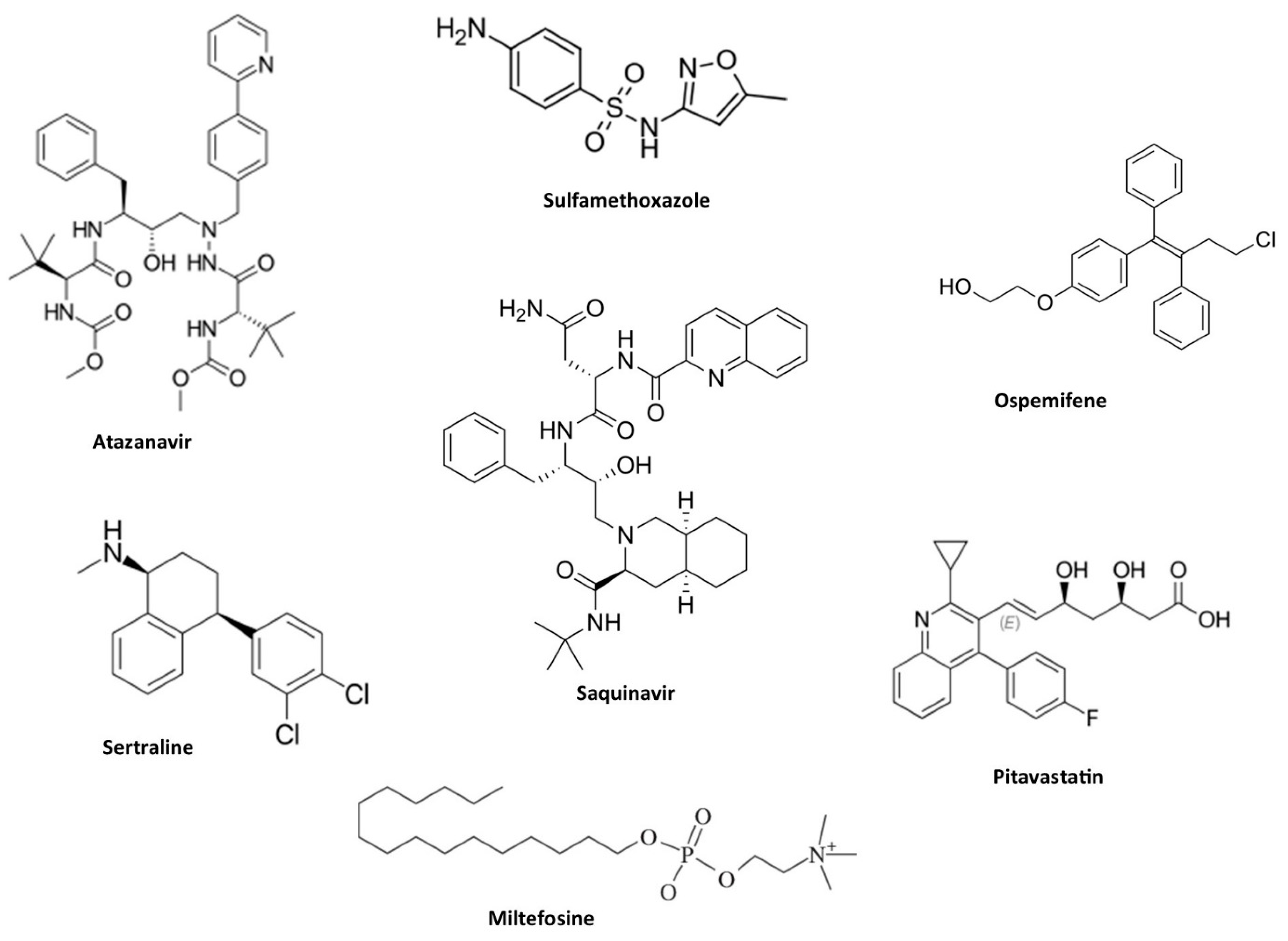
2.8. Repurposing of Drugs with Antifungal Properties
2.9. Nanotechnology Mediated Antifungal Therapy
2.10. Liposomal Technology for Efficient Drug Delivery
3. Conclusion and Future Perspectives
Acknowledgments
References
- Frías-De-león, M.G.; Hernández-Castro, R.; Vite-Garín, T.; Arenas, R.; Bonifaz, A.; Castañón-Olivares, L.; Acosta-Altamirano, G.; Martínez-Herrera, E. Antifungal Resistance in Candida Auris: Molecular Determinants. Antibiotics 2020, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida Auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin Infect Dis 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Yasir, M.; Willcox, M. Candida Auris: An Emerging Antimicrobial-Resistant Organism with the Highest Level of Concern. Lancet Microbe 2023, 4, e482–e483. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Abbasi, A.F.; Prakash, S.; Mangat, J.; Hosein, Z.; Haider, N.; Chan, J. Candida Auris: An Overview of the Emerging Drug-Resistant Fungal Infection. Infect Chemother 2022, 54, 236–246. [Google Scholar] [CrossRef]
- Suphavilai, C.; Ko, K.K.K.; Lim, K.M.; Tan, M.G.; Boonsimma, P.; Chu, J.J.K.; Goh, S.S.; Rajandran, P.; Lee, L.C.; Tan, K.Y.; et al. Discovery of the Sixth Candida Auris Clade in Singapore. medRxiv 2023. [Google Scholar] [CrossRef]
- Adams, E.; Quinn, M.; Tsay, S.; Poirot, E.; Chaturvedi, S.; Southwick, K.; Greenko, J.; Fernandez, R.; Kallen, A.; Vallabhaneni, S.; et al. Candida Auris in Healthcare Facilities, New York, USA, 2013-2017. Emerg Infect Dis 2018, 24, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Abdolrasouli, A.; Armstrong-James, D.; Ryan, L.; Schelenz, S. In Vitro Efficacy of Disinfectants Utilised for Skin Decolonisation and Environmental Decontamination during a Hospital Outbreak with Candida Auris. Mycoses 2017, 60, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Welsh, R.M.; Bentz, M.L.; Shams, A.; Houston, H.; Lyons, A.; Rose, L.J.; Litvintseva, A.P. Survival, Persistence, and Isolation of the Emerging Multidrug-Resistant Pathogenic Yeast Candida Auris on a Plastic Health Care Surface. J Clin Microbiol 2017, 55, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida Auris. Emerg Infect Dis 2017, 23, 328–331. [Google Scholar] [CrossRef]
- Ledwoch, K.; Maillard, J.Y. Candida Auris Dry Surface Biofilm (DSB) for Disinfectant Efficacy Testing. Materials 2018, 12, 18. [Google Scholar] [CrossRef]
- Ruiz-Gaitán, A.; Martínez, H.; Moret, A.M.; Calabuig, E.; Tasias, M.; Alastruey-Izquierdo, A.; Zaragoza, Ó.; Mollar, J.; Frasquet, J.; Salavert-Lletí, M.; et al. Detection and Treatment of Candida Auris in an Outbreak Situation: Risk Factors for Developing Colonization and Candidemia by This New Species in Critically Ill Patients. Expert Rev Anti Infect Ther 2019, 17, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.A.; Ahmad, A. Candida Auris-the Growing Menace to Global Health. Mycoses 2019, 62, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Ademe, M.; Girma, F. Candida Auris: From Multidrug Resistance to Pan-Resistant Strains. Infect Drug Resist 2020, 13, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Sood, P. On the Emergence, Spread and Resistance of Candida Auris: Host, Pathogen and Environmental Tipping Points. J Med Microbiol 2021, 70. [Google Scholar] [CrossRef] [PubMed]
- Lyman, M.; Forsberg, K.; Sexton, D.J.; Chow, N.A.; Lockhart, S.R.; Jackson, B.R.; Chiller, T. Worsening Spread of Candida Auris in the United States, 2019 to 2021. Ann Intern Med 2023, 176, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Almaghrabi, R.S.; Albalawi, R.; Mutabagani, M.; Atienza, E.; Aljumaah, S.; Gade, L.; Forsberg, K.; Litvintseva, A.; Althawadi, S. Molecular Characterisation and Clinical Outcomes of Candida Auris Infection: Single-Centre Experience in Saudi Arabia. Mycoses 2020, 63, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Ahmad, S.; Al-Sweih, N.; Joseph, L.; Alfouzan, W.; Asadzadeh, M. Increasing Prevalence, Molecular Characterization and Antifungal Drug Susceptibility of Serial Candida Auris Isolates in Kuwait. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Al Maani, A.; Paul, H.; Al-Rashdi, A.; Al Wahaibi, A.; Al-Jardani, A.; Al Abri, A.M.A.; Al Balushi, M.A.H.; Al Abri, S.; Al Reesi, M.; Al Maqbali, A.; et al. Ongoing Challenges with Healthcare-Associated Candida Auris Outbreaks in Oman. J Fungi 2019, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida Albicans and Emerging Non- Albicans Candida Species. Front Microbiol, 2017; 7. [Google Scholar] [CrossRef]
- Rybak, J.M.; Muñoz, J.F.; Barker, K.S.; Parker, J.E.; Esquivel, B.D.; Berkow, E.L.; Lockhart, S.R.; Gade, L.; Palmer, G.E.; White, T.C.; et al. Mutations in TAC1B: A Novel Genetic Determinant of Clinical Fluconazole Resistance in Candida Auris. mBio 2020, 11. [Google Scholar] [CrossRef]
- Rybak, J.M.; Barker, K.S.; Muñoz, J.F.; Parker, J.E.; Ahmad, S.; Mokaddas, E.; Abdullah, A.; Elhagracy, R.S.; Kelly, S.L.; Cuomo, C.A.; et al. In Vivo Emergence of High-Level Resistance during Treatment Reveals the First Identified Mechanism of Amphotericin B Resistance in Candida Auris. Clin Microbiol Infect 2022, 28, 838–843. [Google Scholar] [CrossRef]
- Biagi, M.J.; Wiederhold, N.P.; Gibas, C.; Wickes, B.L.; Lozano, V.; Bleasdale, S.C.; Danziger, L. Development of High-Level Echinocandin Resistance in a Patient With Recurrent Candida Auris Candidemia Secondary to Chronic Candiduria. Open Forum Infect Dis 2019, 6. [Google Scholar] [CrossRef]
- Kordalewska, M.; Lee, A.; Park, S.; Berrio, I.; Chowdhary, A.; Zhao, Y.; Perlin, D.S. Understanding Echinocandin Resistance in the Emerging Pathogen Candida Auris. Antimicrob Agents Chemother 2018, 62. [Google Scholar] [CrossRef]
- Rybak, J.M.; Cuomo, C.A.; David Rogers, P. The Molecular and Genetic Basis of Antifungal Resistance in the Emerging Fungal Pathogen Candida Auris. Curr Opin Microbiol 2022, 70. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R. Activity of CD101, a Long-Acting Echinocandin, against Clinical Isolates of Candida Auris. Diagn Microbiol Infect Dis 2018, 90, 196–197. [Google Scholar] [CrossRef]
- Rhodes, J.; Abdolrasouli, A.; Farrer, R.A.; Cuomo, C.A.; Aanensen, D.M.; Armstrong-James, D.; Fisher, M.C.; Schelenz, S. Genomic Epidemiology of the UK Outbreak of the Emerging Human Fungal Pathogen Candida Auris. Emerg Microbes Infect 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pan, J.; Gu, L.; Wang, W.; Wei, B.; Zhang, H.; Chen, J.; Wang, H. Review of Treatment Options for a Multidrug-Resistant Fungus: Candida Auris. Med Mycol 2024, 62. [Google Scholar] [CrossRef] [PubMed]
- Quindós, G.; Miranda-Cadena, K.; San-Millán, R.; Borroto-Esoda, K.; Cantón, E.; Linares-Sicilia, M.J.; Hamprecht, A.; Montesinos, I.; Tortorano, A.M.; Prigitano, A.; et al. In Vitro Antifungal Activity of Ibrexafungerp (SCY-078) Against Contemporary Blood Isolates From Medically Relevant Species of Candida: A European Study. Front Cell Infect Microbiol 2022, 12. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Olivo, M.; Morris, K.N.; Patterson, H.P.; Catano, G.; Patterson, T.F. Ibrexafungerp Demonstrates In Vitro Activity against Fluconazole-Resistant Candida Auris and In Vivo Efficacy with Delayed Initiation of Therapy in an Experimental Model of Invasive Candidiasis. Antimicrob Agents Chemother 2021, 65. [Google Scholar] [CrossRef]
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto-Esoda, K.; et al. The Emerging Pathogen Candida Auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob Agents Chemother 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Berkow, E.L.; Angulo, D.; Lockhart, S.R. In Vitro Activity of a Novel Glucan Synthase Inhibitor, SCY-078, against Clinical Isolates of Candida Auris. Antimicrob Agents Chemother 2017, 61. [Google Scholar] [CrossRef]
- Hodges, M.R.; Ople, E.; Wedel, P.; Shaw, K.J.; Jakate, A.; Kramer, W.G.; van Marle, S.; van Hoogdalem, E.J.; Tawadrous, M. Safety and Pharmacokinetics of Intravenous and Oral Fosmanogepix, a First-in-Class Antifungal Agent, in Healthy Volunteers. Antimicrob Agents Chemother 2023, 67. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Vazquez, J.A.; Oren, I.; Rahav, G.; Aoun, M.; Bulpa, P.; Ben-Ami, R.; Ferrer, R.; Mccarty, T.; Thompson, G.R.; et al. Clinical Safety and Efficacy of Novel Antifungal, Fosmanogepix, for the Treatment of Candidaemia: Results from a Phase 2 Trial. J Antimicrob Chemother 2023, 78, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Vahedi-Shahandashti, R.; Lass-Flörl, C. Novel Antifungal Agents and Their Activity against Aspergillus Species. J Fungi 2020, 6, 213. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P. Review of T-2307, an Investigational Agent That Causes Collapse of Fungal Mitochondrial Membrane Potential. J Fungi 2021, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Santana, D.J.; Anku, J.A.E.; Zhao, G.; Zarnowski, R.; Johnson, C.J.; Hautau, H.; Visser, N.D.; Ibrahim, A.S.; Andes, D.; Nett, J.E.; et al. A Candida Auris-Specific Adhesin, Scf1, Governs Surface Association, Colonization, and Virulence. Science 2023, 381, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Toepfer, S.; Lackner, M.; Keniya, M.V.; Zenz, L.M.; Friemert, M.; Bracher, F.; Monk, B.C. Clorgyline Analogs Synergize with Azoles against Drug Efflux in Candida Auris. J Fungi 2023, 9, 663. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Zhang, H.; Wu, H.; Li, X.; Li, L.; Jiang, Y.; Ni, T. Discovery of Novel Tetrazoles Featuring a Pyrazole Moiety as Potent and Highly Selective Antifungal Agents. ACS Omega 2023, 8, 17103–17115. [Google Scholar] [CrossRef]
- Ni, T.; Chi, X.; Xie, F.; Li, L.; Wu, H.; Hao, Y.; Wang, X.; Zhang, D.; Jiang, Y. Design, Synthesis, and Evaluation of Novel Tetrazoles Featuring Isoxazole Moiety as Highly Selective Antifungal Agents. Eur J Med Chem 2023, 246. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Zhu, T.; Wang, Q.; Yang, W.; Huang, Y.; Xu, D.; Liu, N.; Sheng, C. Discovery of a New Chemical Scaffold for the Treatment of Superbug Candida Auris Infections. Emerg Microbes Infect 2023, 12. [Google Scholar] [CrossRef]
- Li, J.; Coste, A.T.; Bachmann, D.; Sanglard, D.; Lamoth, F. Assessment of the In Vitro and In Vivo Antifungal Activity of NSC319726 against Candida Auris. Microbiol Spectr 2021, 9. [Google Scholar] [CrossRef]
- Lohse, M.B.; Laurie, M.T.; Levan, S.; Ziv, N.; Ennis, C.L.; Nobile, C.J.; DeRisi, J.; Johnson, A.D. Broad Susceptibility of Candida Auris Strains to 8-Hydroxyquinolines and Mechanisms of Resistance. mBio 2023, 14, e0137623. [Google Scholar] [CrossRef]
- Fuchs, F.; Hof, H.; Hofmann, S.; Kurzai, O.; Meis, J.F.; Hamprecht, A. Antifungal Activity of Nitroxoline against Candida Auris Isolates. Clin Microbiol Infect 2021, 27, e7–e1697. [Google Scholar] [CrossRef] [PubMed]
- Maphanga, T.G.; Mpembe, R.S.; Naicker, S.D.; Govender, N.P. In Vitro Antifungal Activity of Manogepix and Other Antifungal Agents against South African Candida Auris Isolates from Bloodstream Infections. Microbiol Spectr 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Kilburn, S.; Kapoor, M.; Chaturvedi, S.; Shaw, K.J.; Chaturvedi, V. In Vitro Activity of Manogepix against Multidrug-Resistant and Panresistant Candida Auris from the New York Outbreak. Antimicrob Agents Chemother 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- John, L.L.H.; Thomson, D.D.; Bicanic, T.; Hoenigl, M.; Brown, A.J.P.; Harrison, T.S.; Bignell, E.M. Heightened Efficacy of Anidulafungin When Used in Combination with Manogepix or 5-Flucytosine against Candida Auris In Vitro. Antimicrob Agents Chemother 2023, 67. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.K.; Kim, J.H.; Parkin, S.; Awuah, S.G.; Garneau-Tsodikova, S. Distorted Gold(I)-Phosphine Complexes as Antifungal Agents. J Med Chem 2020, 63, 2455–2469. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, H.; Eldesouky, H.E.; Hazbun, T.; Mayhoub, A.S.; Seleem, M.N. Identification of a Phenylthiazole Small Molecule with Dual Antifungal and Antibiofilm Activity Against Candida Albicans and Candida Auris. Sci Rep 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.M.; Rovig, J.; Holden, B.S.; Taylor, M.F.; Weber, S.; Wilson, J.; Hilton, B.; Zaugg, A.L.; Ellis, S.W.; Yost, C.D.; et al. Ceragenins Are Active against Drug-Resistant Candida Auris Clinical Isolates in Planktonic and Biofilm Forms. J Antimicrob Chemother 2018, 73, 1537–1545. [Google Scholar] [CrossRef]
- Parker, R.A.; Gabriel, K.T.; Graham, K.D.; Butts, B.K.; Cornelison, C.T. Antifungal Activity of Select Essential Oils against Candida Auris and Their Interactions with Antifungal Drugs. Pathogens 2022, 11, 821. [Google Scholar] [CrossRef]
- Balkrishna, A.; Kharayat, B.; Rastogi, S.; Kabdwal, M.; Haldar, S.; Varshney, A. Withania Somnifera Seed Oil Exhibits Antibiofilm Properties against Drug-Resistant Candida Auris Clinical Isolate through Modulation in Cell Permeability. J Appl Microbiol 2023, 134. [Google Scholar] [CrossRef]
- Di Vito, M.; Garzoli, S.; Rosato, R.; Mariotti, M.; Gervasoni, J.; Santucci, L.; Ovidi, E.; Cacaci, M.; Lombarini, G.; Torelli, R.; et al. A New Potential Resource in the Fight against Candida Auris: The Cinnamomum Zeylanicum Essential Oil in Synergy with Antifungal Drug. Microbiol Spectr 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Rosato, R.; Napoli, E.; Granata, G.; Di Vito, M.; Garzoli, S.; Geraci, C.; Rizzo, S.; Torelli, R.; Sanguinetti, M.; Bugli, F. Study of the Chemical Profile and Anti-Fungal Activity against Candida Auris of Cinnamomum Cassia Essential Oil and of Its Nano-Formulations Based on Polycaprolactone. Plants 2023, 12, 358. [Google Scholar] [CrossRef] [PubMed]
- Shreaz, S.; Wani, W.A.; Behbehani, J.M.; Raja, V.; Irshad, M.; Karched, M.; Ali, I.; Siddiqi, W.A.; Hun, L.T. Cinnamaldehyde and Its Derivatives, a Novel Class of Antifungal Agents. Fitoterapia 2016, 112, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.A.; Stephens, J.C. A Review of Cinnamaldehyde and Its Derivatives as Antibacterial Agents. Fitoterapia 2019, 139. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and Human Health: A Comprehensive Review. Phytother Res 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Srivastava, V.; Marimani, M.; Ahmad, A. Carvacrol Modulates the Expression and Activity of Antioxidant Enzymes in Candida Auris. Res Microbiol 2022, 173. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Fatima, Z.; Hameed, S. Abrogation of Efflux Pump Activity, Biofilm Formation, and Immune Escape by Candidacidal Geraniol in Emerging Superbug, Candida Auris. Int Microbiol 2023, 26, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Marquez, L.; Lee, Y.; Duncan, D.; Whitesell, L.; Cowen, L.E.; Quave, C. Potent Antifungal Activity of Penta-O-Galloyl-β-d-Glucose against Drug-Resistant Candida Albicans, Candida Auris, and Other Non-Albicans Candida Species. ACS Infect Dis 2023, 9, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Ok, S.; Jeong, W.S. Optimization of Extraction Conditions for the 6-Shogaol-Rich Extract from Ginger (Zingiber Officinale Roscoe). Prev Nutr Food Sci 2012, 17, 166–171. [Google Scholar] [CrossRef]
- Kim, H.R.; Eom, Y.B. Antifungal and Anti-Biofilm Effects of 6-Shogaol against Candida Auris. J Appl Microbiol 2021, 130, 1142–1153. [Google Scholar] [CrossRef]
- Zeng, H.; Stadler, M.; Abraham, W.R.; Müsken, M.; Schrey, H. Inhibitory Effects of the Fungal Pigment Rubiginosin C on Hyphal and Biofilm Formation in Candida Albicans and Candida Auris. J Fungi (Basel) 2023, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wu, W.; Liu, Y.; Chen, S.; Li, H.; Yang, X.; Zhu, X.; Chen, X.; Yan, L.; Chu, Z.; et al. Natural Enfumafungin Analogues from Hormonema Carpetanum and Their Antifungal Activities. J Nat Prod 2023, 86, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Haldar, S.; King, J.B.; Mattes, A.O.; Srivastava, S.; Wendt, K.L.; You, J.; Cunningham, C.; Cichewicz, R.H. Persephacin Is a Broad-Spectrum Antifungal Aureobasidin Metabolite That Overcomes Intrinsic Resistance in Aspergillus Fumigatus. J Nat Prod 2023, 86, 1980–1993. [Google Scholar] [CrossRef] [PubMed]
- Jagels, A.; Adpressa, D.A.; Kaweesa, E.N.; McCauley, M.; Philmus, B.; Strother, J.A.; Loesgen, S. Metabolomics-Guided Discovery, Isolation, Structure Elucidation, and Bioactivity of Myropeptins C-E from Myrothecium Inundatum. J Nat Prod 2023, 86, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Takahashi, S.; Ito, S.; Tokiwa, T.; Noguchi, Y.; Azami, H.; Kojima, H.; Higo, M.; Ban, S.; Nagai, K.; et al. Hakuhybotrol, a Polyketide Produced by Hypomyces Pseudocorticiicola, Characterized with the Assistance of 3D ED/MicroED. Org Biomol Chem 2023, 21, 2320–2330. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, R.D.; de Barros, P.P.; Mendonça, I. do C.; Medina, R.P.; Silva, D.H.S.; Fuchs, B.B.; Junqueira, J.C.; Mylonakis, E. The Postbiotic Activity of Lactobacillus Paracasei 28.4 Against Candida Auris. Front Cell Infect Microbiol 2020, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, M.; Braun, D.R.; Ericksen, S.S.; Piotrowski, J.S.; Nelson, J.; Peng, J.; Ananiev, G.E.; Chanana, S.; Barns, K.; et al. A Marine Microbiome Antifungal Targets Urgent-Threat Drug-Resistant Fungi. Science 2020, 370, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, F.; Zarnowski, R.; Barns, K.; Jones, R.; Fossen, J.; Sanchez, H.; Rajski, S.R.; Audhya, A.; Bugni, T.S.; et al. Turbinmicin Inhibits Candida Biofilm Growth by Disrupting Fungal Vesicle-Mediated Trafficking. J Clin Invest 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Peptide Database https://aps.unmc.edu/ (accessed May 8, 2024).
- Perez-Rodriguez, A.; Eraso, E.; Quindós, G.; Mateo, E. Antimicrobial Peptides with Anti- Candida Activity. Int J Mol Sci 2022, 23, 9264. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.; Patel, M.; Ahmad, A. Fungicidal Activity of Human Antimicrobial Peptides and Their Synergistic Interaction with Common Antifungals against Multidrug-Resistant Candida Auris. Int Microbiol 2023, 26, 165–177. [Google Scholar] [CrossRef]
- Rather, I.A.; Sabir, J.S.M.; Asseri, A.H.; Ali, S. Antifungal Activity of Human Cathelicidin LL-37, a Membrane Disrupting Peptide, by Triggering Oxidative Stress and Cell Cycle Arrest in Candida Auris. J Fungi 2022, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, R.U.; Friedman, J.; Norris, H.L.; Salvatori, O.; McCall, A.D.; Kay, J.; Edgerton, M. Fluconazole-Resistant Candida Auris Is Susceptible to Salivary Histatin 5 Killing and to Intrinsic Host Defenses. Antimicrob Agents Chemother 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Kamli, M.R.; Sabir, J.S.M.; Malik, M.A.; Ahmad, A. Characterization of Defensin-like Protein 1 for Its Anti-Biofilm and Anti-Virulence Properties for the Development of Novel Antifungal Drug against Candida Auris. J Fungi 2022, 8, 1298. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Madduri, M.; Rudramurthy, S.M.; Roy, U. Functional Characterization of a Bacillus-Derived Novel Broad-Spectrum Antifungal Lipopeptide Variant against Candida Tropicalis and Candida Auris and Unravelling Its Mode of Action. Microbiol Spectr 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Dal Mas, C.; Rossato, L.; Shimizu, T.; Oliveira, E.B.; Da Silva Junior, P.I.; Meis, J.F.; Hayashi, M.A.F.; Colombo, A.L. Effects of the Natural Peptide Crotamine from a South American Rattlesnake on Candida Auris, an Emergent Multidrug Antifungal Resistant Human Pathogen. Biomolecules 2019, 9, 205. [Google Scholar] [CrossRef]
- Raber, H.F.; Sejfijaj, J.; Kissmann, A.K.; Wittgens, A.; Gonzalez-Garcia, M.; Alba, A.; Vázquez, A.A.; Vicente, F.E.M.; Erviti, J.P.; Kubiczek, D.; et al. Antimicrobial Peptides Pom-1 and Pom-2 from Pomacea Poeyana Are Active against Candidaauris, C. Parapsilosis and C. Albicans Biofilms. Pathogens 2021, 10. [Google Scholar] [CrossRef]
- Pinheiro, T.K.S.; Nicola, A.M.; Bentes, D.B.; Zhang, S.X.; Felipe, M.S.S.; Silva-Pereira, I.; Albuquerque, P. The Antimicrobial Peptide ToAP2 Is Synergic with Caspofungin and Amphotericin B against Candida Auris; 2023.
- dos Reis, T.F.; de Castro, P.A.; Bastos, R.W.; Pinzan, C.F.; Souza, P.F.N.; Ackloo, S.; Hossain, M.A.; Drewry, D.H.; Alkhazraji, S.; Ibrahim, A.S.; et al. A Host Defense Peptide Mimetic, Brilacidin, Potentiates Caspofungin Antifungal Activity against Human Pathogenic Fungi. Nat Commun 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Bugli, F.; Massaro, F.; Buonocore, F.; Saraceni, P.R.; Borocci, S.; Ceccacci, F.; Bombelli, C.; Di Vito, M.; Marchitiello, R.; Mariotti, M.; et al. Design and Characterization of Myristoylated and Non-Myristoylated Peptides Effective against Candida Spp. Clinical Isolates. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Szerencsés, B.; Gácser, A.; Endre, G.; Domonkos, I.; Tiricz, H.; Vágvölgyi, C.; Szolomajer, J.; Howan, D.H.O.; Tóth, G.K.; Pfeiffer, I.; et al. Symbiotic NCR Peptide Fragments Affect the Viability, Morphology and Biofilm Formation of Candida Species. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Vicente, F.E.M.; González-Garcia, M.; Diaz Pico, E.; Moreno-Castillo, E.; Garay, H.E.; Rosi, P.E.; Jimenez, A.M.; Campos-Delgado, J.A.; Rivera, D.G.; Chinea, G.; et al. Design of a Helical-Stabilized, Cyclic, and Nontoxic Analogue of the Peptide Cm-P5 with Improved Antifungal Activity. ACS Omega 2019, 4, 19081–19095. [Google Scholar] [CrossRef]
- Basso, V.; Garcia, A.; Tran, D.Q.; Schaal, J.B.; Tran, P.; Ngole, D.; Aqeel, Y.; Tongaonkar, P.; Ouellette, A.J.; Selsteda, M.E. Fungicidal Potency and Mechanisms of θ-Defensins against Multidrug-Resistant Candida Species. Antimicrob Agents Chemother 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Tóth, L.; Kele, Z.; Borics, A.; Nagy, L.G.; Váradi, G.; Virágh, M.; Takó, M.; Vágvölgyi, C.; Galgóczy, L. NFAP2, a Novel Cysteine-Rich Anti-Yeast Protein from Neosartorya Fischeri NRRL 181: Isolation and Characterization. AMB Express 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Kovács, L.; Virágh, M.; Takó, M.; Papp, T.; Vágvölgyi, C.; Galgóczy, L. Isolation and Characterization of Neosartorya Fischeri Antifungal Protein (NFAP). Peptides (N.Y.) 2011, 32, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Kovács, R.; Holzknecht, J.; Hargitai, Z.; Papp, C.; Farkas, A.; Borics, A.; Tóth, L.; Váradi, G.; Tóth, G.K.; Kovács, I.; et al. In Vivo Applicability of Neosartorya Fischeri Antifungal Protein 2 (NFAP2) in Treatment of Vulvovaginal Candidiasis. Antimicrob Agents Chemother 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Kovács, R.; Nagy, F.; Tóth, Z.; Forgács, L.; Tóth, L.; Váradi, G.; Tóth, G.K.; Vadászi, K.; Borman, A.M.; Majoros, L.; et al. The Neosartorya Fischeri Antifungal Protein 2 (NFAP2): A New Potential Weapon against Multidrug-Resistant Candida Auris Biofilms. Int J Mol Sci 2021, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Jin, C.; Fukushima, H.; Makihira, S.; Hamada, T. Antifungal Activity of Histatin-5 against Non-Albicans Candida Species. Oral Microbiol Immunol 2001, 16, 250–252. [Google Scholar] [CrossRef]
- Peters, B.M.; Zhu, J.; Fidel, P.L.; Scheper, M.A.; Hackett, W.; El Shaye, S.; Jabra-Rizk, M.A. Protection of the Oral Mucosa by Salivary Histatin-5 against Candida Albicans in an Ex Vivo Murine Model of Oral Infection. FEMS Yeast Res 2010, 10, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liu, S.; Wang, H.; Su, H.; Liu, Z. Efficacy of Histatin5 in a Murine Model of Vulvovaginal Candidiasis Caused by Candida Albicans. Pathog Dis 2017, 75. [Google Scholar] [CrossRef]
- Aerts, A.M.; Bammens, L.; Govaert, G.; Carmona-Gutierrez, D.; Madeo, F.; Cammue, B.P.A.; Thevissen, K. The Antifungal Plant Defensin HsAFP1 from Heuchera Sanguinea Induces Apoptosis in Candida Albicans. Front Microbiol, 2011; 2. [Google Scholar] [CrossRef]
- Hayes, B.M.E.; Bleackley, M.R.; Wiltshire, J.L.; Anderson, M.A.; Traven, A.; Van Der Weerden, N.L. Identification and Mechanism of Action of the Plant Defensin NaD1 as a New Member of the Antifungal Drug Arsenal against Candida Albicans. Antimicrob Agents Chemother 2013, 57, 3667–3675. [Google Scholar] [CrossRef]
- Gonçalves, S.; Silva, P.M.; Felício, M.R.; de Medeiros, L.N.; Kurtenbach, E.; Santos, N.C. Ps D1 Effects on Candida Albicans Planktonic Cells and Biofilms. Front Cell Infect Microbiol, 2017; 7. [Google Scholar] [CrossRef]
- García, M.G.; Rodríguez, A.; Alba, A.; Vázquez, A.A.; Vicente, F.E.M.; Pérez-Erviti, J.; Spellerberg, B.; Stenger, S.; Grieshober, M.; Conzelmann, C.; et al. New Antibacterial Peptides from the Freshwater Mollusk Pomacea Poeyana (Pilsbry, 1927). Biomolecules 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Dekkerová, J.; Lopez-Ribot, J.L.; Bujdáková, H. Activity of Anti-CR3-RP Polyclonal Antibody against Biofilms Formed by Candida Auris, a Multidrug-Resistant Emerging Fungal Pathogen. Eur J Clin Microbiol Infect Dis 2019, 38, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Barbarino, A.; Youssef, E.G.; Coleman, D.; Gebremariam, T.; Ibrahim, A.S. Protective Efficacy of Anti-Hyr1p Monoclonal Antibody against Systemic Candidiasis Due to Multi-Drug-Resistant Candida Auris. J Fungi (Basel) 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Uppuluri, P.; Mamouei, Z.; Alqarihi, A.; Elhassan, H.; French, S.; Lockhart, S.R.; Chiller, T.; Edwards, J.E.; Ibrahim, A.S. The NDV-3A Vaccine Protects Mice from Multidrug Resistant Candida Auris Infection. PLoS Pathog 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Di Mambro, T.; Vanzolini, T.; Bruscolini, P.; Perez-Gaviro, S.; Marra, E.; Roscilli, G.; Bianchi, M.; Fraternale, A.; Schiavano, G.F.; Canonico, B.; et al. A New Humanized Antibody Is Effective against Pathogenic Fungi in Vitro. Sci Rep 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Vanzolini, T.; Di Mambro, T.; Magnani, M.; Menotta, M. AFM Evaluation of a Humanized Recombinant Antibody Affecting C. Auris Cell Wall and Stability. RSC Adv 2023, 13, 6130–6142. [Google Scholar] [CrossRef] [PubMed]
- Rosario-colon, J.; Eberle, K.; Adams, A.; Courville, E.; Xin, H. Candida Cell-Surface-Specific Monoclonal Antibodies Protect Mice against Candida Auris Invasive Infection. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nathan, D.; Lustig, S.; Tam, G.; Robinzon, S.; Segal, S.; Rager-Zisman, B. Prophylactic and Therapeutic Efficacy of Human Intravenous Immunoglobulin in Treating West Nile Virus Infection in Mice. J Infect Dis 2003, 188, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Bayry, J.; Lacroix-Desmazes, S.; Kazatchkine, M.D.; Kaveri, S.V. Intravenous Immunoglobulin for Infectious Diseases: Back to the Pre-Antibiotic and Passive Prophylaxis Era? Trends Pharmacol Sci 2004, 25, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Le, V.T.M.; Badiou, C.; Le, H.N.; Pinheiro, M.G.; Duong, A.H.; Wang, X.; Dip, E.C.; Aguiar-Alves, F.; Basuino, L.; et al. IVIG-Mediated Protection against Necrotizing Pneumonia Caused by MRSA. Sci Transl Med 2016, 8. [Google Scholar] [CrossRef]
- Xin, H.; Rosario-Colon, J.A.; Eberle, K. Novel Intravenous Immunoglobulin Therapy for the Prevention and Treatment of Candida Auris and Candida Albicans Disseminated Candidiasis. mSphere 2023, 8. [Google Scholar] [CrossRef]
- Khan, T.; Suleman, M.; Ali, S.S.; Sarwar, M.F.; Ali, I.; Ali, L.; Khan, A.; Rokhan, B.; Wang, Y.; Zhao, R.; et al. Subtractive Proteomics Assisted Therapeutic Targets Mining and Designing Ensemble Vaccine against Candida Auris for Immune Response Induction. Comput Biol Med 2022, 145. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Osmanoglu, Ö.; Minocha, R.; Bandi, S.R.; Bencurova, E.; Srivastava, M.; Dandekar, T. Genome-Wide Scan for Potential CD4+ T-Cell Vaccine Candidates in Candida Auris by Exploiting Reverse Vaccinology and Evolutionary Information. Front Med (Lausanne) 2022, 9. [Google Scholar] [CrossRef]
- Grizante Barião, P.H.; Tonani, L.; Brancini, G.T.P.; Nascimento, E.; Braga, G.Ú.L.; Wainwright, M.; von Zeska Kress, M.R. In Vitro and in Vivo Photodynamic Efficacies of Novel and Conventional Phenothiazinium Photosensitizers against Multidrug-Resistant Candida Auris. Photochem Photobiol Sci 2022, 21, 1807–1818. [Google Scholar] [CrossRef]
- Rodrigues, G.B.; Dias-Baruffi, M.; Holman, N.; Wainwright, M.; Braga, G.U.L. In Vitro Photodynamic Inactivation of Candida Species and Mouse Fibroblasts with Phenothiazinium Photosensitisers and Red Light. Photodiagnosis Photodyn Ther 2013, 10, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bapat, P.S.; Nobile, C.J. Photodynamic Therapy Is Effective Against Candida Auris Biofilms. Front Cell Infect Microbiol 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Cabral, F.V.; Silva, C.R.; Silva, D.F.T.; Freitas, A.Z.; Fontes, A.; Ribeiro, M.S. New Insights in Phenothiazinium-Mediated Photodynamic Inactivation of Candida Auris. J Fungi (Basel) 2023, 9. [Google Scholar] [CrossRef]
- Štefánek, M.; Černáková, L.; Dekkerová, J.; Bujdáková, H. Photodynamic Inactivation Effectively Eradicates Candida Auris Biofilm despite Its Interference with the Upregulation of CDR1 and MDR1 Efflux Genes. J Fungi (Basel) 2022, 8. [Google Scholar] [CrossRef]
- Capoci, I.R.G.; Faria, D.R.; Sakita, K.M.; Rodrigues-Vendramini, F.A.V.; Bonfim-Mendonça, P. de S.; Becker, T.C.A.; Kioshima, É.S.; Svidzinski, T.I.E.; Maigret, B. Repurposing Approach Identifies New Treatment Options for Invasive Fungal Disease. Bioorg Chem 2019, 84, 87–97. [Google Scholar] [CrossRef]
- Kim, J.H.; Cheng, L.W.; Chan, K.L.; Tam, C.C.; Mahoney, N.; Friedman, M.; Shilman, M.M.; Land, K.M. Antifungal Drug Repurposing. Antibiotics (Basel) 2020, 9, 1–29. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat Rev Drug Discov 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Gowri, M.; Jayashree, B.; Jeyakanthan, J.; Girija, E.K. Sertraline as a Promising Antifungal Agent: Inhibition of Growth and Biofilm of Candida Auris with Special Focus on the Mechanism of Action in Vitro. J Appl Microbiol 2020, 128, 426–437. [Google Scholar] [CrossRef]
- Talevi, A.; Bellera, C.L. Challenges and Opportunities with Drug Repurposing: Finding Strategies to Find Alternative Uses of Therapeutics. Expert Opin Drug Discov 2020, 15, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Eldesouky, H.E.; Salama, E.A.; Li, X.; Hazbun, T.R.; Mayhoub, A.S.; Seleem, M.N. Repurposing Approach Identifies Pitavastatin as a Potent Azole Chemosensitizing Agent Effective against Azole-Resistant Candida Species. Sci Rep 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Eldesouky, H.E.; Lanman, N.A.; Hazbun, T.R.; Seleem, M.N. Aprepitant, an Antiemetic Agent, Interferes with Metal Ion Homeostasis of Candida Auris and Displays Potent Synergistic Interactions with Azole Drugs. Virulence 2020, 11, 1466–1481. [Google Scholar] [CrossRef] [PubMed]
- Barreto, T.L.; Rossato, L.; de Freitas, A.L.D.; Meis, J.F.; Lopes, L.B.; Colombo, A.L.; Ishida, K. Miltefosine as an Alternative Strategy in the Treatment of the Emerging Fungus Candida Auris. Int J Antimicrob Agents 2020, 56. [Google Scholar] [CrossRef] [PubMed]
- Aghaei Gharehbolagh, S.; Izadi, A.; Talebi, M.; Sadeghi, F.; Zarrinnia, A.; Zarei, F.; Darmiani, K.; Borman, A.M.; Mahmoudi, S. New Weapons to Fight a New Enemy: A Systematic Review of Drug Combinations against the Drug-Resistant Fungus Candida Auris. Mycoses 2021, 64, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Bidaud, A.L.; Djenontin, E.; Botterel, F.; Chowdhary, A.; Dannaoui, E. Colistin Interacts Synergistically with Echinocandins against Candida Auris. Int J Antimicrob Agents 2020, 55. [Google Scholar] [CrossRef] [PubMed]
- Eldesouky, H.E.; Salama, E.A.; Lanman, N.A.; Hazbun, T.R.; Seleem, M.N. Potent Synergistic Interactions between Lopinavir and Azole Antifungal Drugs against Emerging Multidrug-Resistant Candida Auris. Antimicrob Agents Chemother 2020, 65. [Google Scholar] [CrossRef]
- Salama, E.A.; Eldesouky, H.E.; Elgammal, Y.; Abutaleb, N.S.; Seleem, M.N. Lopinavir and Ritonavir Act Synergistically with Azoles against Candida Auris in Vitro and in a Mouse Model of Disseminated Candidiasis. Int J Antimicrob Agents 2023, 62. [Google Scholar] [CrossRef]
- Elgammal, Y.; Salama, E.A.; Seleem, M.N. Atazanavir Resensitizes Candida Auris to Azoles. Antimicrob Agents Chemother 2023, 67. [Google Scholar] [CrossRef]
- Elgammal, Y.; Salama, E.A.; Seleem, M.N. Saquinavir Potentiates Itraconazole’s Antifungal Activity against Multidrug-Resistant Candida Auris in Vitro Andin Vivo. Med Mycol 2023, 61. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Magnasco, L.; Sepulcri, C.; Mikulska, M.; Koehler, P.; Cornely, O.A.; Bassetti, M. Recent Advances and Future Perspectives in the Pharmacological Treatment of Candida Auris Infections. Expert Rev Clin Pharmacol 2021, 14, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Wang, Y.; Xi, Y.; Yang, Z.; Zhang, H.; Ge, X. Activity of Chlorhexidine Acetate in Combination with Fluconazole against Suspensions and Biofilms of Candida Auris. J Infect Chemother 2022, 28, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ixtepan-Turrent, L.; Jose Yacaman, M.; Lopez-Ribot, J. Inhibition of Candida Auris Biofilm Formation on Medical and Environmental Surfaces by Silver Nanoparticles. ACS Appl Mater Interfaces 2020, 12, 21183–21191. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Lopez, F.D.; Lopez-Ribot, J.L. Silver Nanoantibiotics Display Strong Antifungal Activity Against the Emergent Multidrug-Resistant Yeast Candida Auris Under Both Planktonic and Biofilm Growing Conditions. Front Microbiol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- AlJindan, R.; AlEraky, D.M. Silver Nanoparticles: A Promising Antifungal Agent against the Growth and Biofilm Formation of the Emergent Candida Auris. J Fungi (Basel) 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Batterjee, M.G.; Kamli, M.R.; Alzahrani, K.A.; Danish, E.Y.; Nabi, A. Polyphenol-Capped Biogenic Synthesis of Noble Metallic Silver Nanoparticles for Antifungal Activity against Candida Auris. J Fungi (Basel) 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Kamli, M.R.; Srivastava, V.; Hajrah, N.H.; Sabir, J.S.M.; Hakeem, K.R.; Ahmad, A.; Malik, M.A. Facile Bio-Fabrication of Ag-Cu-Co Trimetallic Nanoparticles and Its Fungicidal Activity against Candida Auris. J Fungi (Basel) 2021, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Munoz, R.; Lopez, F.D.; Lopez-Ribot, J.L. Bismuth Nanoantibiotics Display Anticandidal Activity and Disrupt the Biofilm and Cell Morphology of the Emergent Pathogenic Yeast Candida Auris. Antibiotics (Basel) 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Fayed, B.; Jayakumar, M.N.; Soliman, S.S.M. Caspofungin-Resistance in Candida Auris Is Cell Wall-Dependent Phenotype and Potential Prevention by Zinc Oxide Nanoparticles. Med Mycol 2021, 59, 1243–1256. [Google Scholar] [CrossRef]
- de Alteriis, E.; Maione, A.; Falanga, A.; Bellavita, R.; Galdiero, S.; Albarano, L.; Salvatore, M.M.; Galdiero, E.; Guida, M. Activity of Free and Liposome-Encapsulated Essential Oil from Lavandula Angustifolia against Persister-Derived Biofilm of Candida Auris. Antibiotics (Basel) 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Baldim, I.; Paziani, M.H.; Grizante Barião, P.H.; von Zeska Kress, M.R.; Oliveira, W.P. Nanostructured Lipid Carriers Loaded with Lippia Sidoides Essential Oil as a Strategy to Combat the Multidrug-Resistant Candida Auris. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Jaromin, A.; Zarnowski, R.; Markowski, A.; Zagórska, A.; Johnson, C.J.; Etezadi, H.; Kihara, S.; Mota-Santiago, P.; Nett, J.E.; Boyd, B.J.; et al. Liposomal Formulation of a New Antifungal Hybrid Compound Provides Protection against Candida Auris in the Ex Vivo Skin Colonization Model. Antimicrob Agents Chemother 2024, 68. [Google Scholar] [CrossRef] [PubMed]
| Group | Compound | MIC/MIC range (µg/ml) | Reference |
|---|---|---|---|
| Clorgyline analogs |
M19 | 86.7 - 98.3 µM | Toepfer et al., 2023 [37] |
| M25 | 137 - 228 µM | ||
| Novel Tetrazoles Featuring a Pyrazole Moiety |
8, 11, 15, 24, 25 | < 0.0625 – 4, 0.125 – 8, < 0.0625 – 2, 0.5 – 64, < 0.0625 - 16 | Chi et al., 2023 [38] |
| Novel tetrazoles featuring isoxazole moiety | 10d, 10h, 13r, 13u | 0.008, < 0.008, 0.0313, 0.0313 | Ni et al., 2023 [39] |
| Benzoanilide group | Compound A1 | 0.5 - 2.0 | Tu et al., 2023 [40] |
| Piperidine based 1,2,3-triazolylacetamide derivatives | pta1, pta2, pta3, pta4, pta5, pta6 | 0.48 - 0.97, 0.24-0.48, 0.12-0.24, >250, >250, >250 | Srivastava et al., 2020 [41] |
| Pyrrolidine-based 1,2,3-triazole | P1 – P10 | 0.97 - 62.5 | Wani et al., 2023 [42] |
| Nitroxoline | 0.125 to 1 | Fuchs et al., 2021[43] | |
| NSC319726 is a thiosemicarbazone | 0.125 to 0.25 | Li et al., 2021 [44] | |
| Manogepix |
FluR | 0.002 to 0.063 | Maphanga et al., 2022 [45] |
| Flu and Amp BR | 0.004 to 0.031 | ||
| PanR | 0.004 μg/mL and 0.008 | ||
| Manogepix | PanR - New York | 0.008 to 0.015 | Zhu et al., 2020 [46] |
| Gold(I)−Phosphine Complex 4 | 3.9 to 7.8 | Dennis et al., 2019 [47] |
|
| Gold(I)−Phosphine Complex 6 | 1.95 | ||
| Phenylthiazole - Compound 1 | 0.25–2 | Mohammad et al., 2019[48] | |
| Ceragenins |
CSA-44, CSA-131, CSA-142, CSA-144 | 0.5 – 1, 0.5 – 1, 2 to 8, 0.5 - 2 | Hashemi et al., 2018 [49] |
| Species | Molecule | MIC range (µg/ml) | Ref |
|---|---|---|---|
| Turbinmicin-producing bacterium Micromonospora sp. WMMC-415 | Turbinmicin | 0.125-0.50 | Zhang et al., 2020 [56] |
| Hypoxylon rubiginosum and Hypoxylon texense | Penta-O-galloyl-β-D-Glucose | 1-8 | Marquez et al., 2023 [57] |
|
Hormonema carpetanum |
Enfumafungin | 64 | Cheng et al., 2023 [58] |
| Enfumafungin B | > 64 | ||
| Enfumafungin C | > 64 | ||
| Sphaceloma sp from the leaf of Poplar sp | Persephacin | 2.5 | Du et al., 2023 [59] |
|
Myrothecium inundatum |
Myropeptin C | 16 | Jagels et al., 2023 [60] |
| Myropeptin D | 16 | ||
| Myropeptin E | 16 | ||
| Myropeptin A1 | 4 | ||
|
Hypomyces pseudocorticiicola FKA-73 |
Hakuhybotrol | >128 | Watanabe et al., 2023[61] |
| Cladobotric acids F | >128 | ||
| Pyrenulic acid A | 16 | ||
| F2928-1 | 2 | ||
| Cladobotric acids E | 2 to 4 | ||
| Cladobotric acids H | 16 to 32 | ||
| Cladobotric acids A | 4 to 8 | ||
| Lactobacillus paracasei 28.4 | Culture extract | 3.75 to 7.5 mg/mL | Rossoni et al., 2020 [62] |
| Carvacrol | 125-500 | Ismail et al., 2022 [63] | |
| Geraniol | 225 | Fatima et al., 2023 [64] | |
| Nikkomycin Z | 0.125 to >64 | Bentz et al., 2021[65] | |
| 6-shogaol | 16-32 | Kim & Eom, 2021) [66] |
| S.no | Origin | Name | MIC (µg/ml) | Ref |
|---|---|---|---|---|
| 1 | Homo sapiens | Human β-defensin-3 | 3.125 to 12.5 | Shaban et al., 2023[72] |
| 2 | Homo sapiens | Cathelicidin peptides LL-37 | 25-100 | Rather et al., 2022 [73] |
| 3 | Homo sapiens | Histatin-5 | 7.5 µM | Pathirana et al., 2018[74] |
| 4 | Barley plant | Defensin-like Protein 1 (D-lp1) | 0.047–0.78 mg/mL | Kamli et al., 2022 [75] |
| 5 | Bacillus subtilis | AF4 | 8 | Ramesh et al., 2023[76] |
| 6 | American rattlesnake (Crotalus durissus terrificus) | Crotamine | 40–80 µM which is equal to 0.2 - 0.4 mg/ml | Dal Mas et al., 2019 [77] |
| 7 | Pomacea poeyana - freshwater snail | Pom-1, Pom-2 | 8.5, 8.4 | Raber et al., 2021[78] |
| 8 | Scorpion venom | ToAP1, ToAP2 | >100 µM, 50 - >100 µM | Pinheiro et al., 2023[79] |
| 9 | Brilacidin | Semi synthetic | 80 | Dos Reis et al., 2023 [80] |
| 10 | Chemically prepared, Myristoylated and Non-Myristoylated Peptides | Pep-A, Myr-A, Pep-B, Myr-B, Pep-C, Myr-C | >256, >256, >256, 16-32, >256, 16-64 | Bugli et al., 2022 [81] |
| 11 | Symbiotic NCR Peptide Fragments |
NCR169C 17–38 | 6.25 µM | Szerencsés et al., 2021[82] |
| NCR169C 17–38 ox | 12.5 µM | |||
| 12 | Analogue of the Peptide Cm-p5 | |||
| Monomers | Cm-p5, Cyclic, Hcy | 11, 27, Not active | Vicente et al., 2019[83] |
|
| Dimer | Dimer 1 (parallel) | 30 | ||
| Dimer 2 (anti-parallel) | 31 | |||
| 13 | Rhesus macaque θ-defensin (RTD) | RTD – 1, RTD - 2 | 6.25, 6.25 | Basso et al., 2018[84] |
| Olive baboon θ-defensins (BTD) | BTD – 2, BTD – 4, BTD - 8 | 3.125, > 25, 3.12 - 6.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





