1. Introduction
The yellow-legged gull (
Larus michahellis Naumann, 1840) inhabits the Mediterranean coast of Southern Europe, the Black Sea, and the Caspian Sea, as well as the Azores and Madeira, the Canary Islands, the Iberian Peninsula, the Middle East, and North Africa [
1]. The global population is unknown due to recent taxonomic divisions, but there is an increasing trend both globally and in Europe [
2]. The European population was estimated between 409,000 and 534,000 pairs in 2015 [
3]. Cama and Arcos [
4] calculated the wintering population in Spain at about 230,000 individuals in 2010, and Molina et al. [
5] assessed a minimum of around 125,000 breeding pairs in 2007-2009.
It was classified in the global and European Red Lists as "Least Concern" [
2] due to the population increase in the late 20th and early 21st centuries. This increase is attributed to its adaptability in utilizing new food sources such as organic waste from open landfills, discarded fishery by-products, and processed food from marine aquaculture farms [
3,
4,
6,
7,
8,
9]. However, in the Red List of Spain in 2022, it has been categorized as "Near Threatened" due to a "considerable decline in population size, albeit heterogeneously and more pronounced in the Cantabrian-Galician population of the lusitanius subspecies" [
10], attributed to the closure of numerous landfills [
9,
11,
12].
It is included in Annex I of Royal Decree 1095/89, which designates the species subject to hunting and fishing in Spain. Additionally, the Bern Convention includes it in Annex III, where species whose exploitation will be regulated to keep them out of danger are listed [
4].
The Law 7/1995, of April 21, on wild fauna, hunting, and river fishing in the Region of Murcia (Boletín Oficial del Estado No. 131, June 2, 1995), states that it is a species not classified as threatened. Furthermore, the Law 7/2003, of November 12, on hunting and river fishing in the Region of Murcia, considers the yellow-legged gull a huntable species (Boletín Oficial del Estado No. 47, February 24, 2004).
The nesting population in the Region of Murcia was estimated at around 8,700-10,600 pairs in 2007-2009 [
5], distributed across islands in both the Mediterranean Sea and the Mar Menor, some cliffs, rooftops of coastal buildings, and the Regional Park of Salinas y Arenales de San Pedro del Pinatar. The population has shown a continuous increase since the first two pairs in 1993, reaching a peak of over 300 pairs in 2016. However, it is likely that there would be more than 1,000 pairs if action were not taken each year to eliminate their nests and eggs [
13].
Various studies have been conducted on the impact of the increasing population and distribution area of
L. michahellis on changes in the composition of flora and fauna in the Iberian Peninsula. This impact occurs through predation on chicks of various bird species, both terrestrial (e.g., partridges) and aquatic (waders, herons, gulls, etc.), as well as through competition for nesting sites with other species of waders and gulls [
9,
14,
15,
16,
17]. Studies have also been conducted showing soil changes in locations where colonies and roosts of
L. michahellis are situated due to nutrient inputs, leading to changes in flora composition and consequently habitat [
18,
19,
20,
21,
22].
In some areas, management and nest control programs for
L. michahellis colonies have been implemented through partial elimination and/or eradication, considered successful by some authors [
23,
24,
25,
26]. However, others consider these efforts as of limited efficacy or having a short-term scope, as they often focus on alleviating local consequences without addressing the underlying causes or understanding the demographic consequences of the actions [
10,
27,
28].
Nevertheless, there is a lack of comprehensive studies analyzing the environmental, social, and economic consequences of the increase in the population of L. michahellis in a territory. Additionally, there are no published works evaluating the long-term results of management actions on the reproduction of L. michahellis, such as those carried out jointly in the Salinas de San Pedro del Pinatar. These actions involve government interventions for biodiversity conservation, occupational safety, and salt production, combined with deterrent initiatives by the company managing the salt flats through the placement of deterrent elements to prevent damage in salt extraction and stacking areas.
The Administration of the Region of Murcia initiated the control of L. michahellis in the Salinas de San Pedro del Pinatar in the year 2000 with the aim of maintaining the main breeding areas for shorebirds free from L. michahellis nests and preventing the risk of accidents with saltworks workers. During the breeding season, gulls engage in low flights, and there are even cases of attacks on individuals approaching their nests.
The objectives of this study were: (1) to identify the effects of L. michahellis on nesting waterbirds, workers, and salt production activities, (2) to quantify the results of the control methods implemented by the Administration between 2000-2021, as well as deterrent actions by Salinera Española to prevent damage to salt production, and finally, (3) to develop proposals for controlling the L. michahellis population at its source by adopting measures that hinder its access to anthropogenic food sources, with special attention to organic matter in municipal solid waste landfills, marine aquaculture farms, and fishery discards.
2. Materials and Methods
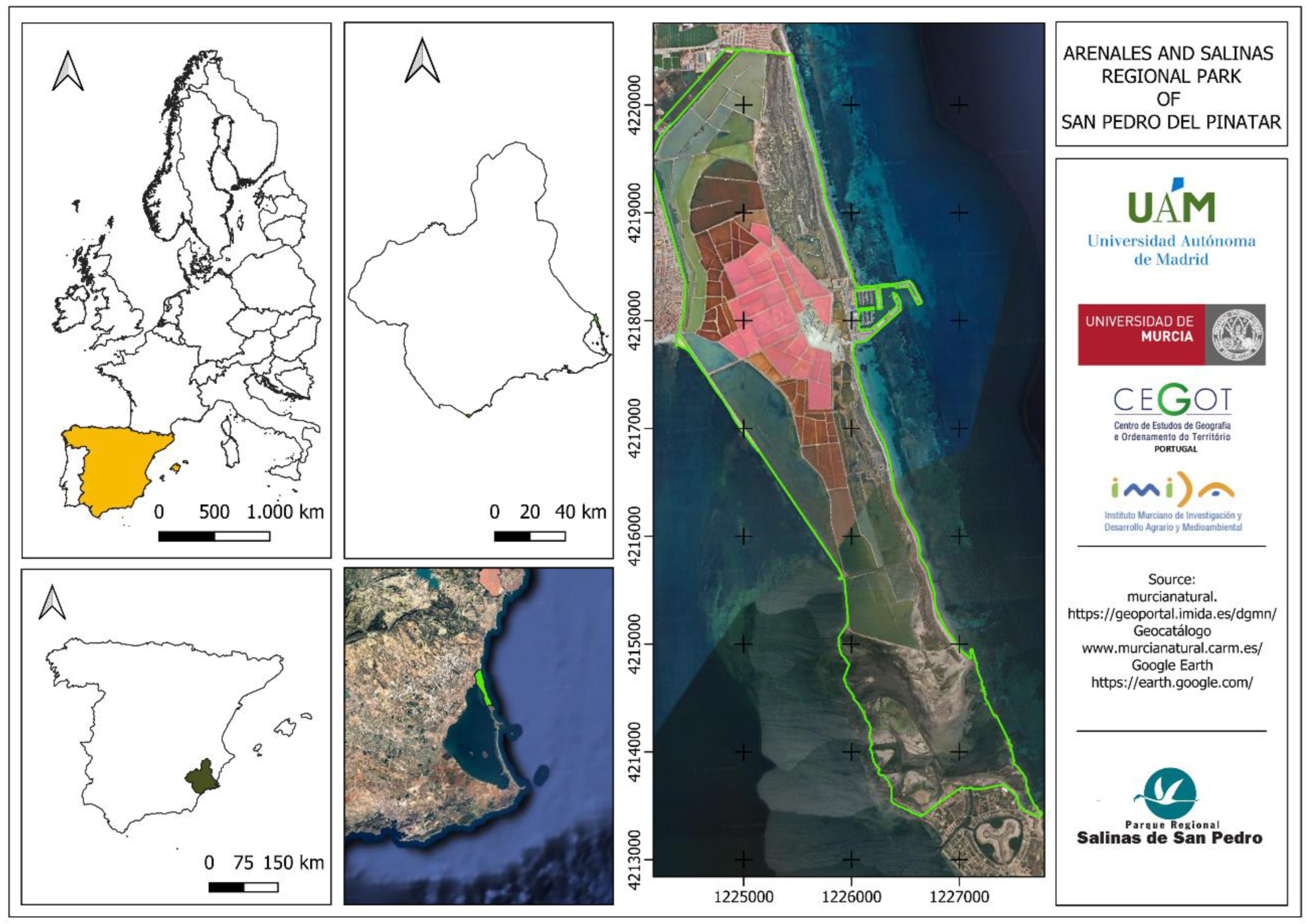
2.1. Study Area
The Regional Park of the Salinas y Arenales de San Pedro del Pinatar covers an area of 856 ha, located in the northernmost coastal area of the Region of Murcia, Spain. It is characterized by a flat morphology and a wide variety of environments. Among these environments are salt ponds, which, depending on their function in the salt production process, are referred to as "storage, heating, or crystallization" pools (
Figure 1).
The total surface area of all salt ponds adapted for salt production is 470 ha, with an annual production of approximately 80-100 thousand tons. The salt pans coexist with sandy areas and salt marshes typical of wetland areas and other sedimentary ecosystems of beaches and lagoon coastlines. Additionally, the park includes the Encañizadas, a pseudomareal area formed by shallow waters and channels between small islands connecting the Mar Menor with the Mediterranean Sea, harboring significant populations of waterbirds at the national, European, and even global levels [
29].
Out of the 170 bird species with regular presence, 32 species stand out as they are listed in Annex I of the Birds Directive (2009/147/EC). The European Union designated the Salinas de San Pedro del Pinatar as a Special Protection Area for Birds (SPA) due to the nesting pairs of common avocet (
Recurvirostra avosetta), black-winged stilt (
Himantopus himantopus), little tern (
Sternula albifrons), and gull-billed tern (
Gelochelidon nilotica). Moreover, the park hosts significant populations in Spain of Audouin's gull (
Ichthyaetus audouinii), Sandwich tern (
Thalasseus sandvicensis), slender-billed gull (
Larus genei), Kentish plover (
Charadrius alexandrinus), and common tern (
Sterna hirundo) [
30].
The sandy dikes separating the salt ponds are constructed and reinforced with sedimentary material from the basins, and their composition varies depending on whether they are "storage," "heating," or "crystallization" ponds. The water flowing through the salt pans is pumped from the Mar Menor and enters the "storage" ponds, where solid particles settle, but salt precipitation is minimal. Subsequently, the water is directed to the "heating" basins, where intense evaporation occurs, leading to an increase in sodium chloride (NaCl) concentration. Before the water passes to the crystallization ponds, part of the sodium chloride (NaCl) deposits in the bottom of the "storage" pond basins, along with significant amounts of calcium carbonate (CaCO
3), calcium sulfate (CaSO
4), magnesium sulfate (MgSO
4), and magnesium chloride (MgCl
2). Therefore, the bottom of the "heating" ponds has a substrate where materials with high NaCl, CaCO
3, CaSO
4, MgSO
4, and MgCl
2 content have accumulated. These compounds are entirely used to build and maintain the dikes separating the salt ponds, hindering vegetation growth and providing optimal habitats for the reproduction of various waterbird colonies [
29].
The sandy dikes separating the salt ponds are constructed and reinforced with sedimentary material from the basins, and their composition varies depending on whether they are "storage," "heating," or "crystallization" ponds. The water flowing through the salt pans is pumped from the Mar Menor and enters the "storage" ponds, where solid particles settle, but salt precipitation is minimal. Subsequently, the water is directed to the "heating" basins, where intense evaporation occurs, leading to an increase in sodium chloride (NaCl) concentration. Before the water passes to the crystallization ponds, part of the sodium chloride (NaCl) deposits in the bottom of the "storage" pond basins, along with significant amounts of calcium carbonate (CaCO
3), calcium sulfate (CaSO
4), magnesium sulfate (MgSO
4), and magnesium chloride (MgCl
2). Therefore, the bottom of the "heating" ponds has a substrate where materials with high NaCl, CaCO
3, CaSO
4, MgSO
4, and MgCl
2 content have accumulated. These compounds are entirely used to build and maintain the dikes separating the salt ponds, hindering vegetation growth and providing optimal habitats for the reproduction of various waterbird colonies [
29].
2.2. Methodology Used
The results of nesting waterbird and yellow-legged gull (
L. michahellis) population control censuses conducted in the Regional Park of the Salinas y Arenales de San Pedro del Pinatar (Murcia, Spain) between 1990 and 2021 have been analyzed. Additionally, winter bird censuses coordinated by the Association of Naturalists of the Southeast [
13,
31,
32,
33,
34] have been considered.
Winter bird censuses, including
L. michahellis, have been carried out in the Salinas de San Pedro del Pinatar since 1990 using a standardized methodology developed by experienced ornithologists. These censuses follow fixed transects covering all salt ponds, conducted in the early hours of the day with suitable optical equipment, counting the total number of individuals present in the wetland [
31]. On the other hand, censuses of nesting waterbirds have been conducted since 1994, involving the direct counting of all nests on the dikes separating the salt ponds [
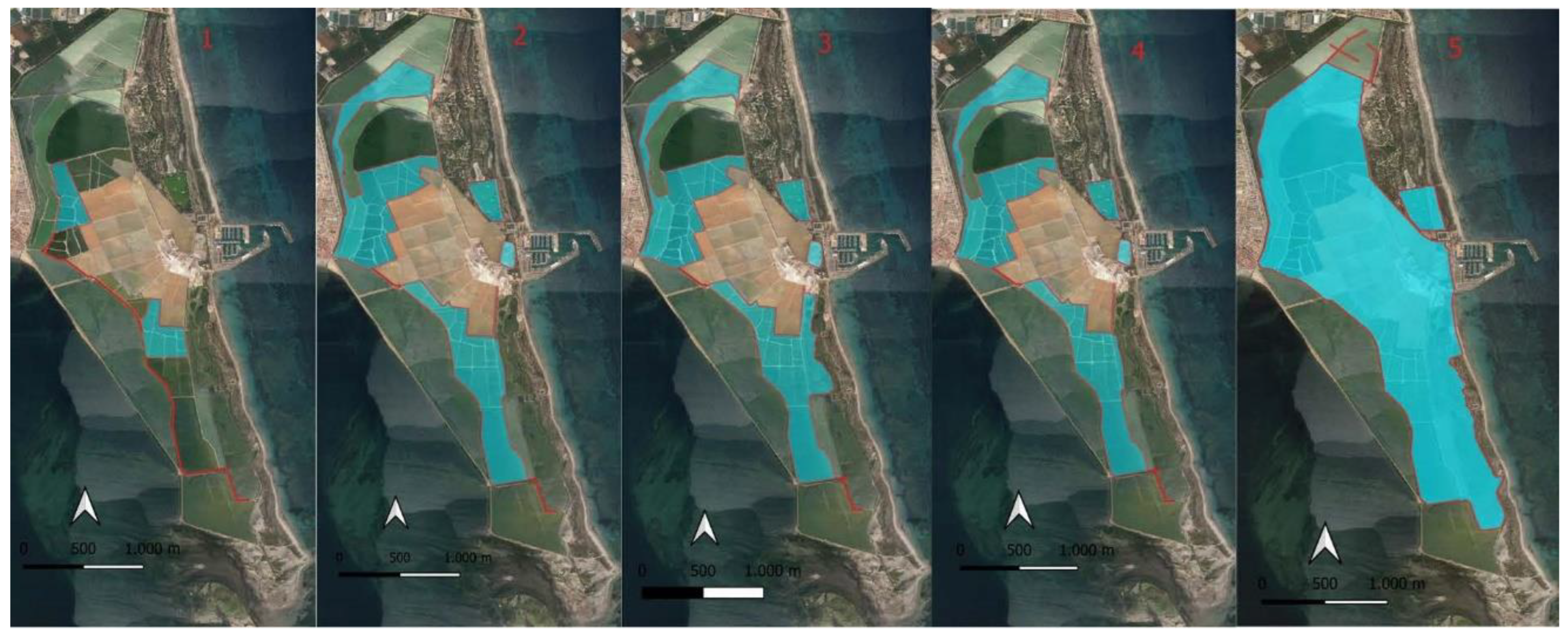
13]. Population control of the breeding yellow-legged gull has been carried out since the year 2000 by eliminating nests and eggs between the last week of March and the first week of June along the dikes separating the salt ponds (
Figure 2).
In the first year of yellow-legged gull control measures (year 2000), nests and eggs were removed along the most frequently used pathway by saltworks workers. This area also housed some colonies of common avocet (
R. avosetta) and common tern (
S. hirundo), which had experienced a reduction in nesting pairs in 1999 due to the increase in the yellow-legged gull population. In 2001, an attempt was made to puncture the eggs of nests located along the pathways, as these paths were traversed daily by saltworks employees. However, the results were not as expected, as many eggs still hatched. Therefore, this trial was not repeated [
24]. Between 2002 and 2005, nest control was expanded to other frequently traveled areas within the Salinas, although breeding pairs were detected on dikes separating salt ponds that were not usually traversed by saltworks workers and were located at a certain distance from colonies of other waterbirds. From 2006 to 2018, the control area for
L. michahellis was expanded again to include all accessible pathways within the Salinas, as well as some dikes separating salt ponds located next to the beaches south of the port. Finally, between 2019 and 2021, as part of the LIFE-SALINAS Project (LIFE17 NAT/ES/000184), comprehensive actions were taken on all pathways and dikes separating salt ponds within the Salinas de San Pedro del Pinatar. This included crystallization ponds, dikes separating salt ponds, and dunes parallel to the beaches south of the port.
3. Results
3.1. Evolución of the Population of L. michahellis
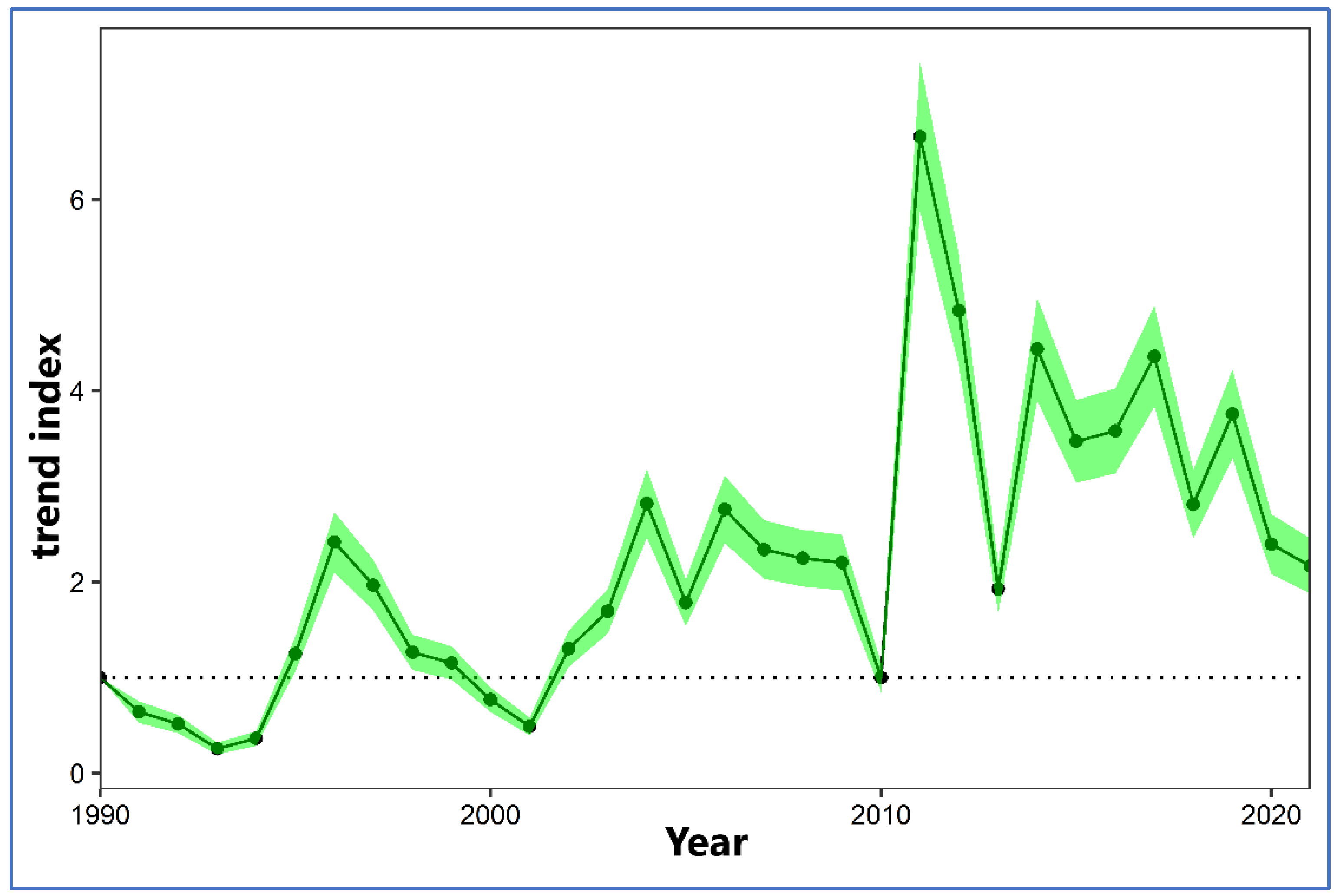
The wintering population of
L. michahellis in the Regional Park of the Salinas y Arenales de San Pedro del Pinatar shows a significant trend with a strong increase (
Figure 3). The average population has been on the rise since the early 1990s when the wintering population was around 100-200 individuals, reaching a peak of 2,113 individuals in 2011, and experiencing a decline in the early 21st century with around a hundred individuals (1990-1999: average 345 individuals; 2000-2009: average 578 individuals; 2020-2021: average 628 individuals).
In the Salinas de San Pedro del Pinatar, two pairs of L. michahellis bred for the first time in 1993, showing a growing trend until reaching 227 pairs in the year 2000. From 2001 onwards, control measures for L. michahellis were initiated, resulting in a reduction in the number of pairs with successful reproduction, ranging between 130 and 302 pairs. Meanwhile, the number of nests and their eggs eliminated varied, with a minimum of 454 nests removed in 2006 and a maximum of 588 nests removed in 2010.
The most extensive nest control coverage for L. michahellis was achieved in 2017, reducing the number of pairs with successful reproduction to 132, mostly located in the Encañizadas area. In this region, control efforts are considered insufficient due to the instability of the marshy, tidal terrain, which is largely inaccessible on foot.
3.2. Landscape and Environmental Impacts
The contribution of organic matter from hundreds of yellow-legged gull specimens, depositing guano as they roost in the salt pans and islets of the Encañizadas since the early nineties, represents a significant increase in nutrients in the soil. This guano deposition leads to modifications in the chemical composition of the substrate, favoring changes in vegetation composition. This effect is more pronounced in those areas of the Salinas where the separation dikes of salt ponds have not been constructed or repaired with material from the saline substrate of the heating ponds.
Although quantitative evaluations of habitat composition variations have not been conducted in the Salinas de San Pedro del Pinatar to date, there is a notable development in vegetation coverage and density. In some sectors where there was little to no vegetation decades ago, the coverage has increased to over 75% of the surface and, in some cases, even 100% of the total area. This represents a significant loss of optimal habitat for the reproduction of waterfowl, which relocate their breeding colonies to areas with less than 50% vegetation coverage.
Nesting waterfowl censuses conducted in the Regional Park of the Salinas y Arenales de San Pedro del Pinatar between 1994 and 2021 indicate that the 8 nesting species listed in Annex I of Directive 2009/147/EC of November 30, 2009, concerning the conservation of wild birds (Birds Directive): Himantopus himantopus, Recurvirostra avosetta, Charadrius alexandrinus, Ichthyaetus audouinii, Gelochelidon nilotica, Sterna hirundo, Sternula albifrons, and Thalasseus sandvicensis, use the separation dikes of salt ponds with sparse vegetation coverage, as well as islets and beaches in the Encañizadas area, which are not frequented by people and/or domestic animals, as nesting sites.
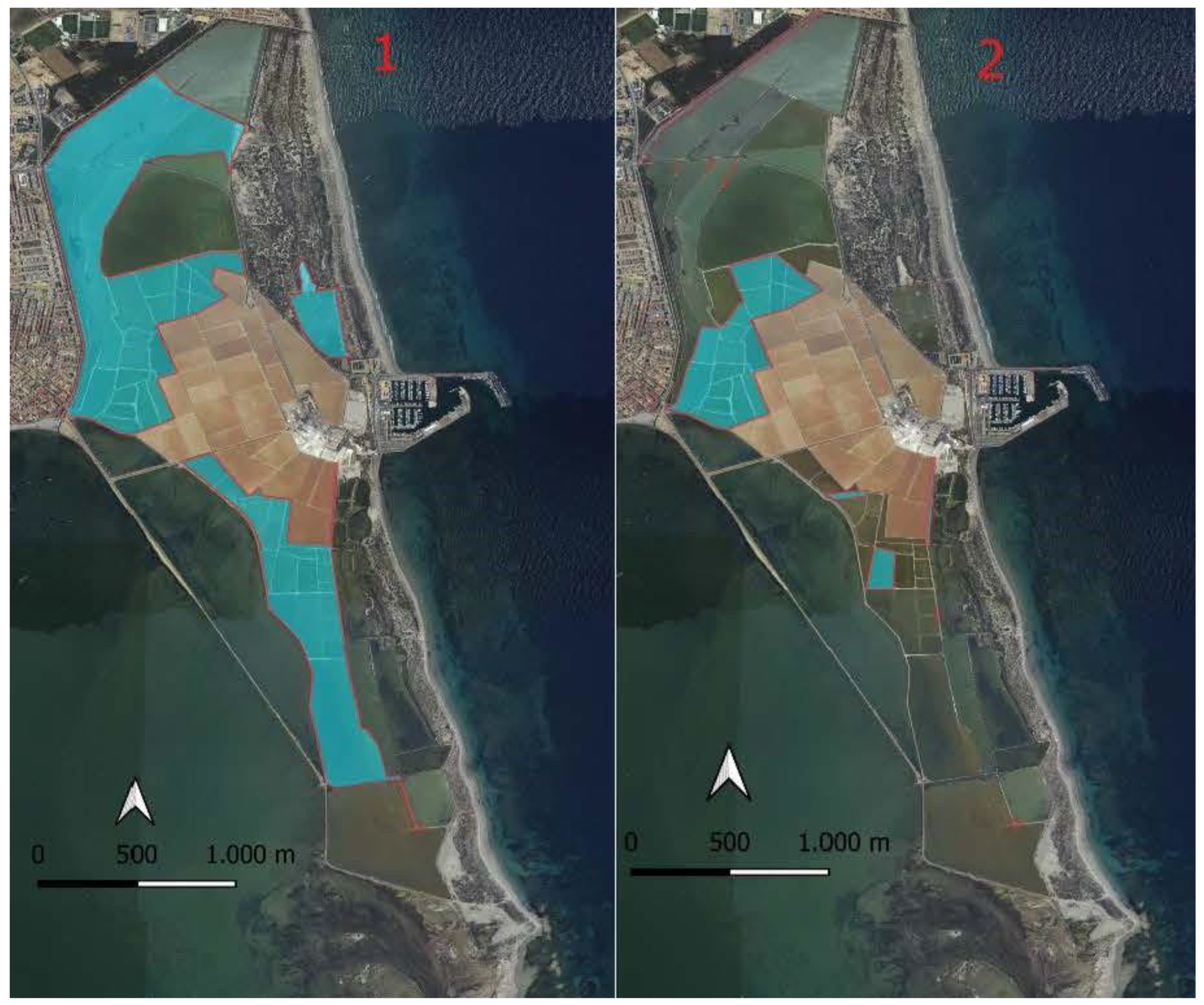
In 1994, nesting waterfowl had a scattered distribution across 406 ha of the Salinas de San Pedro del Pinatar and the Encañizadas. In 2015, a repeat of the 1994 study, using maps to locate the colonies of waterfowl, revealed that the breeding habitat is now concentrated in 85.4 ha. This signifies a reduction of 79% in suitable breeding habitat for waterfowl over 21 years (
Figure 4).
The Regional Park has experienced an increase in the breeding population of L. michahellis since 1993 when 2 pairs bred, up to 191 pairs in 1999, with negative effects on several colonial waterbird species. Notably, there was a 55.6% reduction in the nesting population of S. hirundo compared to the 1992-1995 average. Meanwhile, other species such as R. avosetta, Ch. alexandrinus, and S. albifrons relocated their nests to other areas within the protected space, sometimes in vulnerable zones accessible to humans and predators like rats, cats, and dogs.
Out of the 85.4 ha of suitable habitat for waterbird reproduction in 2015, 71.6 ha (83.9%) are patches of warm puddles, 3.6 ha (4.2%) correspond to storage puddles, and 10.2 ha (11.9%) are in the intertidal zone of the Encañizadas. Therefore, the majority of waterbird colonies have relocated their nests, forming large colonies near patches of warm puddles.
As vegetation covers the patches separating the "storage" puddles, the optimal surface for waterbird reproduction decreases. This leaves the patches separating the "warm" puddles available for waterbirds, where salt management is more intense, and the substrate is composed of precipitated CaSO4, MgSO4, MgCl2 salts that hinder vegetation growth chemically. Meanwhile, in the Encañizadas area, the available habitat for waterbirds is reduced to the zone with barely any land and/or sand deposits that allow vegetation growth, as well as the intertidal zone that emerges during the summer and remains submerged the rest of the year.
The breeding season of L. michahellis begins with nest construction in mid-March on the patches separating the saline puddles covered by halophytic vegetation, especially Suaeda spp. and Arthrocnemum spp. As the breeding period progresses and the number of L. michahellis pairs increases, they install their nests in saline patches with a lower proportion or no vegetative cover, occupying territories that were used by other colonial waterbirds such as R. avosetta, S. hirundo, S. albifrons, etc., until the early 21st century. The reproductive phenology of these species begins in late April, so upon reaching the breeding areas, they found them occupied by colonies of L. michahellis, a predatory species on their offspring. Consequently, they either abandoned the San Pedro del Pinatar Salinas or used areas far from L. michahellis, often in locations easily accessible to humans and predators.
3.3. Social and Economic Impacts
The damages are localized in the sanitary risk due to the introduction of various germs, such as Listeria and Salmonella sp., that seagulls obtain from the landfills where they feed and transfer to the salt pans, potentially affecting wildlife. The entry occurs through two pathways: on one hand, hundreds of resident individuals of L. michahellis throughout the year perch to spend the night on the earth and sand dykes separating saline puddles, on the salt piles, and during the months from September to November, also on the crystallizer ponds once they have dried up for salt extraction, carrying both organic and inorganic residues obtained from their feeding areas, mainly landfills. All of this, combined with excrement, forced the salt-producing company to discard the first layer of salt, located on the surface of the crystallizer ponds. On the other hand, it was common to observe at dawn, apparently to obtain condensed water during the night, up to 120 L. michahellis perched and pecking at plastic salt bags stacked next to the industrial zone warehouses, waiting to be loaded onto trucks for distribution to shopping centers. The bags pecked by the seagulls had to be removed before the arrival of the trucks, resulting in annual losses estimated by Salinera Española at around 120,000 euros.
Regarding the safety of the workers of the salt-producing company, L. michahellis even placed their nests on the sides of transit roads, making low flights with intimidatory purposes over those who approached too close to their nests during the period when the eggs began to hatch and until the moment when the chicks could fly. In the years 1999 and 2000, the seagulls caused two accidents with salt workers due to motorcycle falls during their travels to carry out their routine tasks of regulating the salt concentration in the salt pans and various maintenance works.
3.4. Management of the Population of L. michahellis
The management of L. michahellis nests is yielding the following results: (1) since 2002, there have been no incidents between saltworks workers and L. michahellis, (2) recovery of the population of S. hirundo, (3) return to the usual breeding areas of some waterbird species of conservation interest (Ch. alexandrinus, R. avosetta, S. hirundo, S. albifrons), and (4) facilitating the colonization of two waterbird species that have few breeding locations in Europe: I. audouinii and T. sandvicensis.
The availability of space without
L. michahellis colonies could be one of the reasons that has facilitated the colonization of species listed in Annex I of the European Union Directive on the Conservation of Birds (Directive 49/147/EC), with relevant populations at the national, European, and even global level. Some of these species show a decreasing global trend, such as
S. sandvicensis, which began breeding in 2008 in the Regional Park of Salinas y Arenales de San Pedro del Pinatar, while others have stable populations, such as
I. audouinii, which also began breeding in the Salinas in 2010 (
Table 1).
3.5. Actions to Minimize Damage in Salt Production
Salinera Española has placed life-sized ceramic owls in areas where salt bags are stored to be loaded onto trucks. They have also installed posts with nylon thread tensioned at an approximate height of 1 m along the entire perimeter of crystallizer ponds. In this way, it prevents hundreds of seagulls from perching on salt bags (between late September and November), where they leave all kinds of food remnants from the landfills they use as feeding grounds and a layer of droppings (
Figure 5). Given the success of these deterrent measures, they have become routine tasks incorporated into the rest of the activities related to salt production.
4. Discussion
The population of L. michahellis in the Region of Murcia has shown an increasing trend during the last third of the 20th century and throughout the 21st century, in line with patterns observed globally, due to the growing availability of anthropogenic food sources: municipal solid waste landfills, food provided in aquaculture farms, and discarded fishery bycatch. The population increase of L. michahellis leads to damages to biodiversity, salt production, and incidents with saltworks workers.
In terms of biodiversity, it impacts the floristic composition of the dykes separating 4.saltwater ponds due to the guano contributions from thousands of gulls resting during the day or sleeping at night. This phenomenon aligns with the findings of Otero et al. [
19,
20] and Mouriño and Otero [
18]. 20]The excessive growth of vegetation on the dykes separating saltwater ponds has reduced the breeding habitat for waterbirds by 79% since 1994, increasing predation on chicks and adult nesting waterbirds. These findings are consistent with observations made by Oro [
6].
The impact on salt production occurs through damage to plastic salt bags at dawn, as gulls peck them to obtain condensed fresh water overnight, resulting in economic losses. There is also a safety risk for workers due to low flights over them during routine maintenance and water level regulation tasks. During the breeding season, workers unintentionally approach nests near water passage gates.
These impacts prompted coordinated intervention by environmental authorities and the private company that owns the salt pans. This intervention involved removing nests during the breeding season to alleviate pressure on other nesting waterbird species and reduce the risk of attacks on Salinera workers. Additionally, deterrent elements were installed to prevent gulls from perching on salt bags and crystallizer ponds during the salt harvesting period.
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
5. Conclusions
The control of the nesting population of L. michahellis in the Regional Park of Salinas and Arenales de San Pedro del Pinatar, through the removal of nests and eggs since 2001, has allowed the maintenance of diversity and the number of nesting waterbird pairs, preventing incidents with Salinera Española workers. The deterrent measures implemented by Salinera to prevent gulls from perching on salt bags and crystallizer ponds during the salt harvesting period are also yielding positive results.
If no action were taken to eliminate nests, it would become a magnet for an increasing number of nesting pairs of L. michahellis, likely surpassing a thousand pairs by the second decade of the 21st century. This would lead to increased conservation problems for habitats, waterbirds, and potential social and economic setbacks.
However, measures have not yet been taken to prevent hundreds of L. michahellis individuals from roosting at the Salinas every day and to stop guano contributions from causing changes in habitat composition. This would require a permanent operator to patrol the dykes separating saltwater ponds frequented by gulls, especially during the evening. Additionally, efforts should be made to control colonies of L. michahellis that still breed in the most inaccessible areas of the Regional Park to continue recovering breeding areas for other colonial waterbird species.
Finally, a comprehensive solution needs to be addressed to tackle the problems posed by the L. michahellis population, preventing or impeding access to anthropogenic food sources, especially municipal solid waste landfills, aquaculture farms, and fishery discards. This section is not mandatory but can be added to the manuscript if the discussion is unusually long or complex.
Author Contributions
Conceptualization, G.B-P. and A.A.; methodology, G.B-P. and M.A.S-S.; formal analysis, G.B-P..; investigation, G.B-P., M.A.S-S. and A.A.; resources, G.B-P.; writing—original draft preparation, G.B-P. and A.A.; writing—review and editing, M.A.S-S.; supervision, G.B-P. and A.A.; funding acquisition, G.B-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the LIFE funds of the European Union, through the LIFE-SALINAS Project (LIFE17 NAT/ES/000184) for the Conservation of Habitats and Waterbirds in the SCI and SPA ES0000175 "Salinas y Arenales de San Pedro del Pinatar".
Informed Consent Statement
Not applicable.
Data Availability Statement
All data will be available under reasonable request to the corresponding author.
Acknowledgments
We would like to express our gratitude for the review and improvement of the article conducted by Nerea Martínez Ardal and Antonio Zamora López.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Del Hoyo, J., Elliott, A., Sargatal, J. (1996). Handbook of the Birds of the World. Vol. 3. Hoatzin to Auks. Lynx Edicions. Barcelona. 821 pp.
- IUCN (2024). The IUCN Red List of Threatened Species. Version 2023-13. https://www.iucnredlist.org/.
- BirdLife International, (2021). European Red List of Birds. Office for Official Publications of the European Communities, Luxembourg.
- Cama, A., Arcos, J.M., (2012). Gaviota patiamarilla. En SEO/BirdLife: Atlas de las aves en invierno en España 2007-2010. MAGRAMA-SEO/BirdLife. Madrid. 816: 298-299.
- Molina, B. (Ed.) (2009). Gaviota reidora, sombría y patiamarilla en España. Población en 2007-2009 y método de censo. SEO/BirdLife. Madrid. 166 pp.
- Oro, D., Tavecchia, G. (2008). Gaviota picofina. En: Bertolero, A.; Genovart, M.; Martínez-Abrain, A.; Molina, B.; Mourino, J.; Oro, D.; Tavecchia, G. (Eds.): Gaviotas cabecinegra, picofina, de Audouin y tridáctila, y gavión atlántico en España. Población en 2007 y método de censo, SEO/BirdLife. Madrid, p. 21-42.
- Cantos, J. (2009). Gaviota reidora. En, B. Molina (Ed.): Gaviotas reidora, sombría y patiamarilla en España. Población 2007-2009 y método de censo, p. 17-31. SEO/BirdLife. Madrid.
- Aguado-Giménez, F., Eguía-Martínez, S., Cerezo-Valverde, J., García-García, B., García-García, B. (2018). Spatio-temporal variability of ichthyophagous bird assemblage around western Mediterranean open-sea cage fish farms. Marine environmental research 140: 126-134. [CrossRef]
- Delgado, S. (2021). Impacto del cierre de vertederos en la demografía de especies oportunistas: el caso de la gaviota patiamarilla en el País Vasco. Tesis Doctoral. Departamento de Biología Vegetal y Ecología. Universidad del País Vasco. 163 p.
- Arcos, J.M., Arizaga, J., Barros, A., Fernández-Pajuelo, M., García, D., García-Barcelona, S., López-Jiménez, N., Martín, Blas Molina, G., Mas, R.E., Oro, D., Sanz-Aguilar, A. y Taveccia, G. (2021). Gaviota patiamarilla Larus michahellis. En: López-Jiménez, N. (Ed.): Libro Rojo de las Aves de España, pp. 814-816. SEO/BirdLife. Madrid.
- Zorrozua, N., Delgado, S., Aldalur, A., & Arizaga, J. (2020). Adverse weather reduces the spatial use of an opportunistic gull. Behaviour, 1: 1-15. [CrossRef]
- Steigerwald, E.C., Igual, J.-M., Payo-Payo, A. & Tavecchia, G. (2015). Effects of decreased anthropogenic food availability on an opportunistic gull: evidence for a size-mediated response in breeding females. Ibis, 157, 439448. [CrossRef]
- Ballesteros-Pelegrín, G.A.; Zamora-López, A.; Zamora-Marín, J.M.; Sallent, A.; Hernández-Navarro, A.; Robledano-Aymerich, F.; Fuentes-Marín, A. (2021). Atlas de las Aves Acuáticas del Mar Menor y humedales de su entorno. Natursport. Murcia, 398 pp.
- Bosch, M., Oro, D., Cantos, F. J., & Zabala, M. (2000). Short-term effects of culling on the ecology and population dynamics of the Yellow-legged gull. Journal of Applied Ecololgy, 37: 369-385. [CrossRef]
- Votier, S.C., Crane, J.E., Bearhop, S., De León, A., Mcsorley, C.A., Mínguez, E., Mitchell, I.P., Parsons, M., Phillips, R.A. y Furness, R.W. (2006). Nocturnal foraging by great skuas Stercorarius skua: implications for conservation of storm-petrel populations. J. Ornithol. 147, 405-413. [CrossRef]
- Corbacho, J.M., Sánchez, G., Villegas, M.A. (2009). Pagazas, charranes y fumareles en España. Población reproductora en 2007. SEO/BirdLife. Madrid. 129 pp.
- Oro, D., Pérez-Rodríguez, P. Martínez-Vilalta, A. Bertolero, A. Vidal, F. Genovart, M. (2009). Interference competition in a threatened seabird community: A paradox for a successful conservation. Biological Conservation 142, 1830–1835. [CrossRef]
- Mouriño, J., Otero, P. (2002). Caracterización de la vegetación de los acantilados del Parque Natural Islas Cíes y su relación con Larus michahellis. Cuadernos de la Sociedad Española de Ciencias Forestales 14: 135-142.
- Otero, X. L., Tejada, O., Martín, M., De la Peña, S., Ferreira, T.O., Pérez-Alberti, A. (2015) Phosphorus in seagull colonies and the effect on soil, water and habitats. The case of yellow legged gulls (Larus michahellis) in the Atlantic Islands of Galicia National Park (NW Spain). Science of Total Environment, 532: (1), 383-397. [CrossRef]
- Otero, X.L., De la Peña-Lastra, S., Romero, D.; Nobrega, G.N., Ferreira, T.O., Pérez-Alberti, A. (2018). Trace elements in biomaterials and soils from a yellow-legged gull (Larus michahellis) colony in the Atlantic Islands of Galicia National Park (NW Spain) Marine Pollution Bulletin. Elsevier. pp. 144-149. [CrossRef]
- Mulder, C.P.H., Jones, H., Kameda, K., Palmborg, C., Schmidt, S., Ellis, J.C., Orrock, J.L., Wait, D.A., Wardle, D.A., Yang, L., Young, H., Croll, D.A. and Vidal, E., (2011). Impacts of seabirds on plant and soil properties. In Mulder, C. P. H. , Anderson, W. B. , Towns, D. R. , and Bellingham, P. J. (eds.), Seabird Islands: Ecology, Invasion, and Restoration. New York: Oxford University Press, 135–176.
- Mallory, M.L., Mahon L., Tomlik, M.D., White, Ch., Milton, G.R. and Spooner, I. (2015). Colonial marine birds influence island soil chemistry through biotransport of trace elements. Water, Air, & Soil Pollution, 226: 31. [CrossRef]
- Magella, G., & Brousseau, P. (2001). Does culling predatory gulls enhance the productivity of breeding common terns?. Journal of Applied Ecology, 38(1): 1-8. [CrossRef]
- Eguía, S. (2016). Control de especies oportunistas en las Salinas de San Pedro. Comunidad Autónoma de Murcia. Comunidad Autónoma de la Región de Murcia. 24 pp.
- Paracuellos, M. (2009). El control de la gaviota patiamarilla (Larus michahellis) beneficia a la gaviota de Audouin (Larus audouinii) en la isla de Alborán. Boletín informativo sobre Geodiversidad y Biodiversidad de Andalucía. Nº 003: 1-2.
- Paracuellos, M., & Nevado, J. C. (2010). Culling Yellow-legged Gulls Larus michahellis benefits Audouin's Gulls Larus audouinii at a small and remote colony. Bird Study, 57(1): 26-30. [CrossRef]
- Oro, D. (2008). Adiós al mito de las gaviotas malas. Dudas sobre la utilidad de los controles de gaviota patiamarilla. Quercus 271: 14-20.
- Sanz-Aguilar, A., Martínez-Abraín, A., Tavecchia, G., Mínguez, E., & Oro, D. (2009). Evidence-based culling of a facultative predator: efficacy and efficiency components. Biological conservation, 142(2): 424-431. [CrossRef]
- Ballesteros-Pelegrín, G.A. (2014). El Parque Regional de las Salinas y Arenales de San Pedro del Pinatar: actividades humanas y conservación. Universidad de Murcia. Murcia. 367 p.
- Dirección General de Medio Ambiente 2011. Salinas y Arenales de San Pedro del Pinatar.
- Fernández-Caro, A. (Coordinador) (2015). Revisión histórica del censo invernal de aves acuáticas de la Región de Murcia 1972-2015. Una aproximación a la compilación y unificación de datos y humedales. Asociación de Naturalistas del Sureste. 375 pp.
- Fernández-Caro, A. (Coordinator) (2016). Censo invernal 2016 de aves acuáticas invernantes. Asociación de Naturalistas del Sureste.
- Fernández-Caro, A. (Coordinator) (2017). Censo invernal 2017 de aves acuáticas invernantes. Asociación de Naturalistas del Sureste.
- Fernádez-Caro, A. (Coordinator) (2018). Censo invernal 2018 de aves acuáticas de la Región de Murcia. Asociación de Naturalistas del Sureste.
- Palomino, D., Molina, B. (2009). Aves acuáticas reproductoras en España. Población en 2007 y método de censo. SEO/BirdLife. Madrid. 210 pp.
- SEO/BirdLife (López-Jiménez, N. Ed). (2021). Libro Rojo de las aves de España. https://seo.org/wp-content/uploads/2022/09/LIbro-Rojo-web-3_01.pdf.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).