Submitted:
13 May 2024
Posted:
14 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Body Weight and Biochemical Analysis
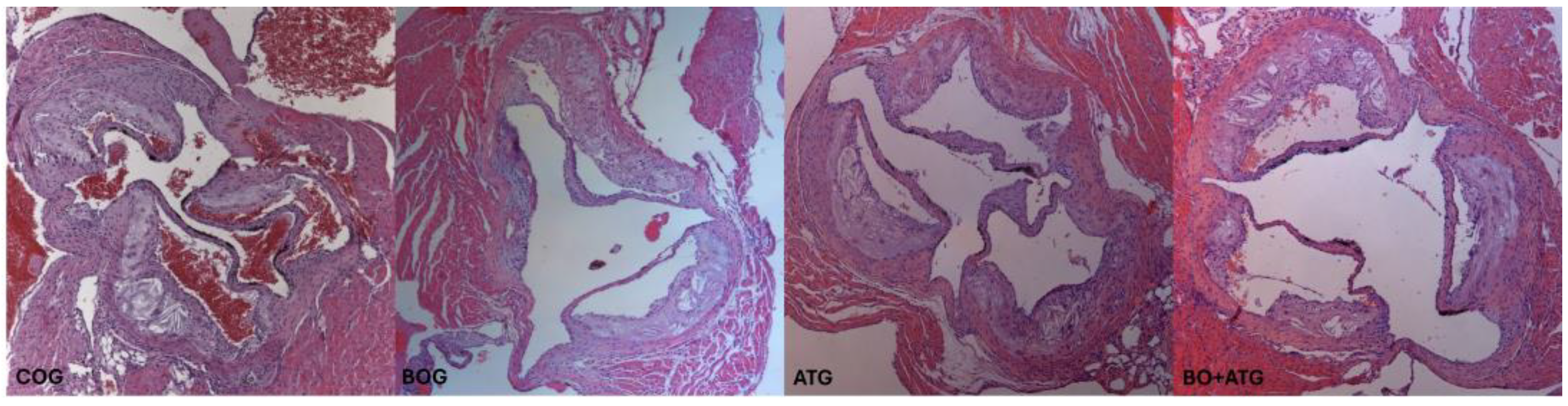
2.2. Morphometry, Collagen, and Elastin
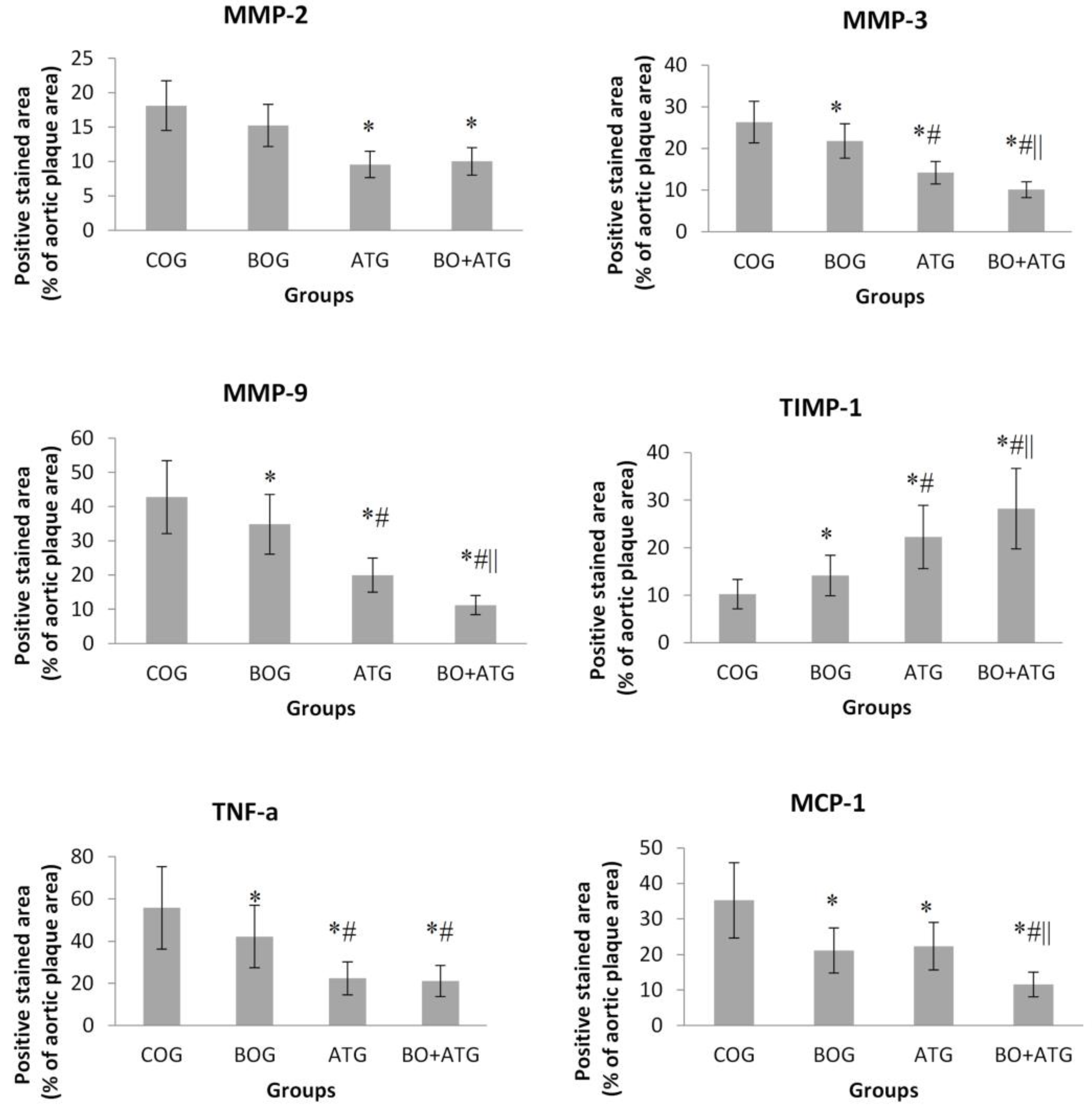
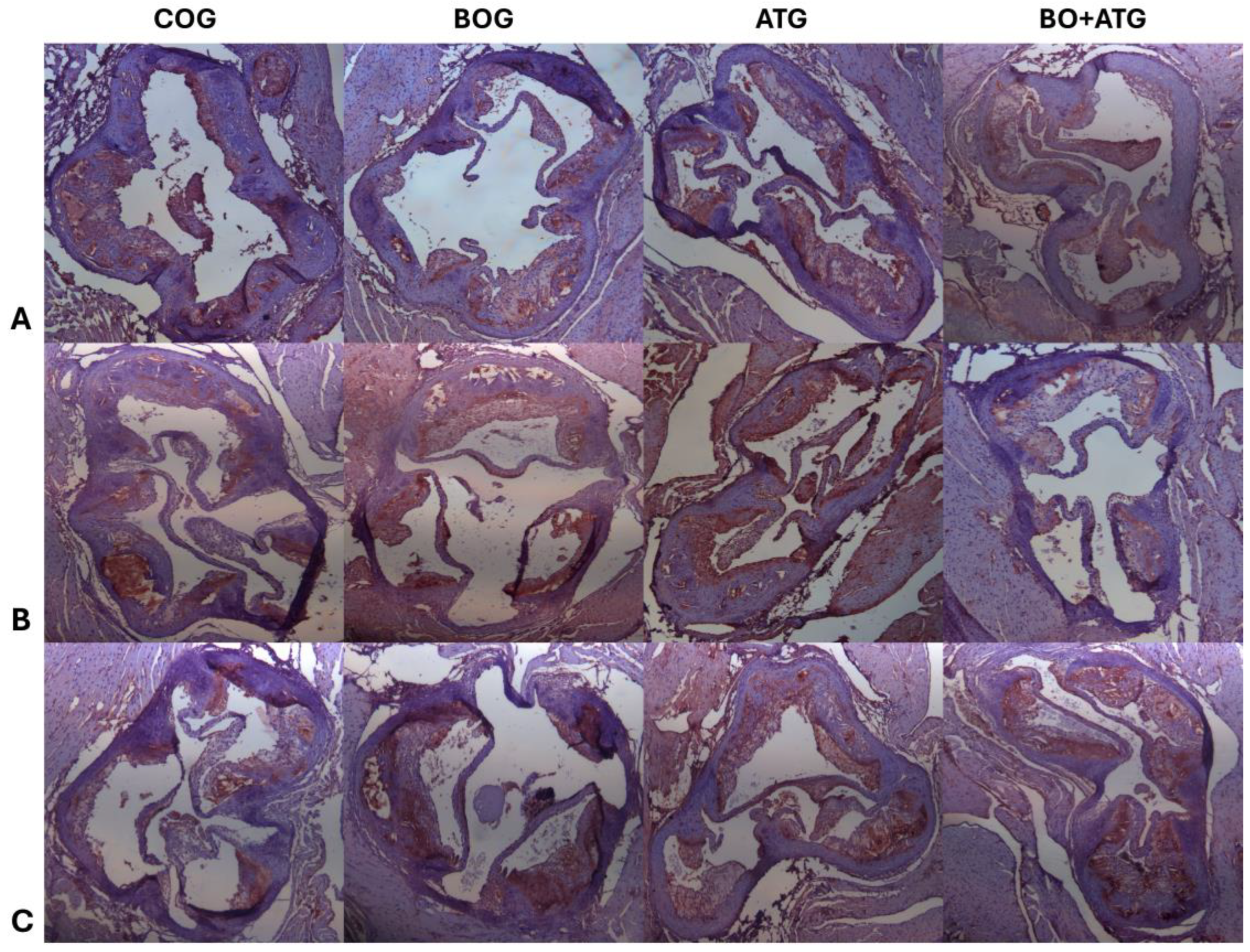
2.3. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Animal Model and Experimental Design
4.2. Histological Parameters
4.3. Histomorphometry
4.4. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libby, P.; Nahrendorf, M.; Swirski, F.K. Monocyte Heterogeneity in Cardiovascular Disease. Semin Immunopathol 2013, 35, 553–562. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P.; Schönbeck, U.; Yan, Z.Q. Innate and Adaptive Immunity in the Pathogenesis of Atherosclerosis. Circ Res 2002, 91, 281–91. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis--an Inflammatory Disease. N Engl J Med 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Sakakura, K.; Nakano, M.; Otsuka, F.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathophysiology of Atherosclerosis Plaque Progression. Heart Lung Circ 2013, 22, 399–411. [Google Scholar] [CrossRef]
- Eckel, R.H.; Bornfeldt, K.E.; Goldberg, I.J. Cardiovascular Disease in Diabetes, beyond Glucose. Cell Metab 2021, 33, 1519–1545. [Google Scholar] [CrossRef]
- Pleus, S.; Tytko, A.; Landgraf, R.; Heinemann, L.; Werner, C.; Müller-Wieland, D.; Ziegler, A.G.; Müller, U.A.; Freckmann, G.; Kleinwechter, H.; et al. Definition, Classification, Diagnosis and Differential Diagnosis of Diabetes Mellitus: Update 2023. Exp Clin Endocrinol Diabetes 2024, 132, 112–124. [Google Scholar] [CrossRef]
- Howard-Alpe, G.; Foëx, P.; Biccard, B. Cardiovascular Protection by Anti-Inflammatory Statin Therapy. Best Pr. Res Clin Anaesthesiol 2008, 22, 111–133. [Google Scholar] [CrossRef]
- Stasinopoulou, M.; Kadoglou, N.P.E.; Christodoulou, E.; Paronis, E.; Kostomitsopoulos, N.G.; Valsami, G.; Liapis, C.D.; Kakisis, J. Statins’ Withdrawal Induces Atherosclerotic Plaque Destabilization in Animal Model-A “Rebound” Stimulation of Inflammation. J Cardiovasc Pharmacol Ther 2019, 24, 377–386. [Google Scholar] [CrossRef]
- Wierzbicki, A.S.; Poston, R.; Ferro, A. The Lipid and Non-Lipid Effects of Statins. Pharmacol Ther 2003, 99, 95–112. [Google Scholar] [CrossRef]
- Gallo, G.; Savoia, C. New Insights into Endothelial Dysfunction in Cardiometabolic Diseases: Potential Mechanisms and Clinical Implications. Int J Mol Sci 2024, 25, 2973. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A Novel Potent Vasoconstrictor Peptide Produced by Vascular Endothelial Cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef]
- Böhm, F.; Pernow, J. The Importance of Endothelin-1 for Vascular Dysfunction in Cardiovascular Disease. Cardiovasc Res 2007, 76, 8–18. [Google Scholar] [CrossRef]
- Masaki, T.; Miwa, S.; Sawamura, T.; Ninomiya, H.; Okamoto, Y. Subcellular Mechanisms of Endothelin Action in Vascular System. Eur J Pharmacol 1999, 375, 133–138. [Google Scholar] [CrossRef]
- Peacock, A.J.; Dawes, K.E.; Shock, A.; Gray, A.J.; Reeves, J.T.; Laurent, G.J. Endothelin-1 and Endothelin-3 Induce Chemotaxis and Replication of Pulmonary Artery Fibroblasts. Am J Respir Cell Mol Biol 1992, 7, 492–499. [Google Scholar] [CrossRef]
- Ito, H.; Hirata, Y.; Hiroe, M.; Tsujino, M.; Adachi, S.; Takamoto, T.; Nitta, M.; Taniguchi, K.; Marumo, F. Endothelin-1 Induces Hypertrophy with Enhanced Expression of Muscle-Specific Genes in Cultured Neonatal Rat Cardiomyocytes. Circ Res 1991, 69, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, L.; Gray, M.; Honbo, N.Y.; Chentoufi, J.; Bergman, M.; Karliner, J.S. Endothelin-1 Stimulates Cardiac Fibroblast Proliferation through Activation of Protein Kinase C. J Mol Cell Cardiol 2000, 32, 565–576. [Google Scholar] [CrossRef]
- Takahashi, K.; Ghatei, M.A.; Lam, H.C.; O’Halloran, D.J.; Bloom, S.R. Elevated Plasma Endothelin in Patients with Diabetes Mellitus. Diabetologia 1990, 33, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Settergren, M.; Pernow, J.; Brismar, K.; Jörneskog, G.; Kalani, M. Endothelin-A Receptor Blockade Increases Nutritive Skin Capillary Circulation in Patients with Type 2 Diabetes and Microangiopathy. J Vasc Res 2008, 45, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Mather, K.J.; Mirzamohammadi, B.; Lteif, A.; Steinberg, H.O.; Baron, A.D. Endothelin Contributes to Basal Vascular Tone and Endothelial Dysfunction in Human Obesity and Type 2 Diabetes. Diabetes 2002, 51, 3517–3523. [Google Scholar] [CrossRef]
- Galiè, N.; Beghetti, M.; Gatzoulis, M.A.; Granton, J.; Berger, R.M.; Lauer, A.; Chiossi, E.; Landzberg, B. Bosentan Randomized Trial of Endothelin Antagonist Therapy-5 (BREATHE-5) Investigators Bosentan Therapy in Patients with Eisenmenger Syndrome: A Multicenter, Double-Blind, Randomized, Placebo-Controlled Study. Circulation 2006, 114, 48–54. [Google Scholar] [CrossRef]
- Gatzoulis, M.A.; Beghetti, M.; Galiè, N.; Granton, J.; Berger, R.M.; Lauer, A.; Chiossi, E.; Landzberg, M. BREATHE-5 Investigators Longer-Term Bosentan Therapy Improves Functional Capacity in Eisenmenger Syndrome: Results of the BREATHE-5 Open-Label Extension Study. Int J Cardiol 2008, 127, 27–32. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Z.; Li, G. The Protective Effect of Bosentan against Atherosclerosis in Apolipoprotein E-Deficient Mice Is Mediated by miRNA-21. Biomed Res Int 2019, 2019, 8348430. [Google Scholar] [CrossRef]
- Mulder, P.; Richard, V.; Derumeaux, G.; Hogie, M.; Henry, J.P.; Lallemand, F.; Compagnon, P.; Macé, B.; Comoy, E.; Letac, B.; et al. Role of Endogenous Endothelin in Chronic Heart Failure: Effect of Long-Term Treatment with an Endothelin Antagonist on Survival, Hemodynamics, and Cardiac Remodeling. Circulation 1997, 96, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, R.M.; Yanagisawa, M. Endothelin System: The Double-Edged Sword in Health and Disease. Annu Rev Pharmacol Toxicol 2001, 41, 851–876. [Google Scholar] [CrossRef] [PubMed]
- Ihling, C.; Szombathy, T.; Bohrmann, B.; Brockhaus, M.; Schaefer, H.E.; Loeffler, B.M. Coexpression of Endothelin-Converting Enzyme-1 and Endothelin-1 in Different Stages of Human Atherosclerosis. Circulation 2001, 104, 864–869. [Google Scholar] [CrossRef]
- Kowala, M.C.; Rose, P.M.; Stein, P.D.; Goller, N.; Recce, R.; Beyer, S.; Valentine, M.; Barton, D.; Durham, S.K. Selective Blockade of the Endothelin Subtype A Receptor Decreases Early Atherosclerosis in Hamsters Fed Cholesterol. Am J Pathol 1995, 146, 819–826. [Google Scholar] [PubMed]
- Best, P.J.; Lerman, A. Endothelin in Cardiovascular Disease: From Atherosclerosis to Heart Failure. J Cardiovasc Pharmacol Ther 2000, 35, S61–63. [Google Scholar] [CrossRef] [PubMed]
- Jabarpour, M.; Rashtchizadeh, N.; Argani, H.; Ghorbanihaghjo, A.; Ranjbarzadhag, M.; Sanajou, D.; Panah, F.; Alirezaei, A. The Impact of Dyslipidemia and Oxidative Stress on Vasoactive Mediators in Patients with Renal Dysfunction. Int Urol Nephrol 2019, 51, 2235–2242. [Google Scholar] [CrossRef]
- Rivera-Gonzalez, O.; Wilson, N.A.; Coats, L.E.; Taylor, E.B.; Speed, J.S. Endothelin Receptor Antagonism Improves Glucose Handling, Dyslipidemia, and Adipose Tissue Inflammation in Obese Mice. Clin. Sci. 2021, 135, 1773–1789. [Google Scholar] [CrossRef]
- Moustardas, P.; Kadoglou, N.P.; Katsimpoulas, M.; Kapelouzou, A.; Kostomitsopoulos, N.; Karayannacos, P.E.; Kostakis, A.; Liapis, C.D. The Complementary Effects of Atorvastatin and Exercise Treatment on the Composition and Stability of the Atherosclerotic Plaques in ApoE Knockout Mice. PLoS One 2014, 9, e108240. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, S.J.; Thuy, N.V.P.; Kim, J.S.; Lee, J.J.; Lee, O.H.; Kim, C.K.; Oh, J.; Park, S.; Lee, O.H.; et al. Synergistic Protective Effects of a Statin and an Angiotensin Receptor Blocker for Initiation and Progression of Atherosclerosis. PLoS One 2019, 14, e0215604. [Google Scholar] [CrossRef]
- Li, D.Q.; Lv, F.F.; Li, Z.C.; Dai, Z.Y.; Wang, H.X.; Han, Y. Anti-Atherosclerotic Effects between a Combined Treatment with Simvastatin plus Hirudin and Single Simvastatin Therapy in Patients with Early Type 2 Diabetes Mellitus. Ann Transl Med 2019, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- van der Vorst, E.P.C.; Weber, C.; Donners, M.M.P.C. A Disintegrin and Metalloproteases (ADAMs) in Cardiovascular, Metabolic and Inflammatory Diseases: Aspects for Theranostic Approaches. Thromb Haemost 2018, 118, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.A.; Wierer, M.; Dang, T.A.; Milic, J.; Moggio, A.; Sachs, N.; von Scheidt, M.; Hinterdobler, J.; Müller, P.; Werner, J.; et al. ADAMTS-7 Modulates Atherosclerotic Plaque Formation by Degradation of TIMP-1. Circ Res 2023, 133, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, L.G.; Yeung, K.; Eldrup, N.; Eiberg, J.P.; Sillesen, H.H.; Davies, M.J. Proteomic Analysis of the Extracellular Matrix of Human Atherosclerotic Plaques Shows Marked Changes between Plaque Types. Matrix Biol Plus 2024, 21, 100141. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Daskalopoulou, S.S.; Perrea, D.; Liapis, C.D. Matrix Metalloproteinases and Diabetic Vascular Complications. Angiology 2005, 56, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Zamilpa, R. Temporal and Spatial Expression of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases Following Myocardial Infarction. Cardiovasc Ther 2012, 30, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Johnson, J.A.; Fulp, A.; Sutton, M.A.; Lessner, S.M. Adhesive Strength of Atherosclerotic Plaque in a Mouse Model Depends on Local Collagen Content and Elastin Fragmentation. J Biomech 2013, 46, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P. The Beneficial Effects of a Direct Thrombin Inhibitor, Dabigatran Etexilate, on the Development and Stability of Atherosclerotic Lesions in Apolipoprotein E-Deficient Mice : Dabigatran Etexilate and Atherosclerosis. Cardiovasc Drugs Ther 2012, 26, 367–374. [Google Scholar] [CrossRef]
- Basiak, M.; Hachula, M.; Kosowski, M.; Machnik, G.; Maliglowka, M.; Dziubinska-Basiak, M.; Krysiak, R.; Okopien, B. The Effect of PCSK9 Inhibition on the Stabilization of Atherosclerotic Plaque Determined by Biochemical and Diagnostic Imaging Methods. Molecules 2023, 28, 5928. [Google Scholar] [CrossRef]
- Basiak, M. Impact of PCSK9 Inhibition on Proinflammatory Cytokines and Matrix Metalloproteinases Release in Patients with Mixed Hyperlipidemia and Vulnerable Atherosclerotic Plaque. Pharm. Basel 2022, 15, 802. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Velidakis, N.; Khattab, E.; Kassimis, G.; Patsourakos, N. The Interplay between Statins and Adipokines. Is This Another Explanation of Statins’ “pleiotropic” Effects? Cytokine 2021, 148, 155698. [Google Scholar] [CrossRef] [PubMed]
- Umebashi, K.; Yamamoto, M.; Tokito, A.; Sudou, K.; Takenoshita, Y.; Jougasaki, M. Inhibitory Effects of Simvastatin on IL-33-Induced MCP-1 via the Suppression of the JNK Pathway in Human Vascular Endothelial Cells. Int J Mol Sci 2023, 24, 13015. [Google Scholar] [CrossRef] [PubMed]
- Vieceli Dalla Sega, F.; Cimaglia, P.; Manfrini, M.; Fortini, F.; Marracino, L.; Bernucci, D.; Pompei, G.; Scala, A.; Trichilo, M.; De Carolis, B.; et al. Circulating Biomarkers of Endothelial Dysfunction and Inflammation in Predicting Clinical Outcomes in Diabetic Patients with Critical Limb Ischemia. Int J Mol Sci 2022, 23, 10641. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Pan, S.; Yu, H.; Hu, H.; Sun, Y.U.; Yang, Z.; Hoffman, R.M.; Yuan, H. Anti-Inflammatory and Anti-Thrombotic Efficacy of Targeted Ultrasound Microbubbles on LPS-Induced HUVEC Cells. Anticancer Res 2021, 41, 4761–4769. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Li, S.H.; Badiwala, M.V.; Weisel, R.D.; Fedak, P.W.; Li, R.K.; Dhillon, B.; Mickle, D.A. Endothelin Antagonism and Interleukin-6 Inhibition Attenuate the Proatherogenic Effects of C-Reactive Protein. Circulation 2002, 105, 1890–1896. [Google Scholar] [CrossRef]
- Yao, E.H.; Wang, H.J.; Xu, C.S. Effects of Tongxinluo on the Neointima Formation and Expression of Inflammatory Cytokines in Rats after Carotid Artery Balloon Injury. Indian J Pharmacol 2014, 46, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Dede, E.; Liapis, D.; Davos, C.; Katsimpoulas, M.; Varela, A.; Mpotis, I.; Kostomitsopoulos, N.; Kadoglou, N.P.E. The Effects of Exercise Training on Cardiac Matrix Metalloproteinases Activity and Cardiac Function in Mice with Diabetic Cardiomyopathy. Biochem Biophys Res Commun 2022, 586, 8–13. [Google Scholar] [CrossRef]
- Kadoglou, N.P. The Anti-Inflammatory Effects of Exercise Training Promote Atherosclerotic Plaque Stabilization in Apolipoprotein E Knockout Mice with Diabetic Atherosclerosis. Eur J Histochem 2013, 57, e3. [Google Scholar] [CrossRef]
| COG (n=12) |
BOG (n=12) |
ATG (n=12) |
BO+ATG (n=12) |
p | |
|---|---|---|---|---|---|
| Weight (g) | 34.4±4.2 | 33.3±4.8 | 32.9±3.9 | 33.5±4.2 | 0.512 |
| FPG (mg/dl) | 281±29 | 289±55 | 295±45 | 309±49 | 0.794 |
| TC (mg/dl) | 759±131 | 758±182 | 511±131* | 502±191* | <0.001 |
| TG (mg/dl) | 155±41 | 145±34 | 129±30 | 131±33 | 0.400 |
| COG (n=12) |
BOG (n=12) |
ATG (n=12) |
BO+ATG (n=12) |
p | |
|---|---|---|---|---|---|
| Lumen Stenosis (%) | 24.6±4.8 | 19.5±2.2 a,c,d | 12.8±4.8a,b,d | 9.1±2.7a,b,c | <0.001 |
| Elastin (%) plaque | 8.12±2.10 | 10.62±6.52c,d | 25.17±6.91a | 31.02±5.23a,b | <0.001 |
| Collagen (%) plaque | 14.21±4.21 | 22.83±4.79 a,c,d | 31.88±5.97 a,b | 40.33±8.72 a,b,c | <0.001 |
| Fibrous cap thickness (μm) | 9.12±3.10 | 13.12±3.23 a,c,d | 23.12±5.44 a,b,d | 48.12±6.21a,b,c | <0.001 |
| a-actin (VSMCs) (%) plaque | 17.13±3.21 | 26.88±6.06a | 20.53±6.97 | 28.10±6.82a | 0.005 |
| Mac-3 (macrophages) (%) plaque | 34.56±10.25 | 26.46±6.82a,c,d | 15.09±3.22a,b,c | 10.12±3.78a,b,c | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





