1. Introduction
Gastric cancer (GC) includes all cancers arising in the stomach. Gastric adenocarcinoma, the predominant form of stomach cancer, originates from the glandular cells of the gastric mucosa [
1]. According to the 2022 National Cancer Center survey data, GC is the second most prevalent cancer in Korea. It has a mortality rate of 8.6%, amounting to 71,747 individuals, and ranks fifth among all cancer deaths. The disease prevalence is 14.5%, affecting 330,217 people, with a leading incidence among men at 21.8% (217,881 individuals) and ranking fourth among women at 8.8% (112,336 individuals) [
2].
Early diagnosis and intervention play pivotal roles in enhancing the survival and quality of life (QoL) of patients with GC [
3]. In Korea, a national cancer screening initiative, initiated in 1999, subjects adults over 40 to upper gastrointestinal endoscopy or upper gastrointestinal tract surgery every 2 years. This effort has contributed to a notable decline in GC mortality, which has dropped to 13.5% in 2021 from 56.3% in 1997. Furthermore, the five-year relative survival rate for GC shows a consistent annual increase. The five-year relative survival rate for GC reached 78.0% in 2016-2020, marking a notable increase of 9.6 percentage points compared to 2006-2010 and a further 2.1 percentage points compared to 2011-2015. Patients with early-stage GC undergoing surgery exhibit a favorable prognosis, boasting a 5-year survival rate exceeding 90% [
2]. As survival rates rise, recent studies highlight the growing significance of patient recovery and nutritional management post-surgery.
With advancements in GC surgical technologies, patients with early GC and low risk of lymph node metastasis undergo endoscopic resection (ER), and those who are ineligible for ER undergo gastrectomy [
4]. Many patients experience post-surgical side effects like dumping syndrome, bile reflux, anastomotic leak, gastritis, and weight loss [
5]. A previous study found that of 134 post-GC surgery patients, 50% experienced a decline in QoL compared to pre-surgery, and 1/3rd witnessed continuous deterioration even 6 months post-surgery [
6].
Recent studies spotlight the role of herbal medicine (HM) in post-surgical gastric cancer treatment. In Taiwan in 2020, Wei-Tai Shih et al. explored HM's impact on the survival of patients with GC undergoing chemotherapy post-surgery. Prolonged HM treatment correlated with higher survival rates and reduced chemotherapy side effects [
7]. Similarly, Japanese research on Rikkunshito, a traditional HM, demonstrated notable enhancements in post-proximal gastrectomy patients' quality of life and gastrointestinal function [
8]. These studies underscore HM's potential to significantly augment recovery and enhance outcomes for post-surgical gastric cancer patients.
However, the landscape of HM in the context of post-surgical recovery in patients with GC remains incompletely explored. While previous systematic review studies have examined HM in conjunction with chemotherapy or other HM treatments like acupuncture and moxibustion after GC surgery, none have exclusively focused on HM post-surgery. This knowledge gap necessitates a systematic review and meta-analysis to assess the effectiveness and safety of HM in fortifying post-surgical recovery among patients with GC. Therefore, our study aims to comprehensively review the impact of HM alone on patients following GC surgery.
2. Methods
2.1. Objective and Strategy
This study aimed to systematically review the research literature, understand Korean Medicine and Western medicine GC treatments, and suggest directions for future clinical guidelines. The study protocol has been registered on PROSPERO with the registration number CRD42022354133.
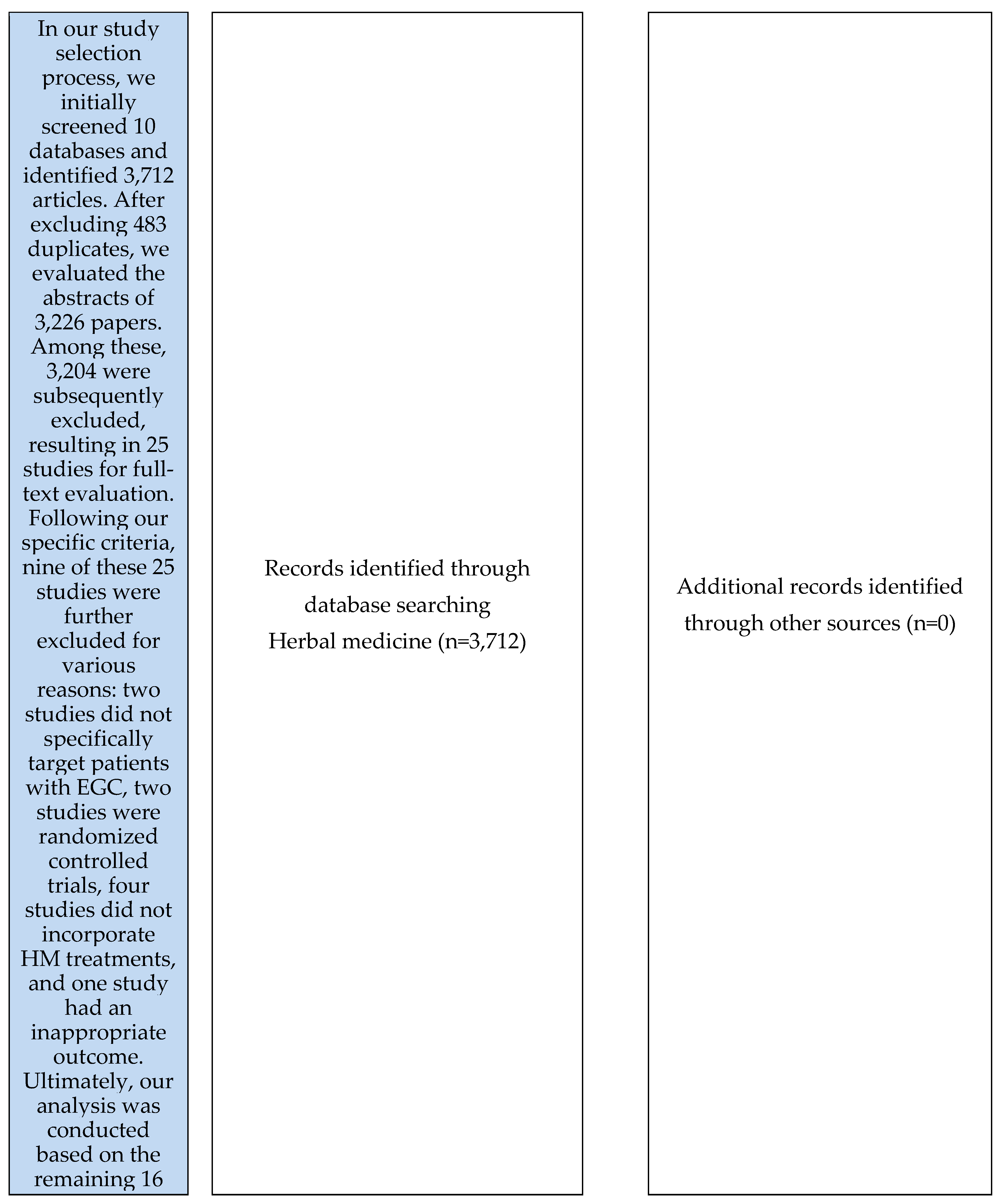
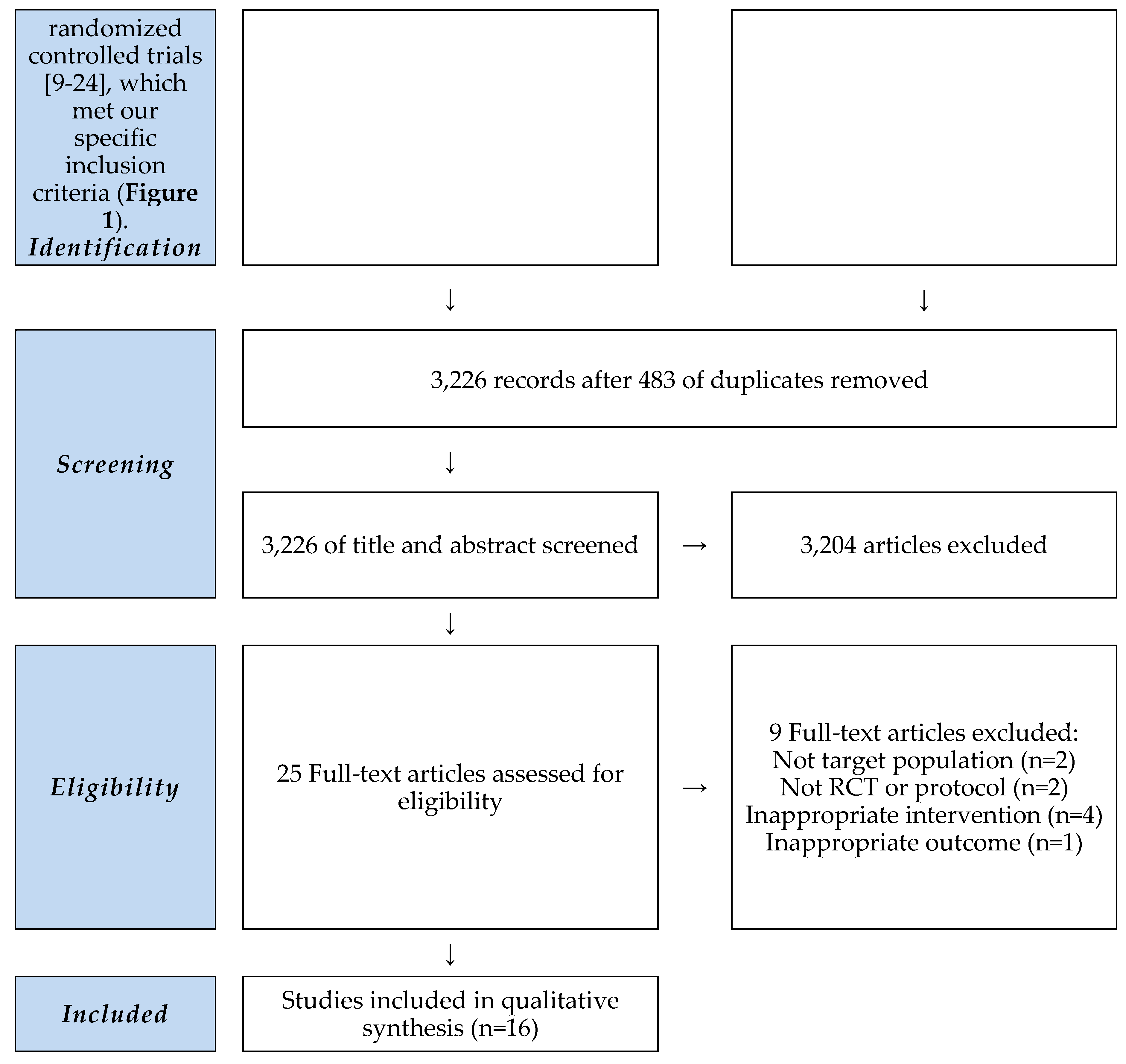
A literature search was conducted for studies related to Korean Medicine GC treatment published until July 31, 2023, on search engines including Korean web databases KMBASE, KISS, OASIS, RISS, and ScienceOn and international web databases such as EMBASE, Medline, Pubmed, CNKI, and CiNii. The search of the PubMed database, which was searched in the corresponding database, yielded the search results provided in
Table 1. This research solely focused on electronic searches. There were 16 papers that satisfied all the inclusion and exclusion conditions.
2.2. Selection of Studies
The collected research papers were organized using a literature management program (EndNote 20) and a spreadsheet program (Excel). The selection and analysis of documents were independently carried out by 2 researchers (SJP, KSD). In instances of disagreement, particularly during the full-text review stage of the final paper, the researchers resolved differences through a thorough discussion and arrived at a consensus on the ultimate selection.
2.3. Quality Assessment
To evaluate the literature's quality, the risk of bias in Randomized Controlled Trials (RCTs) was assessed using Cochrane’s Risk of Bias (RoB) tool. Two researchers (SJP, KSD) independently conducted the risk of bias assessment for the RCT literature. Discrepancies and agreements between evaluators were then analyzed through researcher meetings, where both researchers participated together.
Cochrane’s RoB tool for 25 quantitative papers comprises seven items covering aspects such as random allocation sequence generation, allocation sequence concealment, blinding of study participants and researchers, blinding of outcome evaluation, insufficient outcome data, selective reporting, and other biases. For one qualitative paper, Cochrane’s RoB tool is designed to assess bias risk with three response options: low, high, and uncertain. The tool was translated into Korean to minimize variations in evaluation criteria between evaluators. The overall results were meta-analyzed using Review Manager Software 5.4 (RevMan).
2.4. Data Analysis
Among the literature subjected to analysis, 25 papers presented the mean and standard deviation of outcome variables for the intervention group, where patients with GC were treated with gastrectomy and HM, and a control group without HM treatment. These papers were thoroughly reviewed using RevMan. If the number of included studies was over 10, meta-analysis publication bias was assessed using funnel plots.
In our research, we used the risk ratio (RR) along with its 95% confidence interval (CI) for the analysis of dichotomous data. For continuous data, we utilized mean differences (MD) with a 95% CI, especially when treatment outcomes were measured on an identical scale. In cases where units of measurement varied across studies, we employed the standardized mean difference (SMD) with a 95% CI to ensure a uniform assessment of effect sizes.
To gauge heterogeneity within the study, we calculated Higgins' I². A value of I² exceeding 50% was interpreted as a significant indicator of substantial heterogeneity. The selected evaluation indicators encompassed gastrointestinal recovery, nutritional markers, immune markers, inflammatory markers, quality of life, and adverse effects.
3. Results
3.1. Study Selection
Figure 1.
PRISMA Flowchart. RCT: randomized controlled trial.
Figure 1.
PRISMA Flowchart. RCT: randomized controlled trial.
3.2. Study Characteristics
This meta-analysis, finalized before 2023, collated data from 16 randomized controlled trials involving a total of 1,546 patients diagnosed with GC. These studies examined the efficacy and safety of HM interventions. While participants in the experimental groups were treated with HM, those in the control groups received standard care, placebos, or the established usual care protocols. The treatments spanned a range of durations, from 3 days to months. The study assessed gastrointestinal (GI) recovery, evaluated nutritional markers including TP (Total Protein), PA (Prealbumin), ALB (Albumin), and TRF (Transferrin), measured specific immune markers such as CD4+ and CD8+ cell counts, analyzed the inflammatory marker CRP, gauged participants' QoL using scales such as SF-36 and QLQ-C30, and recorded adverse effects as a measure of safety (
Table 2).
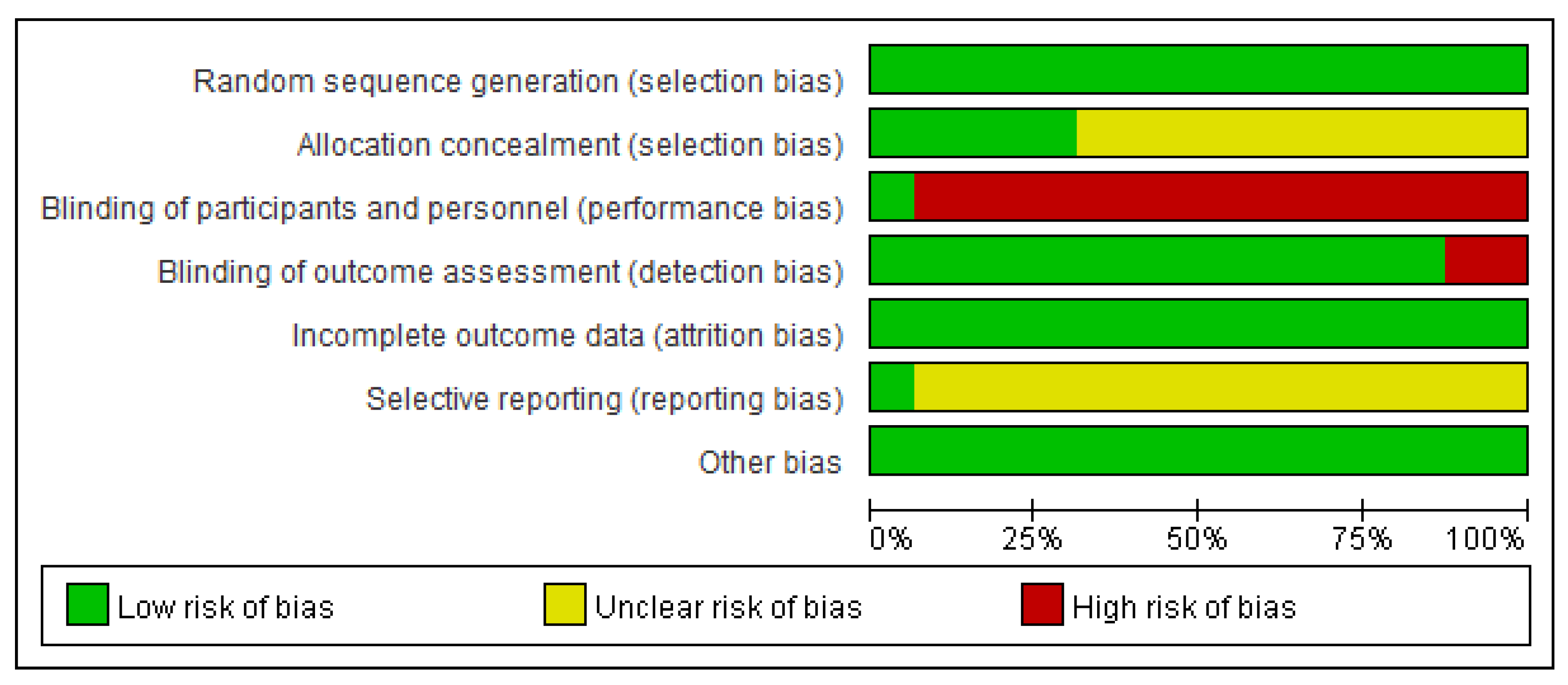
3.3. Risk of Bias in Included Trials
In our review, based on the Cochrane bias risk assessment, we identified a significant risk of bias due to insufficient methodological explanations (
Figure 2). Each study properly utilized random sequence generation [
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24]. However, 10 studies lacked clarity regarding allocation concealment, leading to an uncertain bias risk evaluation [
9,
10,
17,
18,
19,
20,
21,
22,
23,
24]. Aside from one study that implemented a placebo [
14], there was a high risk of performance and detection bias in 15 trials, as only the experimental group was treated with HM [
9,
10,
11,
12,
13,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24]. Except for one study with a confirmed protocol registration [
23], all other studies did not report their pre-registered plans, leading to an unclear risk of reporting bias. Fortunately, we deemed all studies to have minimal risk concerning attrition and other possible biases (
Figure 3).
3.4. Gastrointestinal Recovery
3.4.1. Time to First Flatus (h)
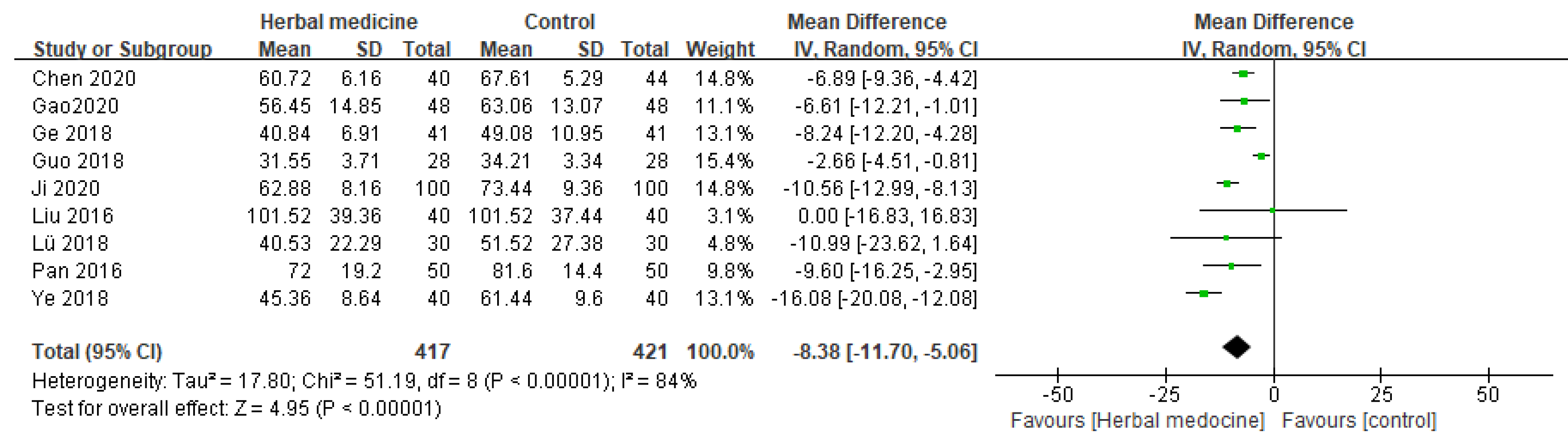
In nine randomized controlled trials, the effect of HM on the time to first flatus after gastric cancer surgery was assessed [
10,
11,
13,
14,
16,
17,
18,
20,
22]. The pooled results from these trials showed that patients who were treated with HM experienced a noticeable enhancement in the time to first flatus. This benefit was highlighted by an MD of -8.38 (95% CI [11.70, -5.06], I² = 84%, P < .00001, n = 838,
Figure 4).
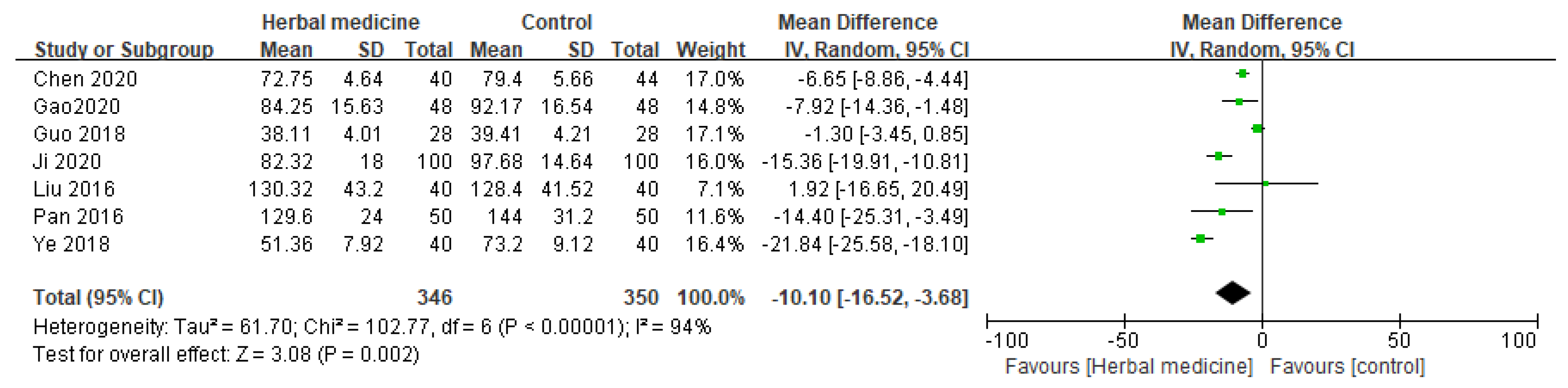
3.4.2. Time to First Bowel Movement (h)
Seven randomized controlled trials investigated the influence of HM on the first bowel movement as a parameter for GI recovery following gastric cancer surgery [
10,
11,
13,
16,
18,
20,
22]. The consolidated data from these studies demonstrated a significant improvement for those patients who received HM treatment with MD -10.10 (95% CI [-16.52, -3.68], I² = 94%, P = .002, n = 696,
Figure 5).
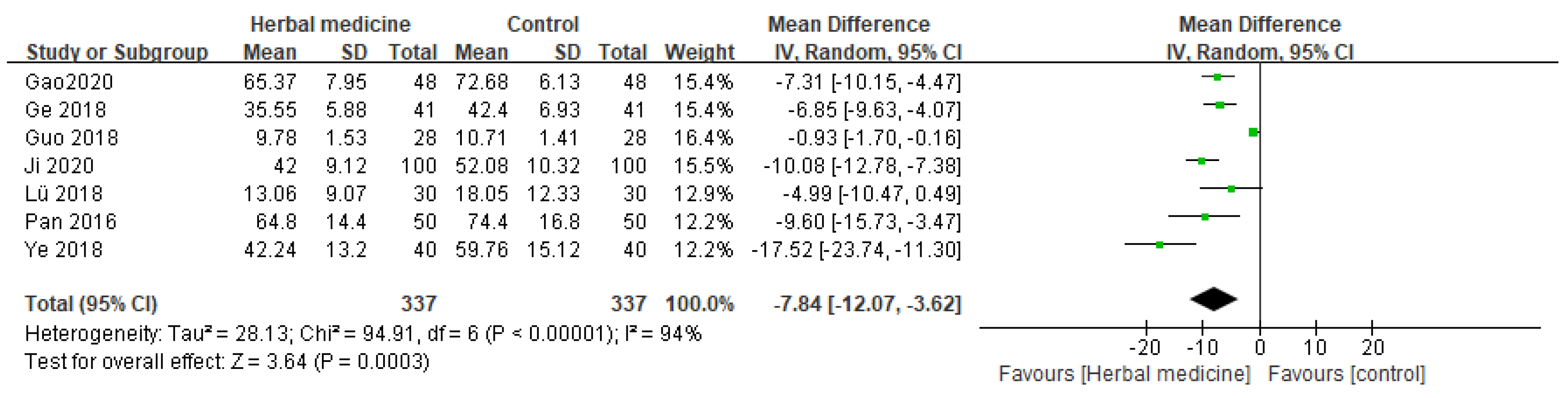
3.4.3. Time to Bowel Sounds Return (h)
Seven randomized controlled trials assessed the effect of HM on the time to bowel sounds return after gastric cancer surgery [
10,
11,
14,
16,
17,
18,
20]. The synthesized results from these studies indicate a significant reduction in the time for bowel sounds to return in patients who received HM treatment. The consolidated data from these studies demonstrated that patients who received HM treatment showed a significant improvement in the time to bowel sounds return, as evidenced by an MD of -7.84 (95%CI [-12.07, -3.62], I² = 94%, P = .0003, n = 674,
Figure 6).
3.5. Nutritional Markers
Several randomized controlled trials evaluated the influence of HM on various nutritional markers in post-gastric cancer surgery patients. The assessment encompassed markers like ALB, TRF, PA, and TP.
3.5.1. Albumin Levels
Six randomized controlled trials assessed the impact of HM on the nutritional markers, specifically ALB, in patients post-gastric cancer surgery [
9,
10,
12,
13,
21,
22]. An analysis of the combined data indicated that patients treated with HM exhibited a notable increase in ALB levels compared to the control group. The compiled results from these investigations indicated that patients administered with HM treatment exhibited a notable enhancement in ALB levels, supported by an MD of 1.83 (95% CI [0.76, 2.90], I² = 80%, P = .0008, n = 532,
Table 3).
3.5.2. Transferrin Levels
The consolidated data from the selected studies indicated a variation in TRF levels in patients treated with HM [
9,
10,
12]. This variation is emphasized by an SMD of 1.89 (95% CI [0.17, 3.96], I² = 98%, P = .07, n = 306,
Table 3). However, there was substantial heterogeneity among the studies, and the results were not significant.
3.5.3. Prealbumin Levels
The compiled data from the studies showcased a pronounced improvement in PA levels in patients treated with HM [
9,
10], even though there was considerable heterogeneity among the studies, with an MD of 36.70 (95% CI [18.53, 54.87], I² = 83%, P < .0001, n = 216,
Table 3).
3.5.4. Total Protein Levels
The gathered data from the included studies highlight a clear elevation of TP levels in patients who were administered HM [
10,
22]. This was demonstrated by an MD of 2.18 (95% CI [0.49, 3.86], I² = 0%, P = .01, n = 176,
Table 3). Notably, the studies showed no heterogeneity, suggesting a consistent positive effect of HM on TP levels.
Table 3.
Nutritional Markers of HM in Post-gastric Cancer Surgery Patients.
Table 3.
Nutritional Markers of HM in Post-gastric Cancer Surgery Patients.
| Nutritional markers |
Studies |
Participants |
Effect Size [95%CI] |
P value |
Study ID references |
| ALB |
6 |
532 |
MD 1.83, 95% CI [0.76, 2.90], I² = 80% |
*** P = 0.0008 |
Chen 2020, Gao 2020, Liu 2016, Liu 2020, Sang 2020, Zou 2016 |
| TRF |
3 |
306 |
SMD 1.89, 95% CI [0.17, 3.96], I² = 98% |
P = 0.07 |
Gao 2020, Liu 2020, Sang 2020 |
| PA |
2 |
216 |
MD 36.70, 95% CI [18.53, 54.87], I² = 83% |
*** P < 0.0001 |
Gao 2020, Sang 2020 |
| TP |
2 |
176 |
MD 2.18, 95% CI [0.49, 3.86], I² = 0% |
** P = 0.01 |
Gao 2020, Liu 2016 |
3.6. Immune Markers
3.6.1. CD3+ Levels
From the available studies on CD3+ levels in post-gastric cancer surgery patients [
10,
15,
24], it was observed that HM had a notable effect on this immune marker. The consolidated data indicates that patients treated with HM exhibited a marked increase in CD3+ levels when compared to the control group. This observation was validated by an MD of 5.01 (95% CI [2.15, 7.87], I² = 88%, P = .0006, n = 311,
Table 4).
3.6.2. CD4+ Levels
The aggregated results from the selected studies showed a significant variation in CD4+ levels in patients administered HM [
9,
10,
15,
16,
24]. This variance is emphasized by an MD of 4.39 (95% CI [1.91, 6.87], I² = 94%, P = .0005, n = 487,
Table 4). The findings suggest a beneficial effect of HM on CD4+ levels in the aforementioned patient group.
3.6.3. CD8+ Levels
The compiled data from the studies indicated no significant difference in CD8+ in patients treated with HM compared to the control [
9,
15,
16]. The pooled findings showed an MD of -1.13 (95% CI [-4.28, 2.02], I² = 97%, P = .48, n = 293,
Table 4), emphasizing the lack of significance and substantial heterogeneity among the studies.
3.6.4. CD4+/CD8+ Ratio
The aggregated data from the studies suggests that there was no statistically significant improvement in the CD4+/CD8+ ratio for patients treated with HM [
9,
10,
15,
16]. The pooled results show a MD of 0.49 (95% CI [0.01, 0.91], I² = 99%, P = .06, n = 389,
Table 4). Despite the considerable heterogeneity among the studies, it is important to note that the results were not significant.
Table 4.
Immune Markers of HM in Post-gastric Cancer Surgery Patients.
Table 4.
Immune Markers of HM in Post-gastric Cancer Surgery Patients.
| Immune markers |
Studies |
Participants |
Effect Size [95%CI] |
P value |
Study ID references |
| CD3+ |
3 |
311 |
MD 5.01, 95% CI [2.15, 7.87], I² = 88% |
*** P = 0.0006 |
Gao 2020, Liu 2018, Ma 2018 |
| CD4+ |
4 |
487 |
MD 4.39, 95% CI [1.91, 6.87], I² = 94% |
*** P = 0.0005 |
Gao 2020, Guo 2018, Liu 2018, Sang 2020 |
| CD8+ |
3 |
293 |
MD -1.13, 95% CI [-4.28, 2.02], I² = 97% |
P = 0.48 |
Guo 2018, Ma 2018, Sang 2020 |
| CD4+/CD8+ |
3 |
389 |
MD 0.49, 95% CI [0.01, 0.91], I² = 99% |
P = 0.06 |
Gao 2020, Guo 2018, Sang 2020 |
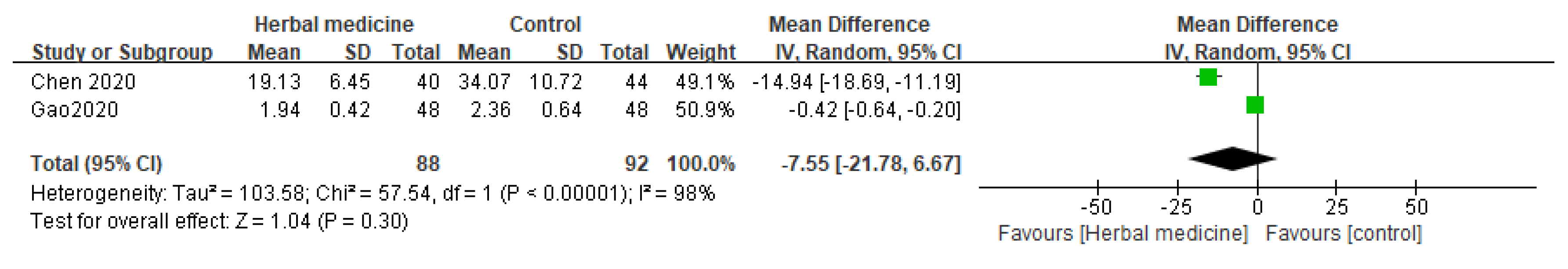
3.7. Inflammatory Markers
Two randomized controlled trials explored the potential effects of HM on inflammatory markers [
10,
13], specifically focusing on CRP levels in the context of medical interventions. The comprehensive data analysis indicates that there was no statistically significant difference in CRP levels for patients in the HM group compared to the control group with an MD of -7.55 (95% CI [-21.78, 6.67], I² = 98%, P = .30, n = 180,
Figure 7).
3.8. Quality of Life Assessments
3.8.1. SF-36 Score
Based on the studies assessing the SF-36 score, which evaluates general health status and QoL, there was no statistically significant enhancement in scores for patients treated with HM compared to the control group [
12,
17]. Although the data showed a mean difference of 11.33 favoring HM, the wide confidence interval (95% CI [3.50, 26.17]) and a high heterogeneity (I² = 96%) suggest variability among studies. The P-value of 13 indicates that the results were not statistically significant (
Table 5).
3.8.2. WHOQOL-BREF Score
The aggregated results from the studies regarding the WHOQOL-BREF score, an essential tool to measure QoL across various dimensions, revealed significant enhancements in scores for patients administered HM [
9]. This is substantiated by an MD of 6.54 (95% CI [5.42, 7.66], P < .00001, n = 60,
Table 5). This confirms the positive effects of HM on patients' QoL as measured by the WHOQOL-BREF.
3.8.3. QLQ-C30 Score
With regards to the QLQ-C30 score, which is specifically designed to assess the QoL in cancer patients, the combined results from the included studies indicated a remarkable difference in scores between the HM group and the control [
19]. The pooled findings revealed an MD of 18.04 (95% CI [15.87, 20.21], P < .00001, n = 60,
Table 5). The results unequivocally favor the HM group, suggesting its efficacy in enhancing the QoL in patients post-gastric cancer surgery.
Table 5.
Quality of Life Assessments of HM in Post-gastric Cancer Surgery Patients.
Table 5.
Quality of Life Assessments of HM in Post-gastric Cancer Surgery Patients.
| Quality of Life Assessment |
Studies |
Participants |
Effect Size [95%CI] |
P value |
Study ID references |
| SF-36 |
2 |
172 |
MD 11.33, 95% CI [3.50, 26.17], I² = 96% |
P = 0.13 |
Ge 2018, Liu 2020 |
| WHOQOL-BREF |
1 |
120 |
MD 6.54, 95% CI [5.42, 7.66] |
*** P < 0.00001 |
Sang 2020 |
| QLQ-C30 |
1 |
120 |
MD 18.04, 95% CI [15.87, 20.21] |
*** P < 0.00001 |
He 2017 |
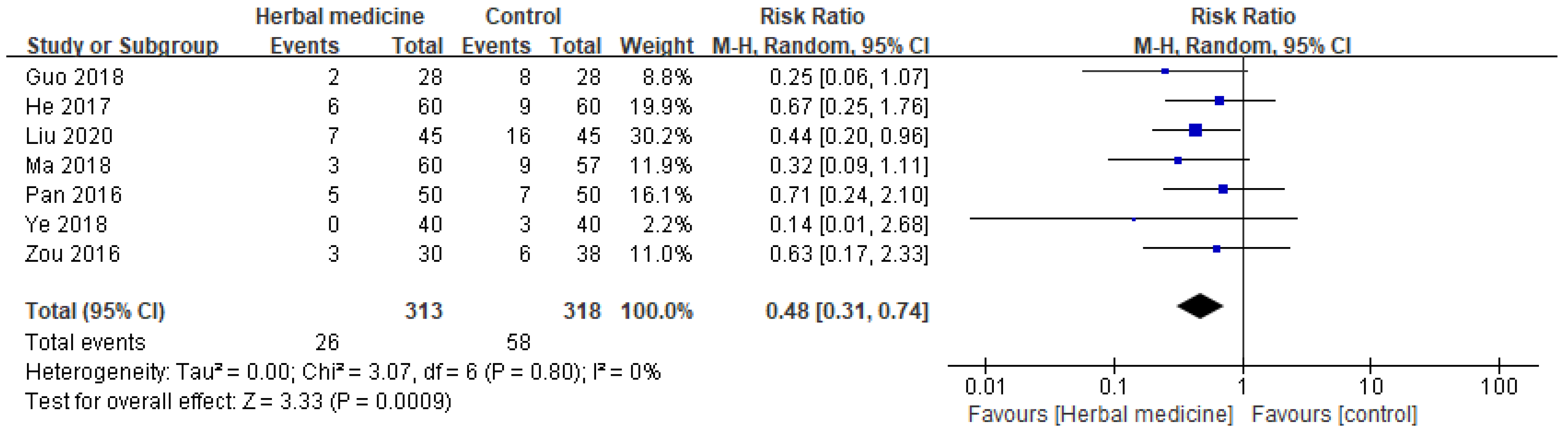
3.9. Safety
3.9.1. Incidence of Adverse Effects
The safety profile of HM was evaluated based on the incidence of adverse effects and the pooled data from various studies suggests that HM is associated with a reduced risk of adverse effects when compared to the control [
12,
15,
16,
18,
19,
20,
21]. The cumulative findings yield an RR of 0.48 with a 95% CI ranging from 0.31 to 0.74. This indicates that patients undergoing HM treatment are approximately half as likely to experience adverse effects as those in the control group (
Figure 8).
Notably, the analysis revealed a consistently low heterogeneity among the studies with an I² value of 0%, signifying minimal variability in the results across different studies. The statistical significance of these findings is further emphasized by a P-value of .0009, suggesting a strong association between HM and a reduced risk of adverse effects.
4. Discussion
In the realm of post-surgical care for patients with GC, the management of GI recovery and nutrition plays a pivotal role in improving survival rates and QoL [
25,
26]. The postoperative hurdles faced by these patients, such as impaired GI function and nutritional deficiencies, profoundly impact their recovery and overall prognosis. This underscores the necessity for innovative and effective treatment approaches [
27,
28]. In this context, the exploration of alternative treatments, particularly HM, emerges as a promising solution. Prior studies indicate that herbal treatments have the potential to significantly enhance GI function, nutritional status, and immune function, while potentially mitigating complications associated with gastric cancer surgery [
29,
30]. This study was undertaken to systematically investigate the efficacy of HM in addressing these challenges and assess its impact on the recovery of patients with early GC. The focus areas include GI recovery, nutrition, immune response, and overall QoL. Through the exploration of these critical aspects, the study aims to provide valuable insights into postoperative care for GC, contributing to the existing body of knowledge and investigating alternative therapies that could markedly improve patient outcomes.
Our study evaluated HM's effects on patients with early GC in detail, focusing on GI recovery, nutrition, immune response, and overall QoL. In the GI recovery assessment, we observed significant improvements in patients treated with HM, particularly in the time to first flatus (P < .00001), time to first bowel movement (P = .002), and time to bowel sounds return (P = .0003), compared to the control group. This improvement is crucial as it directly impacts the overall health and recovery speed post-surgery [
31].
Nutritionally, our study found significant improvements in ALB, PA, and TP levels among patients with early GC treated with HM, with ALB levels (P = .0008) and PA levels (P < .0001) improving markedly, and TP levels also showing significant enhancements (P = .01). Although the results for TRF were not statistically significant, these overall findings underscore the potential of HM in enhancing the postoperative nutritional status in patients with GC. Proper nutrition is essential for the healing and well-being of post-surgical patients [
32].
In terms of immune response, patients with early GC treated with HM showed significant improvements in immune markers, with CD3+ levels (P = .0006) and CD4+ levels (P = .0005) improving significantly. However, CD8+ levels and the CD4+/CD8+ ratio did not show significant changes. indicating a complex and varied impact of HM on the immune system post-surgery. CD4, another T cell surface protein, is mainly used to identify helper T cells. Helper T cells play a role in regulating the immune response by providing instructions to other immune cells. CD8, a surface protein predominantly expressed on cytotoxic T cells, is involved in the direct destruction of infected or abnormal cells. CD8 T cells contribute to immune defense by exerting cytotoxic effects. The CD4/CD8 ratio indicates the relative numbers of helper T cells and cytotoxic T cells. In a healthy immune state, this ratio is balanced. An abnormal ratio may indicate immune dysfunction [
33]. These findings suggest that while HM may enhance certain immune functions in patients with early GC, its effects on different immune markers can vary.
In assessing the QoL of patients with early GC treated with HM, our study showed mixed results. The SF-36 assessment, involving 172 participants, indicated a non-significant improvement in QoL (P = .13), while the WHOQOL-BREF and QLQ-C30 assessments, each with 120 participants, demonstrated significant enhancements in QoL (both with P < .00001). This suggests that HM may have a substantial positive impact on certain QoL aspects in patients with early GC, although the effects vary based on the measurement tool.
In the safety evaluation, the pooled data from eight studies, encompassing a total of 631 participants, revealed an RR of 0.48 with a 95% CI [0.31, 0.74]. This significant result (P = .0009) indicates that patients in the HM group were less likely to experience adverse events compared to those in the control group. The analysis showed no heterogeneity (I² = 0%), suggesting consistent findings across the included studies. This evidence underscores the potential of HM as a safer alternative in the postoperative management of patients with GC, with a reduced risk of adverse effects.
Given that most studies involved a usual care control group compared to the HM treatment group, there are inherent limitations. Furthermore, the unknown efficacy and safety profiles when HM is used in combination with other drugs highlight the need for additional research. To address these challenges, future investigations should focus on reducing heterogeneity through the development of standardized preparations. Additionally, the establishment of a core outcome set is imperative to ensure consistent outcome indicators.
Through this study, we have affirmed the effectiveness of HM treatment in the recovery process following early GC surgery. The significant impact of HM treatment on improving patients' GI function and QoL should be noted. We posit that HM treatment could serve as a viable alternative for the recovery of patients with GC in clinical practice. However, it is crucial to acknowledge the presence of high heterogeneity in the RCTs due to diverse target groups and variations in prescribed HMs.
5. Conclusions
This study substantiates the effectiveness of herbal medicine in enhancing recovery after GC surgery. HM exhibits significant positive effects on improving patients' bowel function and overall quality of life. Our findings indicate that incorporating postoperative HM treatment for patients with GC could be a practical consideration in clinical practice.
Nevertheless, as highlighted earlier, it is crucial to recognize that variations in subjects and prescriptions contribute to considerable heterogeneity. Therefore, we recommend the implementation of long-term clinical trials or follow-ups with rigorous criteria. This aims to observe lasting effects and also to evaluate potential side effects, providing a comprehensive understanding of the enduring impact of herbal medicine treatment in the context of patient recovery after GC surgery. Additional research is deemed essential.
Author Contributions
S.D. Kim and S.J. Pyo are co-first authors and contributed equally to the study design and the drafting of the manuscript. S.D. Kim contributed to the statistical design and analysis. D.H. Kim contributed to the review & editing of the manuscript. H.S. Yoo and S.J. Park conceived the concept of the study and are supervising its implementation. All of the authors have read and approved the final manuscript.
Funding
This study was supported by the Korean Health Industry Development Institutes (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (No. HI19C1046 and HF20C0204).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We would also like to thank Editage (
www.editage.co.kr) for English language editing.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Annual report of cancer statistics in Korea in 2020. National Cancer Center. 2022.
- National Cancer Information Center. 2019. Available online: https://www.cancer.go.kr/ (accessed on 30 October 2023).
- Yang, K.; Lu, L.; Liu, H.; et al. A comprehensive update on early gastric cancer: defining terms, etiology, and alarming risk factors. Expert Rev Gastroenterol Hepatol 2021, 15, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.B.; Agnesi, S.; Scaravaglio, M.; et al. Early Gastric Cancer: Update on Prevention, Diagnosis and Treatment. Int J Environ Res Public Health 2023, 20, 2149. [Google Scholar] [CrossRef] [PubMed]
- Schütte, K.; Schulz, C.; Middelberg-Bisping, K. Impact of gastric cancer treatment on quality of life of patients. Best Pract Res Clin Gastroenterol 2021, 50–51, 101727. [Google Scholar] [CrossRef]
- McCall, M.D.; Graham, P.J.; Bathe, O.F. Quality of life: A critical outcome for all surgical treatments of gastric cancer. World J Gastroenterol 2016, 22, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.T.; Yang, P.R.; Shen, Y.C.; Yang, Y.H.; Wu, C.Y. Traditional Chinese Medicine Enhances Survival in Patients with Gastric Cancer after Surgery and Adjuvant Chemotherapy in Taiwan: A Nationwide Matched Cohort Study. Evid Based Complement Alternat Med 2021, 2021, 7584631. [Google Scholar] [CrossRef] [PubMed]
- Gunji, S.; Ueda, S.; Yoshida, M.; Kanai, M.; Terajima, H.; Takabayashi, A. Effects of rikkunshito, a kampo medicine, on quality of life after proximal gastrectomy. J Surg Res 2013, 185, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.L. Effect of modified Bazhen decoction treatment on the quality of life of patients with gastric cancer after surgery. Journal of Guangzhou University of Traditional Chinese Medicine 2020, 28, 134–136. [Google Scholar]
- Gao, X.; Li, Y.; Wang, N. Effect of Buqi Recipe Combined with Enteral Nutrition on Postoperative Immune Function and Intestinal Function of Patients with Gastric Cancer. Journal of Guangzhou University of Traditional Chinese Medicine 2020, 37, 2103–2108. [Google Scholar]
- Ji, S.W.; Kang, J.Y. Effects of Dachengqi Decoction combined with Mosapride Citrate Tablets on gastrointestinal motility function and gastrointestinal hormone levels in patients after radical resection of gastric cancer. Chinese Folk Therapy 2020, 28, 83–84. [Google Scholar]
- Liu, B.; Wang, Y.M.; Zhou, S.Y. Efficacy of Qixue Shuangbu Formula Combined with Enteral Nutrition in the Treatment of Deficiency of Both Qi and Blood After Laparoscopic Surgery for Gastric Cancer. World Traditional Chinese Medicine 2020, 15, 2941–2944. [Google Scholar]
- Chen, N.; Liu, H.; Ni, Z.Q.; et al. Effects of Modified Chaishao Liujunzi Decoction Combined with Enhanced Recovery after Surgery on Rehabilitation of Laparoscopic Gastric Cancer Surgery. Journal of Hunan University of Traditional Chinese Medicine 2020, 40, 626–629. [Google Scholar]
- Lu, S.J. Clinical study on Jianpiliqi prescription in promoting the recovery of intestinal motility after radical resection of gastric cancer. Electronic Journal of Integrated Traditional Chinese and Western Medicine in Cardiovascular Diseases 2018, 6, 162–164. [Google Scholar]
- Ma, X.L. Influences of Fuyuan Tang Small Intestine Instillation on Fatigue, T-Lymphocyte Subset Complication of Patients with Radical Operation for Stomach Carcinoma. China Traditional Chinese Medicine Science and Technology 2018, 25, 834–835. [Google Scholar]
- Guo, W.B. Clinical observation of 28 cases of enteral nutritional support and traditional Chinese medicine combined after gastric cancer surgery. Chinese Ethnic and Folk Medicine 2018, 27, 71–73. [Google Scholar]
- Ge, S.Y.; Sun, B.; Du, H.W.; et al. Observation on the efficacy of ginseng and dahuang decoction in improving gastrointestinal motility disorder caused by qi stagnation and blood stasis in patients after gastric cancer surgery. Chinese Journal of Chronic Disease Prevention and Control 2018, 26, 617–619. [Google Scholar]
- Ye, Z.; Gu, H.G.; Yang, J.Y.; et al. Effects of Tongfu Huoxue decoction combined with Glutamine on the recovery of oxidative stress, vintestinal mucosal barrier function and gastrointestinal function after radical operation of gastric cancer. Hebei Traditional Chinese Medicine 2018, 40, 68–72. [Google Scholar]
- He, Y.M.; Liu, S.E.; Wang, L.; et al. Research on TCM syndrome differentiation and treatment of early gastric cancer after radical surgery with combined anesthesia. Journal of Modern Integrated Traditional Chinese and Western Medicine 2017, 26, 955–957. [Google Scholar]
- Pan, A.X.; Shi, P.S.; Ye, G.D.; et al. Research on the efficacy of Sini Tongli decoction instilled in the small intestine to promote postoperative intestinal function recovery in patients with gastric cancer. Nursing and Rehabilitation 2016, 15, 783–784. [Google Scholar]
- Zou, L.N.; Mo, D.L.; Yang, S.; et al. Clinical observation on early enteral nutrition combined with traditional Chinese medicine for strengthening the spleen and tonifying the bowels after laparoscopic radical gastrectomy for gastric cancer to promote postoperative gastrointestinal function recovery. New Traditional Chinese Medicine 2016, 48, 192–194. [Google Scholar]
- Liui, Z.W. The Observation of the Fast Track with Shengyangyiwei Decoction after Laparoscopic Distal Gastrectomy. MA Thesis, Guangzhou University of Traditional Chinese Medicine; p. 2016.
- Akamaru, Y.; Takahashi, T.; Nishida, T.; et al. Effects of daikenchuto, a Japanese herb, on intestinal motility after total gastrectomy: a prospective randomized trial. J Gastrointest Surg 2015, 19, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, W. Effects of Xiaoaitang based on the pathogenesis theory of cancerous toxin combined with laparoscopic assisted distal gastrectomy in the treatment of elderly patients with gastric cancer. Journal of Practical Oncology 2018, 33, 222–227. [Google Scholar]
- Wobith, M.; Weimann, A. Postoperative Nutrition Management: Who Needs What? Visc Med 2022, 38, 354–362. [Google Scholar] [CrossRef]
- Martínez-Ortega, A.J.; Piñar-Gutiérrez, A.; Serrano-Aguayo, P.; et al. Perioperative Nutritional Support: A Review of Current Literature. Nutrients 2022, 14, 1601. [Google Scholar] [CrossRef] [PubMed]
- Baji, D.B.; Patel, J.P.; Konanur Srinivasa, N.K.; Gande, A.; Anusha, M.; Dar, H. Nutrition Care in Cancer Surgery Patients: A Narrative Review of Nutritional Screening and Assessment Methods and Nutritional Considerations. Cureus 2022, 14, e33094. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Oh, Y.; Park, S.H.; Kwon, Y.; Park, S. Postoperative nutritional outcomes and quality of life-related complications of proximal versus total gastrectomy for upper-third early gastric cancer: a meta-analysis. Sci Rep 2020, 10, 21460. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, Z.; Tang, X. [Study on early application of Chinese medicinal herbs after total gastrectomy]. Zhongguo Zhong Xi Yi Jie He Za Zhi 1999, 19, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Park, B.; Noh, H.; Choi, D.J. Herbal Medicine for Dumping Syndrome: A Systematic Review and Meta-Analysis. Integr Cancer Ther 2019, 18, 1534735419873404. [Google Scholar] [CrossRef]
- Chapman, S.J.; Thorpe, G.; Vallance, A.E.; Harji, D.P.; Lee, M.J.; Fearnhead, N.S. Systematic review of definitions and outcome measures for return of bowel function after gastrointestinal surgery. BJS Open 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Serra, F.; Pedrazzoli, P.; Brugnatelli, S.; et al. Nutritional support management in resectable gastric cancer. Drugs Context 2022, 11, 2022–5. [Google Scholar] [CrossRef]
- McBride, J.A.; Striker, R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog 2017, 13, e1006624. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).