Submitted:
14 May 2024
Posted:
14 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Reagents
Cell Cultures and Treatments
Cell Viability Study
Immunocytochemistry
Phase-Contrast Microscopy, Measurement of Cells Confluence and Neurites Count
Live-Cell Labeling of Acidic Compartments
TUNEL Assay
Protein Extraction and Western Blot Analysis
Clonogenic Assay
Cell Cycle Analysis
Statistical Analysis
3. Results
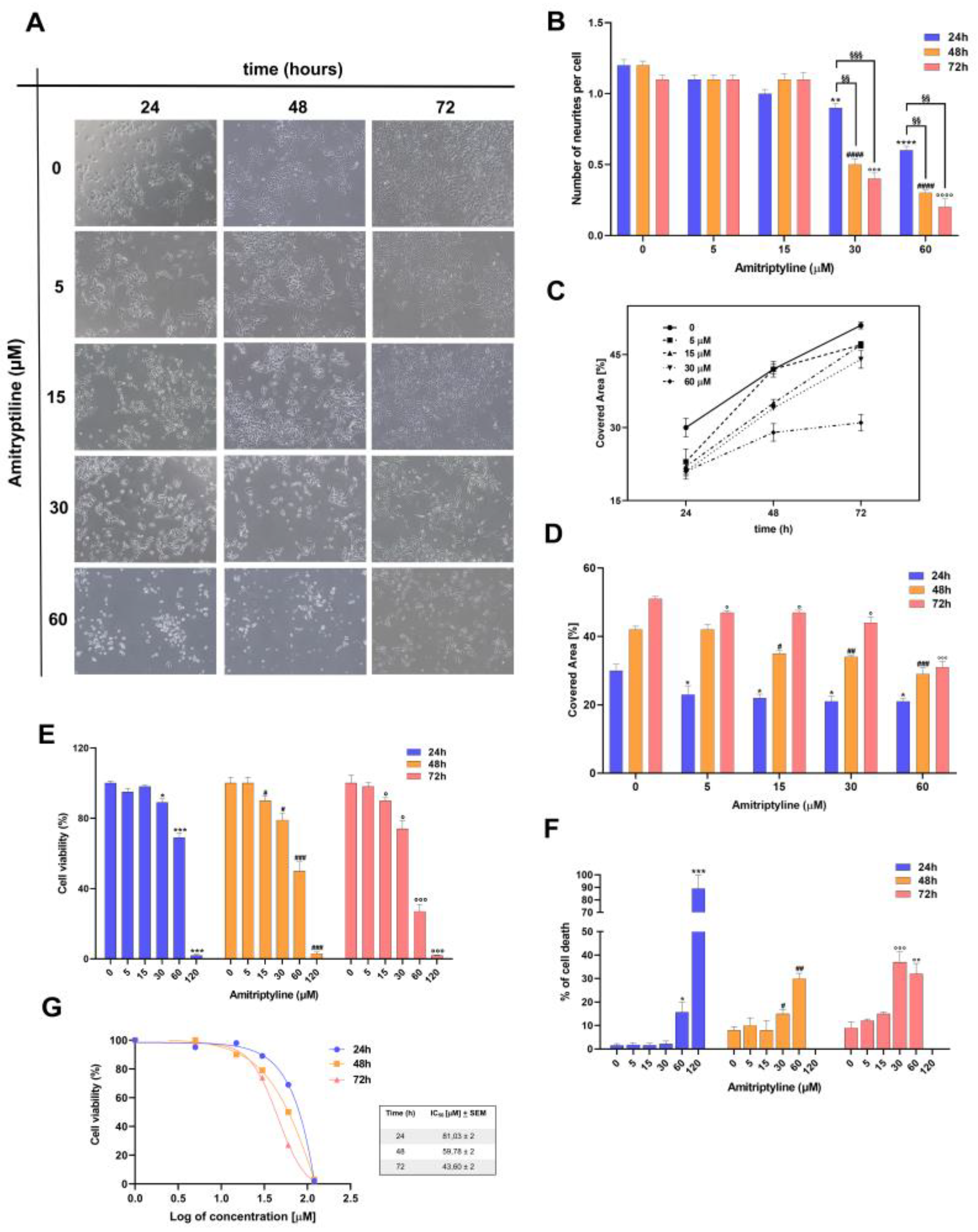
3.1. Amitriptyline Induced a Concentration and Time-Dependent Reduction of Cell Viability in SH-SY5Y Cultures
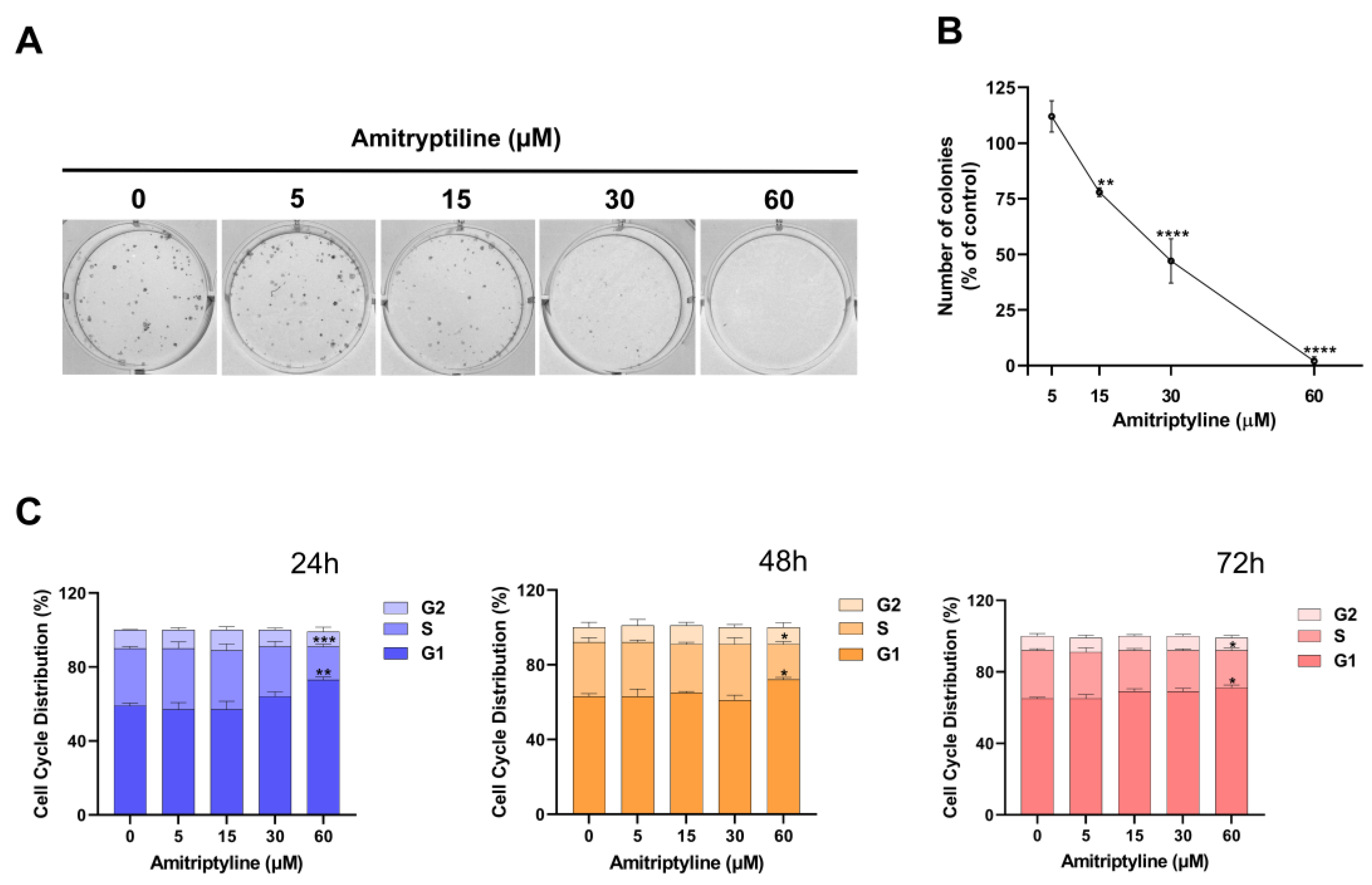
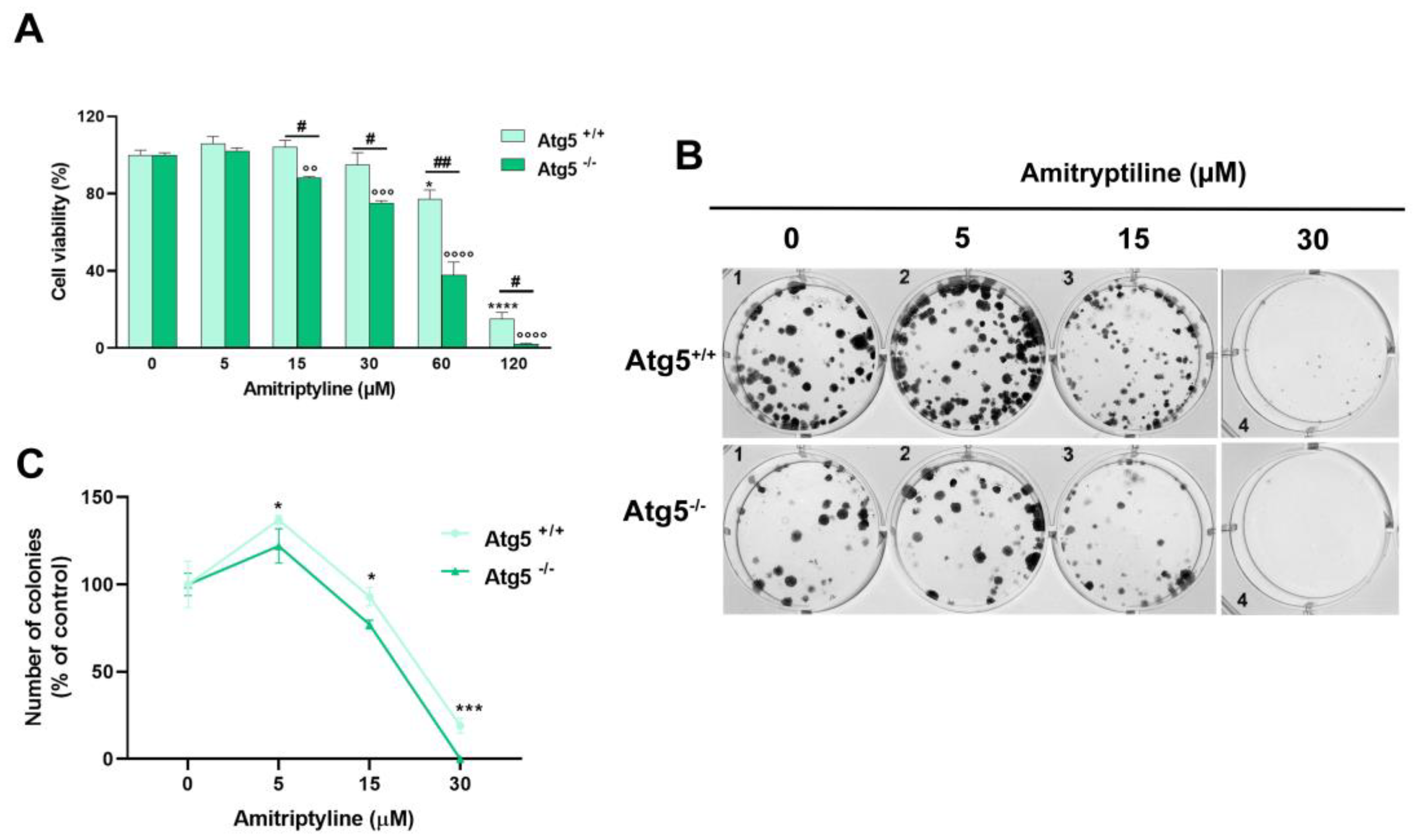
3.2. Amitriptyline Reduced SH-SY5Y Clonogenic Capacity
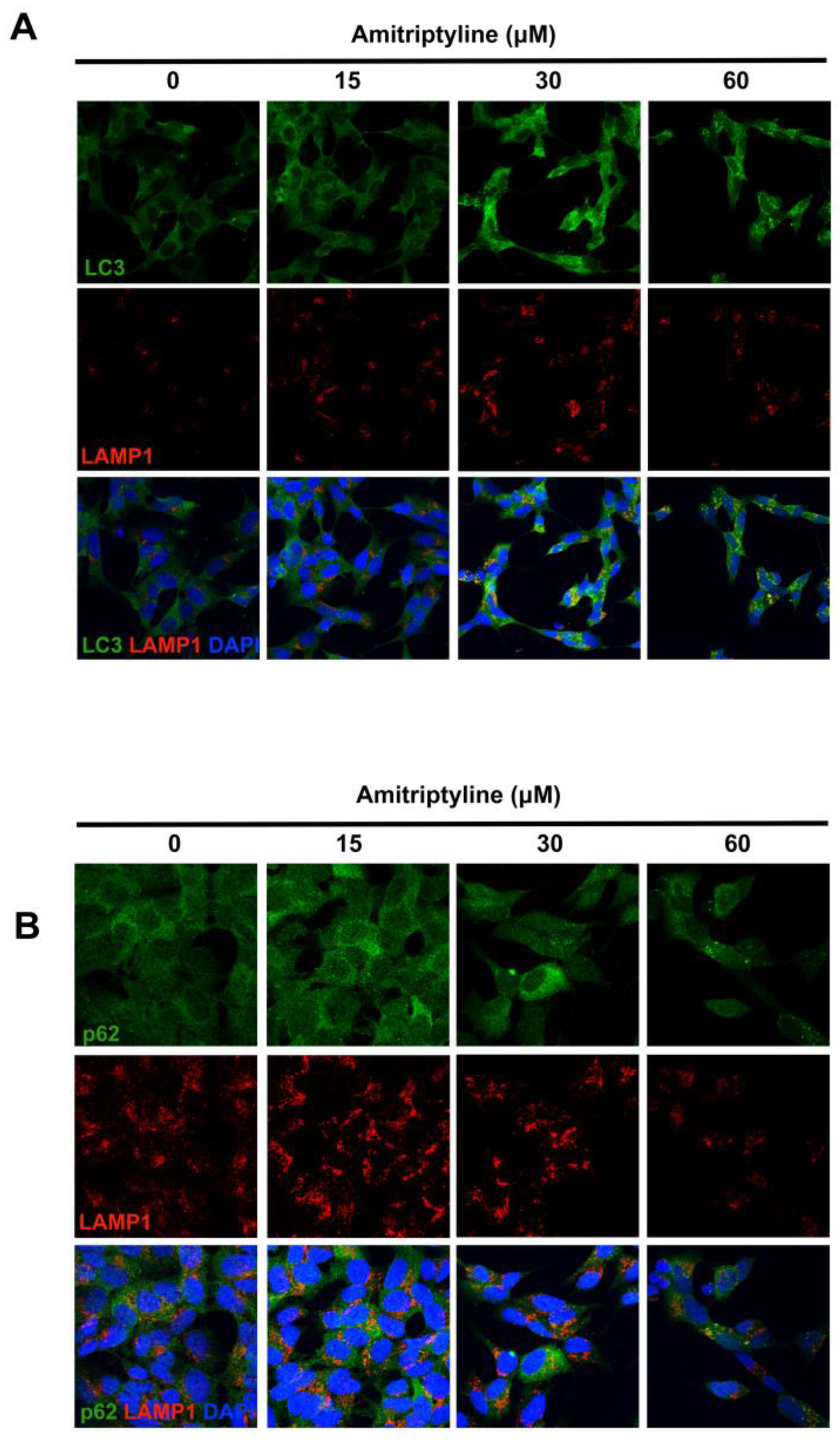
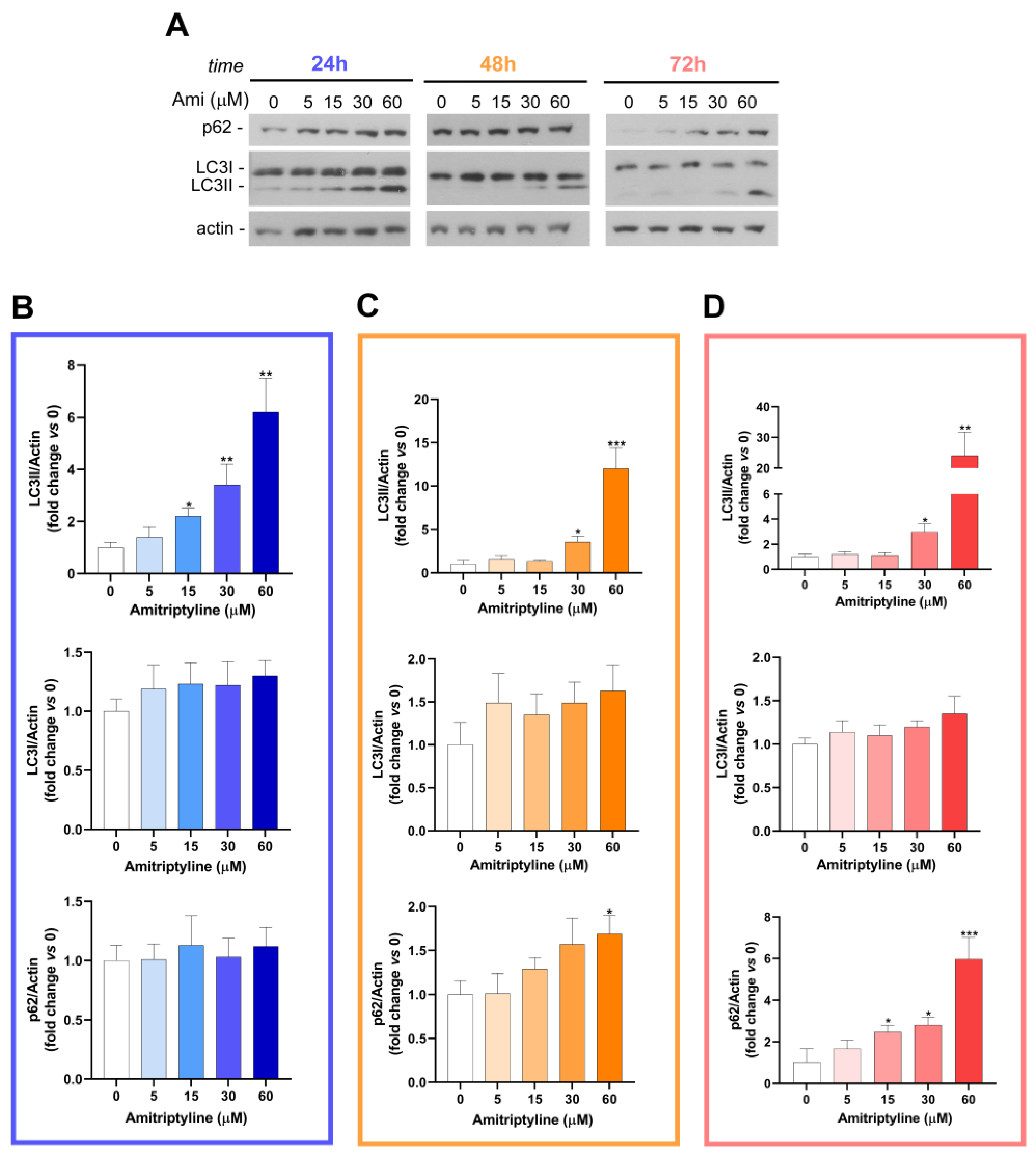
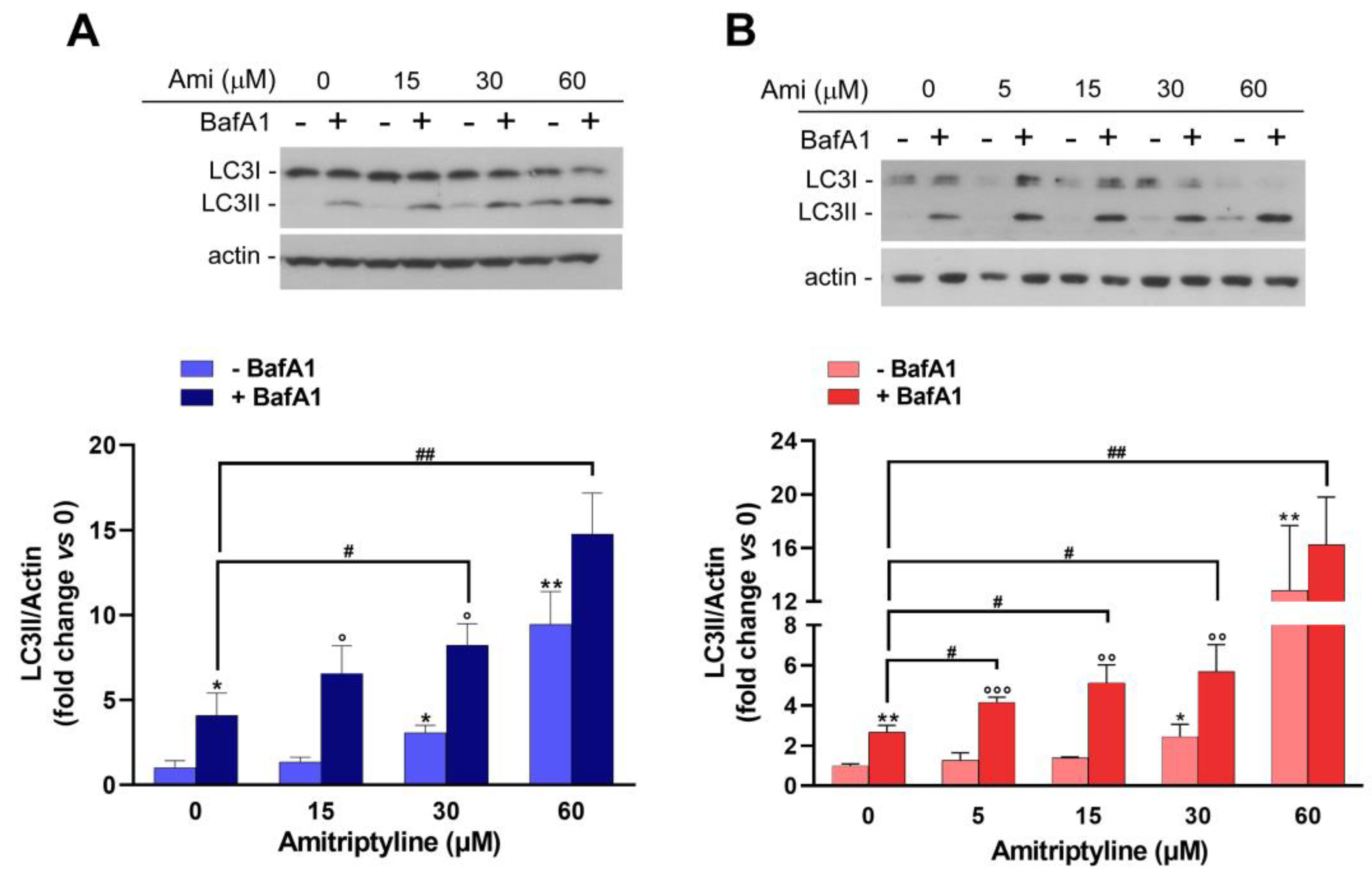
3.3. Autophagy Is Modulated in SH-SY5Y Cell Cultures Exposed to Amitriptyline
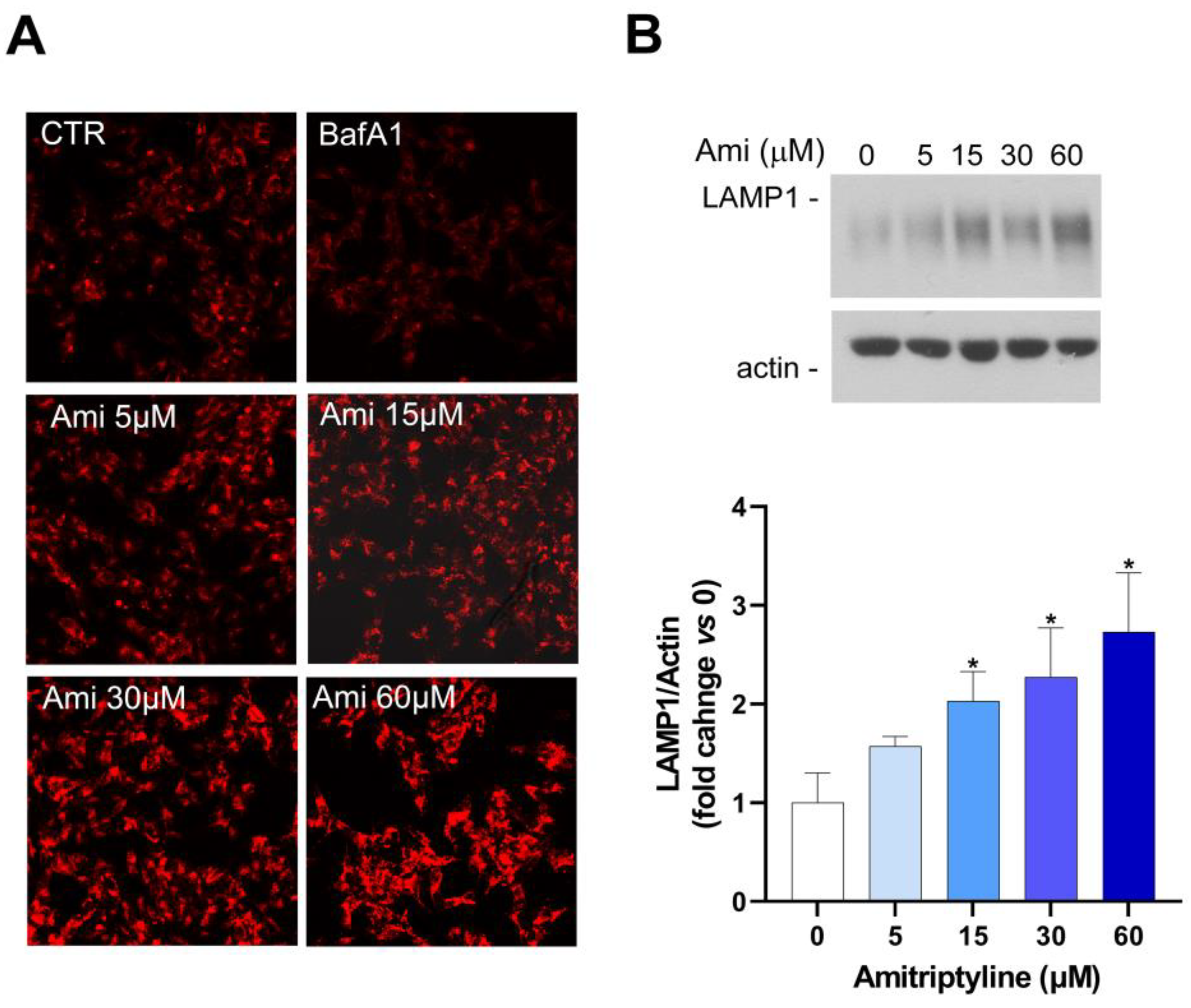
3.4. Amitriptyline Does Not Affect Lysosomal pH but Induces Lysosomes Accumulation
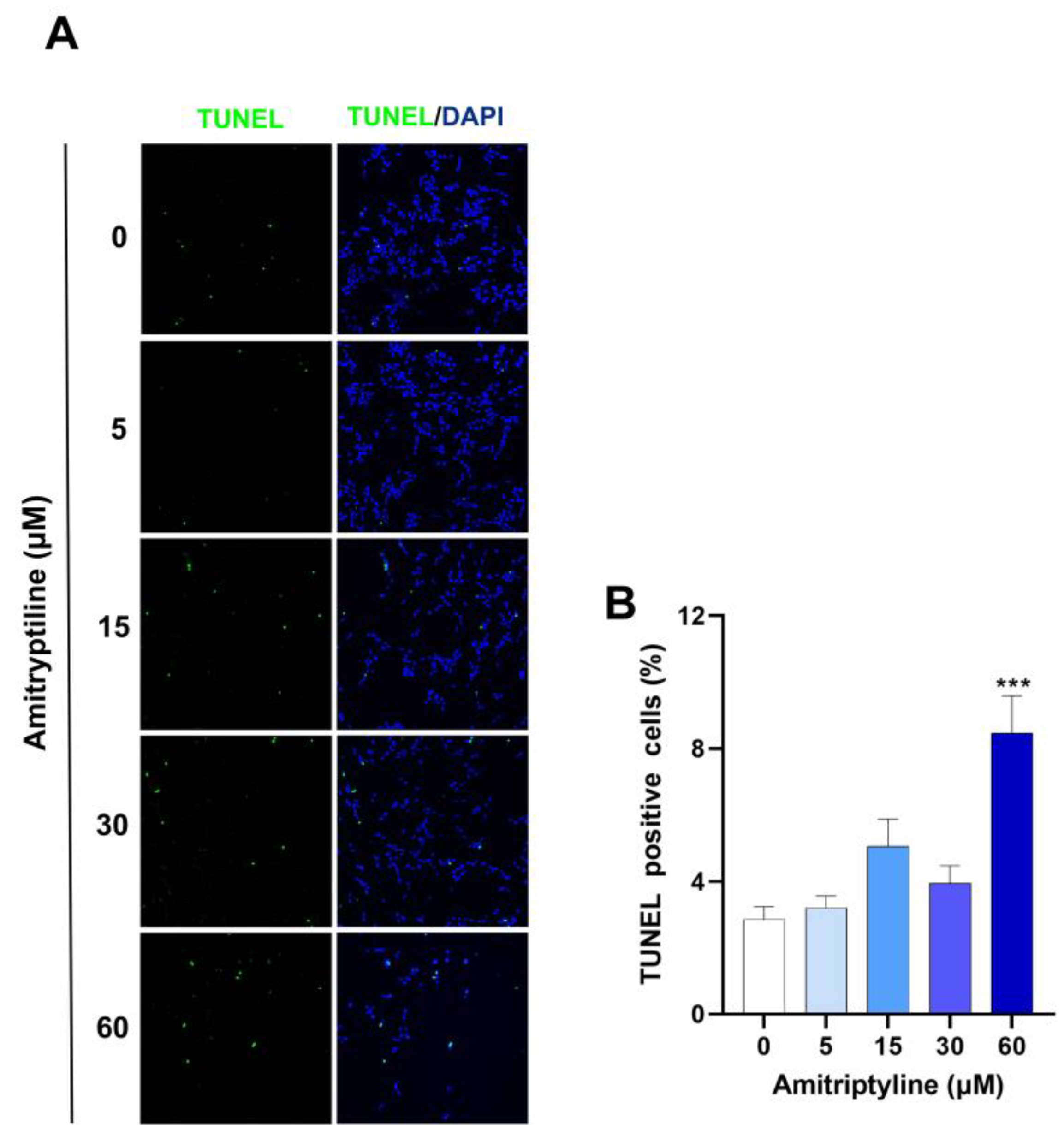
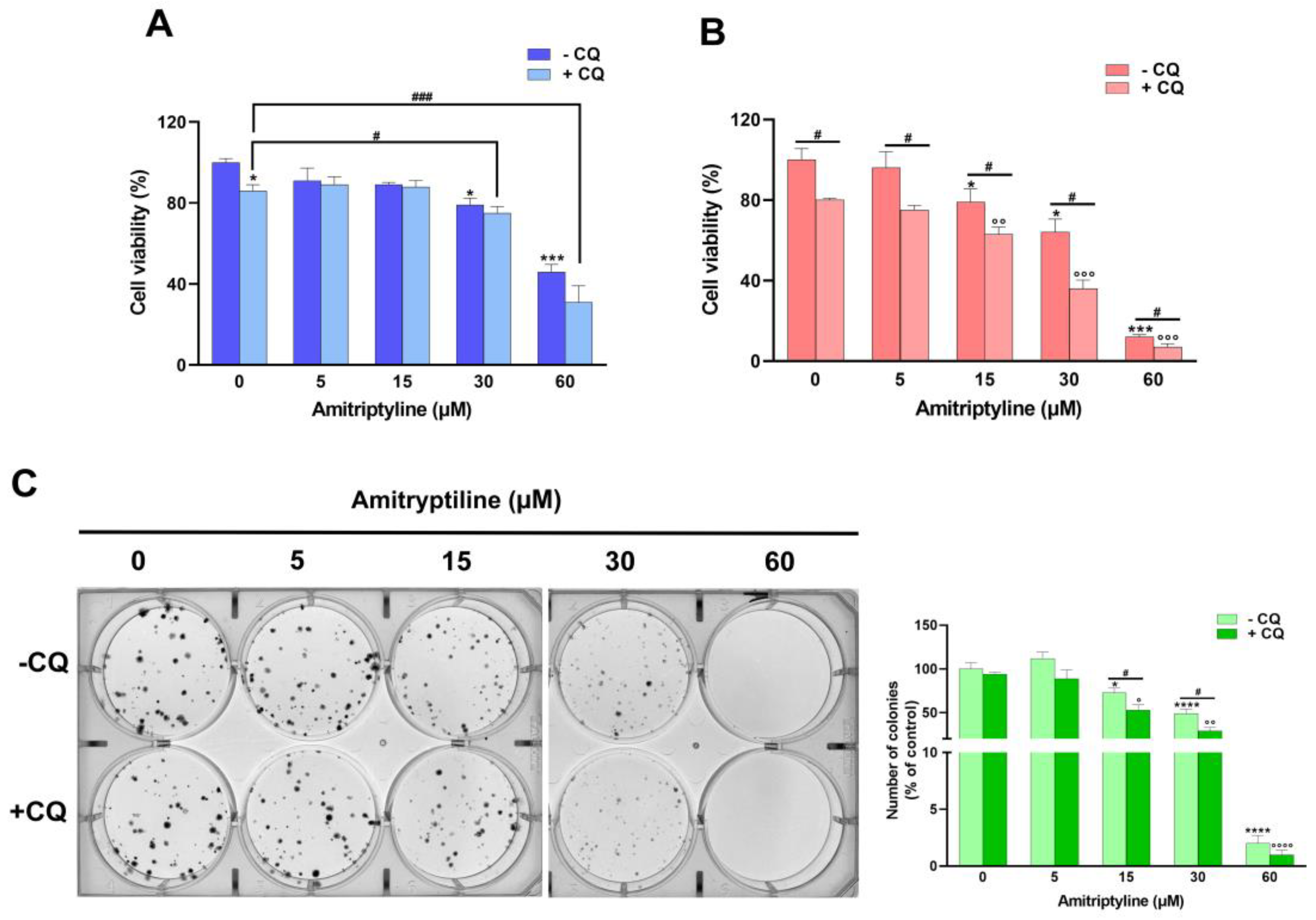
3.5. Autophagy Modulation Does Not Take Part to the Cytotoxic Effects of Amitriptyline
3.6. Reduced Clonogenic Capacity in Amitriptyline Treated Cells Does Not Depend on Autophagy Modulation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedrich, M.J. Depression Is the Leading Cause of Disability Around the World. Jama 2017, 317, 1517. [Google Scholar] [CrossRef] [PubMed]
- Wan-Fei, K.; Hassan, S.T.S.; Sann, L.M.; Ismail, S.I.F.; Raman, R.A.; Ibrahim, F. Depression, anxiety and quality of life in stroke survivors and their family caregivers: A pilot study using an actor/partner interdependence model. Electronic physician 2017, 9, 4924–4933. [Google Scholar] [CrossRef] [PubMed]
- Guekht, A. Epilepsy, Comorbidities and Treatments. Current pharmaceutical design 2017, 23, 5702–5726. [Google Scholar] [CrossRef] [PubMed]
- Brozek, P.; Brachmanska, M.; Rabiczko, K.; Bulska, W.; Ciulkowicz, M.; Krzystanek, E. Depression, sleep disturbances and anxiety in patients with relapsing-remitting multiple sclerosis: a longitudinal cohort observation. Psychiatria Danubina 2017, 29, 464–468. [Google Scholar] [PubMed]
- Baquero, M.; Martín, N. Depressive symptoms in neurodegenerative diseases. World journal of clinical cases 2015, 3, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. The Lancet. Oncology 2011, 12, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, B.; Hyphantis, T.N.; Valpione, S.; Perini, G.; Maes, M.; Morris, G.; Kubera, M.; Köhler, C.A.; Fernandes, B.S.; Stubbs, B.; et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer treatment reviews 2017, 52, 58–70. [Google Scholar] [CrossRef]
- Becker, M.; Weinberger, T.; Chandy, A.; Schmukler, S. Depression During Pregnancy and Postpartum. Current psychiatry reports 2016, 18, 32. [Google Scholar] [CrossRef]
- Martínez-Paredes, J.F.; Jácome-Pérez, N. Depression in Pregnancy. Revista Colombiana de psiquiatria (English ed.) 2019, 48, 58–65. [Google Scholar] [CrossRef]
- Szpunar, M.J.; Malaktaris, A.; Baca, S.A.; Hauger, R.L.; Lang, A.J. Are alterations in estradiol, cortisol, and inflammatory cytokines associated with depression during pregnancy and postpartum? An exploratory study. Brain, behavior, & immunity - health 2021, 16, 100309. [Google Scholar] [CrossRef]
- Comorbid depression in medical diseases. Nature reviews. Disease primers 2020, 6, 70. [CrossRef] [PubMed]
- Karrouri, R.; Hammani, Z.; Benjelloun, R.; Otheman, Y. Major depressive disorder: Validated treatments and future challenges. World journal of clinical cases 2021, 9, 9350–9367. [Google Scholar] [CrossRef] [PubMed]
- Cherrie, M.; Curtis, S.; Baranyi, G.; McTaggart, S.; Cunningham, N.; Licence, K.; Dibben, C.; Bambra, C.; Pearce, J. Use of sequence analysis for classifying individual antidepressant trajectories to monitor population mental health. BMC psychiatry 2020, 20, 551. [Google Scholar] [CrossRef] [PubMed]
- Kleine, R.; Galimov, A.; Hanewinkel, R.; Unger, J.; Sussman, S.; Hansen, J. Impact of the COVID-19 pandemic on young people with and without pre-existing mental health problems. Scientific reports 2023, 13, 6111. [Google Scholar] [CrossRef] [PubMed]
- Ferwana, I.; Varshney, L.R. The impact of COVID-19 lockdowns on mental health patient populations in the United States. Scientific reports 2024, 14, 5689. [Google Scholar] [CrossRef]
- Bonilla-Jaime, H.; Sánchez-Salcedo, J.A.; Estevez-Cabrera, M.M.; Molina-Jiménez, T.; Cortes-Altamirano, J.L.; Alfaro-Rodríguez, A. Depression and Pain: Use of Antidepressants. Current neuropharmacology 2022, 20, 384–402. [Google Scholar] [CrossRef]
- Feighner, J.P. Overview of antidepressants currently used to treat anxiety disorders. The Journal of clinical psychiatry 1999, 60 Suppl 22, 18–22. [Google Scholar]
- Liu, Y.; Xu, X.; Dong, M.; Jia, S.; Wei, Y. Treatment of insomnia with tricyclic antidepressants: a meta-analysis of polysomnographic randomized controlled trials. Sleep medicine 2017, 34, 126–133. [Google Scholar] [CrossRef]
- Burch, R. Antidepressants for Preventive Treatment of Migraine. Current treatment options in neurology 2019, 21, 18. [Google Scholar] [CrossRef]
- Scuteri, D.; Vulnera, M.; Piro, B.; Bossio, R.B.; Morrone, L.A.; Sandrini, G.; Tamburin, S.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Pattern of treatment of behavioural and psychological symptoms of dementia and pain: evidence on pharmacoutilization from a large real-world sample and from a centre for cognitive disturbances and dementia. European journal of clinical pharmacology 2021, 77, 241–249. [Google Scholar] [CrossRef]
- Pearlstein, T. Selective serotonin reuptake inhibitors for premenstrual dysphoric disorder: the emerging gold standard? Drugs 2002, 62, 1869–1885. [Google Scholar] [CrossRef]
- Maidment, I.D. The use of antidepressants to treat attention deficit hyperactivity disorder in adults. Journal of psychopharmacology (Oxford, England) 2003, 17, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.; Vos, R.; Tack, J. The influence of citalopram on interdigestive gastrointestinal motility in man. Alimentary pharmacology & therapeutics 2010, 32, 289–295. [Google Scholar] [CrossRef] [PubMed]
- van Kerrebroeck, P.; Abrams, P.; Lange, R.; Slack, M.; Wyndaele, J.J.; Yalcin, I.; Bump, R.C. Duloxetine versus placebo in the treatment of European and Canadian women with stress urinary incontinence. BJOG : an international journal of obstetrics and gynaecology 2004, 111, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chang, X.; Huang, Y.; He, D. The application of antidepressant drugs in cancer treatment. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2023, 157, 113985. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, X.; Yu, B. Repurposing antidepressants for anticancer drug discovery. Drug discovery today 2022, 27, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Bielecka, A.M.; Obuchowicz, E. Antidepressant drugs as a complementary therapeutic strategy in cancer. Experimental biology and medicine (Maywood, N.J.) 2013, 238, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.B.; Wang, T.T.; Zhu, H.L.; Wang, H.K.; Sun, W.; Zhao, L.F. Vortioxetine induces apoptosis and autophagy of gastric cancer AGS cells via the PI3K/AKT pathway. FEBS open bio 2020, 10, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Qiu, Y.; Yang, L.; Peng, L.; Xia, Z.; Hou, L.N.; Fang, C.; Qi, H.; Chen, H.Z. Desipramine induces apoptosis in rat glioma cells via endoplasmic reticulum stress-dependent CHOP pathway. Journal of neuro-oncology 2011, 101, 41–48. [Google Scholar] [CrossRef]
- Zafir, A.; Ara, A.; Banu, N. Invivo antioxidant status: a putative target of antidepressant action. Progress in neuro-psychopharmacology & biological psychiatry 2009, 33, 220–228. [Google Scholar] [CrossRef]
- Kannen, V.; Hintzsche, H.; Zanette, D.L.; Silva, W.A., Jr.; Garcia, S.B.; Waaga-Gasser, A.M.; Stopper, H. Antiproliferative effects of fluoxetine on colon cancer cells and in a colonic carcinogen mouse model. PloS one 2012, 7, e50043. [Google Scholar] [CrossRef]
- Fang, C.K.; Chen, H.W.; Chiang, I.T.; Chen, C.C.; Liao, J.F.; Su, T.P.; Tung, C.Y.; Uchitomi, Y.; Hwang, J.J. Mirtazapine inhibits tumor growth via immune response and serotonergic system. PloS one 2012, 7, e38886. [Google Scholar] [CrossRef]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Progress in neuro-psychopharmacology & biological psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Thour, A.; Marwaha, R. Amitriptyline. In StatPearls; StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.: Treasure Island (FL) ineligible companies. Disclosure: Raman Marwaha declares no relevant financial relationships with ineligible companies, 2024. [Google Scholar]
- Dopheide, J.A. Recognizing and treating depression in children and adolescents. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists 2006, 63, 233–243. [Google Scholar] [CrossRef]
- Radley, D.C.; Finkelstein, S.N.; Stafford, R.S. Off-label prescribing among office-based physicians. Archives of internal medicine 2006, 166, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Sloan, G.; Petrie, J.; White, D.; Bradburn, M.; Julious, S.; Rajbhandari, S.; Sharma, S.; Rayman, G.; Gouni, R.; et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet (London, England) 2022, 400, 680–690. [Google Scholar] [CrossRef]
- Farag, H.M.; Yunusa, I.; Goswami, H.; Sultan, I.; Doucette, J.A.; Eguale, T. Comparison of Amitriptyline and US Food and Drug Administration-Approved Treatments for Fibromyalgia: A Systematic Review and Network Meta-analysis. JAMA network open 2022, 5, e2212939. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, A.; Guerrero-Peral Á, L.; Arias-Rivas, S.; Silva, L.; Sierra, Á.; Gago-Veiga, A.B.; García-Azorín, D. Amitriptyline for post-COVID headache: effectiveness, tolerability, and response predictors. Journal of neurology 2022, 269, 5702–5709. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: process and function. Genes & development 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Rein, T. Is Autophagy Involved in the Diverse Effects of Antidepressants? Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Zhuang, X.; Zhang, L.; Qiao, T. Antidepressants Fluoxetine Mediates Endoplasmic Reticulum Stress and Autophagy of Non-Small Cell Lung Cancer Cells Through the ATF4-AKT-mTOR Signaling Pathway. Frontiers in pharmacology 2022, 13, 904701. [Google Scholar] [CrossRef] [PubMed]
- Chinnapaka, S.; Bakthavachalam, V.; Munirathinam, G. Repurposing antidepressant sertraline as a pharmacological drug to target prostate cancer stem cells: dual activation of apoptosis and autophagy signaling by deregulating redox balance. American journal of cancer research 2020, 10, 2043–2065. [Google Scholar]
- Hwang, H.Y.; Shim, J.S.; Kim, D.; Kwon, H.J. Antidepressant drug sertraline modulates AMPK-MTOR signaling-mediated autophagy via targeting mitochondrial VDAC1 protein. Autophagy 2021, 17, 2783–2799. [Google Scholar] [CrossRef]
- Cavaliere, F.; Fornarelli, A.; Bertan, F.; Russo, R.; Marsal-Cots, A.; Morrone, L.A.; Adornetto, A.; Corasaniti, M.T.; Bano, D.; Bagetta, G.; et al. The tricyclic antidepressant clomipramine inhibits neuronal autophagic flux. Scientific reports 2019, 9, 4881. [Google Scholar] [CrossRef]
- Kwon, Y.; Bang, Y.; Moon, S.H.; Kim, A.; Choi, H.J. Amitriptyline interferes with autophagy-mediated clearance of protein aggregates via inhibiting autophagosome maturation in neuronal cells. Cell death & disease 2020, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Hong, S.; Kim, N.; Shin, K.S.; Kang, S.J. Tricyclic Antidepressants Amitriptyline and Desipramine Induced Neurotoxicity Associated with Parkinson's Disease. Molecules and cells 2015, 38, 734–740. [Google Scholar] [CrossRef]
- Gentile, D.; Berliocchi, L.; Russo, R.; Bagetta, G.; Corasaniti, M.T. Effects of the autophagy modulators d-limonene and chloroquine on vimentin levels in SH-SY5Y cells. Biochemical and biophysical research communications 2020, 533, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Cassiano, M.G.; Ciociaro, A.; Adornetto, A.; Varano, G.P.; Chiappini, C.; Berliocchi, L.; Tassorelli, C.; Bagetta, G.; Corasaniti, M.T. Role of D-Limonene in autophagy induced by bergamot essential oil in SH-SY5Y neuroblastoma cells. PloS one 2014, 9, e113682. [Google Scholar] [CrossRef]
- Corasaniti, M.T.; Bilotta, A.; Strongoli, M.C.; Navarra, M.; Bagetta, G.; Di Renzo, G. HIV-1 coat protein gp120 stimulates interleukin-1beta secretion from human neuroblastoma cells: evidence for a role in the mechanism of cell death. British journal of pharmacology 2001, 134, 1344–1350. [Google Scholar] [CrossRef]
- Yordanov, Y.I. Hep G2 cell culture confluence measurement in phase-contrast micrographs - a user-friendly, open-source software-based approach. Toxicology mechanisms and methods 2020, 30, 146–152. [Google Scholar] [CrossRef]
- Pemberton, K.; Mersman, B.; Xu, F. Using ImageJ to Assess Neurite Outgrowth in Mammalian Cell Cultures: Research Data Quantification Exercises in Undergraduate Neuroscience Lab. Journal of undergraduate neuroscience education : JUNE : a publication of FUN, Faculty for Undergraduate Neuroscience 2018, 16, A186–a194. [Google Scholar] [PubMed]
- Su, J.M.; Wang, L.Y.; Liang, Y.L.; Zha, X.L. Role of cell adhesion signal molecules in hepatocellular carcinoma cell apoptosis. World journal of gastroenterology 2005, 11, 4667–4673. [Google Scholar] [CrossRef]
- Suzanne, M.; Steller, H. Letting go: modification of cell adhesion during apoptosis. Journal of biology 2009, 8, 49. [Google Scholar] [CrossRef]
- He, L.; Fu, Y.; Tian, Y.; Wang, X.; Zhou, X.; Ding, R.B.; Qi, X.; Bao, J. Antidepressants as Autophagy Modulators for Cancer Therapy. Molecules (Basel, Switzerland) 2023, 28. [Google Scholar] [CrossRef]
- Rossi, M.; Munarriz, E.R.; Bartesaghi, S.; Milanese, M.; Dinsdale, D.; Guerra-Martin, M.A.; Bampton, E.T.; Glynn, P.; Bonanno, G.; Knight, R.A.; et al. Desmethylclomipramine induces the accumulation of autophagy markers by blocking autophagic flux. Journal of cell science 2009, 122, 3330–3339. [Google Scholar] [CrossRef]
- Gassen, N.C.; Hartmann, J.; Schmidt, M.V.; Rein, T. FKBP5/FKBP51 enhances autophagy to synergize with antidepressant action. Autophagy 2015, 11, 578–580. [Google Scholar] [CrossRef]
- Zschocke, J.; Zimmermann, N.; Berning, B.; Ganal, V.; Holsboer, F.; Rein, T. Antidepressant drugs diversely affect autophagy pathways in astrocytes and neurons--dissociation from cholesterol homeostasis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2011, 36, 1754–1768. [Google Scholar] [CrossRef]
- Zschocke, J.; Rein, T. Antidepressants encounter autophagy in neural cells. Autophagy 2011, 7, 1247–1248. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 conjugation system in mammalian autophagy. The international journal of biochemistry & cell biology 2004, 36, 2503–2518. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Elazar, Z.; Seglen, P.O.; Rubinsztein, D.C. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy 2008, 4, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Funk, R.S.; Krise, J.P. Cationic amphiphilic drugs cause a marked expansion of apparent lysosomal volume: implications for an intracellular distribution-based drug interaction. Molecular pharmaceutics 2012, 9, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, I.; Clemmensen, K.K.B.; Fogde, D.L.; Dietrich, T.N.; Giacobini, J.D.; Bilgin, M.; Jäättelä, M.; Maeda, K. Cationic amphiphilic drugs induce accumulation of cytolytic lysoglycerophospholipids in the lysosomes of cancer cells and block their recycling into common membrane glycerophospholipids. Molecular biology of the cell 2024, 35, ar25. [Google Scholar] [CrossRef] [PubMed]
- Vater, M.; Möckl, L.; Gormanns, V.; Schultz Fademrecht, C.; Mallmann, A.M.; Ziegart-Sadowska, K.; Zaba, M.; Frevert, M.L.; Bräuchle, C.; Holsboer, F.; et al. New insights into the intracellular distribution pattern of cationic amphiphilic drugs. Scientific reports 2017, 7, 44277. [Google Scholar] [CrossRef] [PubMed]
- Chazotte, B. Labeling lysosomes in live cells with LysoTracker. Cold Spring Harbor protocols 2011, 2011, pdbprot5571. [Google Scholar] [CrossRef]
- Yoshimori, T.; Yamamoto, A.; Moriyama, Y.; Futai, M.; Tashiro, Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. The Journal of biological chemistry 1991, 266, 17707–17712. [Google Scholar] [CrossRef]
- Frake, R.A.; Ricketts, T.; Menzies, F.M.; Rubinsztein, D.C. Autophagy and neurodegeneration. The Journal of clinical investigation 2015, 125, 65–74. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. International journal of molecular sciences 2018, 19. [Google Scholar] [CrossRef]
- Adornetto, A.; Parisi, V.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G.; Tonin, P.; Russo, R. The Role of Autophagy in Glaucomatous Optic Neuropathy. Frontiers in cell and developmental biology 2020, 8, 121. [Google Scholar] [CrossRef]
- Berliocchi, L.; Maiarù, M.; Varano, G.P.; Russo, R.; Corasaniti, M.T.; Bagetta, G.; Tassorelli, C. Spinal autophagy is differently modulated in distinct mouse models of neuropathic pain. Molecular pain 2015, 11, 3. [Google Scholar] [CrossRef]
- Shi, Q.; Pei, F.; Silverman, G.A.; Pak, S.C.; Perlmutter, D.H.; Liu, B.; Bahar, I. Mechanisms of Action of Autophagy Modulators Dissected by Quantitative Systems Pharmacology Analysis. International journal of molecular sciences 2020, 21. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.L.; Liu, Y.X.; An, N.; Zhao, S.; Bao, J.K. Autophagy modulation as a target for anticancer drug discovery. Acta pharmacologica Sinica 2013, 34, 612–624. [Google Scholar] [CrossRef]
- Gao, L.; Jauregui, C.E.; Teng, Y. Targeting autophagy as a strategy for drug discovery and therapeutic modulation. Future medicinal chemistry 2017, 9, 335–345. [Google Scholar] [CrossRef]
- Arimochi, H.; Morita, K. Characterization of cytotoxic actions of tricyclic antidepressants on human HT29 colon carcinoma cells. European journal of pharmacology 2006, 541, 17–23. [Google Scholar] [CrossRef]
- Xia, Z.; Bergstrand, A.; DePierre, J.W.; Nässberger, L. The antidepressants imipramine, clomipramine, and citalopram induce apoptosis in human acute myeloid leukemia HL-60 cells via caspase-3 activation. Journal of biochemical and molecular toxicology 1999, 13, 338–347. [Google Scholar] [CrossRef]
- Levkovitz, Y.; Gil-Ad, I.; Zeldich, E.; Dayag, M.; Weizman, A. Differential induction of apoptosis by antidepressants in glioma and neuroblastoma cell lines: evidence for p-c-Jun, cytochrome c, and caspase-3 involvement. Journal of molecular neuroscience : MN 2005, 27, 29–42. [Google Scholar] [CrossRef]
- Rácz, B.; Spengler, G. Repurposing Antidepressants and Phenothiazine Antipsychotics as Efflux Pump Inhibitors in Cancer and Infectious Diseases. Antibiotics (Basel, Switzerland) 2023, 12. [Google Scholar] [CrossRef]
- Varga, A.; Nugel, H.; Baehr, R.; Marx, U.; Hevér, A.; Nacsa, J.; Ocsovszky, I.; Molnar, J. Reversal of multidrug resistance by amitriptyline in vitro. Anticancer research 1996, 16, 209–211. [Google Scholar]
- O'Brien, F.E.; Dinan, T.G.; Griffin, B.T.; Cryan, J.F. Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: clinical significance of in vitro and in vivo findings. British journal of pharmacology 2012, 165, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Asensi-Cantó, A.; Rodríguez-Braun, E.; Beltrán-Videla, A.; Hurtado, A.M.; Conesa-Zamora, P. Effects of imipramine on cancer patients over-expressing Fascin1; description of the HITCLIF clinical trial. Frontiers in oncology 2023, 13, 1238464. [Google Scholar] [CrossRef]
- Cordero, M.D.; Sánchez-Alcázar, J.A.; Bautista-Ferrufino, M.R.; Carmona-López, M.I.; Illanes, M.; Ríos, M.J.; Garrido-Maraver, J.; Alcudia, A.; Navas, P.; de Miguel, M. Acute oxidant damage promoted on cancer cells by amitriptyline in comparison with some common chemotherapeutic drugs. Anti-cancer drugs 2010, 21, 932–944. [Google Scholar] [CrossRef]
- Motafeghi, F.; Shahsavari, R.; Mortazavi, P.; Shokrzadeh, M. Anticancer effect of paroxetine and amitriptyline on HT29 and A549 cell lines. Toxicology in vitro : an international journal published in association with BIBRA 2023, 87, 105532. [Google Scholar] [CrossRef]
- Huang, Y.H.; Yeh, C.T. Anticancer Effects of Antidepressants in Hepatocellular Carcinoma Cells. Anticancer research 2023, 43, 1201–1206. [Google Scholar] [CrossRef]
- Mao, X.; Hou, T.; Cao, B.; Wang, W.; Li, Z.; Chen, S.; Fei, M.; Hurren, R.; Gronda, M.; Wu, D.; et al. The tricyclic antidepressant amitriptyline inhibits D-cyclin transactivation and induces myeloma cell apoptosis by inhibiting histone deacetylases: in vitro and in silico evidence. Molecular pharmacology 2011, 79, 672–680. [Google Scholar] [CrossRef]
- Pula, G.; Pistilli, A.; Montagnoli, C.; Stabile, A.M.; Rambotti, M.G.; Rende, M. The tricyclic antidepressant amitriptyline is cytotoxic to HTB114 human leiomyosarcoma and induces p75(NTR)-dependent apoptosis. Anti-cancer drugs 2013, 24, 899–910. [Google Scholar] [CrossRef]
- Zinnah, K.M.A.; Park, S.Y. Sensitizing TRAIL-resistant A549 lung cancer cells and enhancing TRAIL-induced apoptosis with the antidepressant amitriptyline. Oncology reports 2021, 46. [Google Scholar] [CrossRef]
- Evason, K.J.; Francisco, M.T.; Juric, V.; Balakrishnan, S.; Lopez Pazmino Mdel, P.; Gordan, J.D.; Kakar, S.; Spitsbergen, J.; Goga, A.; Stainier, D.Y. Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish. PLoS genetics 2015, 11, e1005305. [Google Scholar] [CrossRef]
- Higgins, S.C.; Pilkington, G.J. The in vitro effects of tricyclic drugs and dexamethasone on cellular respiration of malignant glioma. Anticancer research 2010, 30, 391–397. [Google Scholar]
- Villanueva-Paz, M.; Cordero, M.D.; Pavón, A.D.; Vega, B.C.; Cotán, D.; De la Mata, M.; Oropesa-Ávila, M.; Alcocer-Gomez, E.; de Lavera, I.; Garrido-Maraver, J.; et al. Amitriptyline induces mitophagy that precedes apoptosis in human HepG2 cells. Genes & cancer 2016, 7, 260–277. [Google Scholar] [CrossRef]
- Wu, H.; Che, X.; Zheng, Q.; Wu, A.; Pan, K.; Shao, A.; Wu, Q.; Zhang, J.; Hong, Y. Caspases: a molecular switch node in the crosstalk between autophagy and apoptosis. International journal of biological sciences 2014, 10, 1072–1083. [Google Scholar] [CrossRef]
- Ashoor, R.; Yafawi, R.; Jessen, B.; Lu, S. The contribution of lysosomotropism to autophagy perturbation. PloS one 2013, 8, e82481. [Google Scholar] [CrossRef]
- Guan, Y.; Li, X.; Umetani, M.; Boini, K.M.; Li, P.L.; Zhang, Y. Tricyclic antidepressant amitriptyline inhibits autophagic flux and prevents tube formation in vascular endothelial cells. Basic & clinical pharmacology & toxicology 2019, 124, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, F.; Hensley, T.; Pope, C.; Funk, R.S.; Loewen, G.J.; Buckley, D.B.; Parkinson, A. Lysosomal sequestration (trapping) of lipophilic amine (cationic amphiphilic) drugs in immortalized human hepatocytes (Fa2N-4 cells). Drug metabolism and disposition: the biological fate of chemicals 2013, 41, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Nadanaciva, S.; Lu, S.; Gebhard, D.F.; Jessen, B.A.; Pennie, W.D.; Will, Y. A high content screening assay for identifying lysosomotropic compounds. Toxicology in vitro : an international journal published in association with BIBRA 2011, 25, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Henkel, A.W.; Groemer, T.W.; Städtler, S.; Welzel, O.; Tripal, P.; Rotter, A.; Bleich, S.; Trapp, S. Lipophilic cationic drugs increase the permeability of lysosomal membranes in a cell culture system. Journal of cellular physiology 2010, 224, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Yim, W.W.; Mizushima, N. Lysosome biology in autophagy. Cell discovery 2020, 6, 6. [Google Scholar] [CrossRef]
- Xu, J.; Gu, J.; Pei, W.; Zhang, Y.; Wang, L.; Gao, J. The role of lysosomal membrane proteins in autophagy and related diseases. The FEBS journal 2023. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




