Submitted:
09 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Climatological, Geological, and Hydrological Setting of Sri Lanka
3. Materials and Methods
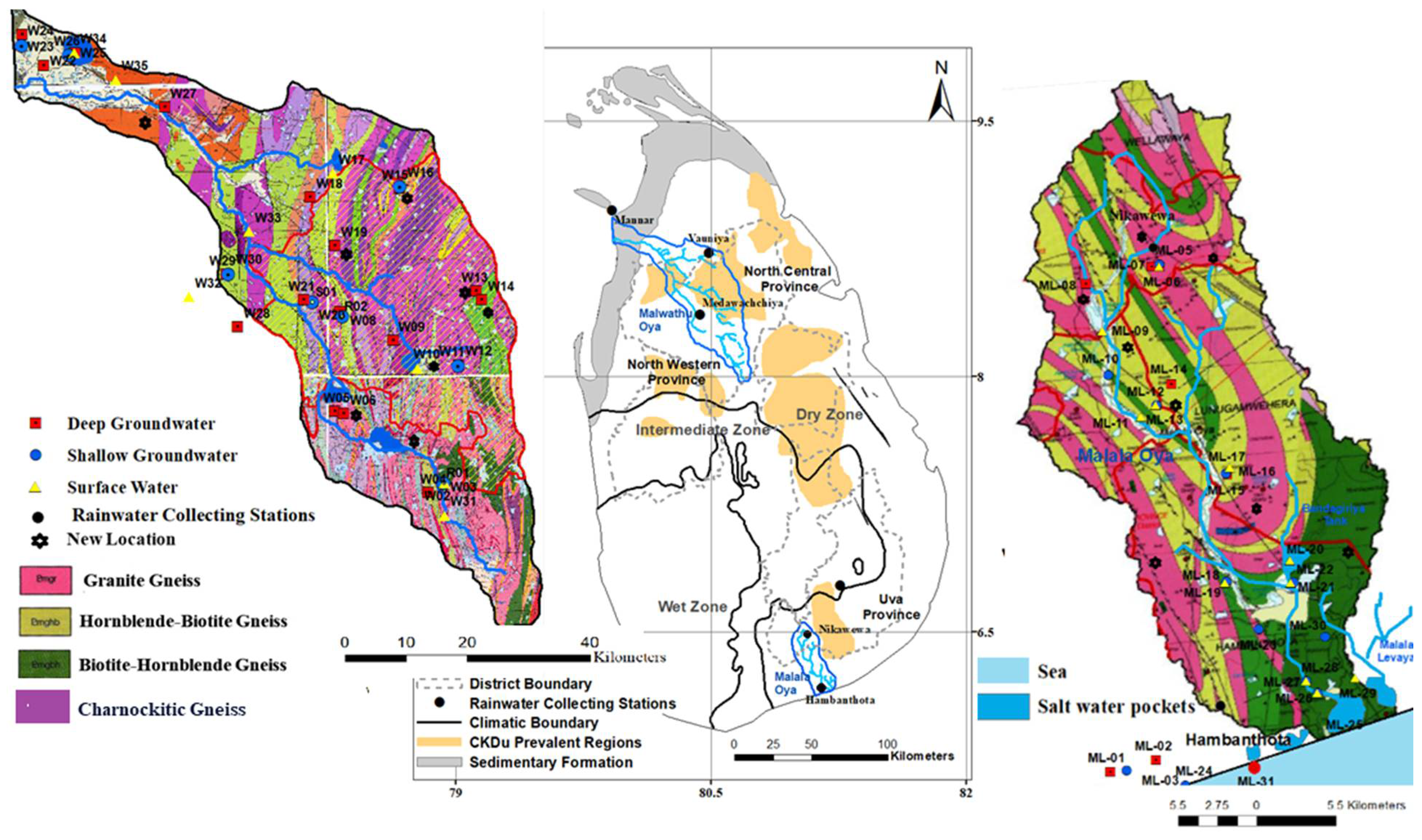
3.1. Study Area
3.2. Sample Collection and Analysis
4. Results and Discussion
4.1. Groundwater Chemistry
| Water Type | pH | TDS | TA | TH | Na+ | Ca2+ | Mg2+ | SO42- | Cl- | NO3- | F- | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/L) | (mg/L as CaCO3) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | |||||||||||||||||||||
| Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | |||||||||
| Malwathu Oya Basin (CKDu endemic area) | ||||||||||||||||||||||||||||||
|
Surface Water |
Min | 7.4 | 7.4 | 304 | 200 | 176 | 136 | 200 | 163 | 68 | 35.4 | 28. | 15.5 | 26.5 | 9.7 | 2.5 | 0 | 94.5 | 29.7 | <0.008 | 0.1 | 0.26 | 0.3 | |||||||
| Max | 9.1 | 8.9 | 427 | 501 | 200 | 321 | 200 | 264 | 103 | 99 | 35 | 52.8 | 30.6 | 30.3 | 7.5 | 18 | 163 | 95.7 | 3.6 | 0.6 | 0.34 | 1.2 | ||||||||
| Avg | 8.3 | 8 | 366 | 338 | 188 | 209 | 200 | 209 | 85 | 63.4 | 31.7 | 28.6 | 28.6 | 18.5 | 5 | 7.4 | 129 | 57.3 | 1.8 | 0.3 | 0.30 | 0.7 | ||||||||
| Crystalline Terrain | ||||||||||||||||||||||||||||||
|
Shallow GW |
Min | 7 | 6.8 | 366 | 215 | 160 | 189 | 208 | 154 | 36.4 | 29.6 | 45.9 | 38.5 | 37.6 | 7.7 | 11.8 | 2 | 43.4 | 23.9 | <0.008 | 0.2 | 0.35 | 0.3 | |||||||
| Max | 7.7 | 8.3 | 1032 | 1394 | 448 | 537 | 820 | 950 | 148 | 192 | 128 | 427 | 114 | 107 | 52.3 | 142 | 390 | 728 | 15.4 | 1.8 | 1.6 | 2.2 | ||||||||
| Avg | 7.4 | 7.7 | 744 | 792 | 301 | 414 | 553 | 453 | 87.5 | 88.9 | 68.4 | 135 | 72.6 | 50.4 | 29.9 | 57.3 | 217 | 226 | 4.5 | 0.9 | 0.8 | 0.9 | ||||||||
|
Deep GW |
Min | 6.6 | 6.7 | 102 | 107 | 104 | 88.2 | 68 | 70.4 | 28.5 | 21.8 | 12.7 | 0.4 | 5.4 | 3.4 | 7.8 | 6 | 7.2 | 3.9 | <0.008 | <0.008 | 0.4 | 0.2 | |||||||
| Max | 7.4 | 8.5 | 663 | 729 | 456 | 473 | 616 | 295 | 157 | 167 | 58 | 88.8 | 67 | 50.2 | 36.6 | 49 | 152 | 153 | 27.6 | 8.5 | 1.3 | 2.7 | ||||||||
| Avg | 7 | 7.5 | 423 | 425 | 266 | 322 | 305 | 201 | 79 | 72.6 | 45 | 35.8 | 36 | 21.4 | 21.3 | 25.8 | 77 | 60 | 5.5 | 1.8 | 0.8 | 0.9 | ||||||||
| Sedimentary Terrain (CKDu non-endemic area) | ||||||||||||||||||||||||||||||
|
Shallow GW |
Min | 7.7 | 7.7 | 904 | 912 | 288 | 393 | 380 | 44 | 239 | 340 | 57.8 | 7.2 | 48.5 | 3.6 | 55 | 62 | 378 | 445 | <0.008 | 0.6 | 0.3 | 0.5 | |||||||
| Max | 8.1 | 8.6 | 2570 | 1289 | 520 | 453 | 420 | 290 | 987 | 590 | 71 | 94.4 | 75 | 23.6 | 220 | 136 | 1246 | 484 | <0.008 | 1.5 | 0.8 | 1.7 | ||||||||
| Avg | 7.9 | 8.1 | 1737 | 1102 | 404 | 423 | 400 | 167 | 613 | 465 | 64.4 | 50.8 | 61.8 | 13.6 | 138 | 99 | 812 | 465 | - | 1.1 | 0.5 | 1.1 | ||||||||
|
Deep GW |
Min | 7.3 | 7.6 | 531 | 530 | 232 | 305 | 204 | 89.2 | 117 | 114 | 52.9 | 49.6 | 34.4 | 15.6 | 25.9 | 29 | 171 | 129 | <0.008 | 0.4 | 0.2 | 0.3 | |||||||
| Max | 7.5 | 7.7 | 1290 | 1294 | 360 | 373 | 336 | 317 | 425 | 360 | 78.8 | 70.8 | 51.9 | 35.4 | 89.4 | 132 | 578 | 380 | <0.008 | 1.1 | 0.3 | 0.6 | ||||||||
| Avg | 7.4 | 7.7 | 925 | 928 | 296 | 329 | 276 | 225 | 264 | 237 | 66 | 60.9 | 43.6 | 25.3 | 56.2 | 76.7 | 376 | 275 | - | 0.9 | 0.3 | 0.4 | ||||||||
| Malala Oya Basin (CKDu control area) | ||||||||||||||||||||||||||||||
| Surface Water | Min | 7.2 | 6.95 | 177 | 130 | 74 | 3.36 | 112 | 52 | 25 | 24 | 1.0 | 18 | 0.4 | 10 | <0.2 | 7 | <0.2 | 16 | <0.2 | <0.2 | <0.2 | 0.4 | |||||||
| Max | 8.2 | 9.19 | 952 | 1810 | 195 | 1831 | 716 | 668 | 305 | 303 | 52.7 | 119 | 44.7 | 102 | 125.1 | 151 | 375 | 705 | 506.5 | 27.6 | 0.6 | 3.1 | ||||||||
| Avg | 7.7 | 7.87 | 470 | 628 | 126 | 850 | 250 | 261 | 139 | 127 | 28.3 | 38 | 23.2 | 31 | 44.7 | 44 | 138 | 177 | 106.6 | 4.8 | 0.1 | 1.1 | ||||||||
|
Shallow GW |
Min | 6.7 | 7.09 | 257 | 143 | 90 | 3.19 | 136 | 132 | 26 | 22 | 17.5 | 14 | 7.0 | 4 | 25.9 | 8 | 27.4 | 20 | <0.2 | <0.2 | <0.2 | 0.2 | |||||||
| Max | 7.8 | 8.38 | 2310 | 2530 | 527 | 1270 | 872 | 980 | 968 | 970 | 69.2 | 129 | 94.8 | 117 | 312.4 | 450 | 1008 | 992 | 6.3 | 26.4 | 2.1 | 5.8 | ||||||||
| Avg | 7.6 | 7.84 | 786 | 851 | 210 | 625 | 381 | 338 | 237 | 304 | 37.2 | 50 | 33.8 | 48 | 112.2 | 132 | 262.9 | 330 | 2.0 | 8.6 | 0.4 | 2.3 | ||||||||
|
Deep GW |
Min | 6.8 | 7.14 | 655 | 493 | 187 | 435 | 268 | 220 | 100 | 149 | 12.1 | 10 | 35.9 | 29 | 34.1 | 41 | 137.2 | 32 | <0.2 | 1.7 | <0.2 | 1.5 | |||||||
| Max | 7.9 | 7.64 | 1354 | 1166 | 357 | 1000 | 728 | 748 | 324 | 350 | 84.8 | 71 | 76.3 | 74 | 169 | 155 | 497 | 363 | 15.7 | 138.7 | 5.9 | 7.8 | ||||||||
| Avg | 7.3 | 7.39 | 901 | 863 | 252 | 620 | 443 | 513 | 187 | 260 | 48.0 | 36 | 53.4 | 50 | 101.6 | 83 | 230 | 205 | 5.9 | 18.4 | 1.5 | 3.7 | ||||||||
| Maximum Permissible Limits | ||||||||||||||||||||||||||||||
| SLS | 6.5-8.5 | 500 | 200 | 250 | 200 | 100 | 30 | 250 | 250 | 50 | 1.0 | |||||||||||||||||||
| WHO | 6.5-8.5 | 600 | 500 | 300 | 200 | 100 | 30 | 250 | 250 | 50 | 1.5 | |||||||||||||||||||
4.2. TA, Total Alkalinity; TDS, Total Dissolved Solids; TH, Total Hardness
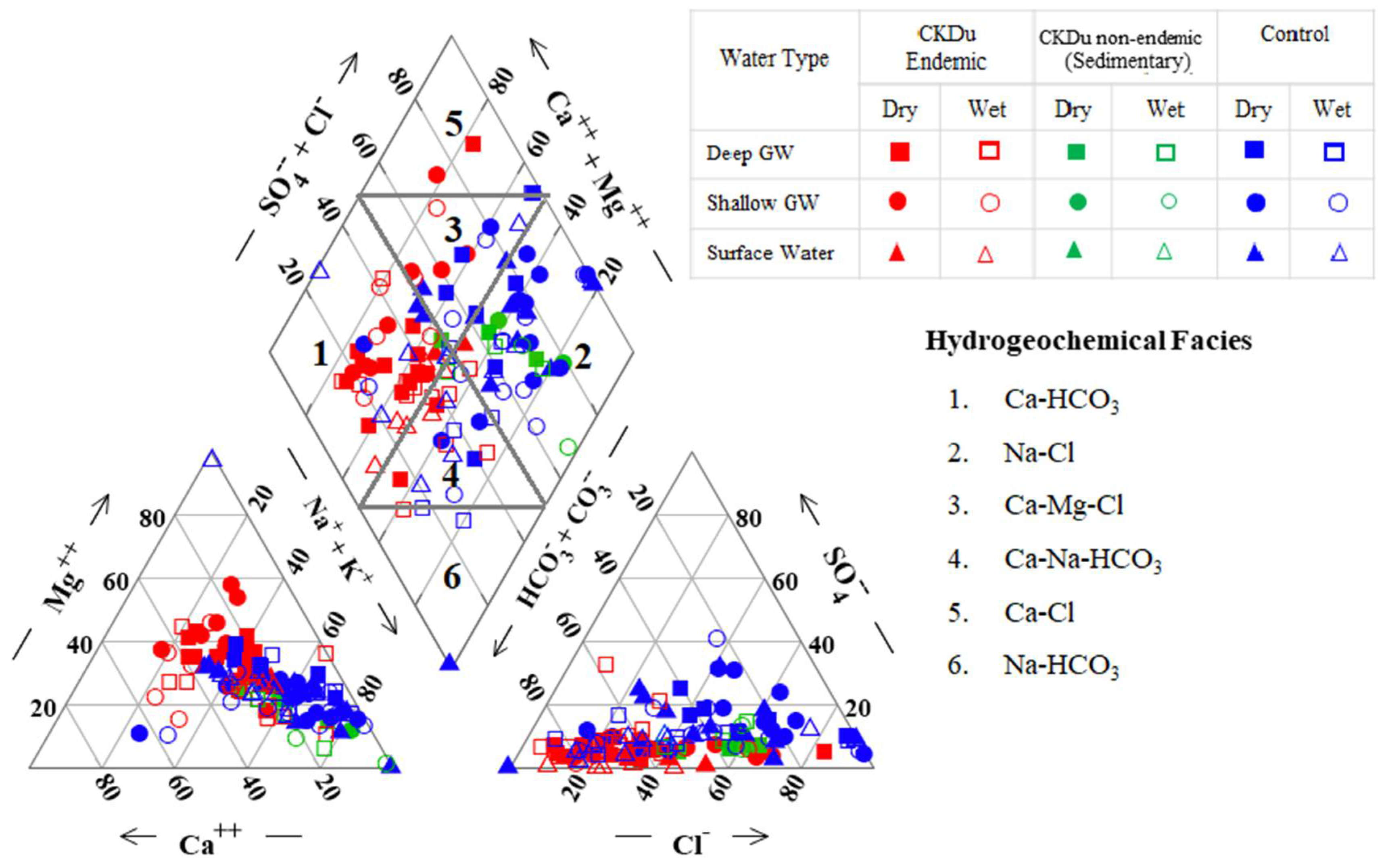
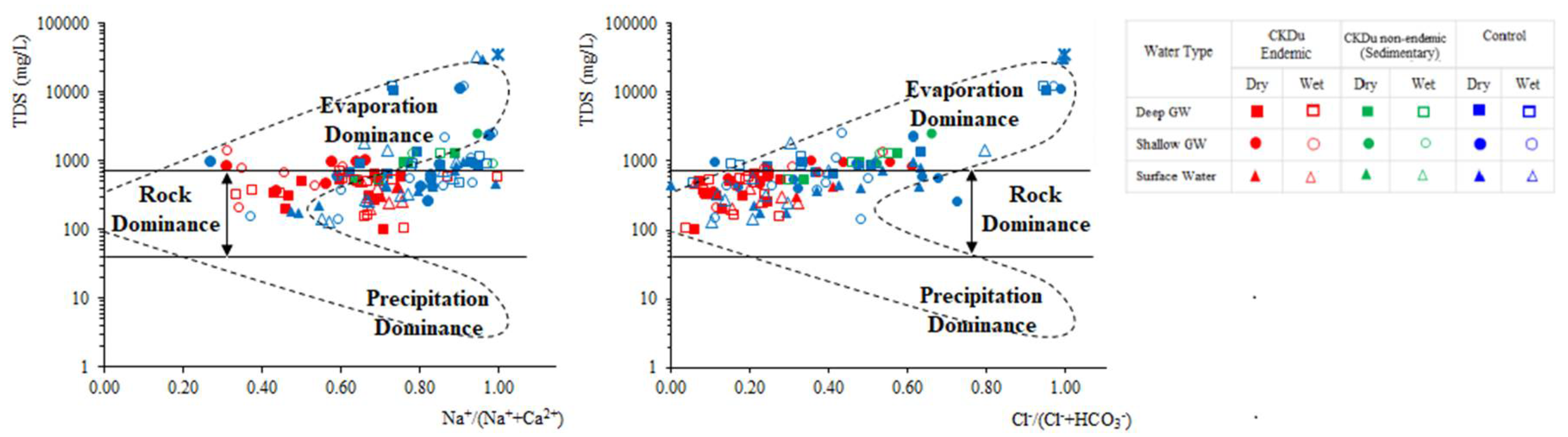
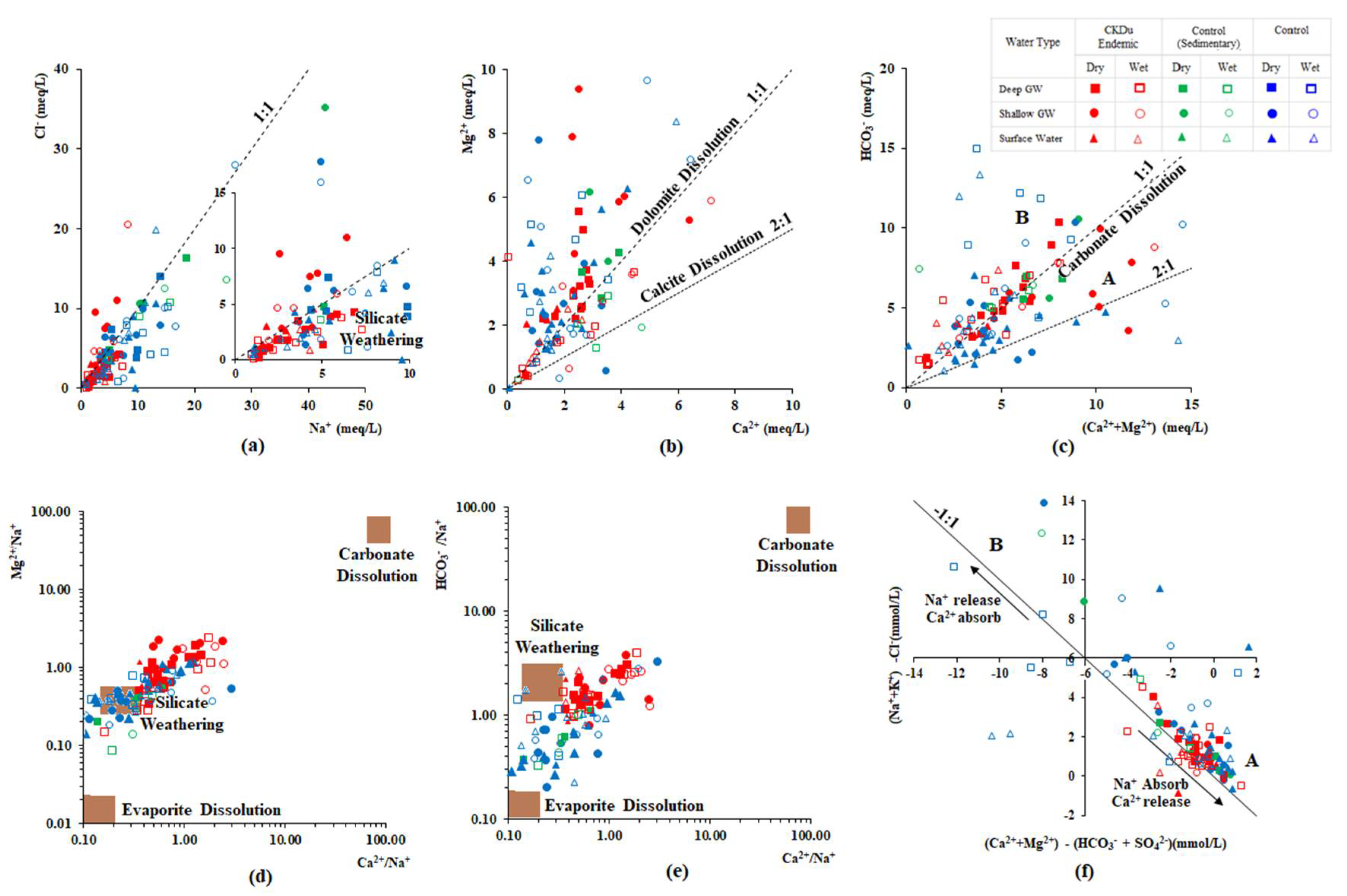
4.3. Hydrogeochemical Evolution of Groundwater
4.4. Groundwater Age
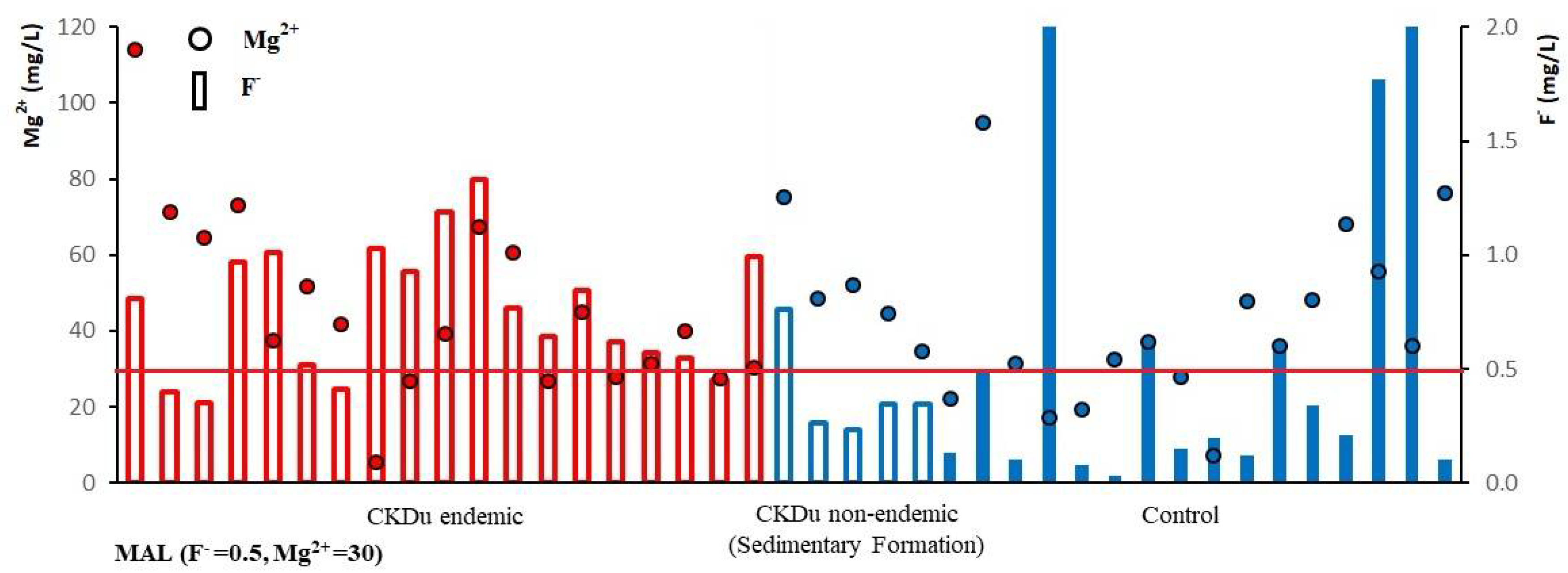
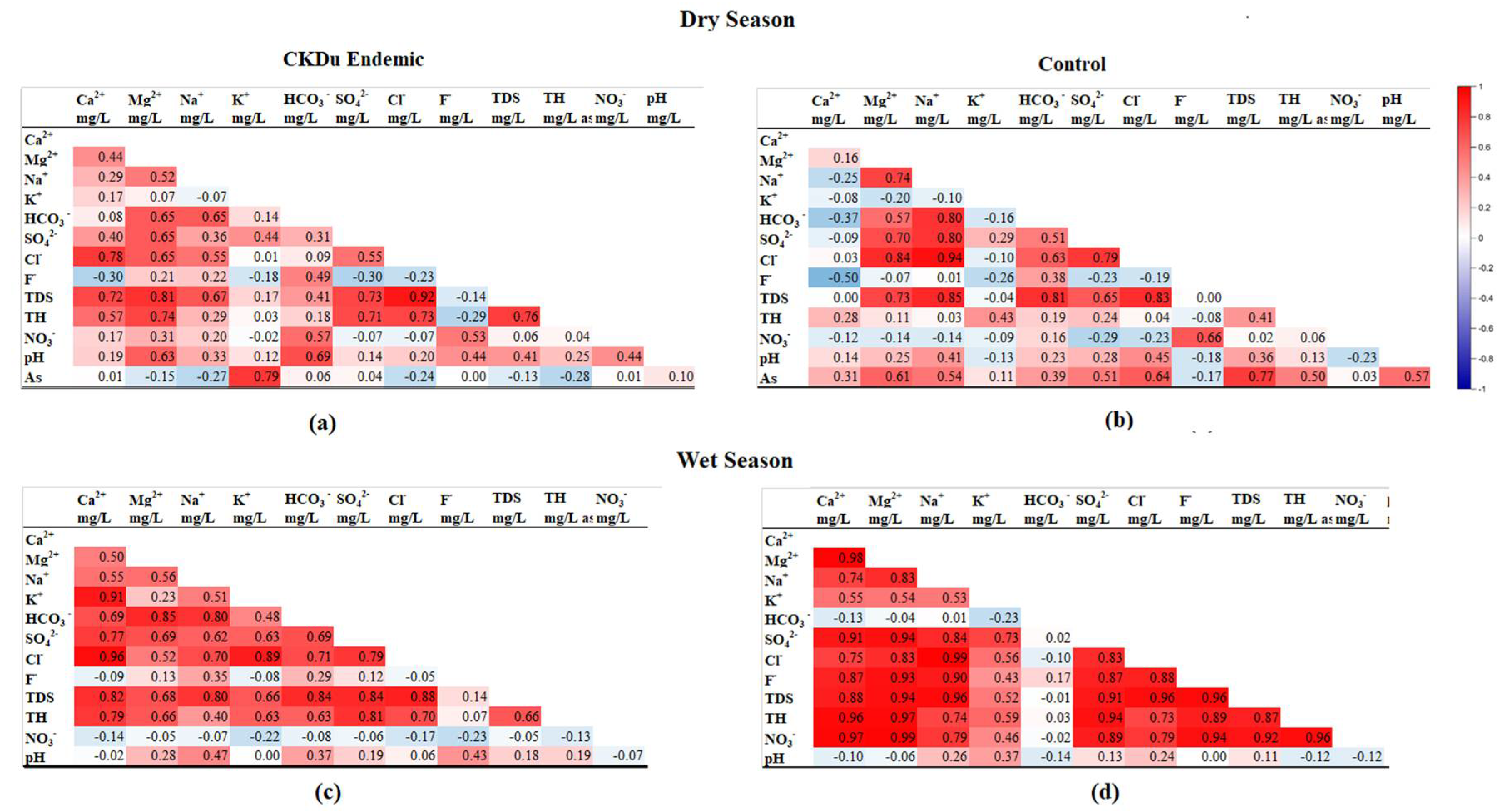
4.5. Correlation between Groundwater Fluoride, Hardness, TDS and Occurrence of CKDu
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balasooriya, B. M. J. K., Chaminda, G. G. T., Weragoda, S. K., Kankanamge, C. E., & Kawakami, T. (2021). Assessment of Groundwater Quality in Sri Lanka Using Multivariate Statistical Techniques. 117–135. [CrossRef]
- Barth, J. A. C., & Veizer, J. (2004). Water mixing in a St. Lawrence river embayment to outline potential sources of pollution. Applied Geochemistry, 19(10), 1637–1641. [CrossRef]
- Bershaw, J., Hansen, D. D., & Schauer, A. J. (2020). Deuterium excess and 17O-excess variability in meteoric water across the Pacific Northwest, USA. Tellus B: Chemical and Physical Meteorology, 72(1), 1–17. [CrossRef]
- Chabuk, A., Abed, S. A., Al-Zubaidi, H. A. M., Al-Ansari, N., Maliki, A. A. A., Ewaid, S. H., & Laue, J. (2021). Application GIS Software to Determine the Distribution of T.D.S. Concentrations along the Tigris River. IOP Conference Series: Earth and Environmental Science, 735(1). [CrossRef]
- Chandrajith, R., Dissanayake, C. B., Ariyarathna, T., Herath, H. M. J. M. K., & Padmasiri, J. P. (2011). Dose-dependent Na and Ca in fluoride-rich drinking water —Another major cause of chronic renal failure in tropical arid regions. Science of The Total Environment, 409(4), 671–675. [CrossRef]
- Chandrajith, R., Diyabalanage, S., & Dissanayake, C. B. (2020). Geogenic fluoride and arsenic in groundwater of Sri Lanka and its implications to community health. Groundwater for Sustainable Development, 10, 100359. [CrossRef]
- Chandrajith, R., Nanayakkara, S., Itai, K., Aturaliya, T. N. C., Dissanayake, C. B., Abeysekera, T., Harada, K., Watanabe, T., & Koizumi, A. (2011). Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: Geographic distribution and environmental implications. Environmental Geochemistry and Health, 33(3), 267–278. [CrossRef]
- Cooray, P. G. (1994). The precambrian of Sri Lanka: a historical review. Precambrian Research, 66(1–4), 3–18. [CrossRef]
- Cooray, T., Wei, Y., Zhong, H., Zheng, L., Weragoda, S. K., & Weerasooriya, R. (2019). Assessment of Groundwater Quality in CKDu Affected Areas of Sri Lanka: Implications for Drinking Water Treatment. International Journal of Environmental Research and Public Health, 16(10). [CrossRef]
- Dharma-wardana, M. W. C. (2018). Chronic kidney disease of unknown etiology and the effect of multiple-ion interactions. Environmental Geochemistry and Health, 40(2), 705–719. [CrossRef]
- Dissanayake, C. B., & Chandrajith, R. (2017). Groundwater fluoride as a geochemical marker in the etiology of chronic kidney disease of unknown origin in Sri Lanka. Article in Ceylon Journal of Science. [CrossRef]
- Dissanayake, C. B., & Weerasooriya, S. V. R. (1986). Fluorine as an indicator of mineralization — Hydrogeochemistry of a Precambrian mineralized belt in Sri Lanka. Chemical Geology, 56(3–4), 257–270. [CrossRef]
- Edirisinghe, E. A. N. V., Pitawala, H. M. T. G. A., Dharmagunawardhane, H. A., & Wijayawardane, R. L. (2017). Spatial and temporal variation in the stable isotope composition (δ18O and δ2H) of rain across the tropical island of Sri Lanka. Isotopes in Environmental and Health Studies, 53(6), 628–645. [CrossRef]
- Edirisinghe, E. A. N. V., Manthrithilake, H., Pitawala, H. M. T. G. A., Dharmagunawardhane, H. A., & Wijayawardane, R. L. (2017). Geochemical and isotopic evidences from groundwater and surface water for understanding of natural contamination in chronic kidney disease of unknown etiology (CKDu) endemic zones in Sri Lanka. Https://Doi.Org/10.1080/10256016.2017.1377704, 54(3), 244–261. [CrossRef]
- Egbueri, J. C. (2019). Evaluation and characterization of the groundwater quality and hydrogeochemistry of Ogbaru farming district in southeastern Nigeria. SN Applied Sciences, 1(8), 1–16. [CrossRef]
- Faleel, R. A., & Jayawardena, U. A. (2020). Progression of potential etiologies of the chronic kidney disease of unknown etiology in Sri Lanka. Journal of Environmental Science and Health. Part C, Toxicology and Carcinogenesis, 38(4), 362–383. [CrossRef]
- Friedman, D., & Luyckx, V. A. (2019). Genetic and Developmental Factors in Chronic Kidney Disease Hotspots. Seminars in Nephrology, 39(3), 244–255. [CrossRef]
- Gangadhara, K. R., & Jayasena. (n.d.). RAINWATER HARVEST BY TANK CASCADES IN SRI LANKA-WAS IT A TECHNICALLY ADAPTED METHODOLOGY BY THE ANCIENTS?
- Gibbs, R. J. (1970). Mechanisms Controlling World Water Chemistry. Science, 170(3962), 1088–1090. https://doi.org/10.1126/SCIENCE.170.3962.1088. [CrossRef]
- Gifford, F. J., Gifford, R. M., Eddleston, M., & Dhaun, N. (2017). Endemic Nephropathy Around the World. Kidney International Reports, 2(2), 282–292. [CrossRef]
- Harris, N. B. W. (1991). P. G. Cooray 1984. An Introduction to the Geology of Sri Lanka (Ceylon), 2nd revised edition. xix + 340 pp. Dated 1984 but only just published. Colombo: National Museums of Sri Lanka. Price £10.00, US $15.00 inc. surface postage; available from National Museums Department, P.O. Box 854, Colombo 7, Sri Lanka. Hard covers. No ISBN. Geological Magazine, 128(1), 85–85. [CrossRef]
- Hettithanthri, O., Sandanayake, S., Magana-Arachchi, D., Wanigatunge, R., Rajapaksha, A. U., Zeng, X., Shi, Q., Guo, H., & Vithanage, M. (2021). Risk factors for endemic chronic kidney disease of unknown etiology in Sri Lanka: Retrospect of water security in the dry zone. Science of The Total Environment, 795, 148839. [CrossRef]
- HM, W., D, A., WM, K., P, W., R, W., & J, B. (2016). Drinking water quality and chronic kidney disease of unknown etiology (CKDu): synergic effects of fluoride, cadmium and hardness of water. Environmental Geochemistry and Health, 38(1), 157–168. [CrossRef]
- Hu, D., Indika, S., Makehelwala, M., Titus, C., Zhu, L., Pang, Z., Zhong, H., Weragoda, S. K., Jinadasa, K. B. S. N., Weerasooriya, R., & Wei, Y. (2023). Chemical characteristics and water stability evaluation of groundwater in the CKDu Zone of Sri Lanka. Journal of Environmental Sciences. [CrossRef]
- Imbulana, K. A. U. S., Droogers, P., & Makin, I. W. (2002). World Water Assessment Programme Sri Lanka case study, Ruhuna basins: proceedings of a Workshop held at Koggala Beach Hotel, Sri Lanka, 7-9 April 2002. [CrossRef]
- Imbulana, S., & Oguma, K. (2021). Groundwater as a potential cause of Chronic Kidney Disease of unknown etiology (CKDu) in Sri Lanka: A review. Journal of Water and Health, 19(3), 393–410. [CrossRef]
- Indika, S., Hu, D., Wei, Y., Yapabandara, I., Cooray, T., Makehelwala, M., Jinadasa, K. B. S. N., Weragoda, S. K., Weerasooriya, R., & Pang, Z. (2023). Spatiotemporal Variation of Groundwater Quality in North Central Province, Sri Lanka. ACS ES and T Water, 3(6), 1687–1698. [CrossRef]
- Jayasena, H. A. H., Chandrajith, R., & Dissanayake, C. B. (2008). Spatial variation of isotope composition in precipitation in a tropical environment: A case study from the Deduru Oya river basin, Sri Lanka. Hydrological Processes, 22(23), 4565–4570. [CrossRef]
- Jayasumana, C., Gunatilake, S., & Senanayake, P. (2014). Glyphosate, Hard Water and Nephrotoxic Metals: Are They the Culprits Behind the Epidemic of Chronic Kidney Disease of Unknown Etiology in Sri Lanka? International Journal of Environmental Research and Public Health, 11(2), 2125. [CrossRef]
- Jayatilake, N., Mendis, S., Maheepala, P., & Mehta, F. R. (2013). Chronic kidney disease of uncertain aetiology: Prevalence and causative factors in a developing country. BMC Nephrology, 14(1). [CrossRef]
- JM, J., DM, D., SB, A., & P, B. (2013). Geographical distribution of chronic kidney disease of unknown origin in North Central Region of Sri Lanka. The Ceylon Medical Journal, 58(1), 6–10. [CrossRef]
- Krishna kumar, S., Logeshkumaran, A., Magesh, N. S., Godson, P. S., & Chandrasekar, N. (2015). Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Applied Water Science, 5(4), 335–343. [CrossRef]
- Kulathunga, M. R. D. L., Ayanka Wijayawardena, M. A., Naidu, R., & Wijeratne, A. W. (2019). Chronic kidney disease of unknown aetiology in Sri Lanka and the exposure to environmental chemicals: a review of literature. Environmental Geochemistry and Health, 41(5), 2329–2338. [CrossRef]
- Kumar, A., & Singh, C. K. (2015). Characterization of Hydrogeochemical Processes and Fluoride Enrichment in Groundwater of South-Western Punjab. Water Quality, Exposure and Health 2015 7:3, 7(3), 373–387. [CrossRef]
- Levine, K. E., Redmon, J. H., Elledge, M. F., Wanigasuriya, K. P., Smith, K., Munoz, B., Waduge, V. A., Periris-John, R. J., Sathiakumar, N., Harrington, J. M., Womack, D. S., & Wickremasinghe, R. (2015). Quest to identify geochemical risk factors associated with chronic kidney disease of unknown etiology (CKDu) in an endemic region of Sri Lanka—a multimedia laboratory analysis of biological, food, and environmental samples. Environmental Monitoring and Assessment, 188(10). [CrossRef]
- Liu, J., Peng, Y., Li, C., Gao, Z., & Chen, S. (2021). A characterization of groundwater fluoride, influencing factors and risk to human health in the southwest plain of Shandong Province, North China. Ecotoxicology and Environmental Safety, 207, 111512. [CrossRef]
- Liyanage, D. N. D., Diyabalanage, S., Dunuweera, S. P., Rajapakse, S., Rajapakse, R. M. G., & Chandrajith, R. (2022). Significance of Mg-hardness and fluoride in drinking water on chronic kidney disease of unknown etiology in Monaragala, Sri Lanka. Environmental Research, 203, 111779. [CrossRef]
- Lyu, M., Pang, Z., Yin, L., Zhang, J., Huang, T., Yang, S., Li, Z., Wang, X., & Gulbostan, T. (2019). The control of groundwater flow systems and geochemical processes on groundwater chemistry: A case study in Wushenzhao Basin, NW China. Water (Switzerland), 11(4). [CrossRef]
- McDonough, L. K., Meredith, K. T., Nikagolla, C., Middleton, R. J., Tan, J. K., Ranasinghe, A. V., Sierro, F., & Banati, R. B. (2020). The water chemistry and microbiome of household wells in Medawachchiya, Sri Lanka, an area with high prevalence of chronic kidney disease of unknown origin (CKDu). Scientific Reports 2020 10:1, 10(1), 1–12. [CrossRef]
- M Pry, J., Jackson, W., Rupasinghe, R., Lishanthe, G., Badurdeen, Z., Abeysekara, T., Chandrajith, R., Smith, W., & Wickramasinghe, S. (2021a). A pilot case-control study using a one health approach to evaluate behavioral, environmental, and occupational risk factors for chronic kidney disease of unknown etiology in Sri Lanka. One Health Outlook, 3(1).a one health approach to evaluate behavioral, environmental, and occupational risk factors for chronic kidney disease of unknown etiology in Sri Lanka. [CrossRef]
- M Pry, J., Jackson, W., Rupasinghe, R., Lishanthe, G., Badurdeen, Z., Abeysekara, T., Chandrajith, R., Smith, W., & Wickramasinghe, S. (2021b). A pilot case-control study using a one health approach to evaluate behavioral, environmental, and occupational risk factors for chronic kidney disease of unknown etiology in Sri Lanka. One Health Outlook 2021 3:1, 3(1), 1–12. [CrossRef]
- Nanayakkara, I., Dissanayake, R. K., & Nanayakkara, S. (2020). The presence of dehydration in paddy farmers in an area with chronic kidney disease of unknown aetiology. Nephrology, 25(2), 156–162. [CrossRef]
- Narsimha Adimalla. (2020). Assessment and Mechanism of Fluoride Enrichment in Groundwater from the Hard Rock Terrain: A Multivariate Statistical Approach. Geochemistry International, 58(4), 456–471. [CrossRef]
- Panabokke, C. R. (2007). Groundwater conditions in Sri Lanka : a geomorphic perspective. National Science Foundation of Sri Lanka.
- Panabokke, C. R., & Perera, A. P. G. R. L. (2005). GROUNDWATER RESOURCES OF SRI LANKA.
- Pathmarajah, S. (2007). USE OF GROUNDWATER FOR AGRICULTURE IN SRI LANKA PROCEEDINGS OF A SYMPOSIUM (Reprint) Editor Symposium sponsors Agricultural Engineering Society of Sri Lanka (AESSL) Postgraduate Institute of Agriculture (PGIA), Peradeniya Faculty of Agriculture, Peradeniya.
- Premadasa, H. K. S., Priyanath, H. M. S., & Walpita, C. N. (2020). The Impact of Social Capital on Socioeconomic Condition of Ckdu Patients in Sri Lanka: An Empirical Investigation. International Journal of Scientific Research and Management, 8(05), 377–388. [CrossRef]
- Priyadarshanee, K. S. G. S., Pang, Z., Edirisinghe, E. A. N. V., Dharmagunawardhane, H. A., Pitawala, H. M. T. G. A., Gunasekara, J. D. C., & Tilakarathna, I. A. N. D. P. (2022). Deep groundwater recharge mechanism in the sedimentary and crystalline terrains of Sri Lanka: A study based on environmental isotope and chemical signatures. Applied Geochemistry, 136, 105174. [CrossRef]
- Rajapakse, S., Shivanthan, M. C., & Selvarajah, M. (2016). Chronic kidney disease of unknown etiology in Sri Lanka. International Journal of Occupational and Environmental Health, 22(3), 259–264. [CrossRef]
- Raja, P., Krishnaraj, S., Selvaraj, G., Kumar, S., & Francis, V. (2021). Hydrogeochemical investigations to assess groundwater and saline water interaction in coastal aquifers of the southeast coast, Tamil Nadu, India. Environmental Science and Pollution Research, 28(5), 5495–5519. [CrossRef]
- Ranasinghe, A. V., Kumara, G. W. G. P., Karunarathna, R. H., De Silva, A. P., Sachintani, K. G. D., Gunawardena, J. M. C. N., Kumari, S. K. C. R., Sarjana, M. S. F., Chandraguptha, J. S., & De Silva, M. V. C. (2019). The incidence, prevalence and trends of Chronic Kidney Disease and Chronic Kidney Disease of uncertain aetiology (CKDu) in the North Central Province of Sri Lanka: An analysis of 30,566 patients. BMC Nephrology, 20(1), 1–11. [CrossRef]
- R, C., S, N., K, I., TN, A., CB, D., T, A., K, H., T, W., & A, K. (2011). Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: geographic distribution and environmental implications. Environmental Geochemistry and Health, 33(3), 267–278. [CrossRef]
- Rubasinghe, R., Gunatilake, S. K., & Chandrajith, R. (2015). Geochemical characteristics of groundwater in different climatic zones of Sri Lanka. Environmental Earth Sciences, 74(4), 3067–3076. [CrossRef]
- Sandanayake, S., Diyabalanage, S., Edirisinghe, E. A. N. V., Guo, H., & Vithanage, M. (2023). Hydrogeochemical characterization of groundwater with a focus on Hofmeister ions and water quality status in CKDu endemic and CKDu non‒endemic areas, Sri Lanka. Environmental Pollution (Barking, Essex : 1987), 328. [CrossRef]
- Saxena, V., & Ahmed, S. (2001). Dissolution of fluoride in groundwater: a water-rock interaction study. Environmental Geology 2001 40:9, 40(9), 1084–1087. [CrossRef]
- Senarathne, S. L., Jayawardana, J. M. C. K., Edirisinghe, E. A. N. V., & Chandrajith, R. (2019). Characterization of groundwater in Malala oya river basin, Sri Lanka using geochemical and isotope signatures. Groundwater for Sustainable Development, 9. [CrossRef]
- Standard methods for the examination of water and wastewater- 22nd edition. APHA - Google Search. (n.d.). Retrieved August 12, 2021, from https://www.google.com/search?q=+Standard+methods+for+the+examination+of+water+and+wastewater-+22nd+edition.+APHA&sxsrf=ALeKk03ixJGzpJO-yKsHCH9Xcy0wlJBB3Q%3A1628741341635&ei=3Z4UYZ-VJqzfz7sP0r6hoAI&oq=+Standard+methods+for+the+examination+of+water+and+wastewater-+22nd+edition.+APHA&gs_lcp=Cgdnd3Mtd2l6EAMyBggAEAgQHkoECEEYAFDFHljFHmCyLGgAcAB4AIAByQGIAckBkgEDMi0xmAEAoAECoAEBwAEB&sclient=gws-wiz&ved=0ahUKEwjf9-SozqryAhWs73MBHVJfCCQQ4dUDCA4&uact=5.
- Su, C., Wang, Y., Xie, X., & Li, J. (2013). Aqueous geochemistry of high-fluoride groundwater in Datong Basin, Northern China. Journal of Geochemical Exploration, 135, 79–92. [CrossRef]
- S, W., S, B., S, D., & R, C. (2017). Tracing environmental aetiological factors of chronic kidney diseases in the dry zone of Sri Lanka-A hydrogeochemical and isotope approach. Journal of Trace Elements in Medicine and Biology : Organ of the Society for Minerals and Trace Elements (GMS), 44, 298–306. [CrossRef]
- Torres, C., Aragón, A., González, M., López, I., Jakobsson, K., Elinder, C. G., Lundberg, I., & Wesseling, C. (2010). Decreased Kidney Function of Unknown Cause in Nicaragua: A Community-Based Survey. American Journal of Kidney Diseases, 55(3), 485–496. [CrossRef]
- Vaheesar, K. (n.d.). NITRATE AND FLUORIDE CONTENT IN GROUND WATER IN THE BATTICALOA DISTRICT. 2, 9–15.
- Vlahos, P., Schensul, S. L., Nanayakkara, N., Chandrajith, R., Haider, L., Anand, S., Silva, K. T., & Schensul, J. J. (2019). Kidney progression project (KiPP): Protocol for a longitudinal cohort study of progression in chronic kidney disease of unknown etiology in Sri Lanka. Global Public Health, 14(2), 214–226. [CrossRef]
- Water Scarcity Variations Within a Country: A Case Study of Sri Lanka - Upali Ananda Amarasinghe, Lal Mutuwatta, R. Sakthivadivel - Google Books. (n.d.). Retrieved October 11, 2023, from https://books.google.com.tw/books?hl=en&lr=&id=A2I3I6RizqwC&oi=fnd&pg=PR5&dq=Amarasinghe+1999&ots=J6DZyBb7bj&sig=jWehWT3X46DytrJxesDQbiqe93M&redir_esc=y#v=onepage&q=Amarasinghe%201999&f=false.
- Wei, C., Guo, H., Zhang, D., Wu, Y., Han, S., An, Y., & Zhang, F. (2016). Occurrence and hydrogeochemical characteristics of high-fluoride groundwater in Xiji County, southern part of Ningxia Province, China. Environmental Geochemistry and Health, 38(1), 275–290. [CrossRef]
- Wickramarathna, S., Balasooriya, S., Diyabalanage, S., & Chandrajith, R. (2017). Tracing environmental aetiological factors of chronic kidney diseases in the dry zone of Sri Lanka—A hydrogeochemical and isotope approach. Journal of Trace Elements in Medicine and Biology, 44, 298–306. [CrossRef]
- Wimalawansa, S. J. (2015). The role of ions, heavy metals, fluoride, and agrochemicals: critical evaluation of potential aetiological factors of chronic kidney disease of multifactorial origin (CKDmfo/CKDu) and recommendations for its eradication. Environmental Geochemistry and Health 2015 38:3, 38(3), 639–678. [CrossRef]
- Xu, S., Li, S. L., Yue, F., Udeshani, C., & Chandrajith, R. (2021). Natural and anthropogenic controls of groundwater quality in sri lanka: Implications for chronic kidney disease of unknown etiology (ckdu). Water (Switzerland), 13(19), 2724. [CrossRef]
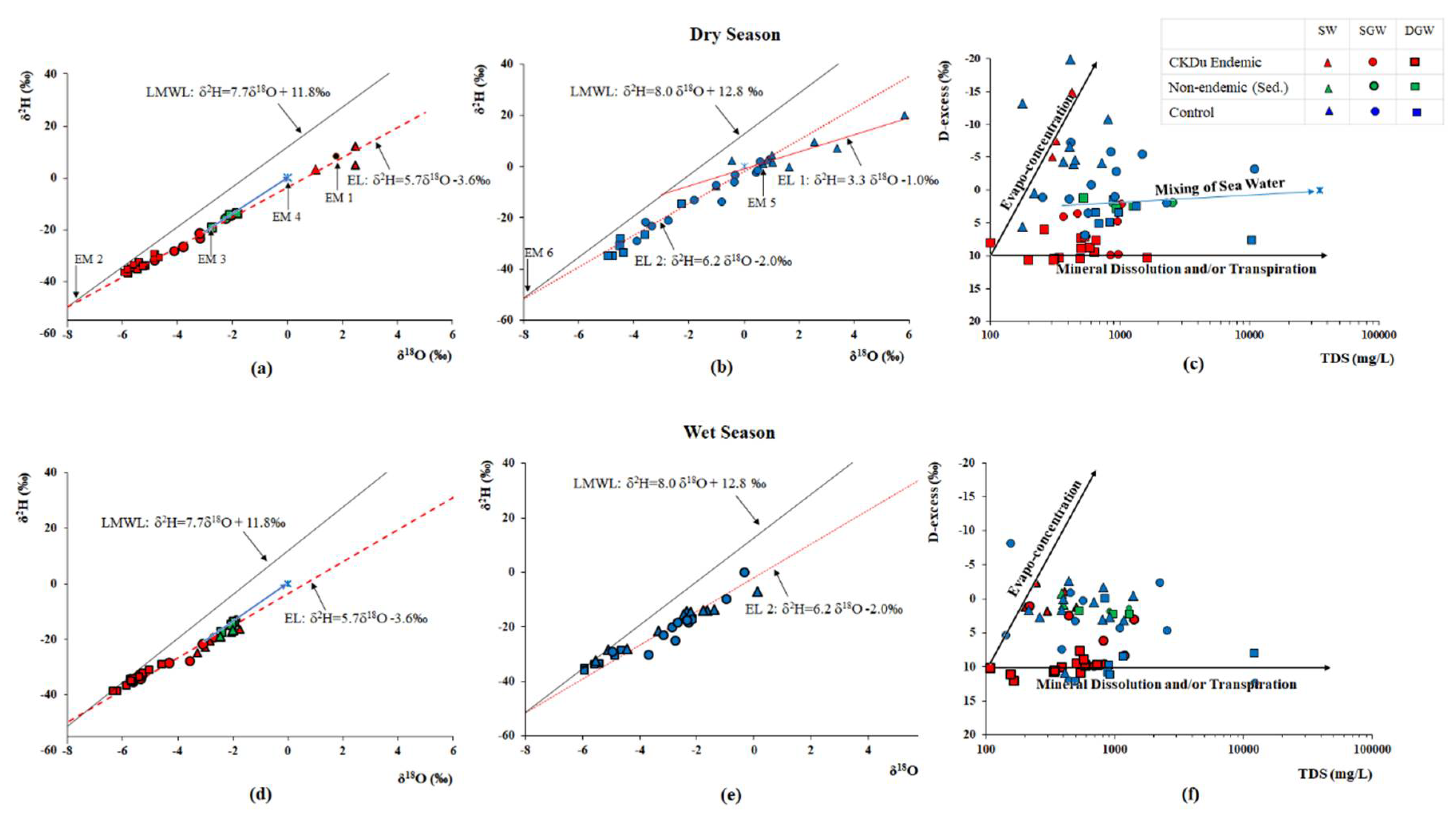
| Water Type | δ18O(‰) | δ2H(‰) | D-excess | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Minimum | Maximum | Minimum | Maximum | |||||||
| Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | |
| Malwathu Oya | ||||||||||||
| Surface Water | 1.0 | -3.3 | 2.5 | -1.8 | 3.2 | -25.0 | 12.2 | -16.4 | -14.8 | -2.4 | -5.1 | 1.8 |
| Metamorphic Granite Area (CKDu endemic) | ||||||||||||
| Shallow Groundwater | -5.6 | -5.6 | -3.2 | -2.7 | -34.5 | -35.2 | -21.5 | -19.1 | 2.1 | 1.0 | 9.9 | 9.8 |
| Deep Groundwater | -5.9 | -6.3 | -4.6 | -4.6 | -36.8 | -38.8 | -29.6 | 29.0 | 6.0 | 8.9 | 10.7 | 11.9 |
| Sedimentary Limestone Area (CKDu non-endemic) | ||||||||||||
| Shallow Groundwater | -2.2 | -2.4 | -2.0 | -1.9 | -15.8 | -17.5 | -14.4 | -13.0 | 1.9 | 1.5 | 2.0 | 1.8 |
| Deep Groundwater | -2.8 | -2.4 | -1.9 | -2.0 | -19.2 | -17.3 | -13.5 | -13.7 | 1.3 | 1.8 | 2.9 | 2.2 |
| Malala Oya (CKDu control) | ||||||||||||
| Surface Water | -1.0 | -5.0 | 5.8 | -1.0 | -7.7 | -30.2 | 19.8 | -9.6 | -26.8 | -2.6 | 0.5 | 11.0 |
| Shallow Groundwater | -3.9 | -5.6 | 0.6 | 0.1 | -29.2 | -32.3 | 1.8 | -7.1 | -7.2 | -8.1 | 6.9 | 12.4 |
| Deep Groundwater | -5.0 | -6.0 | -2.3 | -2.1 | -34.8 | -36.3 | -14.7 | -17.0 | 1.4 | 0.0 | 7.6 | 12.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





