Submitted:
15 May 2024
Posted:
16 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
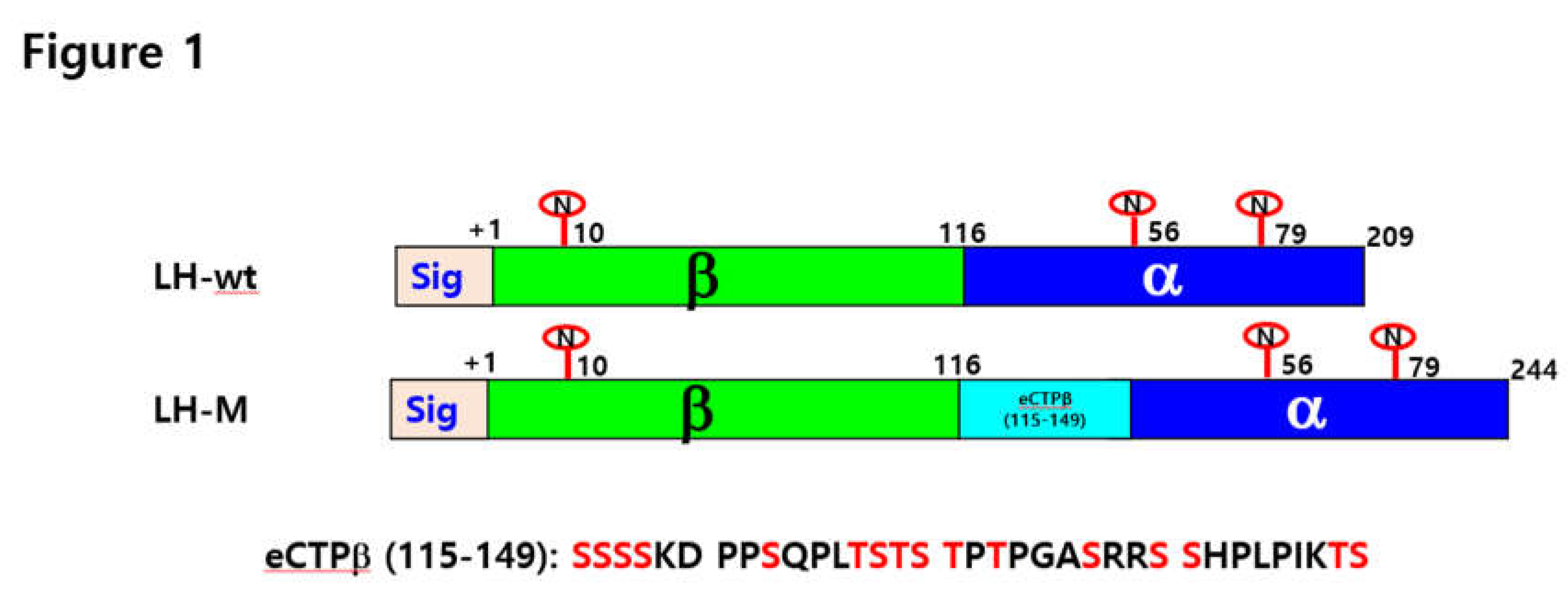
2.2. Vector Construction of Eel LH-wt and LH-M
2.3. Transfection into CHO DG44 Cells
2.4. Single Cell Isolation and Production of the Rec-Eel LH-wt and LH-M Proteins
2.5. Quantitation and Western Blotting of the rec-Eel LH Proteins
2.6. Analysis of cAMP Levels via Homogenous Time-Resolved Fluorescence Assays
2.7. Phospho-ERK1/2 Time Course
2.8. Data Analysis
3. Results
3.1. Isolation of Single Cells after Transfection
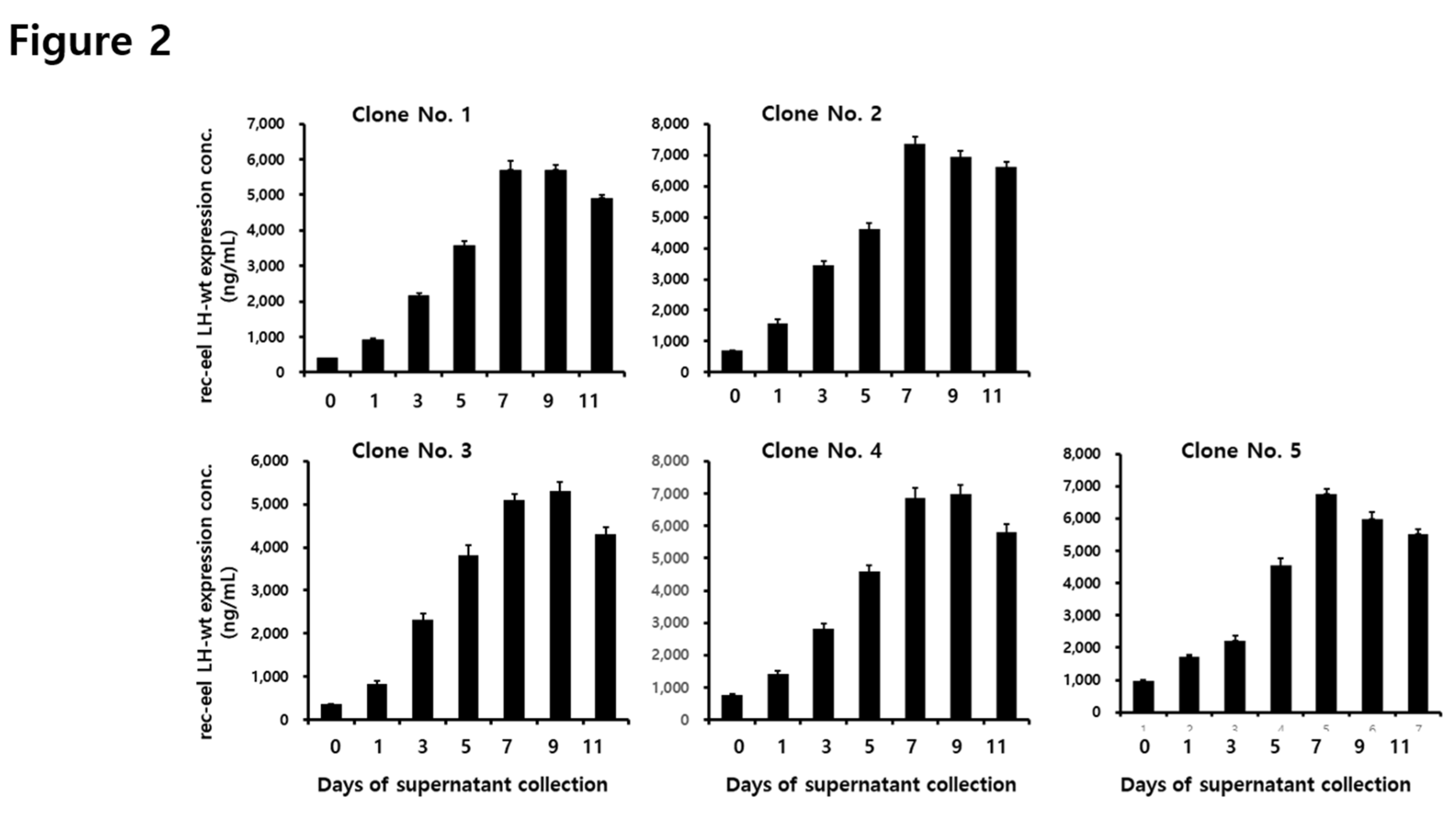
3.2. Secreted Quantity of the rec-eel LH-wt Protein
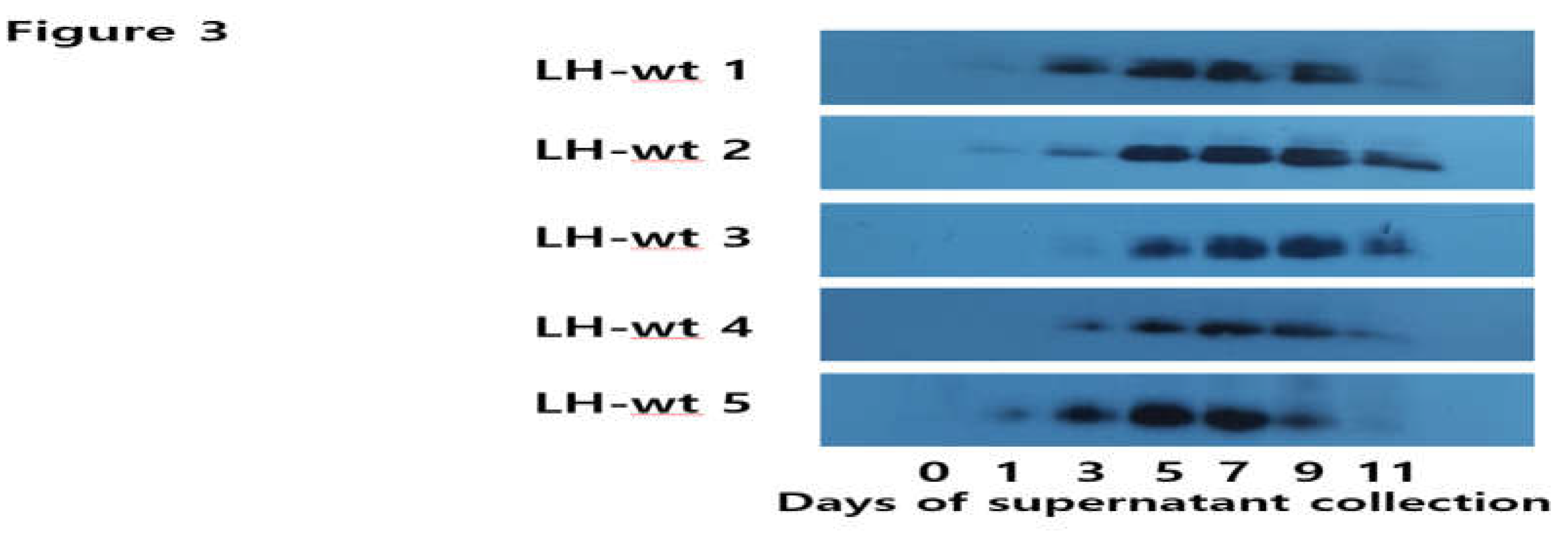
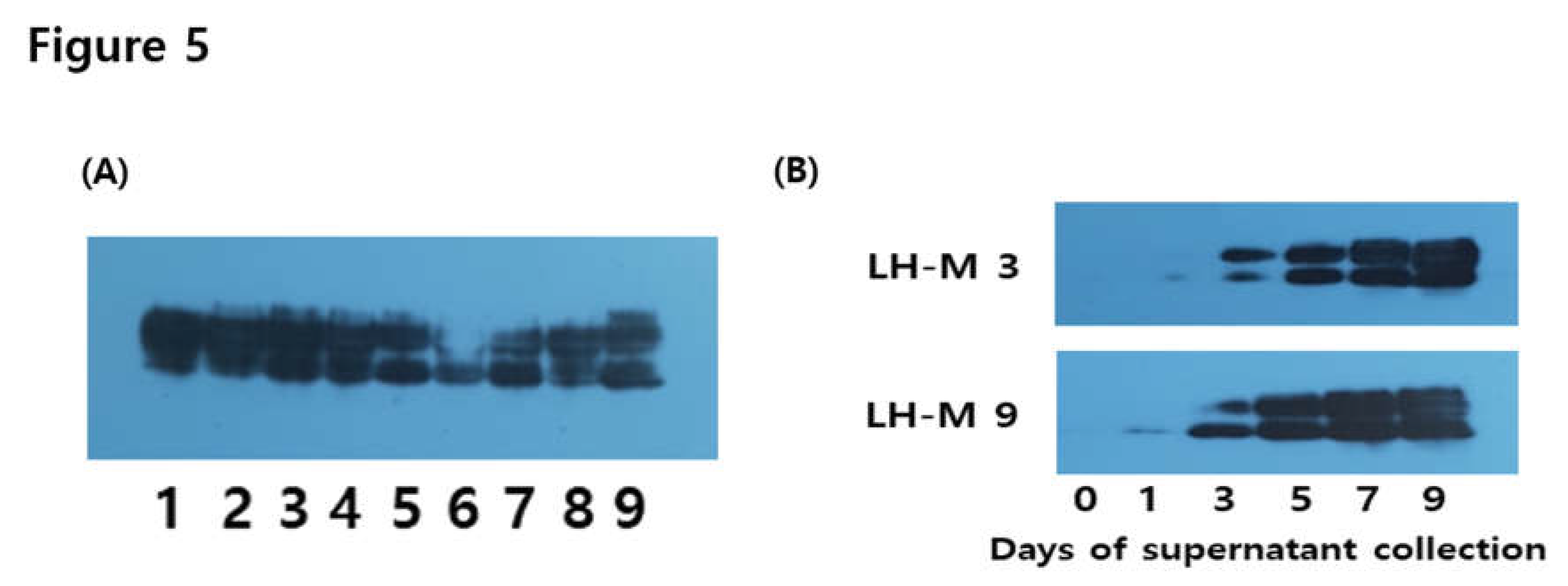
3.3. Western Blotting for rec-eel LH-wt
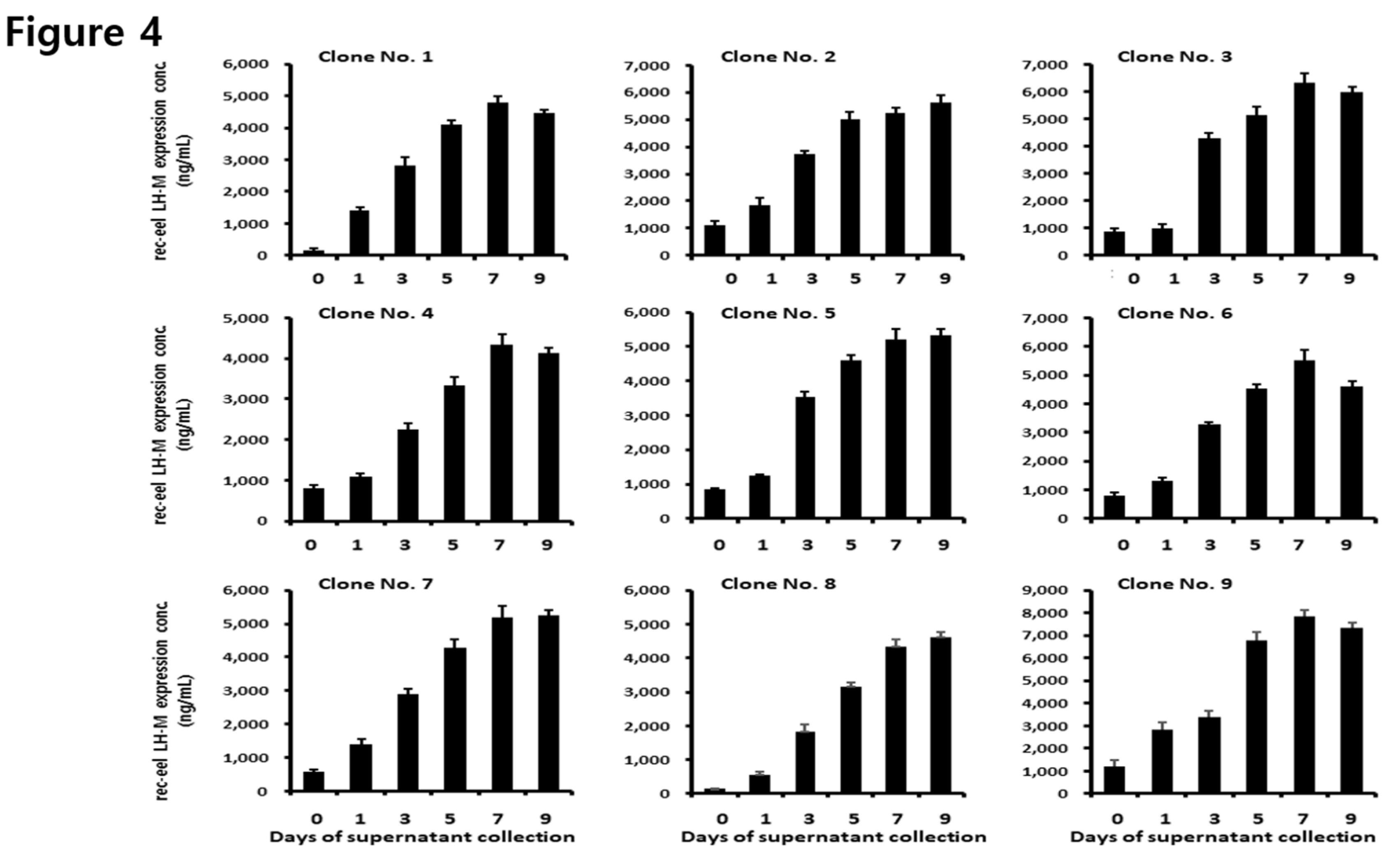
3.4. Secreted Quantity of the rec-eel LH-M Protein
3.5. Western Blotting for eel FSH-M
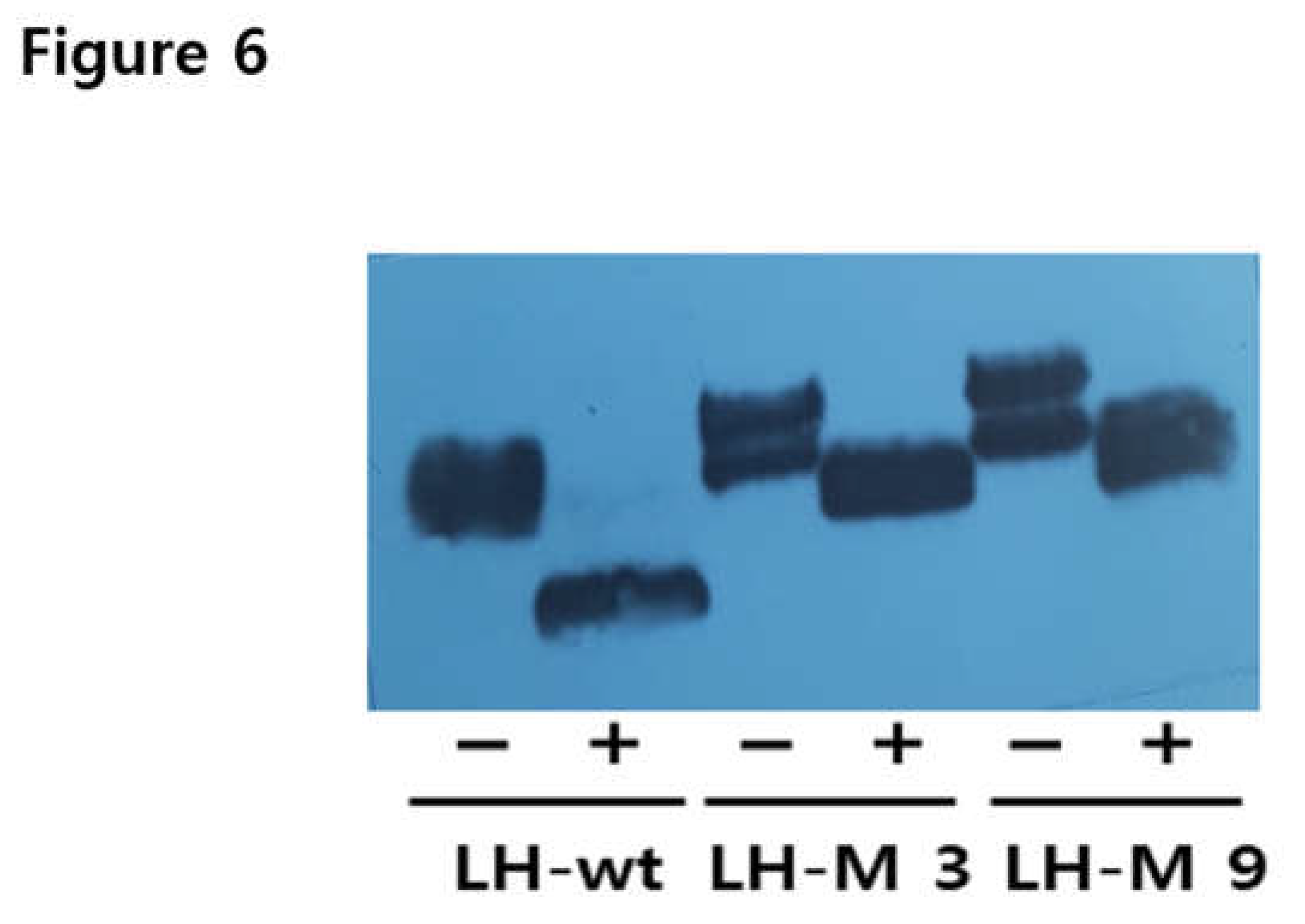
3.6. Deglycosylation of rec-eel LH-wt and LH-M Proteins
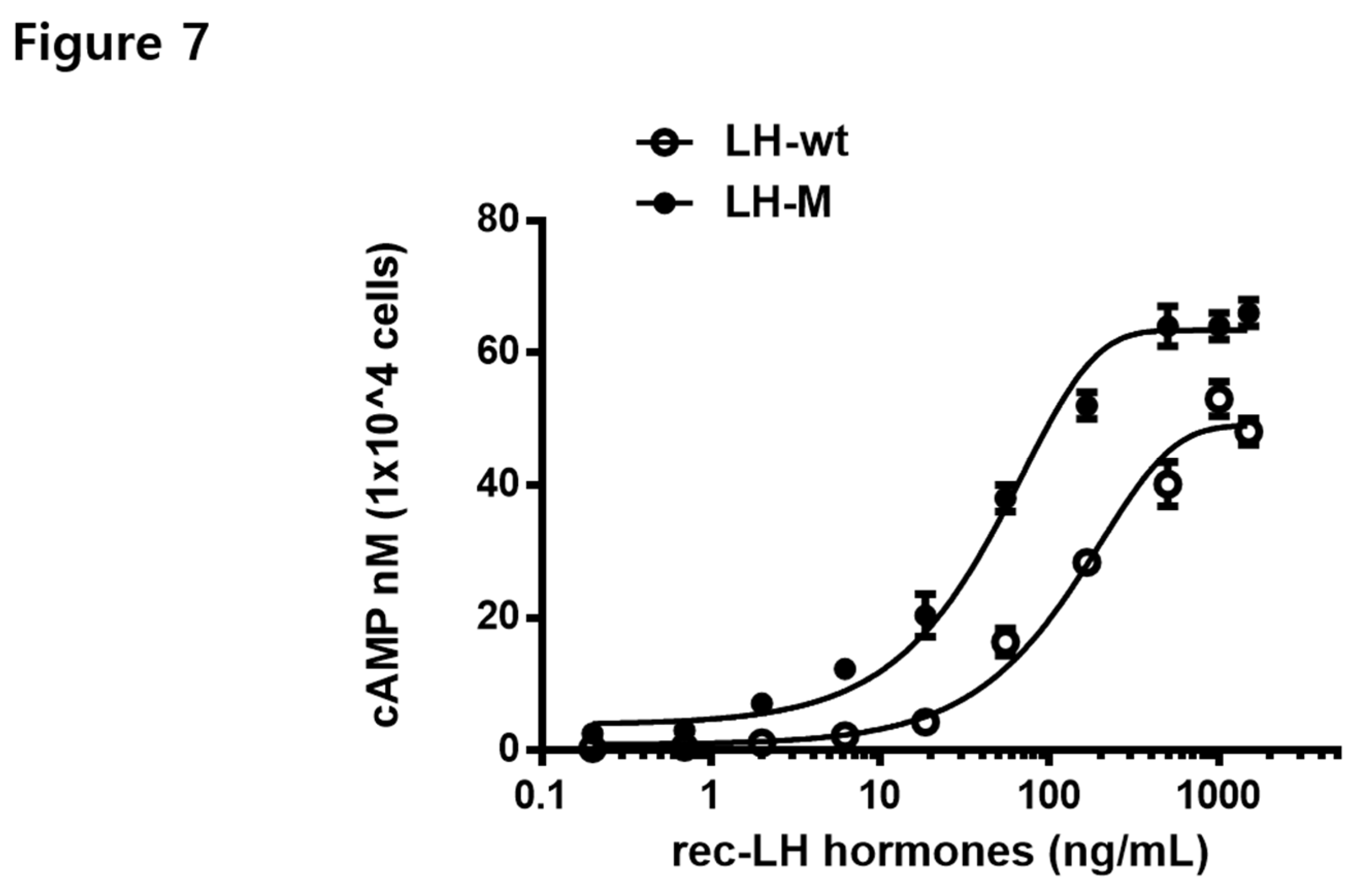
3.7. Biological Activities of the rec-eel LH-wt and LH-M Proteins
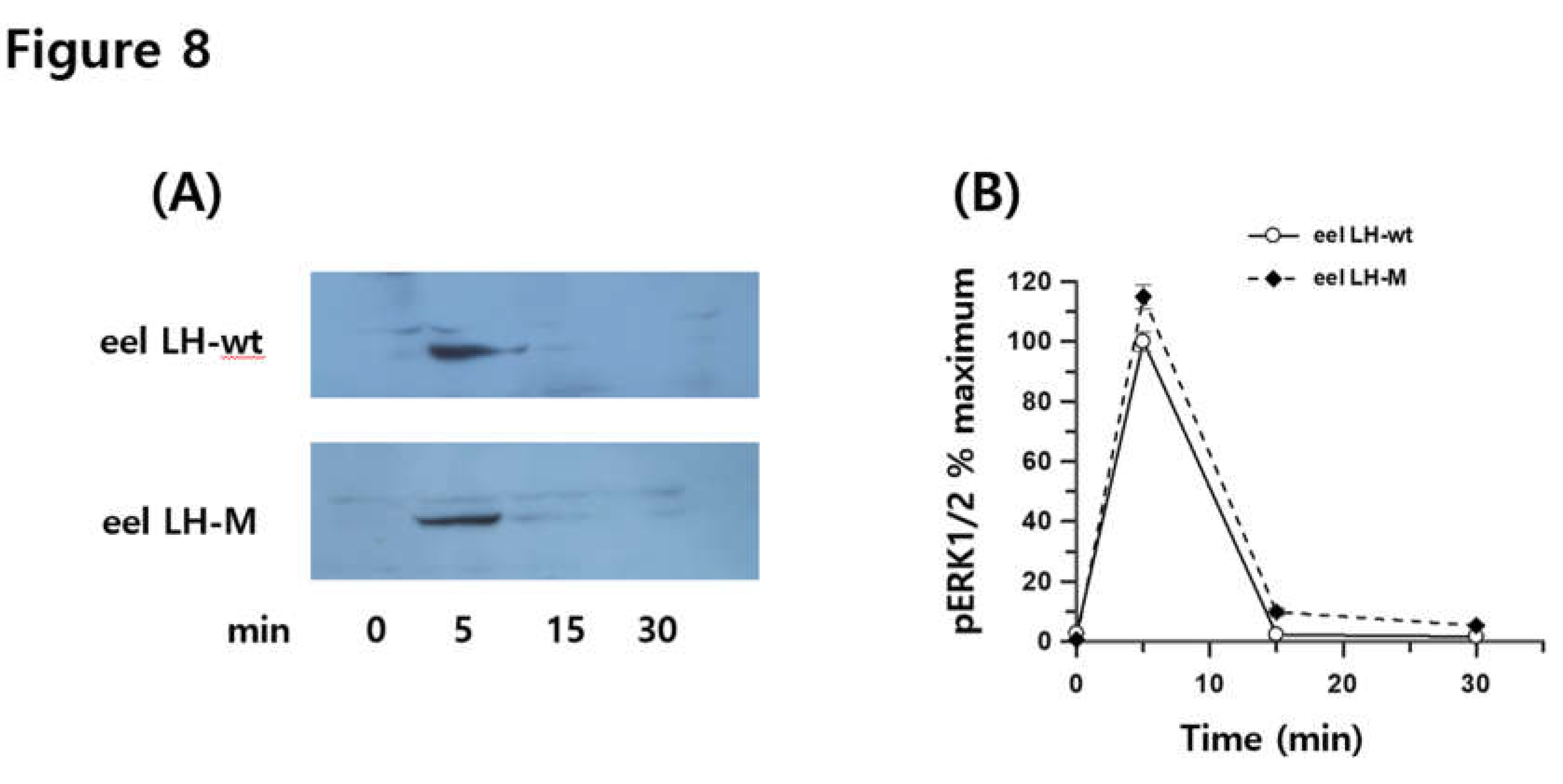
3.8. Phospho-ERK1/2 Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Min, K.S.; Park, J.J.; Byambaragchaa, M.; Kang, M.H. Characterization of tethered equine chorionic gonadotropin and its deglycosylated mutants by ovulation stimulation in mice. BMC Biotechnol. 2019, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Park, J.J.; Lee, S.Y.; Byambragchaa, M.; Kang, MH. Comparative gene expression profiling of mouse ovaries upon stimulation with natural equine chorionic gonadotropin (N-eCG) and tethered recombinant-eCG (R-eCG). BMC Biotechnol. 2020, 20, 59. [Google Scholar] [CrossRef]
- Talmadge, K.; Vamvakopoulos, N.C.; Fiddes, J. Evolution of the genes for the β subunits of human chorionic gonadotropin and luteinizing hormone. Nature 1984, 307, 37–40. [Google Scholar] [CrossRef]
- Bousfield, G.R.; Sugino, H.; Ward, D. Structural studies on equine glycoprotein hormones: Amino acid sequence of equine lutropin β-subunit. J. Biol. Chem. 1985, 260, 9531–9533. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.J.; Tregear, G.W.; Niall, H.D. The nucleotide sequences of baboon chorionic gonadotropin β-subunit genes have diverged from the human. Gene 1986, 46, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Sugino, H.; Bousefield, G.; Moore, W.T.; Ward, D.N. Structural studies on equine glycoprotein hormones: Amino acid sequence of equine chorionic gonadotropin β subunit. J. Biol. Chem. 1987, 262, 8603–8609. [Google Scholar] [CrossRef]
- Sherman, G.B.; Wolfe, M.W.; Farmerie, T.A.; Clay, C.M.; Threadgill, D.S.; Sharp, D.C.; Nilson, J.H. A single gene encodes the β-subunits of equine luteinizing hormone and chorionic gonadotropin. Mol. Endocrinol. 1992, 6, 951–959. [Google Scholar]
- Min, K.S.; Hattori, N.; Aikawa, J.I.; Shiota, K.; Ogawa, T. Site-directed mutagenesis of recombinant equine chorionic gonadotropin/luteinizing hormone: differential role of oligosaccharides in luteinizing hormone- and follicle-stimulating hormone-like activities. Endocrine J. 1996, 43, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Hiyama, T.; Seong, H.W.; Hattori, N.; Tanaka, S.; Shiota, K. Biological activities of tethered equine chorionic gonadotropin (eCG) and its deglycosylated mutants. J. Reprod. Dev. 2004, 50, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.C.; Parsons, T.F. Glycoprotein hormones: structure and function. Annu. Rev. Biochem. 1981, 50, 465–495. [Google Scholar] [CrossRef] [PubMed]
- Byambaragchaa, M.; Kim, D.J.; Kang, M.H.; Min, K.S. Site specificity of eel luteinizing hormone N-linked oligosaccharides in signal transduction. Gen. Comp. Endocrinol. 2018, 268, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Park, C.W.; Kim, D.W.; Park, H.K.; Byambaragchaa, M.; Lee, N.S.; Hong, S.M.; Kang, M.H.; Min, K.S. Production and characterization of monoclonal antibodies against recombinant tethered follicle-stimulating hormone from Japanese eel Anguilla japonica. Gen. Comp. Endocrinol. 2016, 233, 8–15. [Google Scholar] [CrossRef]
- Bishop, L.A.; Robertson, D.M.; Cahir, N.; Schofield, P.R. Specific roles for the asparagine-linked carbohydrate residues of recombinant human follicle stimulating hormone in receptor binding and signal transduction. Mol. Endocrinol. 1994, 8, 722–731. [Google Scholar] [PubMed]
- Flack, M.R.; Froehlich, J.; Bennet, A.P.; Anasti, J.; Nisula, B.C. Site-directed mutagenesis defines the individual roles of the glycosylation sites on follicle-stimulating hormone. J. Biol. Chem. 1994, 269, 14015–14020. [Google Scholar] [CrossRef] [PubMed]
- Valove, F.M.; Finch, C.; Anasti, J.N.; Froehlich, J.; Flack, M.R. Receptor binding and signal transduction are dissociable functions requires different sites on follicle-stimulating hormone. Endocrinology 1994, 135, 2657–2661. [Google Scholar] [CrossRef] [PubMed]
- Fares, F. The role of O-linked and N-linked oligosaccharides on the structure-function of glycoprotein hormones: Development of agonists and antagonists. Biochim. Biophys. Acta 2006, 1760, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Boeta, M.; Zarco, L. Luteogenic and luterotropic effects of eCG during pregnancy in the mare. Anim. Reprod. Sci. 2012, 130, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Munkhuu, O.; Bambaragchaa, M.; Lee, B.I.; Kim, S.K.; Kang, M.H.; Kim, D.J.; Min, K.S. Site-specific roles of N-linked oligosaccharides in recombinant eel follicle-stimulating hormone for secretion and signal transduction. Gen. Comp. Endocrinol. 2019, 276, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Byambaragchaa, M.; Kang, H.J.; Choi, S.H.; Kang, M.H.; Min, K.S. Specific roles of N- and O-linked oligosaccharide sites on biological activity of equine chorionic gonadotropin (eCG) in cells expressing rat luteinizing hormone/chorionic gonadotropin receptor (LH/CGR) and follicle-stimulating hormone receptor (FSHR). BMC Biotechnol. 2021, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Hayakawa, Y.; Park, W.; Banba, A.; Yoshizaki, G.; Kumamura, K.; Kagawa, H.; Nagaya, H.; Sohn, Y.C. Production of recombinant Japanese eel gonadotropins by baculovirus in silkworm larvae. Gen. Comp. Endocrinol. 2010, 167, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.M.; Choi, J.H.; Jo, S.J.; Min, K.S.; Kim, D.J.; Lee, J.M.; Kusakabe, T. Heterologous production and glycosylation of Japanese eel follitropin using silkworm. Biotechnol. Bioprocess Eng. 2019, 24, 745–753. [Google Scholar] [CrossRef]
- Kazeto, Y.; Kohara, M.; Miura, T.; Miura, C.; Yamaguchi, S.; Trant, J.M.; Adachi, S.; Yamaguchi, K. Japanese eel follicle-stimulating hormone (fsh) and luteinizing hormone (lh)): production of biologically active recombinant fsh and lh by Drosophila S2 cells and their differential actions on the reproductive biology. Biol. Reprod. 2008, 79, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Kamei, H.; Ohira, T.; Yoshiura, Y.; Uchida, N.; Nagasawa, H.; Aida, K. Expression of a biologically active recombinant follicle stimulating hormone of Japanese eel Anguilla japonica using methylotropic yeast, Pichia pastoris. Gen. Comp. Endocrinol. 2003, 134, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Miyake, H.; Miura, C.; Kamei, H.; Aida, K.; Miura, T. Follicle-stimulating hormone induces spermatogenesis mediated by androgen production in Japanese eel, Anguilla japonica. Biol. Reprod. 2007, 77, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Byambaragchaa, M.; Kim, S.G.; Park, S.H.; Shin, M.G.; Kim, S.K.; Kang, M.H.; Min, K.S. Production of recombinant single-chain eel luteinizing hormone and follicle-stimulating hormone analogs in Chinese hamster ovary suspension cell culture. Curr. Issues Mol. Biol. 2024, 46, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Byambaragchaa, M.; Park, A.; Gil, S.J.; Lee, H.W.; Ko, Y.J.; Choi, S.H.; Kang, M.H.; Min, K.S. Luteinizing hormone-like and follicle-stimulating hormone-like activities of equine chorionic gonadotropin β-subunit mutants in cells expressing rat luteinizing hormone/chorionic gonadotropin receptor and rat follicle-stimulating hormone receptor. Anim. Cells Syst. 2021, 25, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Fares, F.; Suganuma, N.; Hishimori, K.; Lapolt, P.S.; Hsueh, A.J.W.; Boime, I. Design of a long-acting follitropin agonist by fusing the C-terminal sequence of the chorionic gonadotropin β subunit to the follitropin β subunit. Proc. Natl. Acad. Sci. USA 1992, 89, 4304–4308. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Shikone, T.; Fares, F.A.; Sugahara, T.; Hsueh, A.J.W.; Boime, I. Construction of gonadotropin analogs fusing the carboxylterminal peptide of the CGβ subunit to the common α-subunit: O-linked glycosylation and in vivo bioactivity of chimeric hCG. Mol. Endocrinol. 1995, 9, 54–63. [Google Scholar]
- Joshi, L.; Murata, Y.; Wondisford, F.E.; Szkudlinski, M.W.; Desai, R.; Weintraub, B.D. Recombinant thyrotropin containing a β-subunit chimera with the human chorionic gonadotropin-β carboxy terminal is biologically active with a prolonged plasma half-life: role of carbohydrate in bioactivity and metabolic clearance. Endocrinology 1994, 136, 3839–3848. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Grootenhuis, P.D.J.; Sato, A.; Kudo, M.; Ben-Menahem, D.; Pixley, M.R.; Hsueh, A.J.W.; Boime, I. Expression of biological active fusion genes encoding the common α subunit and either the CGβ or FSHβ subunits: role of a linker sequence. Mol. Cell. Endocrinol. 1996, 125, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Sato, A.; Kudo, M.; Ben-Menahem, D.; Pixley, M.R.; Hsueh, A.J.W.; Boime, I. Expression of biological active fusion genes encoding the common α subunit and the follicle-stimulating hormone β subunits. J. Biol. Chem. 1996, 271, 10445–10448. [Google Scholar] [CrossRef] [PubMed]
- Fares, F.; Ganem, S.; Hajouj, T.; Agai, E. Development of a long-acting erythropoietin by fusing the carboxyl-terminal peptide of human chorionic gonadotropin β subunit to the coding sequence of human erythropoietin. Endocrinology 2007, 148, 5081–5087. [Google Scholar] [CrossRef] [PubMed]
- Fares, F.; Guy, R.; Bar-Ilan, A.; Felikman, Y.; Fima, E. Designing a long-acting human growth hormone (hGH) by fusing the carboxyl-terminal peptide of human chorionic gonadotropin β-subunit to the coding sequence of hGH. Endocrinology 2010, 151, 4410–4417. [Google Scholar] [CrossRef] [PubMed]
- Hershkovitz, O.; Bar-Ilan, A.; Guy, R.; Felikman, Y.; Moschcovich, L.; Hwa, V.; Rosenfeld, R.G.; Fima, E.; Hart, G. In vitro and in vivo characterization of MOD-4023, a long-acting carboxyl-terminal peptide (CTP)-modified human growth hormone. Mol. Pharmaceutics. 2016, 13, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Strasburger, C.J.; Vanuga, P.; Payer, J.; Pfeifer, M.; Popovic, V.; Bajnok, L.; Goth, M.; Olsovska, V.; Trejbalova, L.; Vadasz, J.; Fima, E.; Koren, R.; Amitzi, L.; Bidlingmaier, M.; Hershkovitz, O.; Hart, G.; Biller, B.M.K. MOD-4023, a long-acting carboxyl-terminal peptide-modified human growth hormone: results of a Phase 2 study in growth hormone-deficient adults. European J. Endocrinol. 2017, 176, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Zelinska, N.; Lotova, V.; Skorodok, J.; Maliesky, O.; Peterkova, V.; Samsonova, L.; Rosenfeld, R.G.; Zadik, Z.; Jaron-Mendelson, M.; Koren, R.; Amitzi, L.; Raduk, D.; Hershkovitz, O.; Hart, G. Long-acting C-terminal peptide-modified hGH (MOD-4023): results of a safety and dose-finding study in GHD children. J. Clin. Endocrinol. Metab. 2017, 102, 1578–1587. [Google Scholar] [CrossRef]
- Tao, Y.X.; Abell, A.N.; Liu, X.; Nakamura, K.; Segaloff, DL. Constitutive activation of G protein-coupled receptors as a result of selective substitution of a conserved leucine residue in transmembrane helix III. Mol. Endocrinol. 2000, 14, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, R.S.; Ascoli, M. The post-endocytotic fate of the gonadotropin receptors is an important determinant of the desensitization of gonadotropin responses. J. Mol. Endocrinol. 2005, 34, 447–457. [Google Scholar] [CrossRef]
- Martinelle, N.; Holst, M.; Soder, O.; Svechnikov, K. Extracellular signal-regulated kinases are involved in the acute activation of steroidogenesis in immature rat Leydig cells by human chorionic gonadotropin. Endocrinology 2004, 145, 4629–4639. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X. Inactivation mutations of G protein-couped receptors and disease: Structure-function insights and therapeutic implications. Pharmacol. Ther. 2006, 111, 949–973. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Ascoli, M. Lutropin/choriogonadotropin stimulate the proliferation of primary cultures of rat Leydig cells through a pathway that involves activation of the extracellularly regulated kinase 1/2 cascade. Endocrinology 2007, 148, 3214–3225. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.K.; Barak, L.S.; Xiao, K.; Ahn, S.; Berthouze, M.; Shukla, A.K.; Luttrell, L.M.; Lefkowitz, R.J. Ubiquitination of β-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J. Biol. Chem. 2007, 282, 29549–29562. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.K.; Draka, M.T.; Nelson, C.D.; Houtz, D.A.; Xiao, K.; Madabushi, S.; Reiter, E.; Premont, R.T.; Lichtarge, O.; Lefkowitz, R.J. β-arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J. Biol. Chem. 2006, 281, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Slosky, L.M.; Bai, Y.; Toth, K.; Ray, C.; Rochelle, L.K.; Badea, A.; Chandrasekhar, R.; Pogorelov, V.M.; Abraham, D.M.; Atluri, N.; Peddibhotla, S.; Hedrick, M.P.; Hershberger, P.; Maloney, P.; Yuan, H.; Li, Z.; Wetsel, W.C.; Pinkerton, A.B.; Barak, L.S.; Caron, M.G. β-arrestin-biased allosteric modulated of NTSR1 selectively attenuates addictive behaviors. Cell 2020, 181, 1364–1379. [Google Scholar] [CrossRef]
- Kara, E.; Crepieux, P.; Gauthier, C.; Martinat, N.; Piketty, V.; Guillou, F.; Reiter, E. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for β-arrestin-mediated ERK activation. Mol. Endocrinol. 2006, 20, 3014–3026. [Google Scholar] [CrossRef] [PubMed]
- Piketty, V.; Kara, E.; Guillou, F.; Reiter, E.; Crepiux. P. Follicle-stimulating hormone (FSH) activates extracellular signal-regulated kinase phosphorylation independently of beta-arrestin- and dynamin-mediated FSH receptor internalization. RBE. 2006, 4, 33.
- Banerjee, A.A.; Dupakuntla, M.; Pathak, B.R.; Mahale, S.D. FSH receptor-specific residues L501 and I505 in extracellular loop 2 are essential for its function. J. Mol. Endocrinol. 2015, 54, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.A.; Mahale, S.D. Extracellular loop 3 substitutions K589N and A590S in FSH receptor increase FSH-induced receptor internalization and along with S588T substitution exhibit impaired ERK1/2 phosphorylation. Arch. Biochem. Biophys. 2018, 659, 57–65. [Google Scholar] [CrossRef]
- Metta, M.K.; Kunaparaju, R.K.; Tantravaji, S. Rapid amplification system for recombinant protein production in Chinese hamster ovary (CHO) cells. Cell. Mol. Biol. 2016, 62, 101–106. [Google Scholar] [PubMed]
- Meta. A.; Hirashima, M.; Imamura, T.; Kawamura, R.; Yano, K.; Uehara, K.; Nakashima, T. High-yield preparation of recombinant human α-thrombin for therapeutic use. J. Biosci. Bioeng. 2015, 120, 432–437.
- Chin, C.L.; Chin, H.K.; Chin, C.S.H.; Lai, E.T.; Ng, S.K. Engineering selection stringency on expression vector for the production of recombinant human alpha 1-antitrypsin using Chinese hamster ovary cells. BMC Biotechnol. 2015, 15, 44. [Google Scholar] [CrossRef]
- Jazayeri, S.H.; Amiri-Yekta, A.; Gourabi, H.; Emami, B.A.; Halfinezhad, Z.; Abolghasemi, S.; Fatemi, N.; Daneshipour, A.; Ghahremani, M.H.; Sanati, M.H.; Khorramizadeh, M.R. Comparative assessment on the expression level of recombinant human follicle-stimulating hormone (FSH) in serum-containing versus protein-free culture media. Mol. Biotechnol. 2017, 59, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Thennati, R.; Singh, S.K.; Nage, N.; Patel, Y.; Bose, S.K.; Burade, V.; Ranbhor, R.S. Analytical characterization of recombinant hCG and comparative studies with reference product. Biologics 2018, 12, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Crispo, M.; Meikle, M.N.; Schlapp, G.; Menchaca, A. Ovarian superstimulatory response and embryo development using a new glycoprotein with eCG-like activity in mice. Theriogenology 2021, 164, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.C.; Mussio, P.E.; Villarraza, J.; Tardivo, M.B.; Antuna, S.; Fontana, D.; Ceaglio, N.; Prieto, C. Physiochemical characterization of a recombinant eCG and comparative studies with PMSG commercial preparations. The Protein J, 2023, 42, 24–36.
- Kamei, H.; Kawazoe, I.; Kanekko, T.; Aida, K. Purification of follicle-stimulating hormone from immature Japanese eel Anguilla japonica, and its biochemical properties and steroidogenic activities. Gen. Comp. Endocrinol. 2005. 143, 257–256. [CrossRef]
- Fares, F.; Yamada, S.; Ben-Menahem, D.; Pixley, M.; Hsueh, A.J.W.; Boime, I. Conversion of thyrotropin heterodimer to a biologically active single-chain. Endocrinology 1998, 139, 2459–2468. [Google Scholar] [CrossRef]
- Kramer, W.G.; Jaron-Mendelson, M.; Koren, R.; Hershkovitz, O.; Hart, G. Pharmacokinetics, pharmacodynamics, and safety of a long-acting human growth hormone (MOD-4023) in healthy Japanese and Caucasian adults. Clin. Pharmacol. Drug Dev. 2018, 7, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; Hsueh, A.J.; Lapolt, P.; Tsafriri, A.; Keene, J.L.; Boime, I. The biological role of the carboxyl-terminal extension of human chorionic gonadotropin beta-subunit. Endocrinology 1990, 126, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, R.J.; Shenoy, S.K. Transduction of receptor signals by β-arrestins. Science 2005, 308, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Reiter, E.; Lefkowitz, R.J. GRKs and β-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. 2006, 17, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Ascoli, M. Potential Leydig cell mitogenic signals generated by the wild-type and constitutively active mutants of the lutropin/choriogonadotropin receptor (LHR). Mol. Cell. Endocrinol. 2007, 260-262, 244–248.
- Shiraishi, K.; Ascoli, M. A co-coculture system reveals the involvement of intercellular pathways as mediators of the lutropin receptor (LHR)-stimulated ERK1/2 phosphorylation in Leydig cells. Exp. Cell Res. 2008, 314, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.R.; Reiter, E.; Ahn, S.; Kim, J.; Chen, W.; Lefkowitz, R.J. Different G protein-coupled receptor kinases govern G protein and β-arrestin-mediated signaling of V2 vasopressin receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Gesty-Palmer, D.; Chen, M.; Reiter, E.; Ahn, S.; Nelson, C.D.; Wang, S.; Eckhardt, A.E.; Cowan, C.L.; Spurney, R.F.; Luttrell, L.M.; Lefkowitz, R.J. Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-mediated ERK1/2 activation. J. Biol. Chem. 2006, 281, 10856–10864. [Google Scholar] [CrossRef] [PubMed]
- Moller, T.C.; Pedersen, M.F.; van Senten, J.R.; Seiersen, S.D.; Mathiesen, J.M.; Bouvier, M.; Brauner-Osborne, H. Dissecting the roles of GRK2 and GRK3 in mu-opioid receptor internalization and β-arrestin2 recruitment using CRISPR/Cas9-edited HEK293 cells. Sci. Rep. 2020, 10, 173955. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; McGlone, E.R.; Fang, Z.; Pickford, P.; Correa, I.R.Jr.; Oishi, A.; Jockers, R.; Inoue, A.; Kumar, S.; Gorlitz, F.; Dunsby, C.; French, P.M.W.; Rutter, G.A.; Tan, T.; Bloom, S.R. Genetic and biased agonist-mediated reductions in β-arrestin recruitment prolong cAMP signaling at glucagon family receptors. J. Biol. Chem. 2021, 296, 100133. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Shenoy, K.K.; Wei, H.; Lefkowitz, R.J. Differential kinetic and spatial patterns of β-arrestin and G protein mediated ERK activation by the angiotensin II receptor. J. Biol. Chem. 2004, 279, 35518–35525. [Google Scholar] [CrossRef] [PubMed]
|
rec-LH hormones |
cAMP responses | |||||
| Basala (nM / 104 cells) |
Log (EC50) (ng/mL) |
Rmaxb (nM / 104 cells) |
||||
| LH-wt | 0.9 ± 0.5 |
138.8 (116.6 to 171.4)c |
49.0 ± 1.2 |
|||
| LH-M | 2.1 ± 0.8 |
47.8 (40.1 to 57.4) |
63.4 ± 1.5 |
|||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





