Submitted:
14 May 2024
Posted:
17 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introducción
2. Materials and Methods
2.1. Herbicides, solvents and Reagents
| Herbicide1 | Structure | Formula | MW2 | SH2O3 | log Kow4 | H5 |
|---|---|---|---|---|---|---|
| IsoproturonPU |  |
C₁₂H₁₈N₂O | 206.3 | 70 | 2.5 | 1.5×10-5 |
| TerbuthylazineTZ |  |
C₉H₁₆ClN₅ | 229.7 | 7 | 3.4 | 2.3×10-3 |
2.2. Experimental Setup
2.3. Sample Preparation and Analytical Determinations
| Trials | pH | aCE | bSO4= | bCl- | bHCO3- | |
|---|---|---|---|---|---|---|
| 1 | UV (1 x 254 nm + 1 x 366 nm) | 6.7 | < 5 | - | - | - |
| 2 | UV (2 x 366 nm) | 6.7 | < 5 | - | - | - |
| 3 | Na2S2O8 | 5.1 | 240 | - | - | - |
| 4 | Na2S2O8/UV (254/366 nm) | 5.1 | 242 | - | - | - |
| 5 | Na2S2O8/UV (366 nm) | 5.0 | 246 | - | - | - |
| 6 | TiO2/UV (366 nm) | 5.3 | < 5 | - | - | - |
| 7 | Na2S2O8/UV (366 nm) | 4.7 | 760 | 250 | - | - |
| 8 | Na2S2O8/UV (366 nm) | 8.3 | 377 | - | - | 125 |
| 9 | Na2S2O8/UV (366 nm) | 5.2 | 610 | - | 150 | - |
| 10 | Na2S2O8/UV (366 nm) | 8.4 | 1200 | 250 | 150 | 125 |
3. Results and Discussion
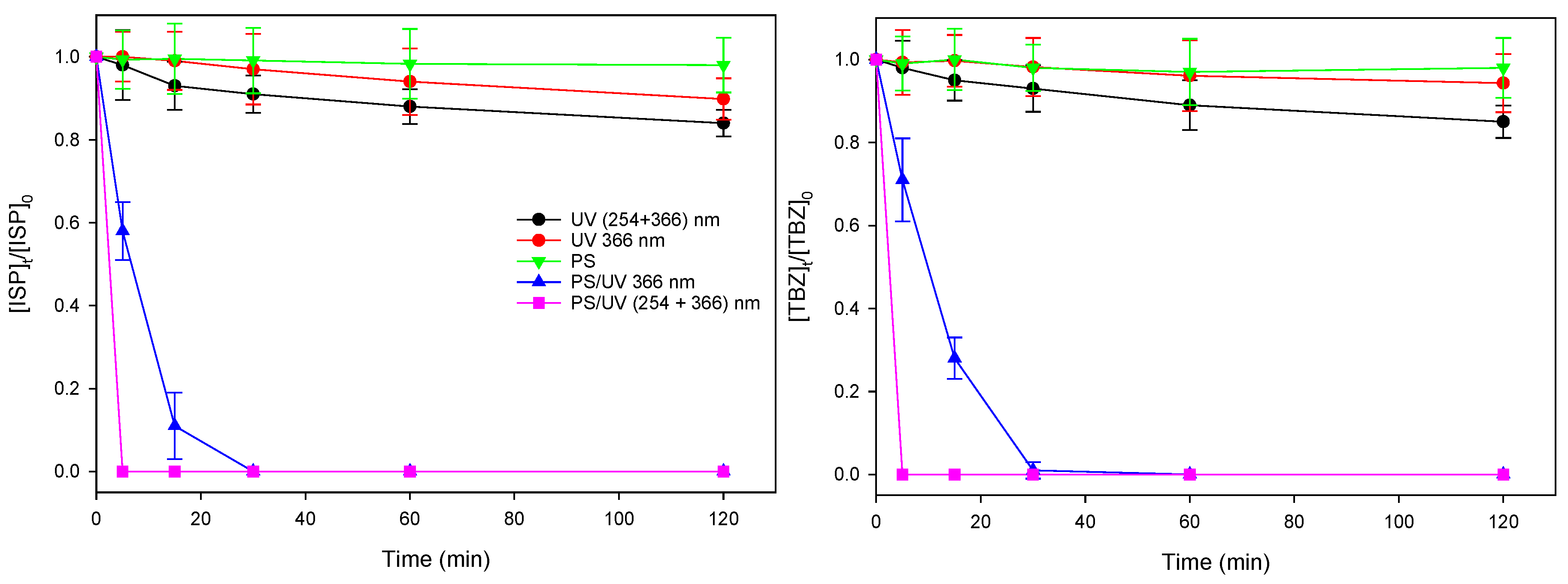
3.1. Photodegradation of herbicides by UV, Na2S2O8 and UV/Na2S2O8
3.2. Comparing UV/TiO2 and UV/Na2S2O8 Efficiency
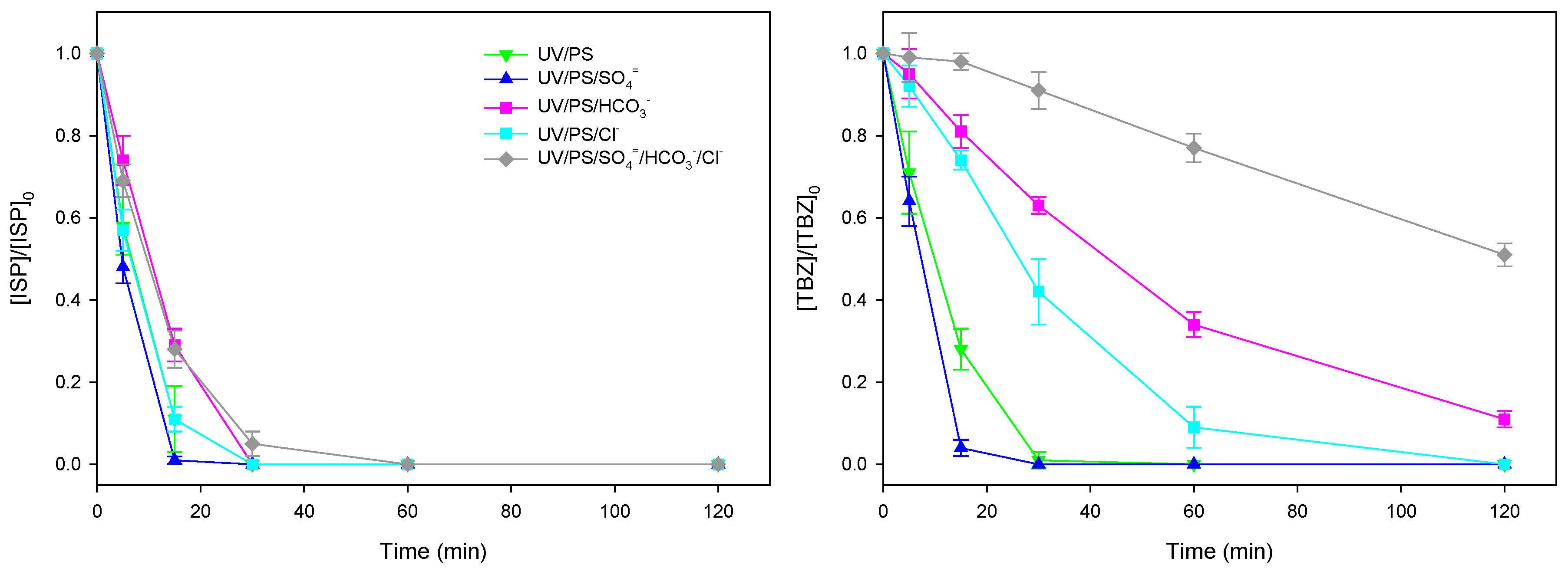
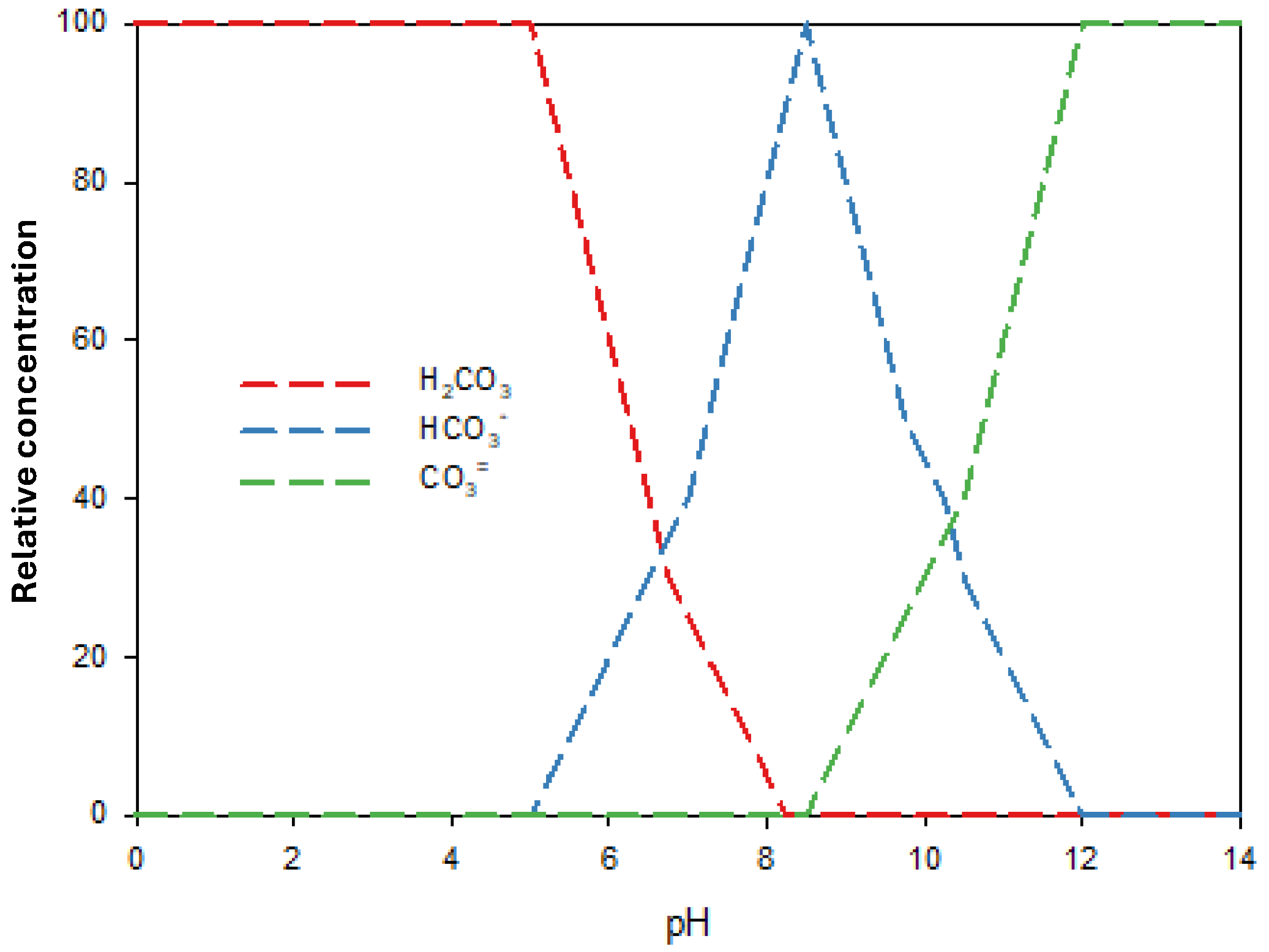
3.3. Effect of Inorganic Anion Content on Herbicide Photodegradation Using Na2S2O8/UV
3.4. Degradation Pathway of Herbicides with UV/Na2S2O8
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- EU. 2009. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. Off. J. Eur. Union, L309, 71-86. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009L0128.
- EC. 2019. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions - the European Green Deal, COM/2019/640. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN.
- EU. 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Union, L327, 1-73. https://eur-lex.europa.eu/eli/dir/2000/60/oj.
- EU. 2006. Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the protection of groundwater against pollution and deterioration. Off. J. Eur. Union, L372, 19-31. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006L0118.
- EU. 2013. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union, L226, 1-17. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:en:PDF.
- EU. 2022. Proposal for a Directive of the European Parliament and of the Council amending Directive 2000/60/EC establishing a framework for Community action in the field of water policy, Directive 2006/118/EC on the protection of groundwater against pollution and deterioration and Directive 2008/105/EC on environmental quality standards in the field of water policy. COM/2022/540. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52022PC0540.
- EU. 2001. Decision No 2455/2001/EC of the European Parliament and of the Council of 20 November 2001 establishing the list of priority substances in the field of water policy and amending Directive 2000/60/EC. Off. J. Eur. Union, L331, 1-5. https://faolex.fao.org/docs/pdf/eur36180.pdf.
- EU. 2008 Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/ EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council. Off. J. Eur. Union, L348, 84-97. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0105.
- EC. 2015. Commission Implementing Decision (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union, L78, 40-42. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015D0495.
- EC. 2018. Commission Implementing Decision (EU) 2018/840 of 5 June 2018 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing Commission Implementing Decision (EU) 2015/495. Off. J. Eur. Union, L141, 9-12. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0840.
- EC. 2020. Commission Implementing Decision (EU) 2020/1161 of 4 August 2020 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union, L257, 32-35. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020D1161.
- EC. 2022. Commission Implementing Decision (EU) 2022/1307 of 22 July 2022 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council Off. J. Eur. Union, L197, 117-120. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022D1307.
- EU. 2020. EU Parliament Council Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption (recast). Off. J. Eur. Union, L435, 1-62. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184.
- EU. 1991. Council Directive of 21 May 1991 concerning urban wastewater treatment (91/271/EEC). Off. J. Eur. Union, L135, 40-91. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31991L0271.
- EEA, 2018, European waters- assessment of status and pressures 2018, EEA Report No 7/2018, European Environment Agency. https://www.eea.europa.eu/publications/state-of-water/.
- Mohaupt, V.; Völker, J.; Altenburger, R.; Birk, S.; Kirst, I.; Kühnel, D.; Küster, E.; Semeradova, S.; Šubelj, G.; Whalley, C. 2020. Pesticides in European rivers, lakes and groundwaters - Data assessment. European Topic Centre on Inland, Coastal and Marine Waters (European Environment Agency). Magdeburg, Germany. Technical Report 1/2020. Magdeburg, Germany. [CrossRef]
- Serrano-Valera, M.; Vela, N.; Piuvezam, G.; Mateo-Ramírez, F.; Santiago-Fernandes, I.D.; Martínez-Alcalá, I. 2024. Prevalence and concentration of pesticides in European waters: A protocol for systematic review and meta-analysis. PLoS ONE, 19, e0282386. [CrossRef]
- EC. 2022. Proposal for a Directive of the European Parliament and of the Council concerning urban wastewater treatment (recast). SWD/2022/541. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52022SC0541.
- Parida, V.K.; Saidulu, D.; Majumder, A.; Srivastava, A.; Gupta, B.; Gupta, A.K. 2021. Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. J. Environ. Chem. Eng.; 9, e105966. [CrossRef]
- EU. 2020. Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on minimum requirements for water reuse. Off. J. Eur. Union, L177, 32-55. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0741.
- Alvarino, T.; Suarez, S.; Lema, J.; Omil, F. 2018. Understanding the sorption and biotransformation of organic micropollutants in innovative biological wastewater treatment technologies. Sci. Total Environ.; 615, 297-306. [CrossRef]
- Zhang, W.; Liang, W.; Zhang, Z.; Hao, T. 2021. Aerobic granular sludge (AGS) scouring to mitigate membrane fouling: Performance, hydrodynamic mechanism and contribution quantification model. Water Res. 188, e116518. [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. 2021. Occurrence and fate of emerging pollutants in water environment and options for their removal. Water, 13, e181. [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. 2021. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ.; 753, e141990. [CrossRef]
- Saha, M.P. 2021. Advanced oxidation processes for effluent treatment plants. Elsevier, Amsterdam.
- Soto-Verjel, J.; Maturana, A.Y.; Villamizar, S.E. 2022. Advanced catalytic oxidation coupled to biological systems to treat pesticide contaminated water: A review on technological trends and future challenges. Water Sci. Technol.; 85, 1263-1294. [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. 2018. Evaluation of advanced oxidation processes for water and wastewater treatment - A critical review. Water Res.; 139, 118-131. [CrossRef]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. 2019. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J.; 378, e122149. [CrossRef]
- Lee, J.; Von Gunten, U.; Kim, J.H. 2020. Persulfate-based advanced oxidation: critical assessment of opportunities and roadblocks. Environ. Sci. Technol.; 54, 3064-3081. [CrossRef]
- Brillas, E. 2023. Activation of persulfate and peroxymonosulfate for the removal of herbicides from synthetic and real waters and wastewaters. J. Environ. Chem. Eng.; 110380. [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I.; 2013. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol. Chem. Eng. J,. 224, 10-16. [CrossRef]
- Oh, W.D.; Dong, Z.; Lim, T.T.; 2016. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. App. Catal. B Environ.; 194, 169-201. [CrossRef]
- Wang, J.; Wang, S. 2018. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J.; 334, 1502-1517. [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D.; 2017. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J.; 330, 44-62. [CrossRef]
- Matzek, L.W.; Carter, K.E.; 2016. Activated persulfate for organic chemical degradation: a review. Chemosphere, 151, 178-188. doi10.1016/j.chemosphere.2016.02.055.
- Ren, W.; Huang, X.; Wang, L.; Liu, X.; Zhou, Z.; Wang, Y.; Lin, C.; He, M.; Ouyang, W. 2021. Degradation of simazine by heat-activated peroxydisulfate process: A coherent study on kinetics, radicals and models. Chem. Eng. J.; 426, e131876. [CrossRef]
- Serrano, K.G. 2018. Indirect Electrochemical Oxidation Using Hydroxyl Radical, Active Chlorine, and Peroxodisulfate. In: Electrochemical Water and Wastewater Treatment. (Martínez-Huitle, C.A.; Rodrigo, M.A.; Scialdone, O. (Eds.), Elsevier, Amsterdam. [CrossRef]
- Ribeiro, A.R.L.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. 2019. Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem. Eng. J.; 363, 155-173. [CrossRef]
- Vela, N.; Fenoll, J.; Garrido, I.; Navarro, G.; Gambín, M.; Navarro, S. 2015. Photocatalytic mitigation of triazinone herbicide residues using titanium dioxide in slurry photoreactor. Catal. Today, 252, 70-77. [CrossRef]
- Orellana-Garcia, F.; Alvarez, M.A.; Lopez-Ramon, V.; Rivera-Utrilla, J.; Sanchez-Polo, M.; Mota, A.J. 2014. Photodegradation of herbicides with different chemical natures in aqueous solution by ultraviolet radiation. Effects of operational variables and solution chemistry. Chem. Eng. J.; 255, 307-315. [CrossRef]
- ECHA. 2014. Proposal for Harmonised Classification and Labelling. CLH-227-637-9 Report for terbuthylazine. European Chemicals Agency. Merseyside, UK. https://echa.europa.eu/substance-information/-/substanceinfo/100.025.125.
- Lin, C.C.; Wu, M.S.; 2014. UV/S2O82− process for degrading polyvinyl alcohol in aqueous solutions. Chem. Eng. Process.; 85, 209-215. [CrossRef]
- Liu, B.; Zhao, X.; Terashima, C.; Fujishima, A.; Nakata, K.; 2014. Thermodynamic and kinetics analysis of heterogeneous photocatalysis for semiconductor systems. Phys. Chem. Chem. Phys.; 16, 8751-8760. [CrossRef]
- Nafradi, M.; Alapi, T.; Bencsik, G.; Janaky, C. 2022. Impact of reaction parameters and water matrices on the removal of organic pollutants by TiO2/LED and ZnO/LED heterogeneous photocatalysis using 365 and 398 nm radiation. Nanomaterials, 12, e5. [CrossRef]
- Mack, J.; Bolton, J.R.; 1999. Photochemistry of nitrite and nitrate in aqueous solution: a review. J. Photochem. Photobiol. A. Chem. 128, 1-13. [CrossRef]
- Ghauch, A.; Baalbaki, A.; Amasha, M.; El Asmar, R.; Tantawi, O. 2017. Contribution of persulfate in UV-254 nm activated systems for complete degradation of chloramphenicol antibiotic in water. Chem. Eng. J.; 317, 1012–1025. [CrossRef]
- Lin, C.C.; Wu, M.S.; 2014. Degradation of ciprofloxacin by UV/S2O82− process in a large photoreactor. J. Photochem. Photobiol. A: Chem.; 285, 1-6. [CrossRef]
- Cabrera-Reina, A.; Aliste, M.; Polo-López, M.I.; Malato, S.; Oller, I. 2023. Individual and combined effect of ions species and organic matter on the removal of microcontaminants by Fe3+-EDDS/solar-light activated persulfate. Water Res.; 230, e119566. [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B.; 1988. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals in aqueous solution. J. Phys. Chem. Ref. Data, 17, 513-886. [CrossRef]
- Armstrong, D.A.; Huie, R.E.; Koppenol, W.H.; Lymar, S.V.; Merényi, G.; Neta, P.; Ruscic, B.; Stanbury, D.M.; Steenken, S.; Wardman, P. 2015. Standard electrode potentials involving radicals in aqueous solution: inorganic radicals (IUPAC Technical Report). Pure Appl. Chem.; 87, 1139-1150. [CrossRef]
- Bennedsen, L.R.; Muff, J.; Søgaard, E.G.; 2012. Influence of chloride and carbonates on the reactivity of activated persulfate. Chemosphere, 86, 1092–1097. [CrossRef]
- Canonica, S.; Kohn, T.; Mac, M.; Real, F.J.; Wirz, J.; von Gunten, U. 2005. Photosensitizer method to determine rate constants for the reaction of carbonate radical with organic compounds. Environ. Sci. Tech.; 39, 9182-9188. [CrossRef]
- Acero, J.L.; Benítez, F.J.; Real, F.J.; Rodríguez, E.; 2018. Degradation of selected emerging contaminants by UV-activated persulfate: kinetics and influence of matrix constituents. Sep. Purif. Technol.; 201, 41-50. [CrossRef]
- Lebik-Elhadi, H.; Frontistis, Z.; Ait-Amar, H.; Madjene, F.; Mantzavinos, D.; 2020. Degradation of pesticide thiamethoxam by heat–activated and ultrasound–activated persulfate: effect of key operating parameters and the water matrix. Process Saf. Environ. Prot.; 134, 197–207. [CrossRef]
- Sbardella, L.; Gala, I.V.; Comas, J.; Layret, R.R.; Gernjak, W. 2019. The impact of wastewater matrix on the degradation of pharmaceutically active compounds by oxidation processes including ultraviolet radiation and sulfate radicals, J. Hazard. Mat.; 380, e120869. [CrossRef]
- Lin, C.C.; Lee, L.T.; Hsu, L.J. 2013. Performance of UV/S2O82- process in degrading polyvinyl alcohol in aqueous solutions. J. Photochem. Photobiol. A-Chem. 252, 1-7. [CrossRef]
- Manaham, S.E. 2010. Environmental chemistry, 9th Edition, CRC Press.
- Zuo, Z.; Cai, Z.; Katsumura, Y.; Chitose, N.; Muroya, Y. Reinvestigation of the acid−base equilibrium of the (bi) carbonate radical and pH dependence of its reactivity with inorganic reactants. Radiat. Phys. Chem.; 1999, 55, 15-23. [CrossRef]
- Ma, J.; Yang, Y.; Jiang, X.; Xie, Z.; Li, X.; Chen, C.; Chen, H. 2018. Impacts of inorganic anions and natural organic matter on thermally activated persulfate oxidation of BTEX in water, Chemosphere, 190, 296-306. [CrossRef]
- Xiao, R.; Meng, Y.; Fu, Y.; Wacławek, S.; Wei, Z.; Spinney, R.; Dionysiou, D.; Hu, W.P. 2023. The overlooked carbonate radical in micropollutant degradation: An insight into hydration interaction. Chem. Eng. J.; 474, e145245. [CrossRef]
- Mikhaylin, S.; Bazinet, L. 2016. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci.; 229, 34-56. [CrossRef]
- Haghsheno, R.; Mohebbi, A.; Hashemipour, H.; Sarraf, A.; 2009. Study of kinetic and fixed bed operation of removal of sulfate anions from an industrial wastewater by an anion exchange resin. J. Hazard. Mat.; 166, 961-966. [CrossRef]
- Haque, M.M.; Muneer, M. 2003. Heterogeneous photocatalysed degradation of an herbicide derivative, isoproturon in aqueous suspension of titanium dioxide. J. Environ. Manag.; 69, 169-176. [CrossRef]
- Fenoll, J.; Sabater, P.; Navarro, G.; Pérez-Lucas, G.; Navarro, S. 2013. Photocatalytic transformation of sixteen substituted phenylurea herbicides in aqueous semiconductor suspensions: Intermediates and degradation pathways. J. Hazard. Mat.; 244, 370-379. [CrossRef]
- Fenoll, J.; Hellin, P.; Martinez, C.M.; Flores, P.; Navarro, S. 2012. Semiconductor-sensitized photodegradation of s-triazine and chloroacetanilide herbicides in leaching water using TiO2 and ZnO as catalyst under natural sunlight. J. Photochem. Photobiol. A: Chem.; 238, 81-87. [CrossRef]
- Roberts, T.; Hutson, D. 1998. Metabolic Pathways of Agrochemicals. Part one: Herbicides and Plant Growth Regulators. The Royal Society of Chemistry. Cambridge, UK.



| Test | Terbuthylazine | Isoproturon | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | C/C0 | 1k | 2Sy/x | 3DT50 | R | C/C0 | 1k | 2Sy/x | 3DT50 | |
| UV/PS | 0.9952 | 1.01 | 0.1257 | 0.03 | 5.51 | 0.9953 | 1.01 | 0.1267 | 0.03 | 5.47 |
| UV/TiO2 | 0.9736 | 0.94 | 0.0409 | 0.07 | 16.94 | 0.9935 | 1.02 | 0.1137 | 0.04 | 6.09 |
| Anion | Terbuthylazine | Isoproturon | ||||||
|---|---|---|---|---|---|---|---|---|
| R | 1k | 2Sy/x | 3DT50 | R | 1k | 2Sy/x | 3DT50 | |
| UV/PS | 0.9952 | 0.1257 | 0.03 | 5.5 | 0.9953 | 0.1267 | 0.03 | 5.5 |
| UV/PS/SO4= | 0.9755 | 0.1287 | 0.08 | 5.4 | 0.9917 | 0.1672 | 0.04 | 4.1 |
| UV/PS/HCO3- | 0.9963 | 0.0175 | 0.02 | 40 | 0.9875 | 0.0844 | 0.05 | 8.2 |
| UV/PS/Cl- | 0.9829 | 0.0307 | 0.06 | 23 | 0.9961 | 0.1284 | 0.03 | 5.4 |
| UV/PS/SO4=+HCO3-+Cl- | 0.9755 | 0.0054 | 0.03 | 128 | 0.9979 | 0.0849 | 0.03 | 8.2 |
| Time (min) | pH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trial | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 0 | 6.70 | 6.74 | 5.09 | 5.11 | 5.04 | 5.33 | 4.69 | 8.29 | 5.20 | 8.39 |
| 5 | 6.53 | 6.56 | 4.97 | 5.02 | 5.00 | 5.15 | 4.60 | 8.20 | 4.79 | 8.36 |
| 15 | 6.42 | 6.41 | 4.81 | 4.88 | 4.84 | 4.93 | 4.46 | 8.23 | 4.46 | 8.39 |
| 30 | 6.29 | 6.33 | 4.58 | 4.60 | 4.64 | 4.84 | 4.29 | 8.24 | 4.20 | 8.40 |
| 60 | 6.15 | 6.19 | 4.23 | 4.34 | 4.38 | 4.75 | 4.01 | 8.21 | 3.93 | 8.35 |
| 120 | 6.07 | 6.14 | 3.86 | 3.88 | 3.95 | 4.61 | 3.77 | 8.07 | 3.66 | 8.25 |
| Time (min) | EC (µS cm-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trial | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 0 | < 5 | < 5 | 242 | 238 | 242 | < 5 | 760 | 377 | 610 | 1200 |
| 5 | < 5 | < 5 | 248 | 245 | 249 | 7 | 764 | 380 | 620 | 1234 |
| 15 | < 5 | < 5 | 255 | 252 | 254 | 10 | 769 | 383 | 644 | 1253 |
| 30 | < 5 | < 5 | 261 | 263 | 260 | 12 | 773 | 386 | 662 | 1259 |
| 60 | < 5 | < 5 | 273 | 270 | 266 | 18 | 795 | 389 | 674 | 1262 |
| 120 | < 5 | < 5 | 285 | 287 | 280 | 23 | 825 | 395 | 700 | 1265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





