Submitted:
13 May 2024
Posted:
14 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
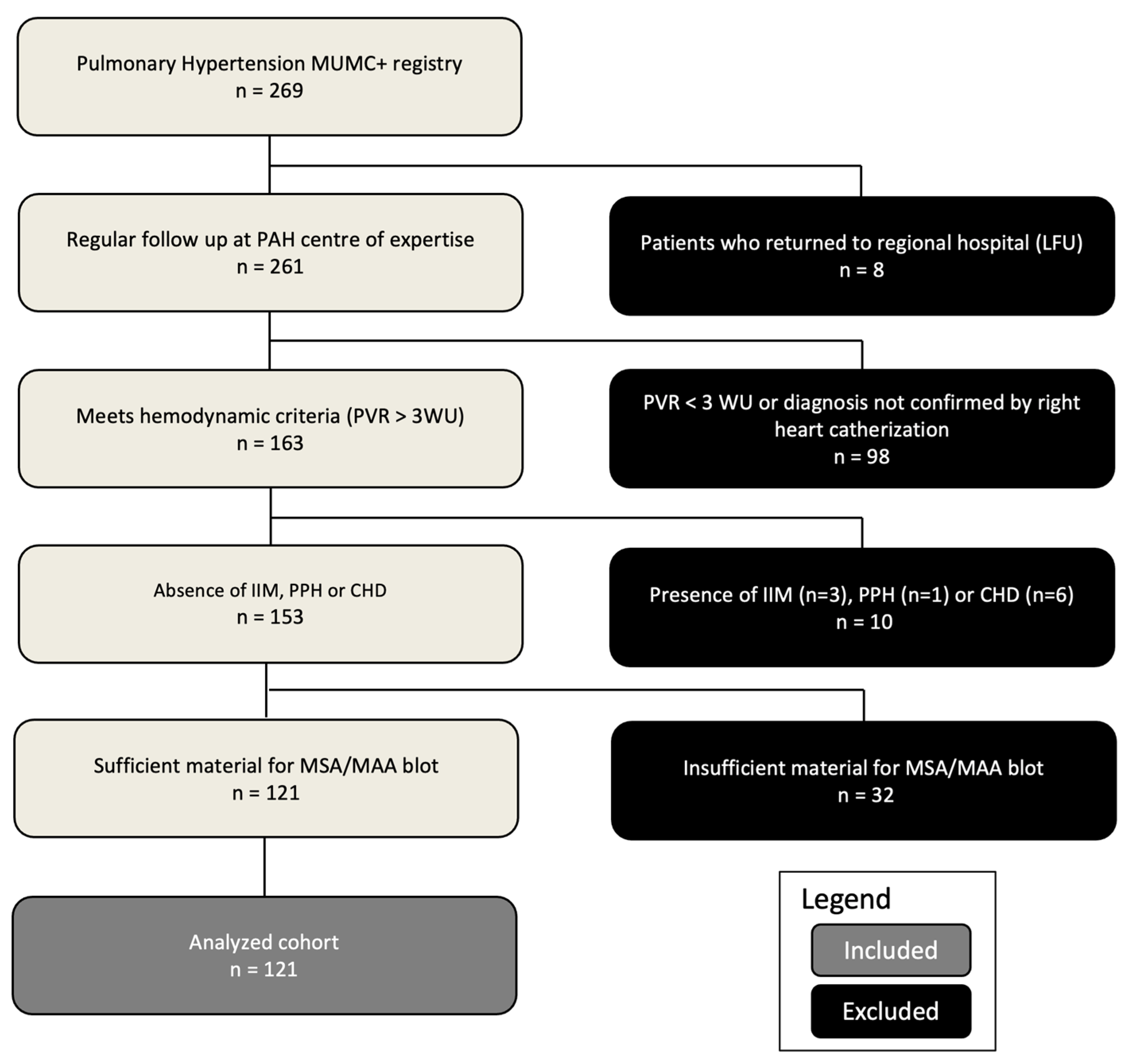
2.1. Patient Selection
2.2. Determination of Antibodies
2.3. Statistical Analysis
3. Results
3.1. Study Population
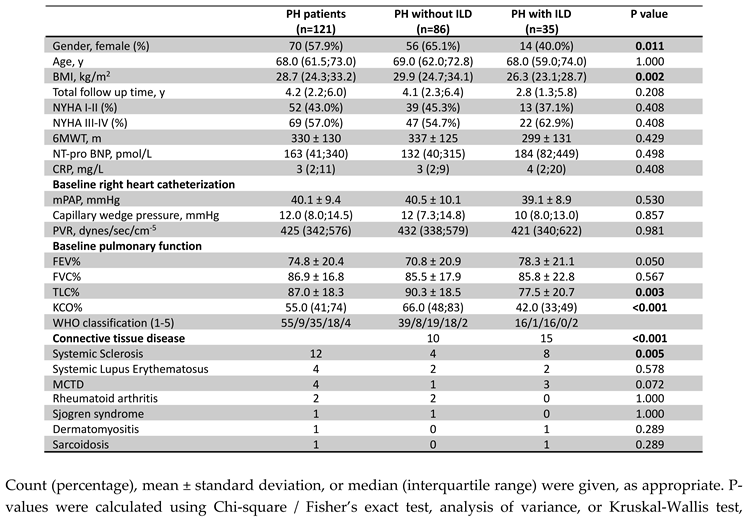

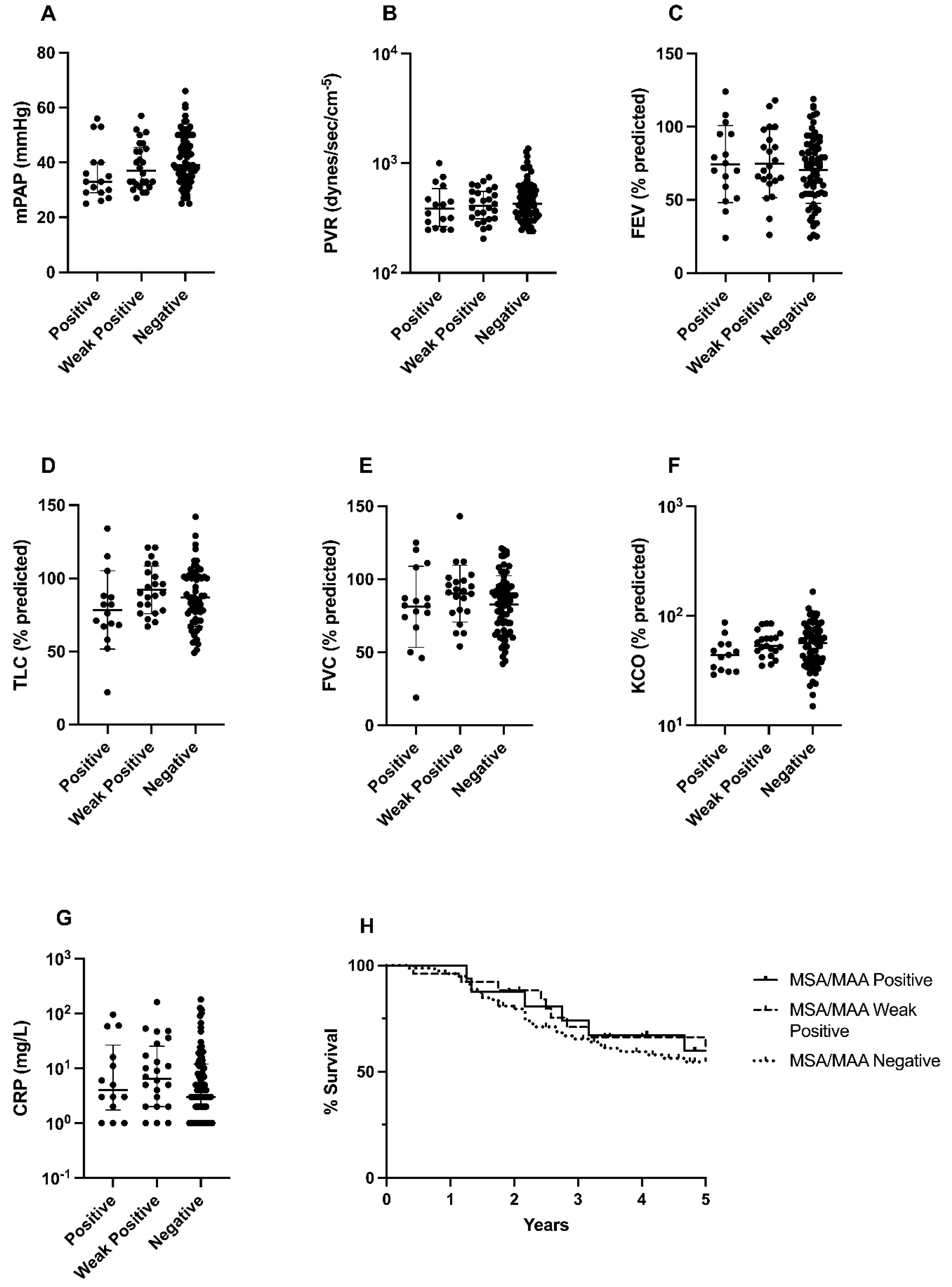
3.2. Cardiopulmonary Characteristics
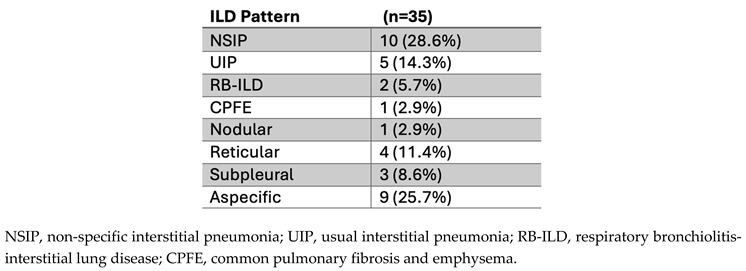
3.3. Prevalence of Myositis-Specific Antibodies (MSA) and Myositis-Associated Antibodies (MAA) in PH Patients
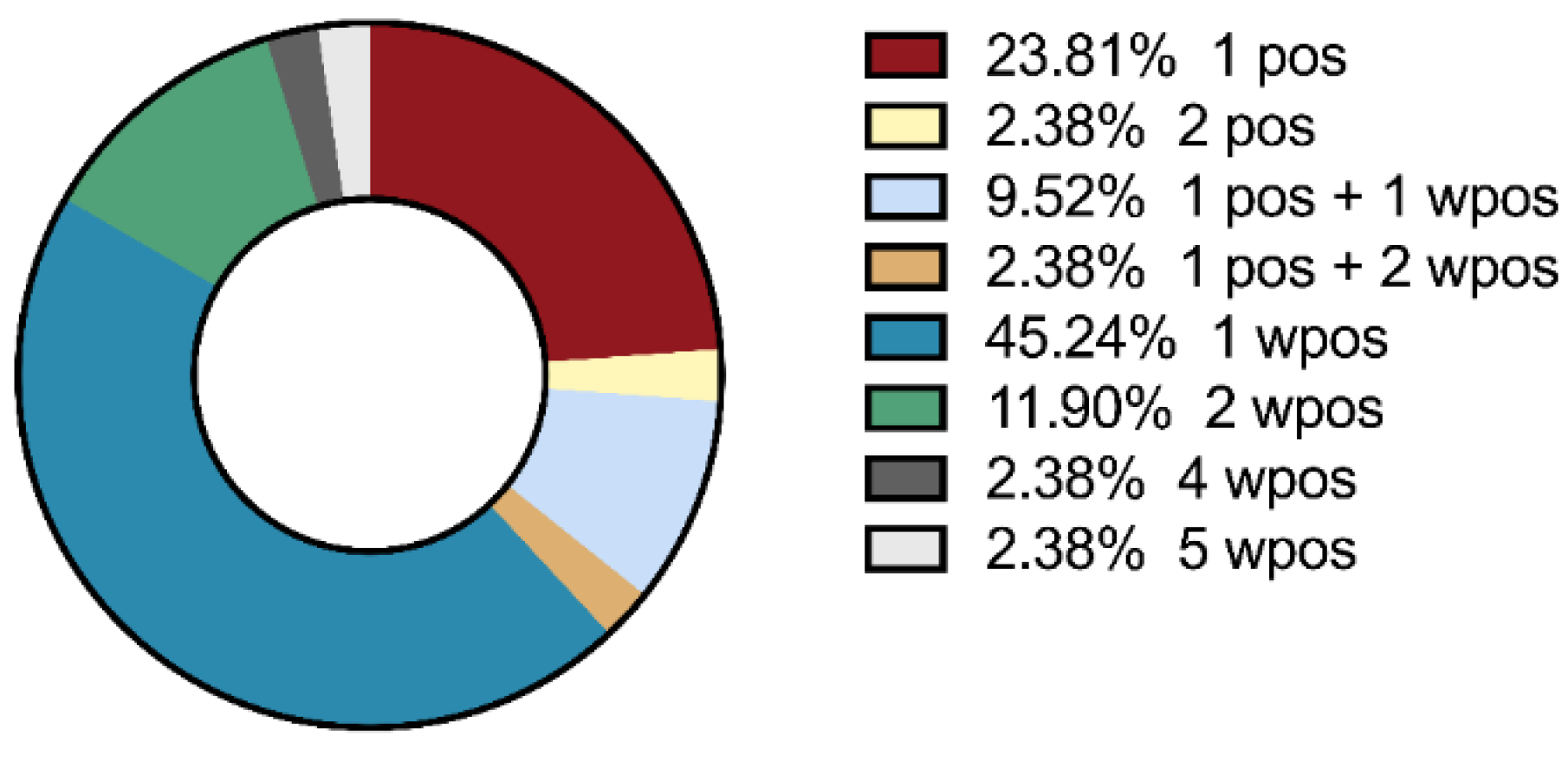
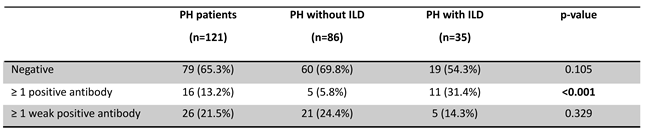
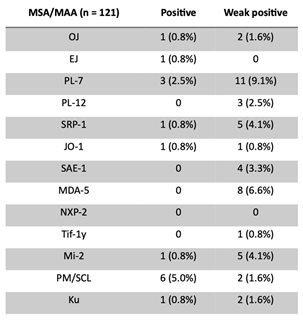
- Prevalence of myositis specific antibodies (MSA) and myositis associated antibodies (MAA) in PH patients with or without radiological signs of ILD
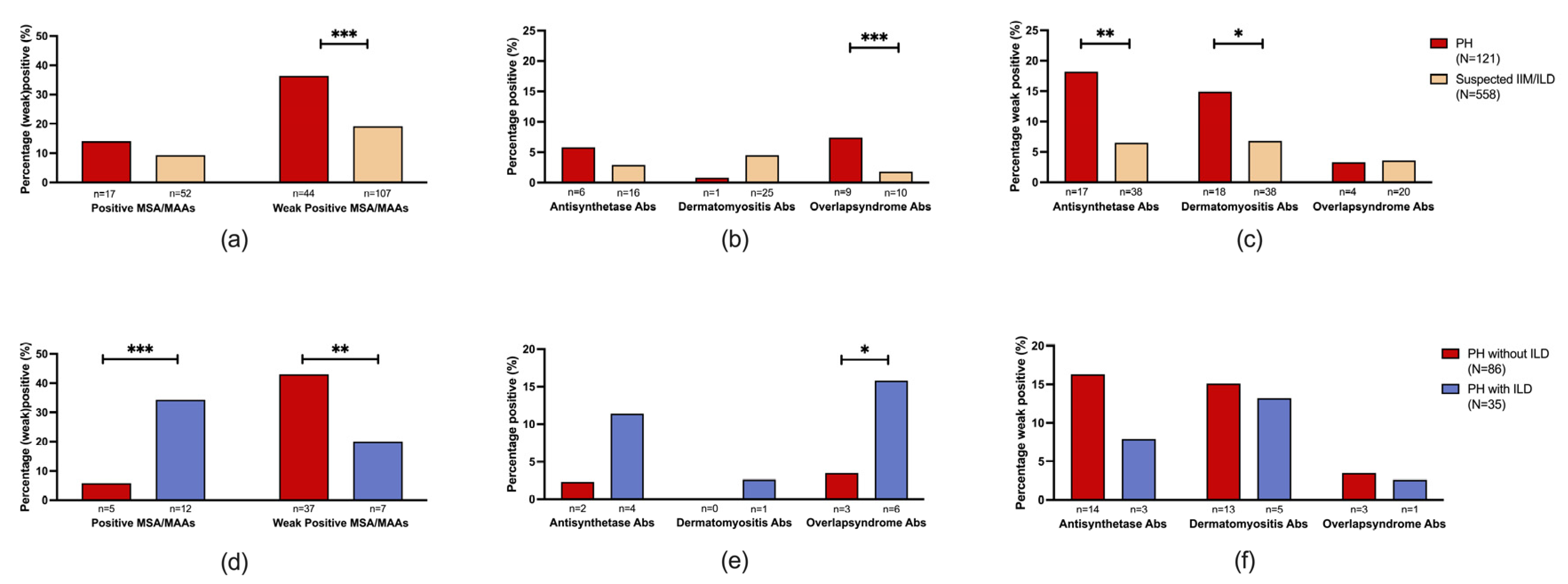
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Patient ID | Sex m/f | Age at inclusion | PH WHO type | Radiological signs of ILD y/n | MMA/MSA |
| 1 | 0 | 64 | 1 | y | neg |
| 2 | 0 | 73 | 4 | n | wpos Jo1 |
| 3 | 1 | 56 | 5 | y | pos PM-Scl |
| 4 | 0 | 54 | 1 | y | pos PM-Scl |
| 5 | 0 | 59 | 3 | y | neg |
| 6 | 0 | 77 | 2 | n | pos EJ |
| 7 | 0 | 65 | 1 | y | neg |
| 8 | 0 | 59 | 1 | y | pos Jo1 |
| 9 | 1 | 82 | 2 | n | neg |
| 10 | 1 | 78 | 3 | y | pos PM-Scl |
| 12 | 0 | 75 | 3 | y | neg |
| 13 | 1 | 56 | 1 | y | neg |
| 15 | 1 | 77 | 3 | y | neg |
| 17 | 1 | 67 | 1 | n | neg |
| 18 | 1 | 53 | 1 | n | neg |
| 20 | 1 | 60 | 5 | y | wpos OJ, wpos Mi2 |
| 21 | 0 | 44 | 1 | n | neg |
| 23 | 0 | 79 | 1 | n | neg |
| 24 | 0 | 61 | 1 | n | neg |
| 26 | 0 | 66 | 4 | n | neg |
| 27 | 0 | 71 | 1 | n | wpos PL7 wpos MDA5 |
| 29 | 0 | 66 | 2 | n | wpos PL7 |
| 30 | 0 | 69 | 4 | n | wpos MDA5 |
| 31 | 0 | 63 | 1 | y | neg |
| 32 | 1 | 70 | 3 | n | neg |
| 33 | 0 | 75 | 3 | n | neg |
| 37 | 1 | 73 | 4 | n | neg |
| 38 | 1 | 65 | 3 | y | neg |
| 39 | 0 | 75 | 2 | n | wpos SRP1 wpos PL12 wpos Mi2 wpos MDA5 wpos Ku |
| 40 | 0 | 69 | 4 | n | pos PM-Scl wpos Mi2 |
| 41 | 1 | 75 | 4 | n | wpos PL7 |
| 43 | 0 | 68 | 1 | y | pos Ku wpos SAE1 wpos MDA5 |
| 46 | 0 | 66 | 1 | n | neg |
| 47 | 0 | 60 | 1 | n | neg |
| 50 | 0 | 59 | 3 | n | wpos SRP1 |
| 52 | 0 | 74 | 1 | n | neg |
| 53 | 0 | 69 | 1 | n | neg |
| 54 | 1 | 57 | 5 | n | wpos MDA5 |
| 56 | 1 | 67 | 1 | y | neg |
| 57 | 0 | 74 | 1 | y | pos PM-Scl |
| 64 | 0 | 59 | 1 | n | neg |
| 66 | 0 | 73 | 3 | y | pos OJ, |
| 67 | 0 | 80 | 2 | y | wpos Mi2 |
| 69 | 1 | 66 | 3 | n | neg |
| 70 | 0 | 62 | 5 | n | neg |
| 73 | 0 | 72 | 1 | n | wpos MDA5 |
| 78 | 1 | 40 | 1 | n | neg |
| 81 | 1 | 75 | 3 | y | neg |
| 83 | 0 | 64 | 4 | n | neg |
| 85 | 1 | 80 | 4 | n | neg |
| 86 | 0 | 65 | 3 | n | neg |
| 87 | 1 | 60 | 1 | y | neg |
| 88 | 0 | 75 | 1 | n | neg |
| 91 | 0 | 63 | 3 | n | neg |
| 93 | 1 | 68 | 3 | y | pos SRP1 |
| 94 | 0 | 75 | 2 | n | wpos SRP1 wpos PL7 |
| 95 | 1 | 77 | 3 | n | wpos OJ |
| 103 | 0 | 79 | 1 | n | wpos PL7 |
| 104 | 0 | 56 | 4 | n | neg |
| 106 | 0 | 64 | 1 | n | neg |
| 107 | 1 | 79 | 3 | n | pos Ku wpos PL7 |
| 108 | 1 | 50 | 1 | y | wpos Ku |
| 111 | 0 | 47 | 1 | n | neg |
| 119 | 0 | 70 | 4 | n | wpos PL7 wpos PM-Scl |
| 120 | 1 | 46 | 1 | n | wpos PL7 |
| 124 | 1 | 72 | 2 | n | neg |
| 126 | 1 | 39 | 1 | y | neg |
| 127 | 0 | 87 | 2 | n | pos PL7 wpos PM-Scl |
| 134 | 0 | 66 | 1 | n | wpos MDA5 |
| 141 | 0 | 66 | 4 | n | neg |
| 162 | 1 | 89 | 3 | n | neg |
| 164 | 1 | 23 | 4 | n | neg |
| 165 | 0 | 71 | 1 | n | pos PM-Scl |
| 168 | 1 | 76 | 3 | y | neg |
| 175 | 1 | 68 | 3 | y | neg |
| 181 | 0 | 81 | 4 | n | neg |
| 186 | 1 | 71 | 3 | y | neg |
| 191 | 0 | 73 | 1 | n | neg |
| 194 | 0 | 63 | 1 | n | neg |
| 195 | 1 | 72 | 1 | y | neg |
| 197 | 0 | 46 | 1 | n | neg |
| 198 | 0 | 66 | 1 | n | neg |
| 202 | 0 | 27 | 1 | n | neg |
| 205 | 0 | 71 | 1 | n | neg |
| 208 | 0 | 59 | 3 | y | neg |
| 213 | 0 | 63 | 1 | n | neg |
| 217 | 0 | 70 | 3 | n | neg |
| 220 | 1 | 75 | 3 | y | neg |
| 223 | 0 | 71 | 3 | n | neg |
| 228 | 0 | 70 | 3 | n | neg |
| 233 | 0 | 70 | 3 | n | neg |
| 246 | 0 | 64 | 1 | y | pos PL7 pos Ku |
| 250 | 1 | 72 | 1 | n | neg |
| 254 | 1 | 71 | 4 | n | neg |
| 271 | 1 | 74 | 3 | n | neg |
| 273 | 0 | 46 | 1 | n | wpos SAE1 |
| 279 | 1 | 57 | 1 | n | neg |
| 285 | 0 | 74 | 3 | n | neg |
| 293 | 1 | 78 | 3 | n | neg |
| 301 | 1 | 72 | 3 | y | wpos PL7 |
| 306 | 1 | 66 | 4 | n | neg |
| 309 | 1 | 55 | 2 | n | neg |
| 310 | 1 | 73 | 1 | y | pos Mi2, wpos PL12 |
| 311 | 0 | 71 | 1 | n | neg |
| 312 | 0 | 66 | 4 | n | wpos PL7 |
| 313 | 1 | 74 | 1 | n | neg |
| 314 | 1 | 75 | 3 | y | neg |
| 315 | 0 | 49 | 3 | n | neg |
| 316 | 1 | 57 | 1 | n | wpos Tif1y |
| 317 | 1 | 68 | 3 | y | wpos SAE1 wpos MDA5 |
| 318 | 0 | 69 | 4 | n | wpos SAE1 |
| 319 | 1 | 72 | 1 | n | wSAE1 |
| 320 | 0 | 63 | 1 | n | neg |
| 321 | 0 | 50 | 1 | y | pos PL7 |
| 322 | 1 | 73 | 1 | n | neg |
| 323 | 1 | 57 | 1 | n | neg |
| 324 | 0 | 64 | 3 | n | neg |
| 325 | 0 | 53 | 1 | n | neg |
| 326 | 1 | 65 | 4 | n | neg |
|
327 |
0 | 62 | 1 | n | wpos SRP1 wpos PL7 wpos PL12 wpos Mi-2 |
| 328 | 1 | 69 | 3 | n | neg |
References
- Chang KY, Duval S, et al. Mortality in Pulmonary Arterial Hypertension in the Modern Era: Early Insights From the Pulmonary Hypertension Association Registry. J Am Heart Assoc. 2022;11(9):e024969. [CrossRef]
- Humbert M, Kovacs G, et al. [2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension]. G Ital Cardiol (Rome). 2023;24(4):1e-116e. [CrossRef]
- Tobal R, Potjewijd J, et al. Vascular Remodeling in Pulmonary Arterial Hypertension: The Potential Involvement of Innate and Adaptive Immunity. Front Med (Lausanne). 2021;8:806899. [CrossRef]
- Thoreau B, Mouthon L. Pulmonary arterial hypertension associated with connective tissue diseases (CTD-PAH): Recent and advanced data. Autoimmun Rev. 2023:103506. [CrossRef]
- Dhala A. Pulmonary arterial hypertension in systemic lupus erythematosus: current status and future direction. Clin Dev Immunol. 2012;2012:854941. [CrossRef]
- Parperis K, Velidakis N, Khattab E, Gkougkoudi E, Kadoglou NPE. Systemic Lupus Erythematosus and Pulmonary Hypertension. Int J Mol Sci. 2023;24(6). [CrossRef]
- Naranjo M, Hassoun PM. Systemic Sclerosis-Associated Pulmonary Hypertension: Spectrum and Impact. Diagnostics (Basel). 2021;11(5). [CrossRef]
- Arends SJ, Damoiseaux JG, et al. Functional implications of IgG anti-endothelial cell antibodies in pulmonary arterial hypertension. Autoimmunity. 2013;46(7):463-70. [CrossRef]
- Koudstaal T, Boomars KA. Inflammatory biomarkers in pulmonary arterial hypertension: ready for clinical implementation? Eur Respir J. 2023;61(3).
- Koudstaal T, Boomars KA, Kool M. Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension: An Immunological Perspective. J Clin Med. 2020;9(2). [CrossRef]
- Koudstaal T, van Uden D, et al. Plasma markers in pulmonary hypertension subgroups correlate with patient survival. Respir Res. 2021;22(1):137. [CrossRef]
- El Kasmi KC, Pugliese SC, et al. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol. 2014;193(2):597-609. [CrossRef]
- Heukels P, Corneth OBJ, et al. Loss of immune homeostasis in patients with idiopathic pulmonary arterial hypertension. Thorax. 2021;76(12):1209-18. [CrossRef]
- Price LC, Wort SJ, et al. Inflammation in pulmonary arterial hypertension. Chest. 2012;141(1):210-21. [CrossRef]
- Arends SJ, Damoiseaux JG, et al. Immunoglobulin G anti-endothelial cell antibodies: inducers of endothelial cell apoptosis in pulmonary arterial hypertension? Clin Exp Immunol. 2013;174(3):433-40.
- Arends SJ, Damoiseaux J, et al. Prevalence of anti-endothelial cell antibodies in idiopathic pulmonary arterial hypertension. Eur Respir J. 2010;35(4):923-5. [CrossRef]
- Stenmark KR, Nozik-Grayck E, et al. The adventitia: Essential role in pulmonary vascular remodeling. Compr Physiol. 2011;1(1):141-61.
- Marsh LM, Jandl K, et al. The inflammatory cell landscape in the lungs of patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2018;51(1). [CrossRef]
- Johnson SR, Granton JT. Pulmonary hypertension in systemic sclerosis and systemic lupus erythematosus. Eur Respir Rev. 2011;20(122):277-86.
- Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med. 2016;280(1):8-23. [CrossRef]
- Damoiseaux J, Mammen AL, Piette Y, Benveniste O, Allenbach Y, Group EtWS. 256(th) ENMC international workshop: Myositis specific and associated autoantibodies (MSA-ab): Amsterdam, The Netherlands, 8-10 October 2021. Neuromuscul Disord. 2022;32(7):594-608.
- Gasparotto M, Gatto M, Saccon F, Ghirardello A, Iaccarino L, Doria A. Pulmonary involvement in antisynthetase syndrome. Curr Opin Rheumatol. 2019;31(6):603-10. [CrossRef]
- Ghirardello A, Gatto M, et al. Detection of Myositis Autoantibodies by Multi-Analytic Immunoassays in a Large Multicenter Cohort of Patients with Definite Idiopathic Inflammatory Myopathies. Diagnostics (Basel). 2023;13(19). [CrossRef]
- Moll SA, Platenburg M, et al. Prevalence and clinical associations of myositis antibodies in a large cohort of interstitial lung diseases. PLoS One. 2022;17(11):e0277007. [CrossRef]
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A Comprehensive Overview on Myositis-Specific Antibodies: New and Old Biomarkers in Idiopathic Inflammatory Myopathy. Clin Rev Allergy Immunol. 2017;52(1):1-19. [CrossRef]
- . Foris V, Kovacs G, Matucci-Cerinic M, Olschewski H. PL-7 positive antisynthetase syndrome and pulmonary hypertension. J Rheumatol. 2013;40(10):1777-9. [CrossRef]
- Hervier B, Meyer A, et al. Pulmonary hypertension in antisynthetase syndrome: prevalence, aetiology and survival. Eur Respir J. 2013;42(5):1271-82. [CrossRef]
- Sanges S, Yelnik CM, et al. Pulmonary arterial hypertension in idiopathic inflammatory myopathies: Data from the French pulmonary hypertension registry and review of the literature. Medicine (Baltimore). 2016;95(39):e4911. [CrossRef]
- Lega JC, Reynaud Q, Belot A, Fabien N, Durieu I, Cottin V. Idiopathic inflammatory myopathies and the lung. Eur Respir Rev. 2015;24(136):216-38. [CrossRef]
- Ghigna MR, Mooi WJ, Grunberg K. Pulmonary hypertensive vasculopathy in parenchymal lung diseases and/or hypoxia: Number 1 in the Series "Pathology for the clinician" Edited by Peter Dorfmuller and Alberto Cavazza. Eur Respir Rev. 2017;26(144).
- Dhont S, Zwaenepoel B, Vandecasteele E, Brusselle G, De Pauw M. Pulmonary hypertension in interstitial lung disease: an area of unmet clinical need. ERJ Open Res. 2022;8(4). [CrossRef]
- Platteel ACM, Wevers BA, et al. Frequencies and clinical associations of myositis-related antibodies in The Netherlands: A one-year survey of all Dutch patients. J Transl Autoimmun. 2019;2:100013. [CrossRef]
- Vulsteke JB, De Langhe E, et al. Detection of myositis-specific antibodies. Ann Rheum Dis. 2019;78(1):e7.
- Espinosa-Ortega F, Holmqvist M, et al. Comparison of autoantibody specificities tested by a line blot assay and immunoprecipitation-based algorithm in patients with idiopathic inflammatory myopathies. Ann Rheum Dis. 2019;78(6):858-60. [CrossRef]
- Garcia-Fernandez A, Quezada-Loaiza CA, de la Puente-Bujidos C. Antisynthetase syndrome and pulmonary hypertension: report of two cases and review of the literature. Mod Rheumatol Case Rep. 2021;5(1):152-5. [CrossRef]
- Lopes AJ, Capone D, Mogami R, Lanzillotti RS, Melo PL, Jansen JM. Severity classification for idiopathic pulmonary fibrosis by using fuzzy logic. Clinics (Sao Paulo). 2011;66(6):1015-9. [CrossRef]
- Parikh R, Konstantinidis I, O'Sullivan DM, Farber HW. Pulmonary hypertension in patients with interstitial lung disease: a tool for early detection. Pulm Circ. 2022;12(4):e12141. [CrossRef]
- Parikh R, O'Sullivan DM, Farber HW. The PH-ILD Detection tool: External validation and use in patients with ILD. Pulm Circ. 2023;13(3):e12273. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





