Submitted:
10 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
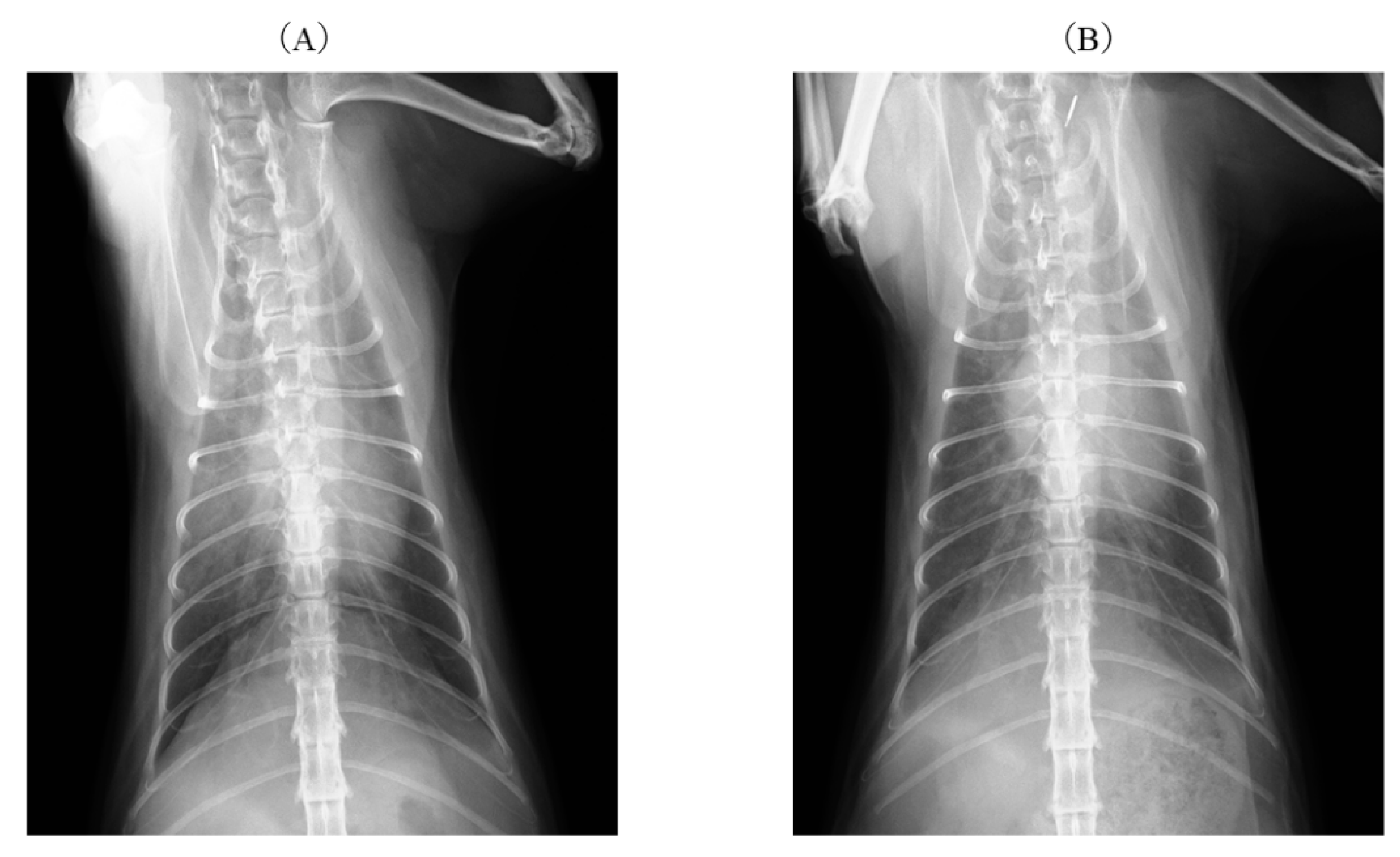
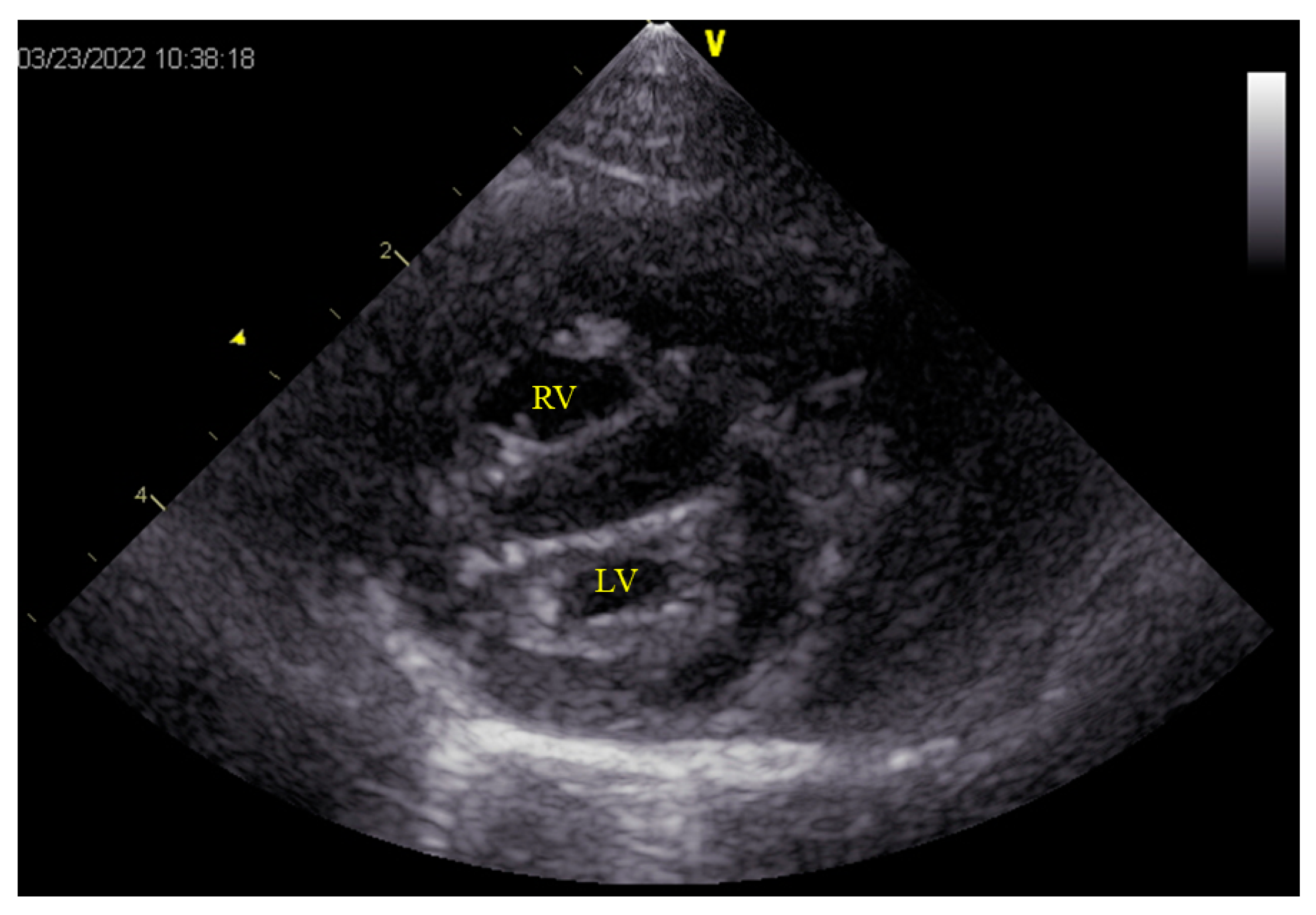
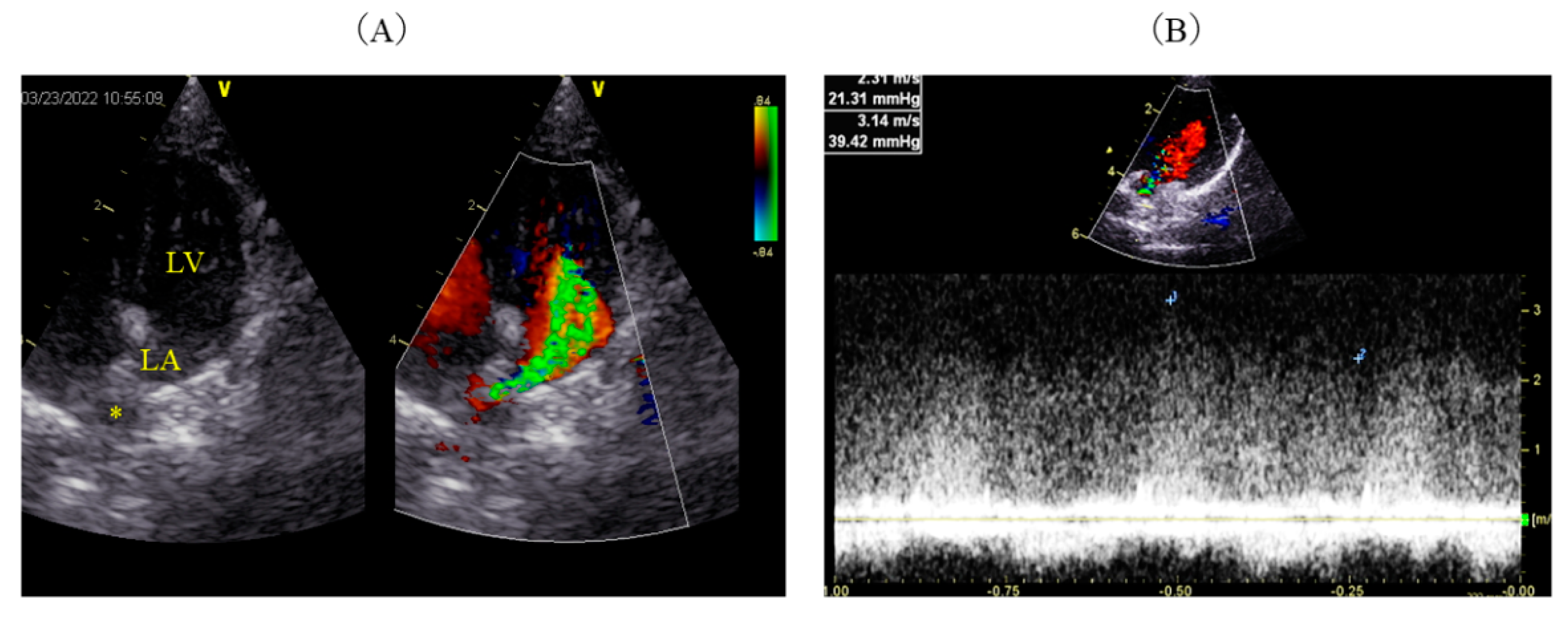
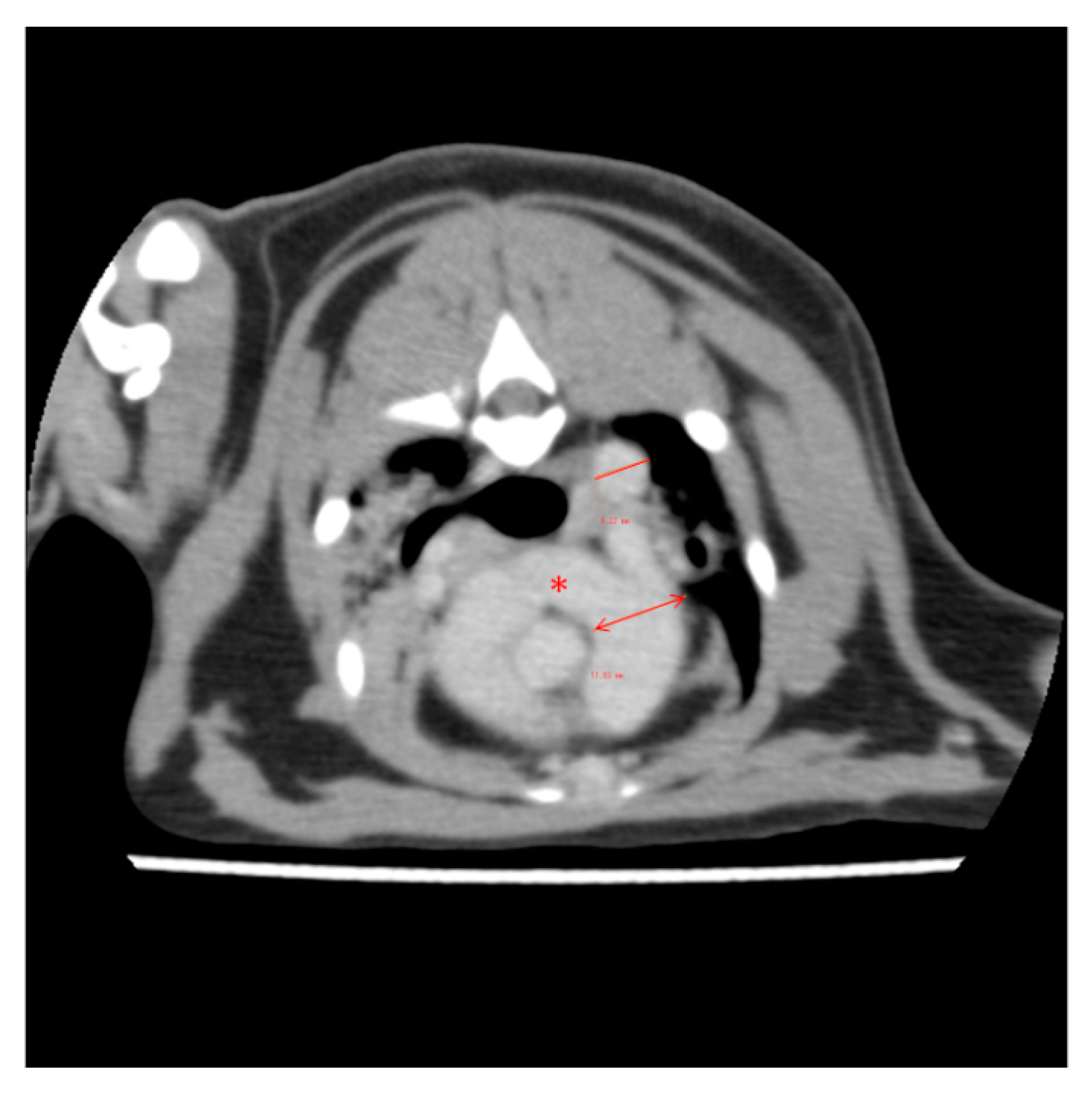
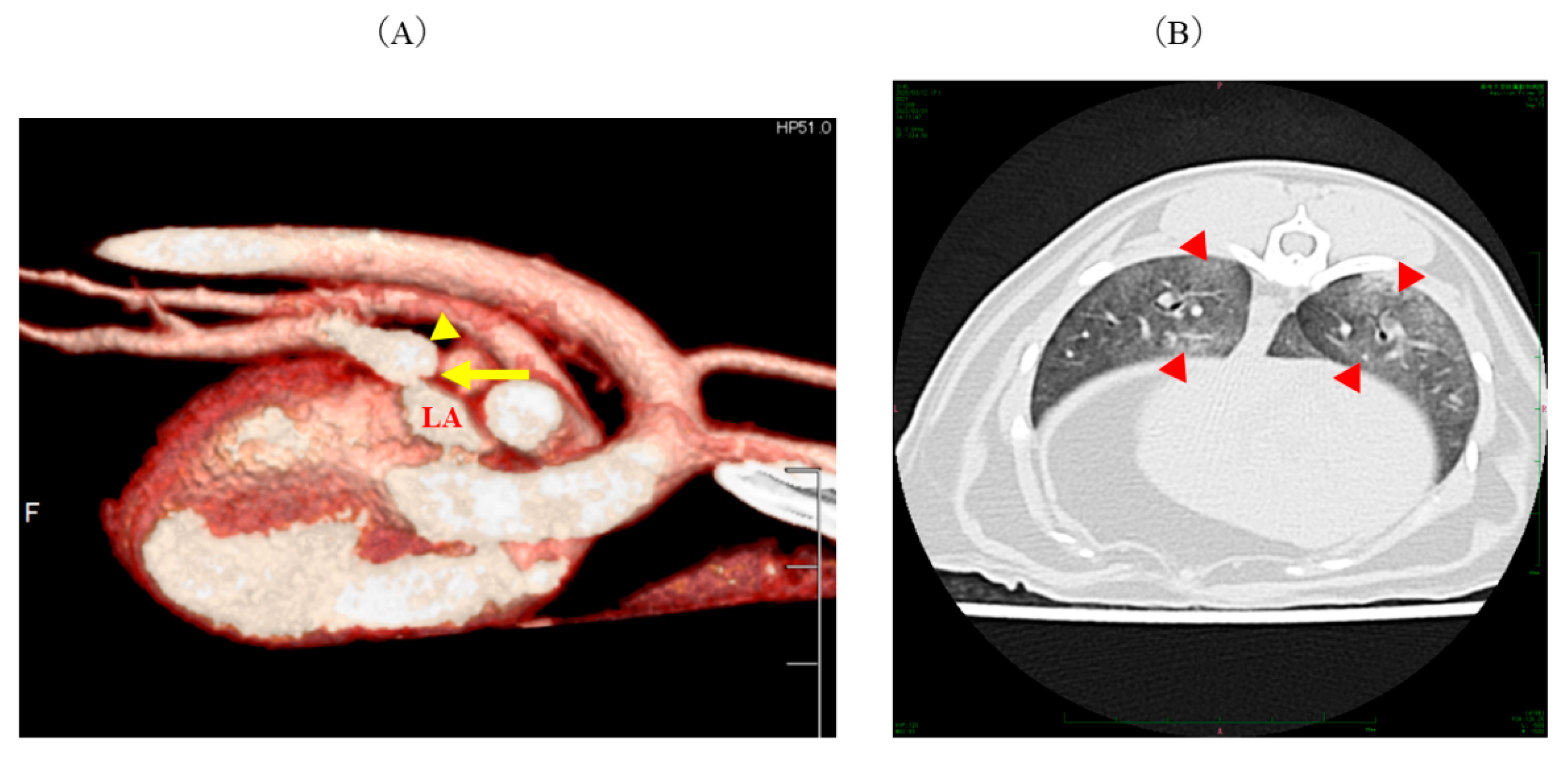
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.; Borgarelli, M.; Scansen, B.A. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J Vet Intern Med 2020, 34, 549–573. [Google Scholar] [CrossRef] [PubMed]
- Rolph, K.E.; Cavanaugh, S.M. Feline pulmonary hypertension: Are we overlooking an important comorbidity? J Feline Med Surg 2022, 24, e636–e646. [Google Scholar] [CrossRef] [PubMed]
- Kriström, K.; Karlstam, E.; Nielsen, T.; Lagerqvist, A.; Dirven, M. A case of congenital pulmonary vein stenosis with secondary post-capillary pulmonary hypertension and left sided congestive heart failure in a cat. Vet Sci 2022, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Günther, S.; Dorfmüller, P.; Perros, F.; Girerd, B.; Garcia, G.; Jaïs, X.; Savale, L.; Artaud-Macari, E.; Price, L.C.; et al. Pulmonary arterial hypertension. Orphanet J Rare Dis 2013, 8, 97. [Google Scholar] [CrossRef]
- Vyas-Read, S.; Varghese, N.P.; Suthar, D.; Backes, C.; Lakshminrusimha, S.; Petit, C.J.; Levy, P.T. Prematurity and pulmonary vein stenosis: The role of parenchymal lung disease and pulmonary vascular disease. Children (Basel) 2022, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Latson, L.A.; Prieto, L.R. Congenital and acquired pulmonary vein stenosis. Circulation 2007, 115, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Nealon, E.; Armstrong, A.K.; Cua, C.L.; Mitchell, C.; Krishnan, U.; Vanderlaan, R.D.; Song, M.K.; Viola, N.; Smith, C.V.; et al. Pulmonary vein stenosis in infants: A systematic review, meta-analysis, and meta-regression. J Pediatr 2018, 198, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Northway, W.H. Jr.; Moss, R.B.; Carlisle, K.B.; Parker, B.R.; Popp, R.L.; Pitlick, P.T.; Eichler, I.; Lamm, R.L.; Brown, B.W. Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med 1990, 323, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Suntharos, P.; Prieto, L.R. Treatment of congenital and acquired pulmonary vein stenosis. Curr Cardiol Rep 2020, 22, 153. [Google Scholar] [CrossRef]
- Panopoulos, I.; Auriemma, E.; Specchi, S.; Diana, A.; Pietra, M.; Papastefanou, A.; Zini, E.; Cipone, M. 64-multidetector CT anatomical assessment of the feline bronchial and pulmonary vascular structures. J Feline Med Surg 2019, 21, 893–901. [Google Scholar] [CrossRef]
- Thomas, C.A.; Cruz Morel, K.J.C.; Viswanathan, M.N.; de Jesus Perez, V.A. Pulmonary vein stenosis and pulmonary hypertension following a catheter-based radiofrequency ablation for atrial fibrillation: A case report. Am J Case Rep 2020, 21, e924709. [Google Scholar] [CrossRef] [PubMed]
- Pazos-López, P.; García-Rodríguez, C.; Guitián-González, A.; Paredes-Galán, E.; Álvarez-Moure, M.Á.D.L.G.; Rodríguez-Álvarez, M.; Baz-Alonso, J.A.; Teijeira-Fernández, E.; Calvo-Iglesias, F.E.; Íñiguez-Romo, A. Pulmonary vein stenosis: Etiology, diagnosis and management. World J Cardiol 2016, 8, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.C.; Im, M.K.; Johnson, L.R.; Stern, J.A. Diagnostic value of right pulmonary artery distensibility index in dogs with pulmonary hypertension: Comparison with doppler echocardiographic estimates of pulmonary arterial pressure. J Vet Intern Med 2016, 30, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, K.; Diller, G.P.; Opotowsky, A.R.; D’Alto, M.; Gu, H.; Giannakoulas, G.; Budts, W.; Broberg, C.S.; Veldtman, G.; Swan, L.; et al. Definition and management of segmental pulmonary hypertension. J Am Heart Assoc 2018, 7, e008587. [Google Scholar] [CrossRef] [PubMed]
- Guihaire, J.; Haddad, F.; Noly, P.E.; Boulate, D.; Decante, B.; Dartevelle, P.; Humbert, M.; Verhoye, J.P.; Mercier, O.; Fadel, E. Right ventricular reserve in a piglet model of chronic pulmonary hypertension. Eur Respir J 2015, 45, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Sottiaux, J.; Franck, M. Pulmonary embolism and cor pulmonale in a cat. J Small Anim Pract 1999, 40, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Fontijn, S.; Balink, S.J.A.; Bonte, M.; Andrinopoulou, E.R.; Duijts, L.; Kroon, A.A.; Ciet, P.; Pijnenburg, M.W. Chest computed tomography in severe bronchopulmonary dysplasia: Comparing quantitative scoring methods. Eur J Radiol 2023, 169, 111168. [Google Scholar] [CrossRef]
- Milne, M.E.; McCowan, C.; Landon, B.P. Spontaneous feline pneumothorax caused by ruptured pulmonary bullae associated with possible bronchopulmonary dysplasia. J Am Anim Hosp Assoc 2010, 46, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Reinero, C.R.; Jutkowitz, L.A.; Nelson, N.; Masseau, I.; Jennings, S.; Williams, K. Clinical features of canine pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis. J Vet Intern Med 2019, 33, 114–123. [Google Scholar] [CrossRef]
- Montani, D.; Price, L.C.; Dorfmüller, P.; Achouh, L.; Jais, X.; Yaici, A.; Sitbon, O.; Musset, D.; Simonneau, G.; Humbert, M. Pulmonary veno-occlusive disease. Eur Respir J 2009, 33, 189–200. [Google Scholar] [CrossRef]
- den Toom, M.L.; Grinwis, G.; van Suylen, R.J.; Boroffka, S.A.; de Jong, P.; van Steenbeek, F.G.; Szatmári, V. Pulmonary veno-occlusive disease as a cause of severe pulmonary hypertension in a dog. Acta Vet Scand 2018, 60, 78. [Google Scholar] [CrossRef] [PubMed]
- Jaffey, J.A.; Williams, K.J.; Masseau, I.; Krueger, M.; Reinero, C. Vasoproliferative process resembling pulmonary capillary hemangiomatosis in a cat. BMC Vet Res 2017, 13, 72. [Google Scholar] [CrossRef]
- Vezzosi, T.; Schober, K.E. Doppler-derived echocardiographic evidence of pulmonary hypertension in cats with left-sided congestive heart failure. J Vet Cardiol 2019, 23, 58–68. [Google Scholar] [CrossRef]
- Borgarelli, M.; Abbott, J.; Braz-Ruivo, L.; Chiavegato, D.; Crosara, S.; Lamb, K.; Ljungvall, I.; Poggi, M.; Santilli, R.A.; Haggstrom, J. Prevalence and prognostic importance of pulmonary hypertension in dogs with myxomatous mitral valve disease. J Vet Intern Med 2015, 29, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.A.; Munroe, M.L.; Tucker, A.; Reeves, J.T. Histamine H1- and H2-receptors in the cat and their roles during alveolar hypoxia. Respir Physiol 1977, 29, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Reinero, C. Interstitial lung diseases in dogs and cats part I: The idiopathic interstitial pneumonias. Vet J 2019, 243, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015, 46, 903–975. [Google Scholar] [CrossRef] [PubMed]
- Jaffey, J.A.; Wiggen, K.; Leach, S.B.; Masseau, I.; Girens, R.E.; Reinero, C.R. Pulmonary hypertension secondary to respiratory disease and/or hypoxia in dogs: Clinical features, diagnostic testing and survival. Vet J 2019, 251, 105347. [Google Scholar] [CrossRef]
- Johnson, L.R.; Stern, J.A. Clinical features and outcome in 25 dogs with respiratory-associated pulmonary hypertension treated with sildenafil. J Vet Intern Med 2020, 34, 65–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



