Submitted:
21 May 2024
Posted:
21 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnOH-LSH, ZnAl-LDH, ZnCuAl-LDH in Nitrate Form
2.3. Intercalation of Cinnamate and Salicylate in ZnOH-LSH, ZnAl-LDH and ZnCuAl-LDH
2.4. Coating Resin Composite of PVA/LDH and PVA/LSH Preparation
2.5. Deposition of Resin Composite on PET Substrate
2.6. Microorganisms
2.7. Antimicrobial Susceptibility Test
2.8. Analytical Procedures and Instrumentation
2.8.1. Inductively Coupled Plasma-Optical Emission Spectrometer
2.8.2. X-Rays Diffraction Spectroscopy
2.8.3. FT-IR Analysis
2.8.4. Thermal Analysis
2.8.5. UV-Vis Analysis
3. Results and Discussion
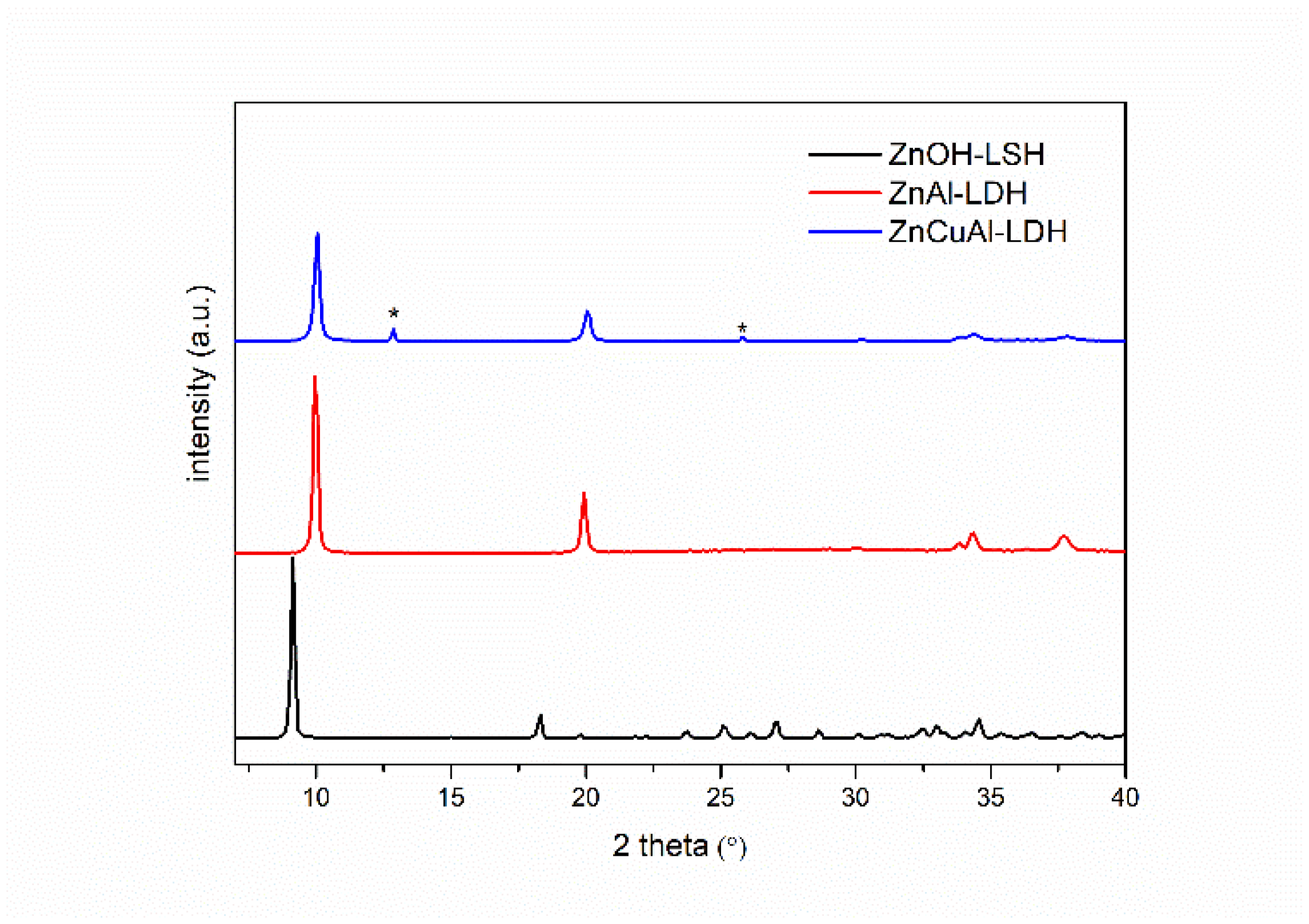
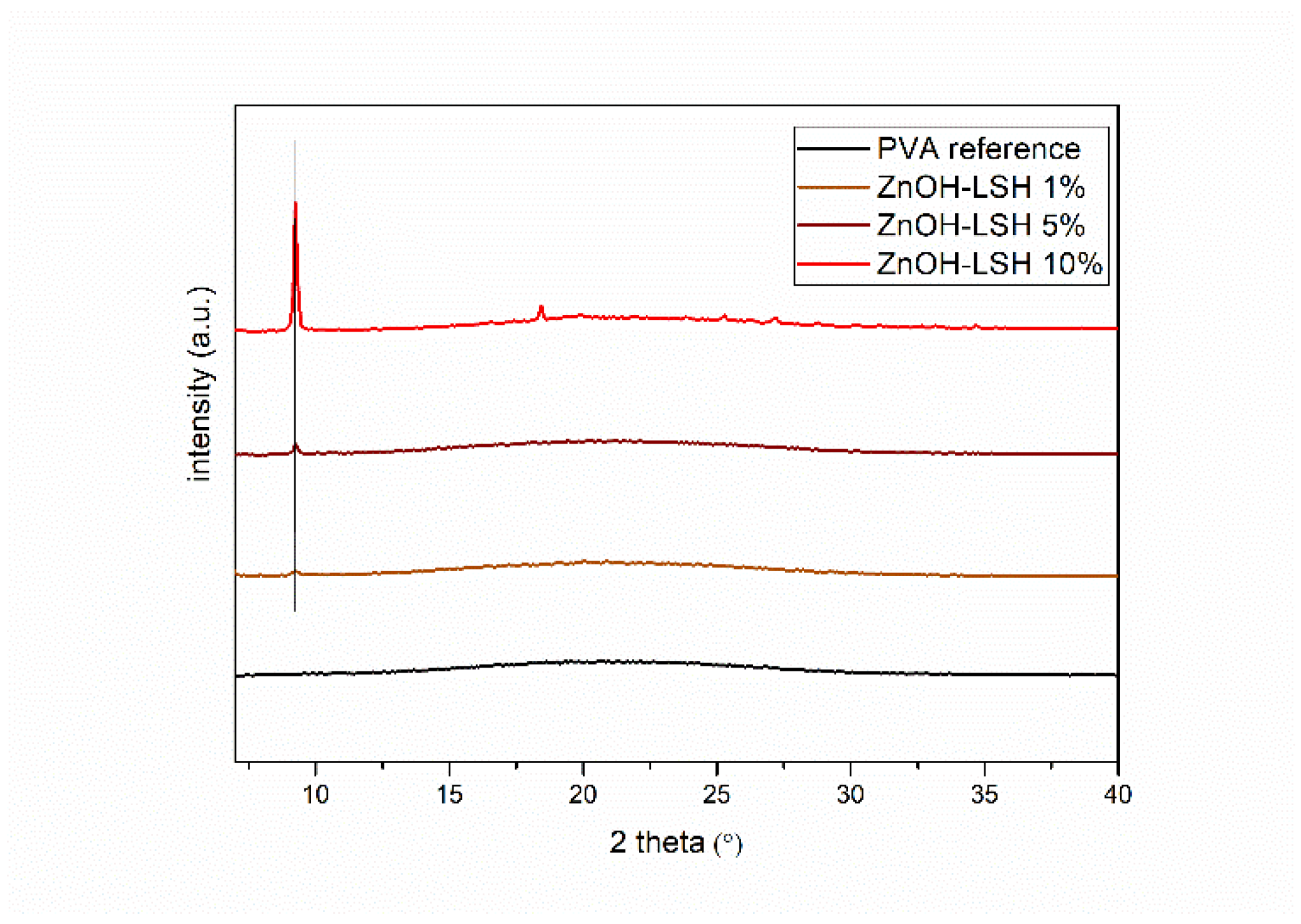
3.1. Characterization of the Pristine Powders
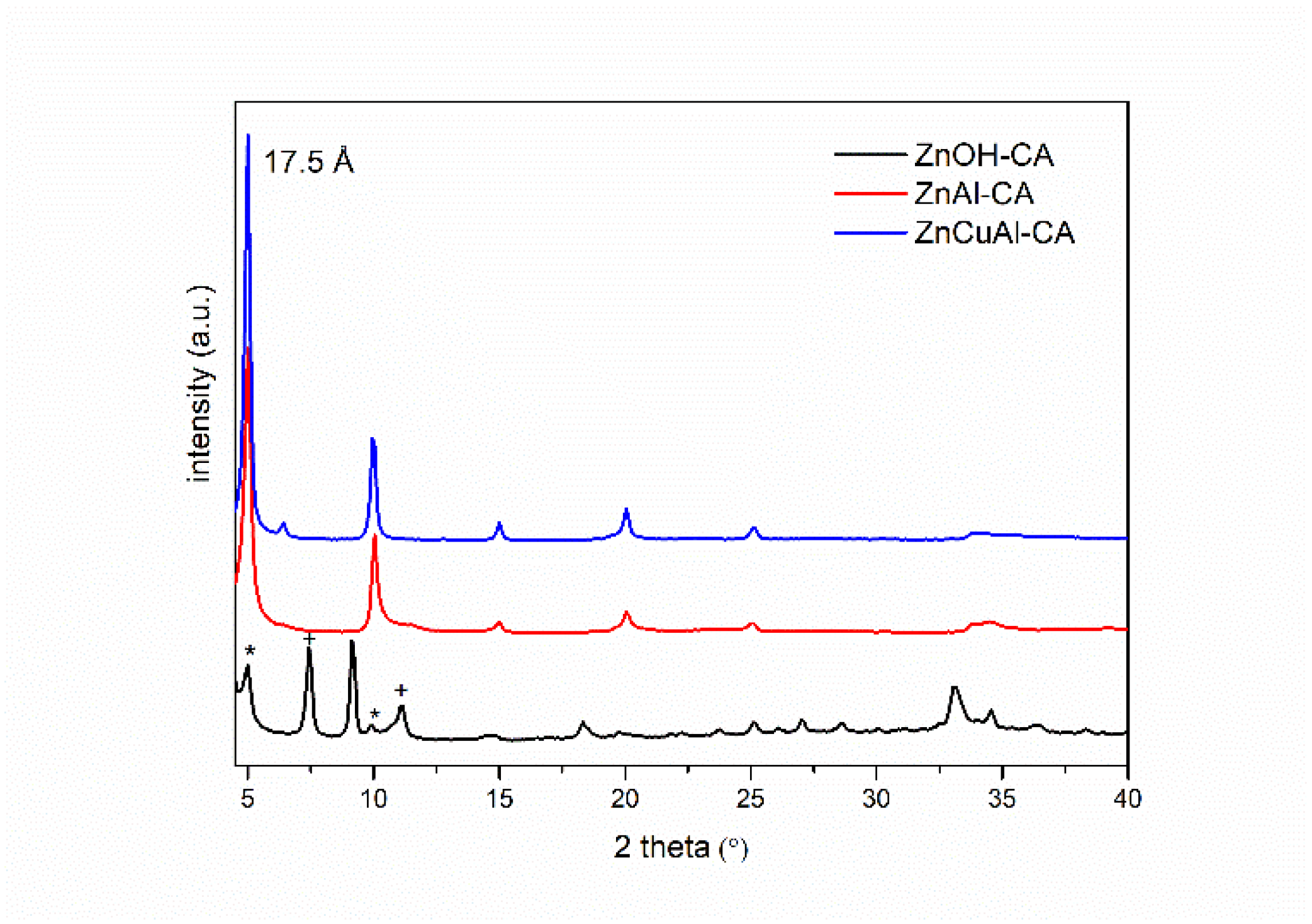
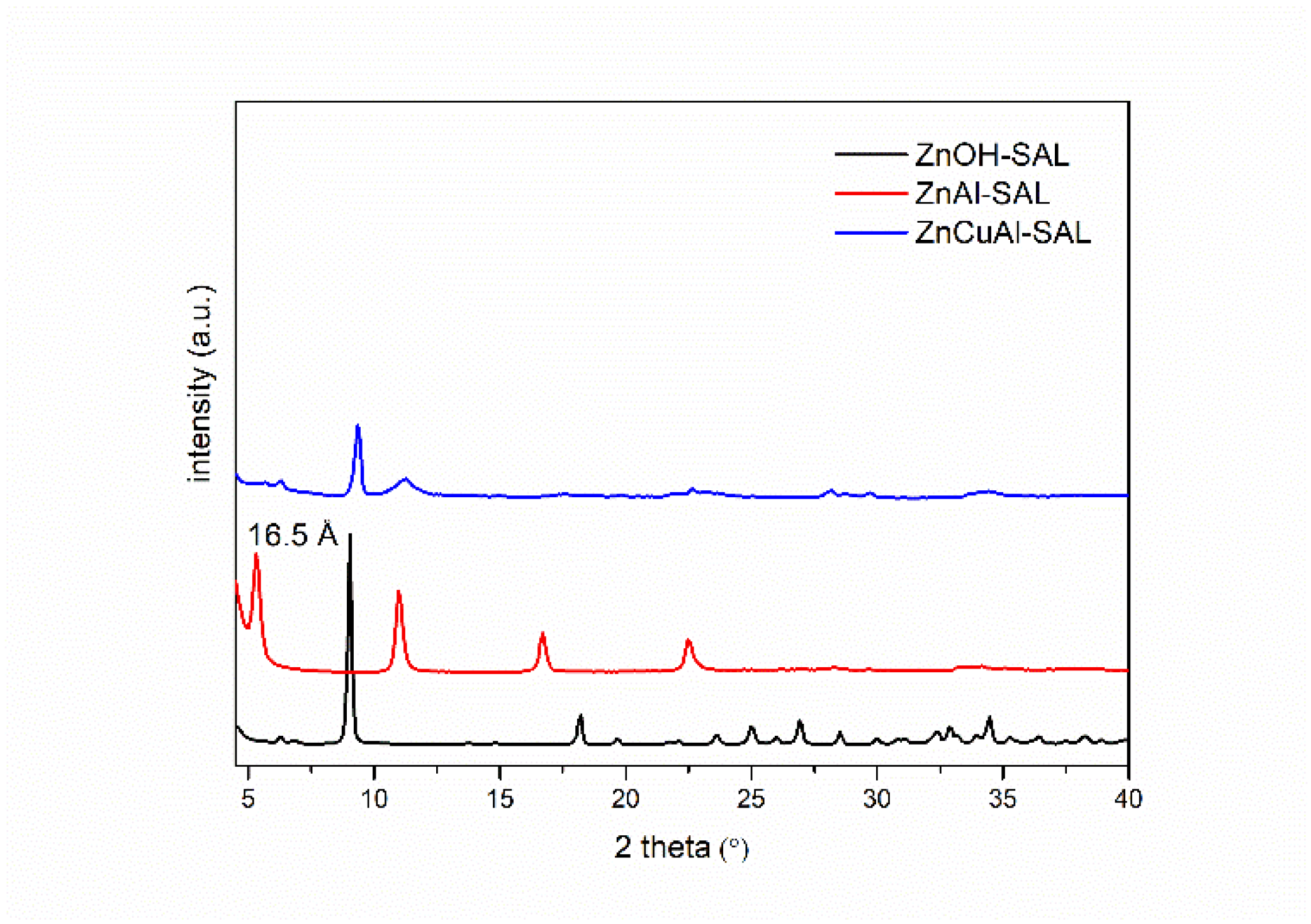
3.2. Characterization of the Intercalated Powders
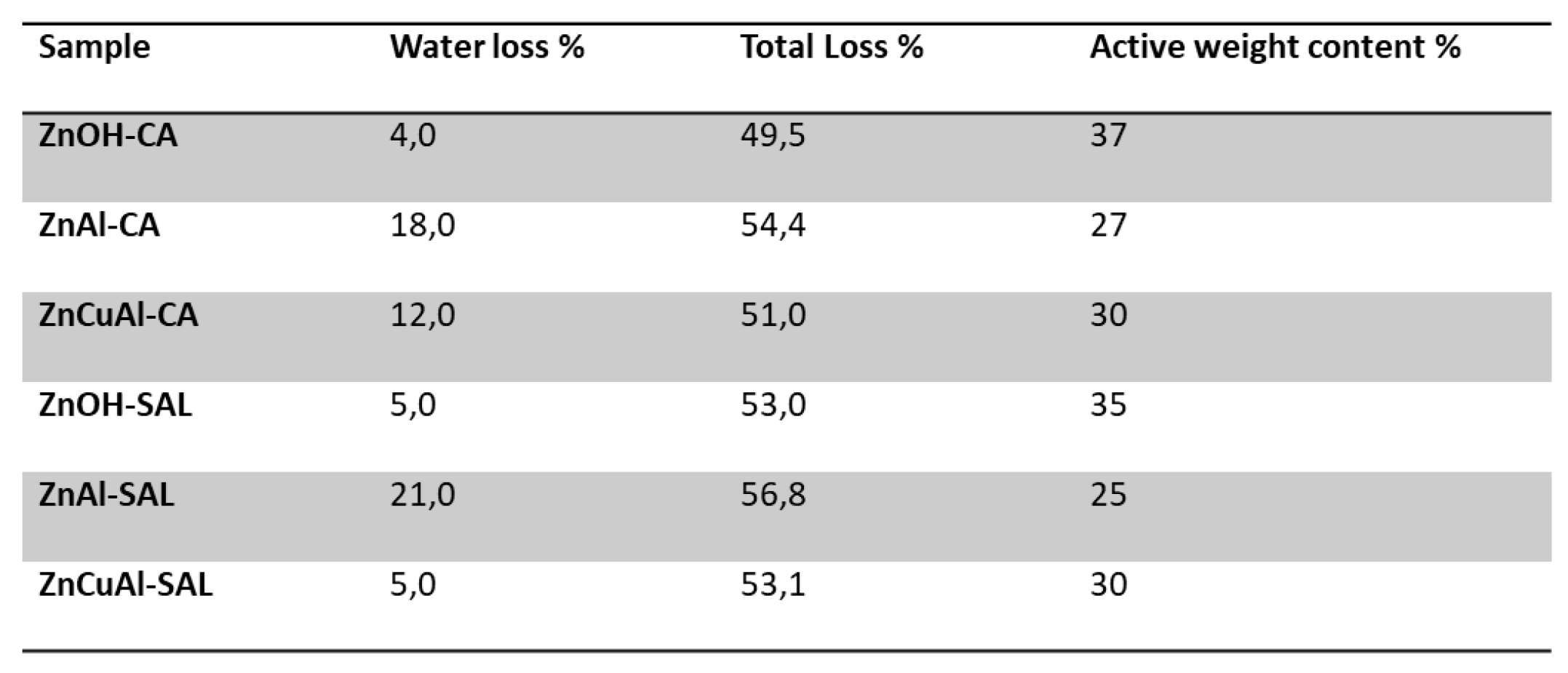
3.3. Chemical-Physical Characterization of the Coatings
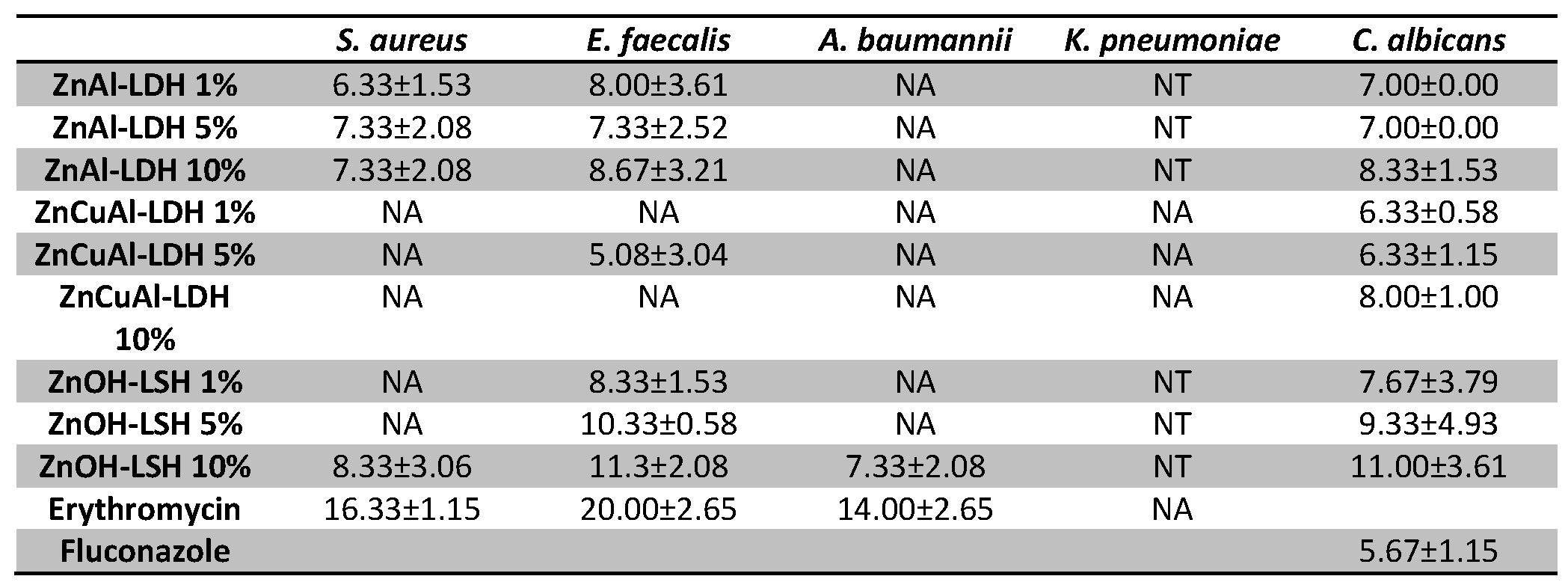
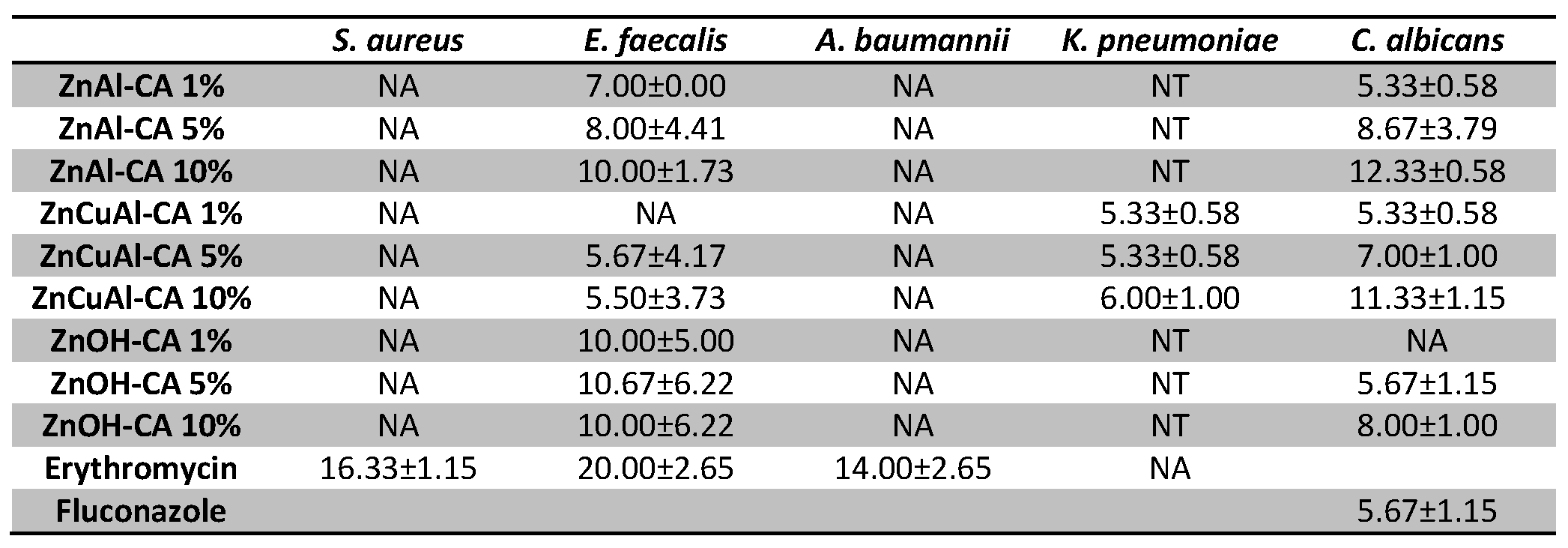
3.4. Antimicrobial Tests on the Coatings
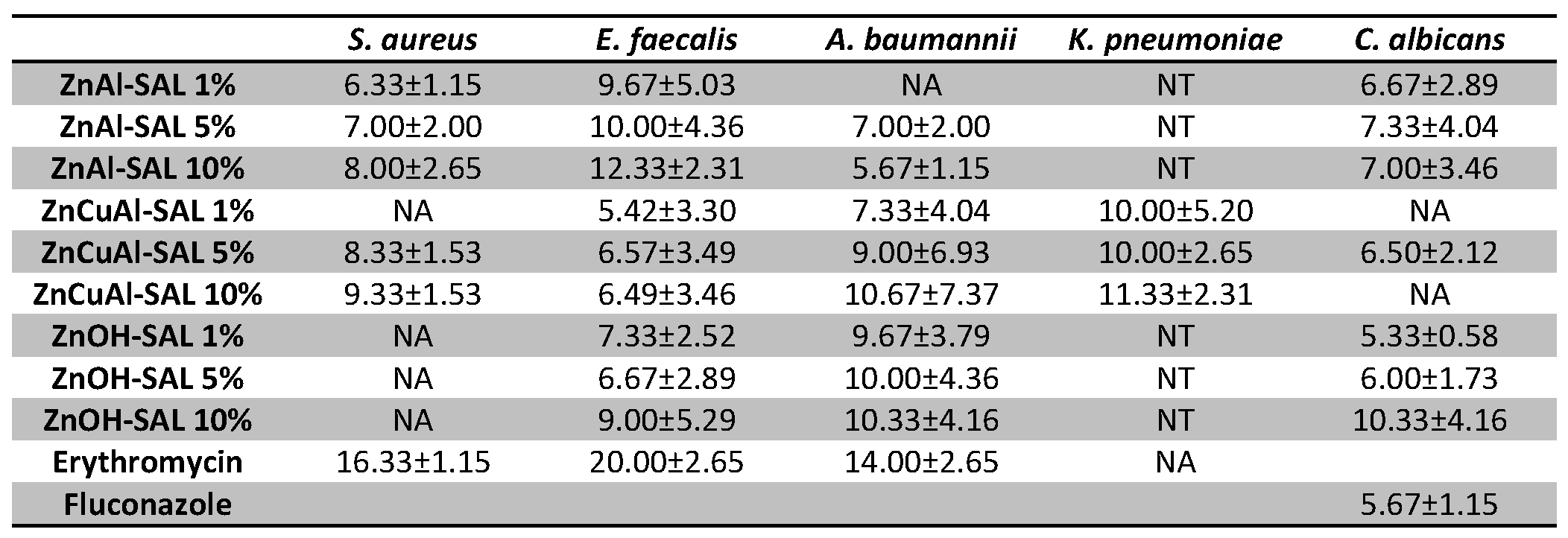
5. Conclusions
Supplementary Materials
Funding
Acknowledgements
Conflicts of interest
References
- Zhang, Y.; Fan, W.; Sun, Y. ; Chen, W. ; Zhang, Y.; Application of antiviral materials in textiles: A review. Nanotechnol. Rev. 2021, 10, 1092–1115. [CrossRef]
- Mingoia, M. ; Conte, C. ; Rienzo, Di A. ; Dimmito, M.P. ; Marinucci, L. ; Magi, G. ; Turkez, H. ; Cufaro, M.C. ; Del Boccio, P.; Di Stefano, A.; Synthesis and Biological Evaluation of Novel Cinnamic Acid-Based Antimicrobials. Pharmaceuticals 2022, 15, 228. [CrossRef] [PubMed]
- Mulani, M. S. ; Kamble, E. E. ; Kumkar, S. N. ; Tawre, M. S; Pardesi, K. R. ; Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10. [CrossRef] [PubMed]
- Birkett, M.; Dover, L.; Cherian Lukose, C.; Wasy Zia, A.; Tambuwala, M.M.; Serrano-Aroca, Á.; Recent Advances in Metal-Based Antimicrobial Coatings for High-Touch Surfaces. Int. J. Mol. Sci. 2022, 23, 1162.
- Bastianini, M.; Scatto, M.; Sisani, M.; Scopece, P.; Patelli, A.; Petracci, A.; Innovative Composites Based on Organic Modified Zirconium Phosphate and PEOT/PBT Copolymer. J.Compos.Sci. 2018, 2, 31. [CrossRef]
- Coiai, S.; Cicogna, F.; Pinna, S; Spiniello, R.; Onor, M.; Oberhauser, W.; Coltelli, M.-B.; Passaglia, E.; Antibacterial LDPE-based nanocomposites with salicylic and rosmarinic acid-modified layered double hydroxides. Appl. Clay Sci. 2021, 214, 106276. [CrossRef]
- Puccetti, M. ; Donnadio, A.; Ricci, M.; Latterini, L.; Quaglia, G.; Pietrella, D.; Di Michele A.; Ambrogi, V.; Alginate Ag/AgCl Nanoparticles Composite Films for Wound Dressings with Antibiofilm and Antimicrobial Activities. J. Funct. Biomater. 2023, 14. [CrossRef]
- Guzman, J. D.; Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules, 2014, 19. [CrossRef]
- Sykes, E. M. E.; White, D.; McLaughlin, S.; Kumar, A.; Salicylic acids and pathogenic bacteria: New perspectives on an old compound. Can. J. Microbiol. 2023, 14, 1–14. [CrossRef]
- Moulay, S.; Review: Poly(vinyl alcohol) Functionalizations and Applications. Polym. - Plast. Technol. Eng. 2015, 54, 1289–1319. [CrossRef]
- Yeun, J. H.; Bang, G. S.; Park, B. J.; Ham, S. K.; Chang, J. H. Poly(vinyl alcohol) nanocomposite films: Thermooptical properties, morphology, and gas permeability. J. Appl. Polym. Sci. 2006, 101, 591–596. [Google Scholar] [CrossRef]
- Bastianini, M.; Sisani, M.; Naryyev, E.; Petracci, A.; Di Guida, I.; Narducci. R. Composite membranes based on polyvinyl alcohol and lamellar solids for water decontamination. New J. Chem., 2024, 48, 2128. [CrossRef]
- Ruiz, C. V.; López-González, M.; Giraldo, O.; Composite films based on salicylic acid-layered zinc hydroxide and polyvinyl alcohol: Preparation, characterization, properties and potential applications. Polym. Test. 2021, 94. [CrossRef]
- Li, P.; Xu, Z. P.; Hampton, M. A.; Vu, D. T.; Huang, L.; Rudolph, V.; Nguyen, A. V.; Control preparation of zinc hydroxide nitrate nanocrystals and examination of the chemical and structural stability. J. Phys. Chem. C, 2012, 116, 10325–10332. [CrossRef]
- Bastianini, M.; Vivani, R.; Nocchetti, M.; Costenaro, D.; Bisio, C.; Oswald, F.; Meyer, T.B.; Marchese, L. Effect of iodine intercalation in nanosized layered double hydroxides for the preparation of quasi-solid electrolyte in DSSC devices. Sol. Energy 2014, 107, 692–699. [Google Scholar] [CrossRef]
- Costantino, U.; Marmottini, F.; Sisani, M.; Montanari, T.; Ramis, G.; Busca, G.; Turco, M.; Bagnasco, G. Cu-Zn-Al hydrotalcites as precursors of catalysts for the production of hydrogen from methanol. Solid State Ionics 2005, 176, 2917–2922. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Clinical and Laboratory Standards Institute Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Standard, Approval CDM-A.; M07 Methods dilution Antimicrob. Susceptibility Tests Bact. That Grow Aerob. 2018, 91.
- Costantino, U.; Bugatti, V.; Gorrasi, G.; Montanari, F.; Nocchetti, M.; Tammaro, L.; and Vittoria, V. New polymeric composites based on poly(ε-caprolactone) and layered double hydroxides containing antimicrobial species. ACS Appl. Mater. Interfaces 2009, 1, 668–677. [CrossRef]
- Bastianini, M.; Costenaro, D.; Bisio, C.; Marchese, L.; Costantino, U.; Vivani, R.; Nocchetti. M.; On the intercalation of the iodine-iodide couple on layered double hydroxides with different particle sizes. Inorg. Chem. 2012, 51. [CrossRef] [PubMed]
- Bovio, B.; Locchi, S.; Crystal structure of the orthorhombic basic copper nitrate, Cu2(OH)3NO3. Journal of Crystallographic and Spectroscopic Research, 1982, 12, 6. [CrossRef]
- Ruiz, C. V.; Rodríguez-Castellón, E.; Giraldo, O.; Hybrid materials based on a layered zinc hydroxide solid and gallic acid: Structural characterization and evaluation of the controlled release behavior as a function of the gallic acid content. Appl. Clay Sci. 2019, 181, 105228. [CrossRef]
- Nabipour, H.; Sadr, M. H.; Thomas, N. Synthesis, controlled release and antibacterial studies of nalidixic acid-zinc hydroxide nitrate nanocomposites. New J. Chem. 2016, 40, 238–244. [Google Scholar] [CrossRef]
- Adam, N.; Mohd Ghazali, S. A. I. S.; Dzulkifli, N. N.; Hak, C. R. C.; Sarijo. S. H. Intercalations and characterization of zinc/aluminium layered double hydroxide-cinnamic acid. Bull. Chem. React. Eng. Catal. 2019, 14, 165–172. [CrossRef]
- Pérez, M.R.; Pavlovic, I.; Barriga, C.; Cornejo, J.; Hermosín, M.C.; Ulibarri, M.A.; Uptake of Cu2+, Cd2+ and Pb2+ on Zn-Al layered double hydroxide intercalated with edta. Appl. Clay Sci. 2006, 32, 245–251. [CrossRef]
- Palacio, L. A.; Velásquez, J.; Echavarría, A.; Faro, A.; Ribeiro, F. R.; Ribeiro, M. F. Total oxidation of toluene over calcined trimetallic hydrotalcites type catalysts. J. Hazard. Mater. 2010, 177, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.M.N.; Hussein, M.Z.; Sarijo, S.H.; Fakurazi, S.; Arulselven, P.; Yun Hin, T.-Y. Synthesis of (cinnamate-zinc layered hydroxide) intercalation compound for sunscreen application. Chem. Cent. J. 2013, 7, 1–12. [Google Scholar] [CrossRef]
- Arizaga, G. G. C.; Satyanarayana, K. G.; F. Wypych. Layered hydroxide salts: Synthesis, properties and potential applications. Solid State Ionics 2007, 178, 1143–1162. [CrossRef]
- Silion, M.; Hritcu, D.; Popa, M. I. Intercalation of salicylic acid into ZnAl layered double hydroxides by ionic-exchange method. Optoelectron. Adv. Mater. Rapid Commun. 2009, 3, 817–820. [Google Scholar]
- Shamraiz, U.; Badshah, A.; Hussain, R. A.; Nadeem, M. A.; Saba, S. Surfactant-Free Fabrication of Copper Sulfides (CuS, Cu2S) via Hydrothermal Method. J. Clust. Sci. 2013, 24, 1181–1191. [Google Scholar] [CrossRef]
- Cheng, H. M.; Gao, X.W.; Kang Zhang, K.; Xin-Rui Wang, X.-R.; Zhou, W.; Li, S.-H.; Cao, X.-L.; Yan, D.-P. A novel antimicrobial composite: ZnAl-hydrotalcite with: P -hydroxybenzoic acid intercalation and its possible application as a food packaging material. New J. Chem. 2019, 43, 19408–19414. [Google Scholar] [CrossRef]
- Effah, C. Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Congradyova, A.; Jomová, K.; Kucková, L.; Kozísek, J.; Moncol, J.; Valko, M. Antimicrobial activity of copper (II) complexes. The Journal of Microbiology, Biotechnology and Food Sciences 2014, 3, 67. [Google Scholar]
| Film | PVA amount (g) | Water (mL) | Filler (g) |
|---|---|---|---|
| 1% | 9.9 | 100 | 0.1 |
| 5% | 9.5 | 100 | 0.5 |
| 10% | 9.0 | 100 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





