Submitted:
10 May 2024
Posted:
10 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
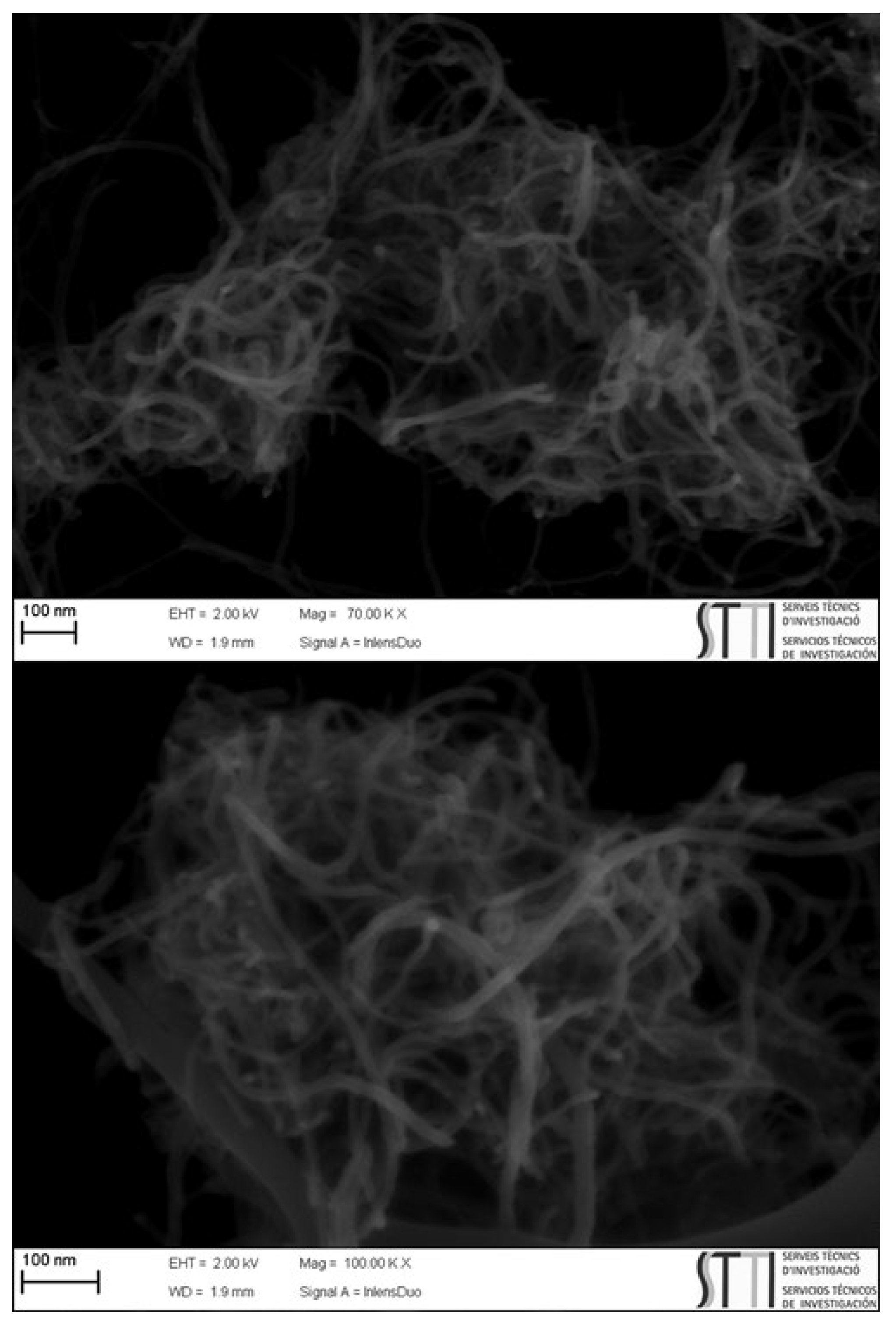
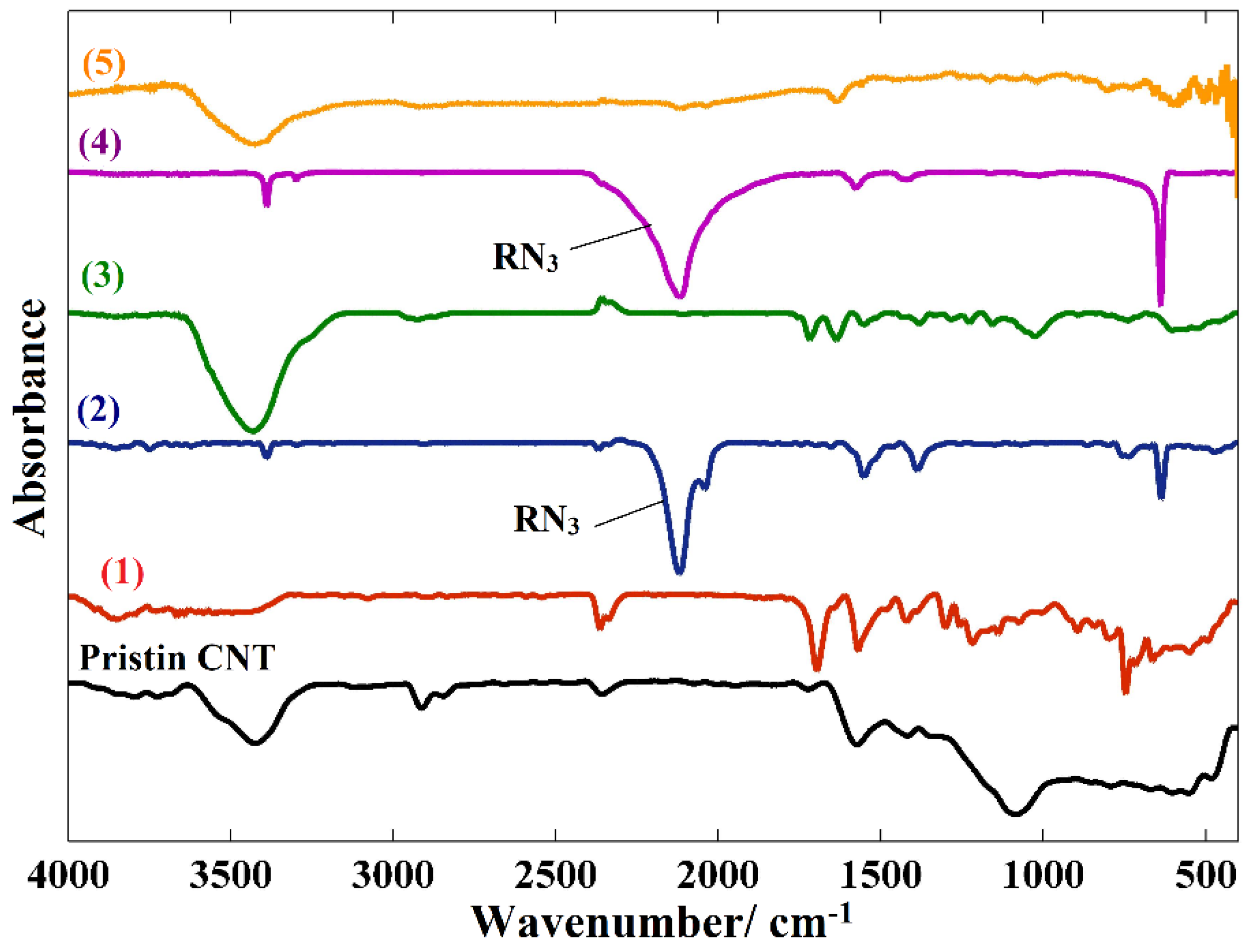
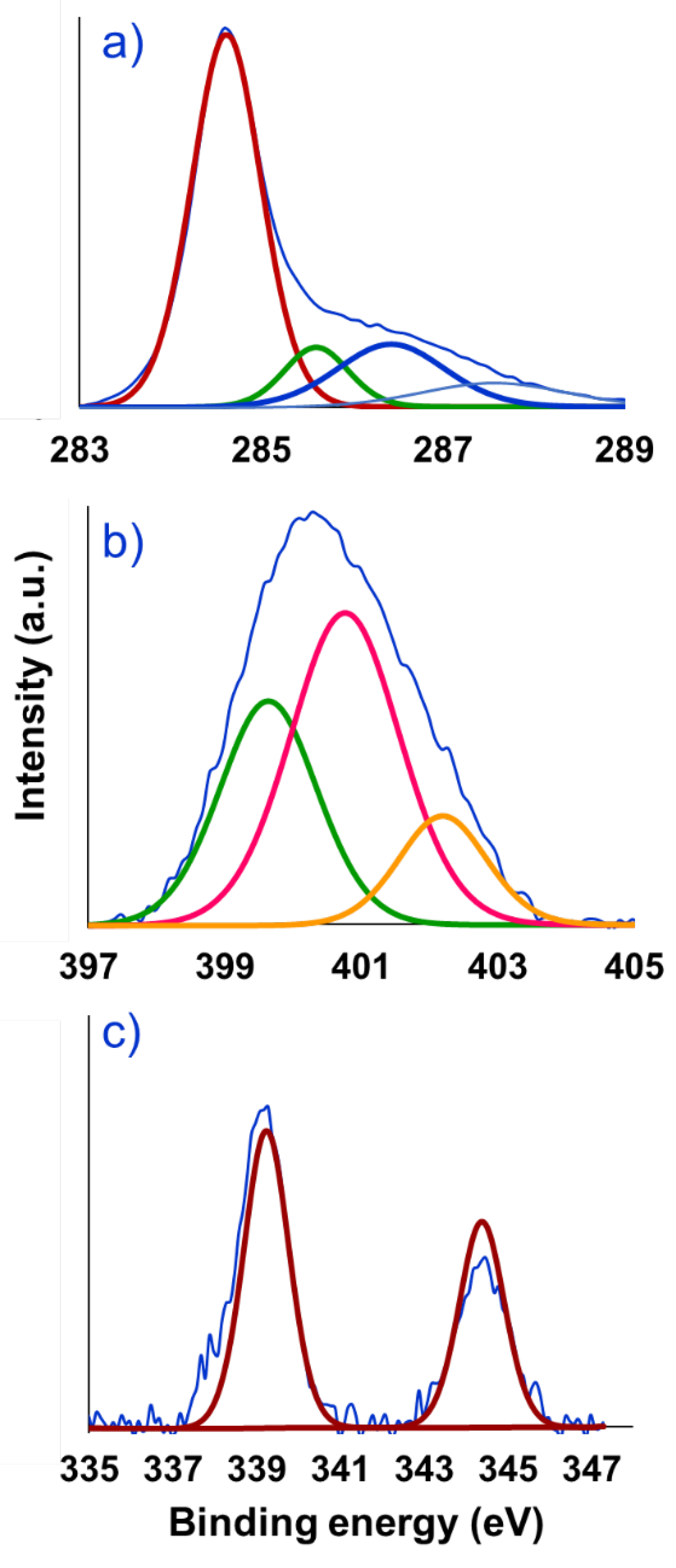
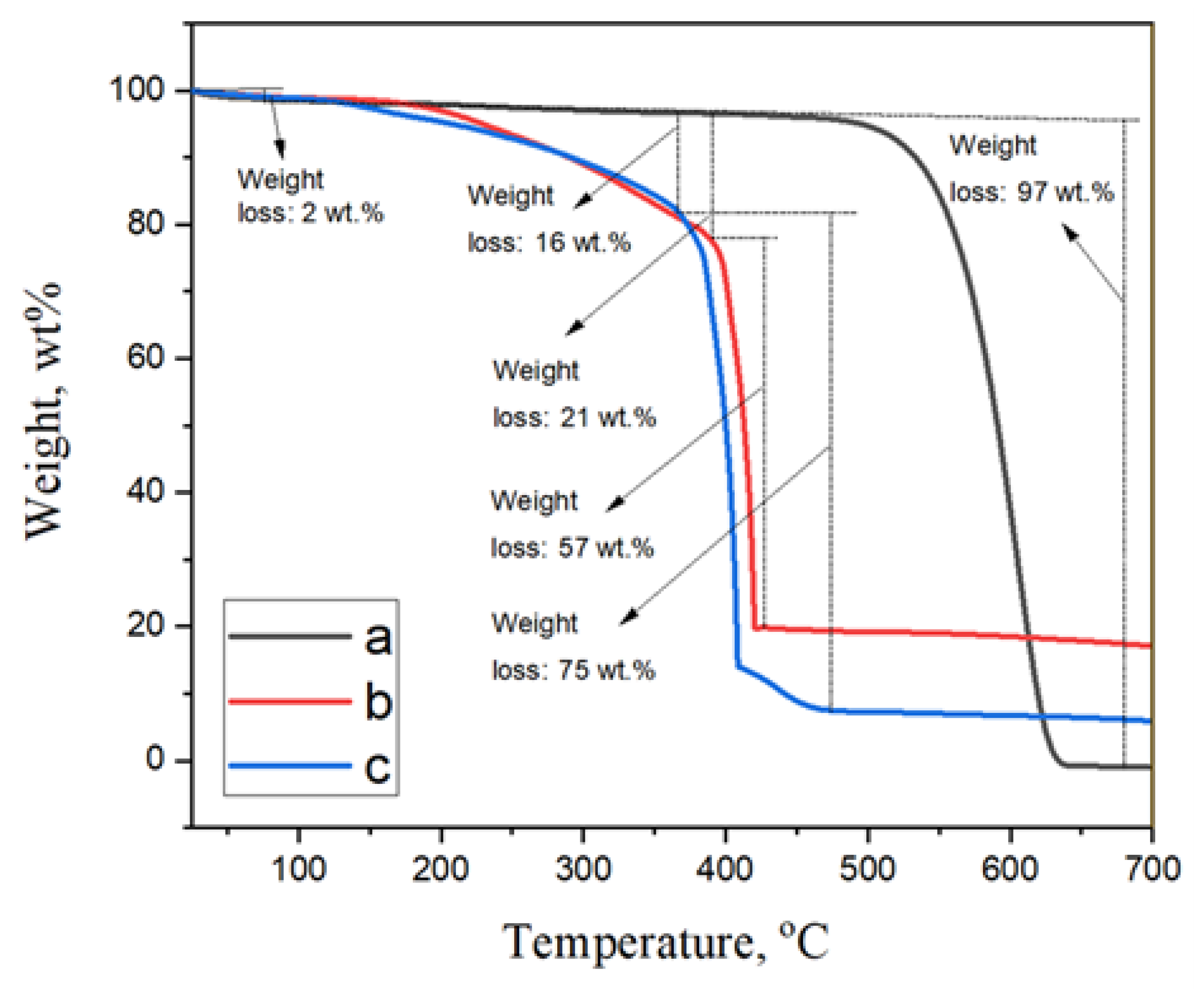
2. Results and Discussions
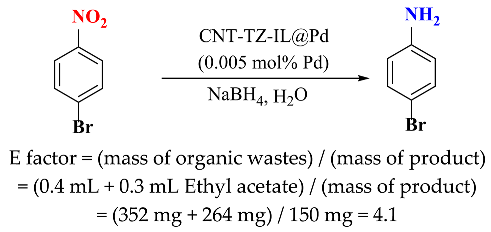
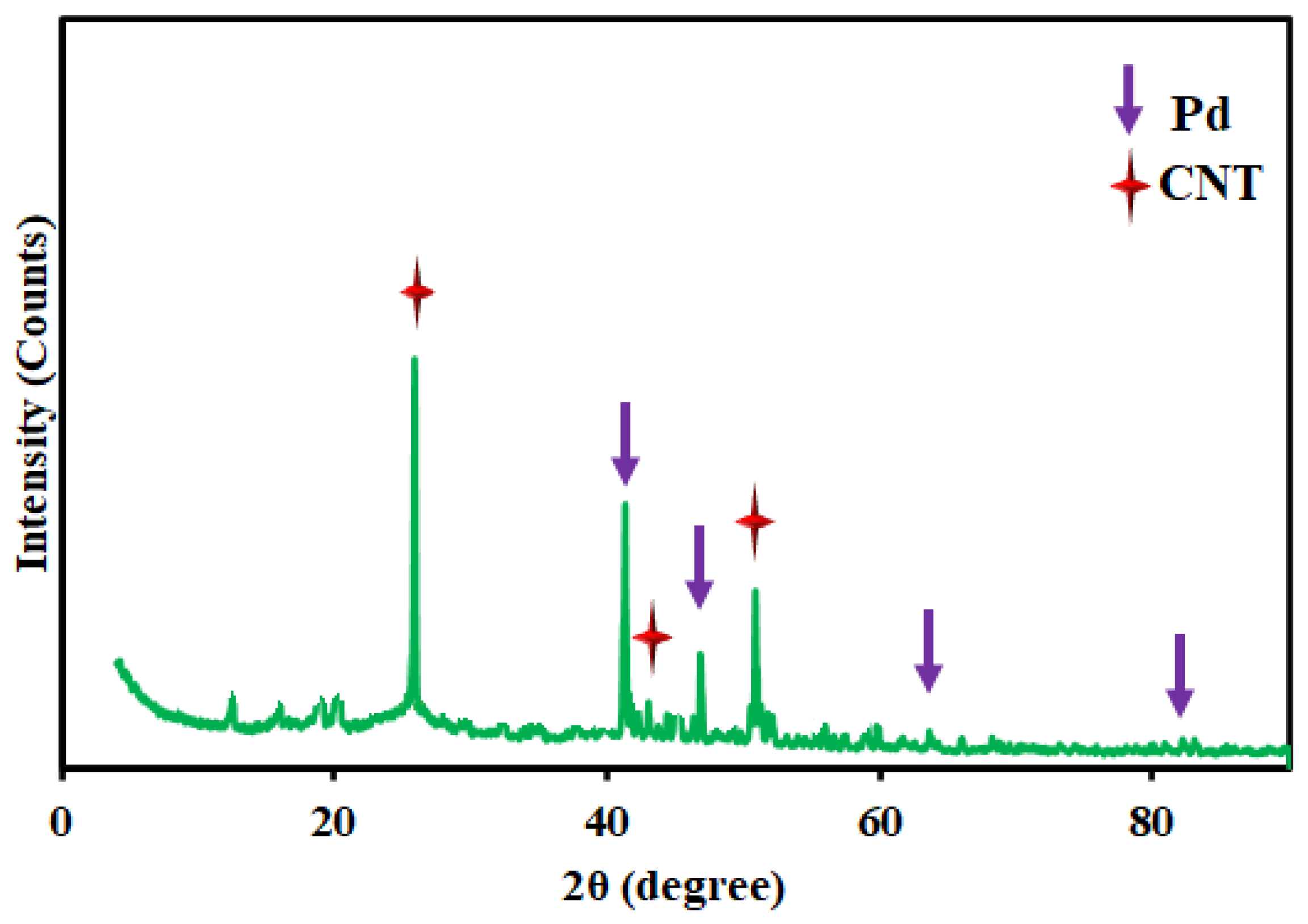
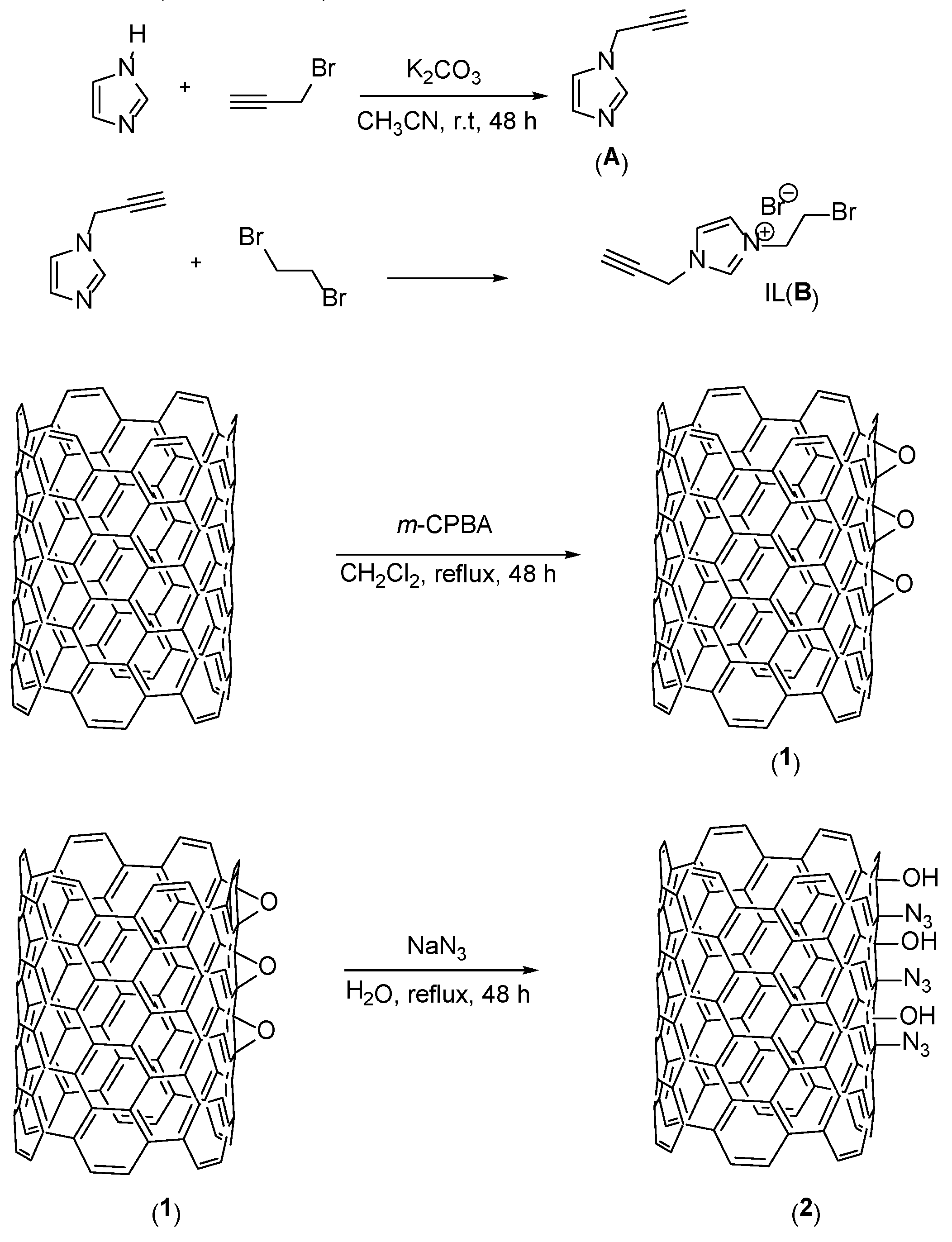
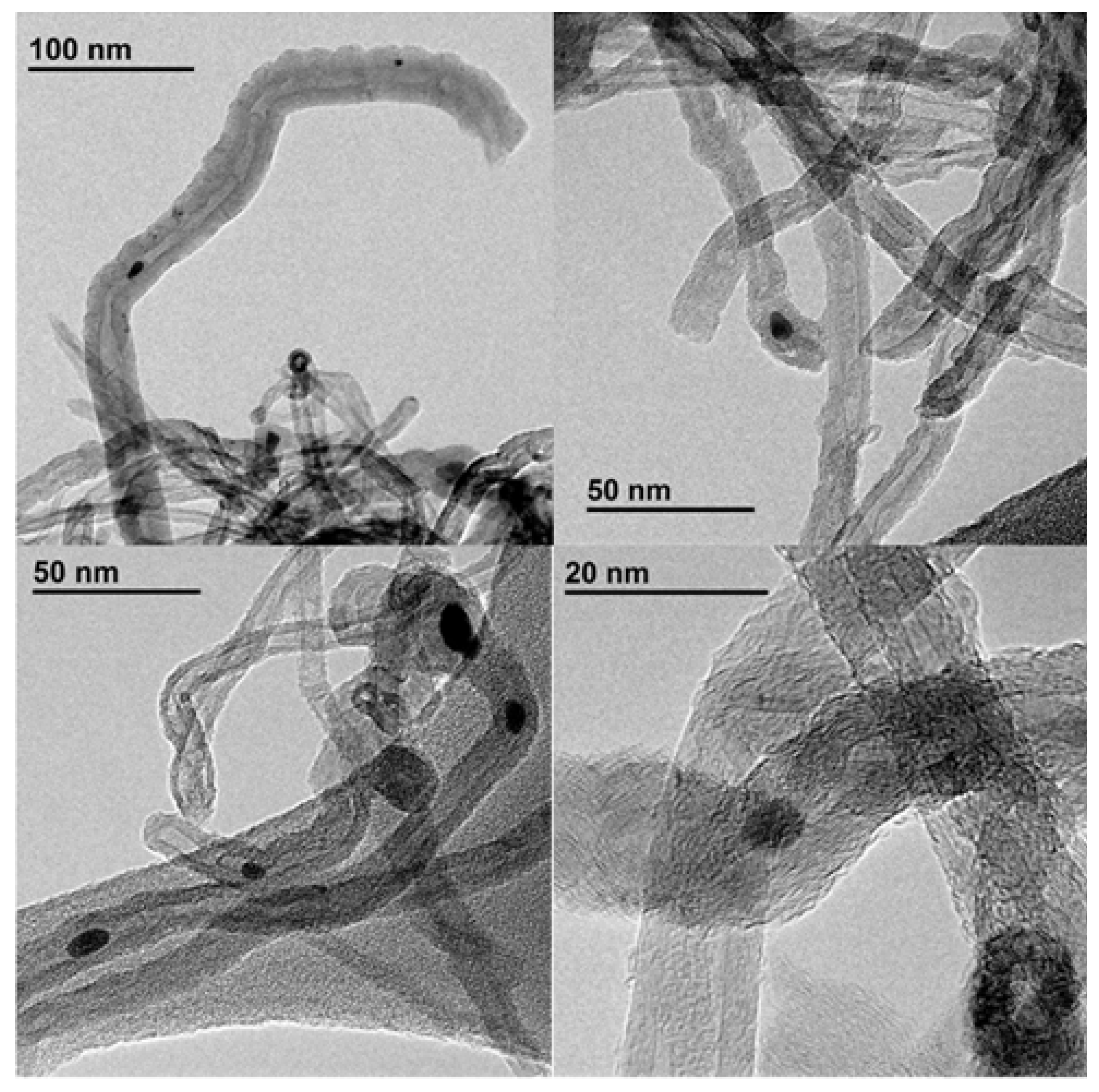
3. Conclusion
Acknowledgments
References
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B. 2021, 268, 115095. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, X.; Shang, Y.; Chang, S.; Dai, L.; Cao, A. Application-Driven Carbon Nanotube Functional Materials. ACS Nano 2021, 15, 7946–7974. [Google Scholar] [CrossRef]
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.X.; Yang, H.B.; Liu, B.; Yang, Y. Carbon nanotube catalysts: recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef] [PubMed]
- Gholinejad, M.; Naghshbandi, Z.; Nájera, C. Carbon-Derived Supports for Palladium Nanoparticles as Catalysts for Carbon-Carbon Bonds Formation. ChemCatChem 2019, 11, 1792–1823. [Google Scholar] [CrossRef]
- Rodríguez-Flórez, L.V.; Retamosa, M.d.G.; Martínez-Sánchez, B.; Cazorla-Amorós, D.; Nájera, C.; Yus, M.; Sansano, J.M. Microwave assisted functionalization of SWCNT as platform of a supported iridium catalyst. Synlett 2024, 35, 930–934. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 10–6633. [Google Scholar] [CrossRef]
- Steinrück, H.P.; Libuda, J.; Wasserscheid, P.; Cremer, T.; Kolbeck, C.; Laurin, M.; Maier, F.; Sobota, M.; Schulz, P.S.; Stark, M. Surface Science and Model Catalysis with Ionic Liquid-Modified Materials. Adv. Mater. 2011, 23, 2571–2587. [Google Scholar] [CrossRef] [PubMed]
- Gholinejad, M.; Zareh, F.; Sheibani, H.; Nájera, C.; Yus, M. Magnetic ionic liquids as catalysts in organic reactions. J. Mol. Liq. 2022, 367, 120395. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R. Nitroaromatic compounds: Environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. J. Appl. Toxicol. 2014, 34, 810–824. [Google Scholar] [CrossRef]
- Tchieno, F.M.M.; Tonle, I.K. p-Nitrophenol determination and remediation: an overview” Rev. Anal. Chem. 2018, 37, 20170019. [Google Scholar] [CrossRef]
- Kadam, H.K.; Tilve, S.G. Advancement in methodologies for reduction of nitroarenes. RSC Adv., 2015, 5, 83391–83407. [Google Scholar] [CrossRef]
- Hu, Z.H.; Liang, J.; Ding, K.; Ai, Y.; Liang, Q.; Sun, H.B. Insight into the selectivity of nano-catalytic nitroarenes reduction over other active groups by exploring hydrogen sources and metal components, Appl. Catal. A: Gen. 2021, 626, 118339. [Google Scholar] [CrossRef]
- Tanimu, A.; Alhooshani, K. Advanced Hydrodesulfurization Catalysts: A Review of Design and Synthesis. Energy Fuels 2019, 33, 2810–2838. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, T.; Jia, J. Indirect hydrodesulfurization of gasoline via sodium borohydride reduction with nickel catalysis under ambient conditions. RSC Advances, 2012, 2, 3123–3132. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, H.; Ding, R.; Wang, L.; Liu, Z.; Lv, B. Highly efficient oxidative desulfurization of dibenzothiophene using Ni modified MoO3 catalyst. Appl. Catal. A: Gen. 2020, 589, 117308. [Google Scholar] [CrossRef]
- Hasanbeik, N.Y.; Pourmadadi, M.; Ghadami, A.; Yazdian, F.; Rahdar, A.; Kyzas, G.Z. Biodesulfurization of Dibenzothiophene by Decorating Rhodococcus erythropolis IGTS8 Using Montmorillonite/Graphitic Carbon Nitride. Catalysts 2022, 12, 1450. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.L.; Kumar, R.; Rajak, P.K.; Singh, P.K. Desulphurization of Dibenzothiophene by Different Bacterial Strains: An Eco-Friendly Approach to Obtain Clean Fuel from Coal. Geomicrobiol. J. 2022, 39, 477–486. [Google Scholar] [CrossRef]
- Muhammad, Y.; Li, C. Dibenzothiophene hydrodesulfurization using in situ generated hydrogen over Pd promoted alumina-based catalysts. Fuel Process. Technol. 2011, 92, 624–630. [Google Scholar] [CrossRef]
- Muhammad, Y.; Rahman, A.U.; Rashid, H.U.; Sahibzada, M.; Subhan, S.; Tong, Z. Hydrodesulfurization of dibenzothiophene using Pd-promoted Co–Mo/Al2O3 and Ni–Mo/Al2O3 catalysts coupled with ionic liquids at ambient operating conditions. RSC Adv. 2019, 9, 10371–10385. [Google Scholar] [CrossRef]
- Yaragalla, S.; Anilkumar, G.; Kalarikkal, N.; Thomas, S. Structural and optical properties of functionalized multi-walled carbon nanotubes. Mater. Sci. Semicond. Process. 2016, 41, 491–496. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, Y.; Zhu, Z.; Guo, L.; Cao, Z.; Xia, Y.; Yang, H.; Gong, F.; Zhong, J. Synthesis of Poly(methyl methacrylate) Grafted Multiwalled Carbon Nanotubes via a Combination of RAFT and Alkyne-Azide Click Reaction. Appl. Sci. 2019, 9, 603. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Rigana, F.; Balaji, S.; Selvamani, A.; Sarojadevi, M. New polytriazoleimides containing green synthesized titanium dioxide using Artemisia pallens plant extract: optical, dielectric, thermal and mechanical properties. New J. Chem. 2017, 41, 2815–2825. [Google Scholar] [CrossRef]
- Mahouche Chergui, S.; Ledebt, A.; Mammeri, F.; Herbst, F.; Carbonnier, B.; Ben Romdhane, H.; Delamar, M.; Chehimi, M.M. Hairy Carbon Nanotube@Nano-Pd Heterostructures: Design, Characterization, and Application in Suzuki C−C Coupling Reaction. Langmuir 2010, 26, 16115–16121. [Google Scholar] [CrossRef]
- Lawal, I.A.; Lawal, M.M.; Akpotu, S.O.; Azeez, M.A.; Ndungu, P.; Moodley, B. Theoretical and experimental adsorption studies of sulfamethoxazole and ketoprofen on synthesized ionic liquids modified CNTs. Ecotoxicol Environ Saf. 2018, 161, 542–552. [Google Scholar] [CrossRef]
- Kuba, A.G.; Smolin, Y.Y.; Soroush, M.; Lau, K.K. Synthesis and integration of poly(1-vinylimidazole) polymer electrolyte in dye sensitized solar cells by initiated chemical vapor deposition. Chem. Eng. Sci. 2016, 154, 136–142. [Google Scholar] [CrossRef]
- Okpalugo, T.; Papakonstantinou, P.; Murphy, H.; McLaughlin, J.; Brown, N. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005, 43, 153–161. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, J.; Fang, M.; Tan, L.; Chen, C.; Alharbi, N.S.; Hayat, T.; Tan, X. Synthesis of a core–shell magnetic Fe3O4–NH2@PmPD nanocomposite for efficient removal of Cr(VI) from aqueous media. RSC Advances 2017, 7, 36231–36241. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, S.; Zhang, J.; Li, W.; Fu, Y. Peroxidase Mimicking Activity of Palladium Nanocluster Altered by Heparin. https://doi.org/10.1007/s1056 2-021-03530-x; Bai,C.; Jian,S.; Yao, X.; Li, Y.; Carbonylative Sonogashira coupling of terminal alkynes with aryl iodides under atmospheric pressure of CO using Pd(II)@MOF as the catalyst. Catal. Sci. Technol. 2014, 4, 326. https://doi.org/10.1039/C4CY00488D.
- Awadallah-F, A.; Al-Muhtaseb, S. Carbon Nanoparticles-Decorated Carbon Nanotubes. Sci Rep. 2020, 10, 4878. [Google Scholar] [CrossRef]
- Sheldon, R.A. ; The E factor 25 years on: the rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Oh, D.G.; Aleksandrov, H.A.; Kim, H.; Koleva, I.Z.; Khivantsev, K.; Vayssilov, G.N.; Kwak, J.H. Understanding of Active Sites and Interconversion of Pd and PdO during CH4 Oxidation. Molecules 2023, 28, 1957. [Google Scholar] [CrossRef] [PubMed]


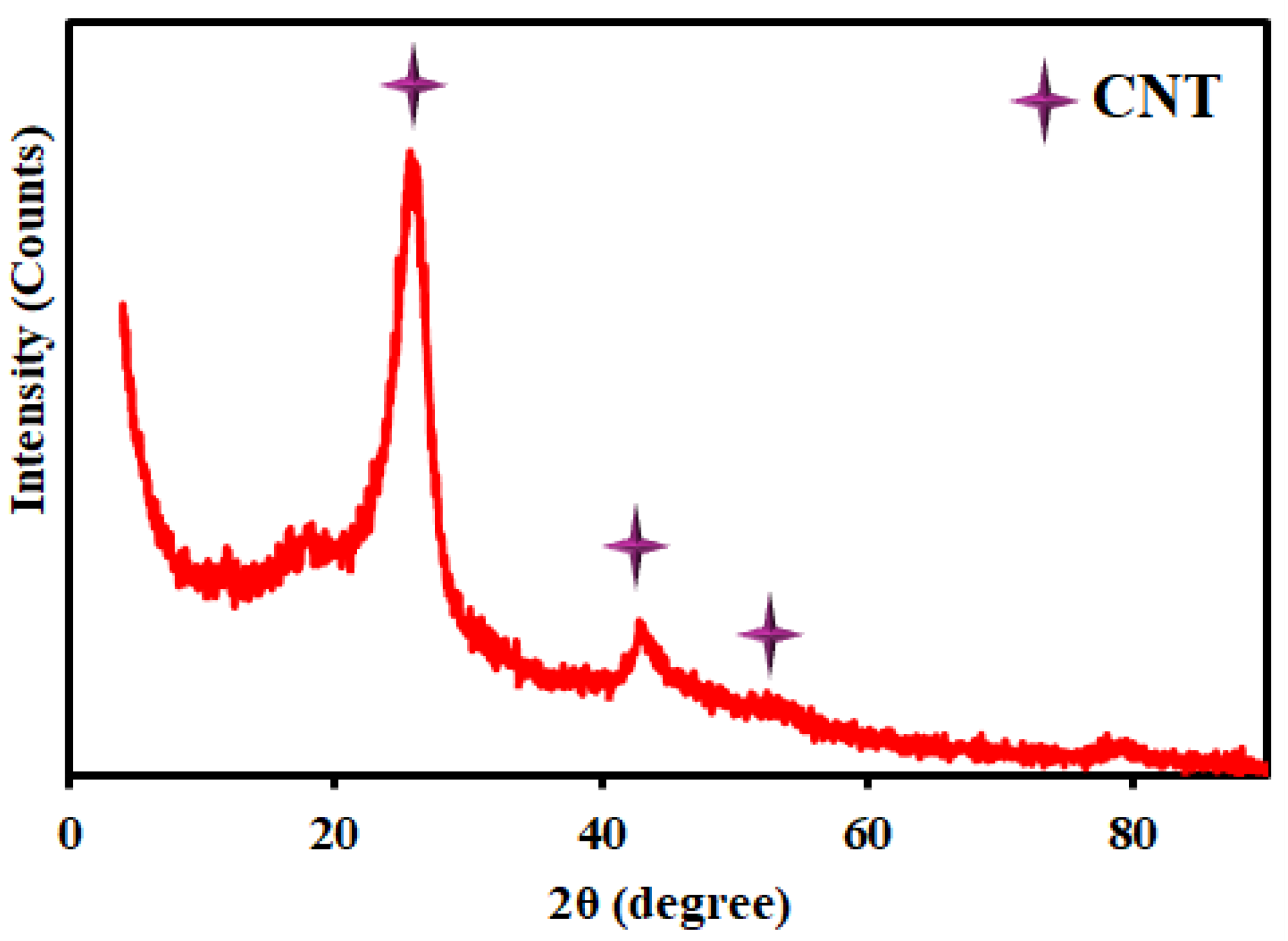
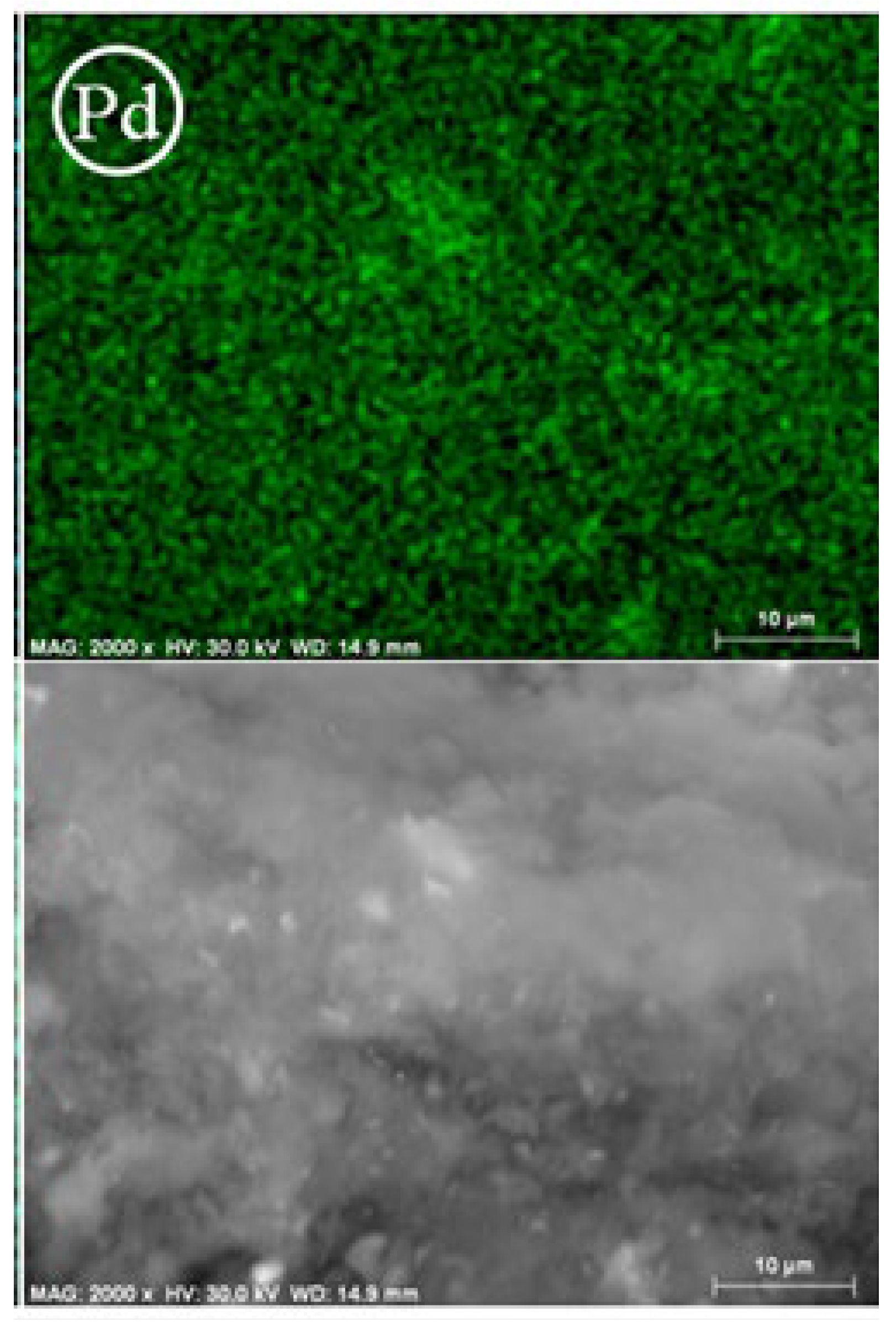
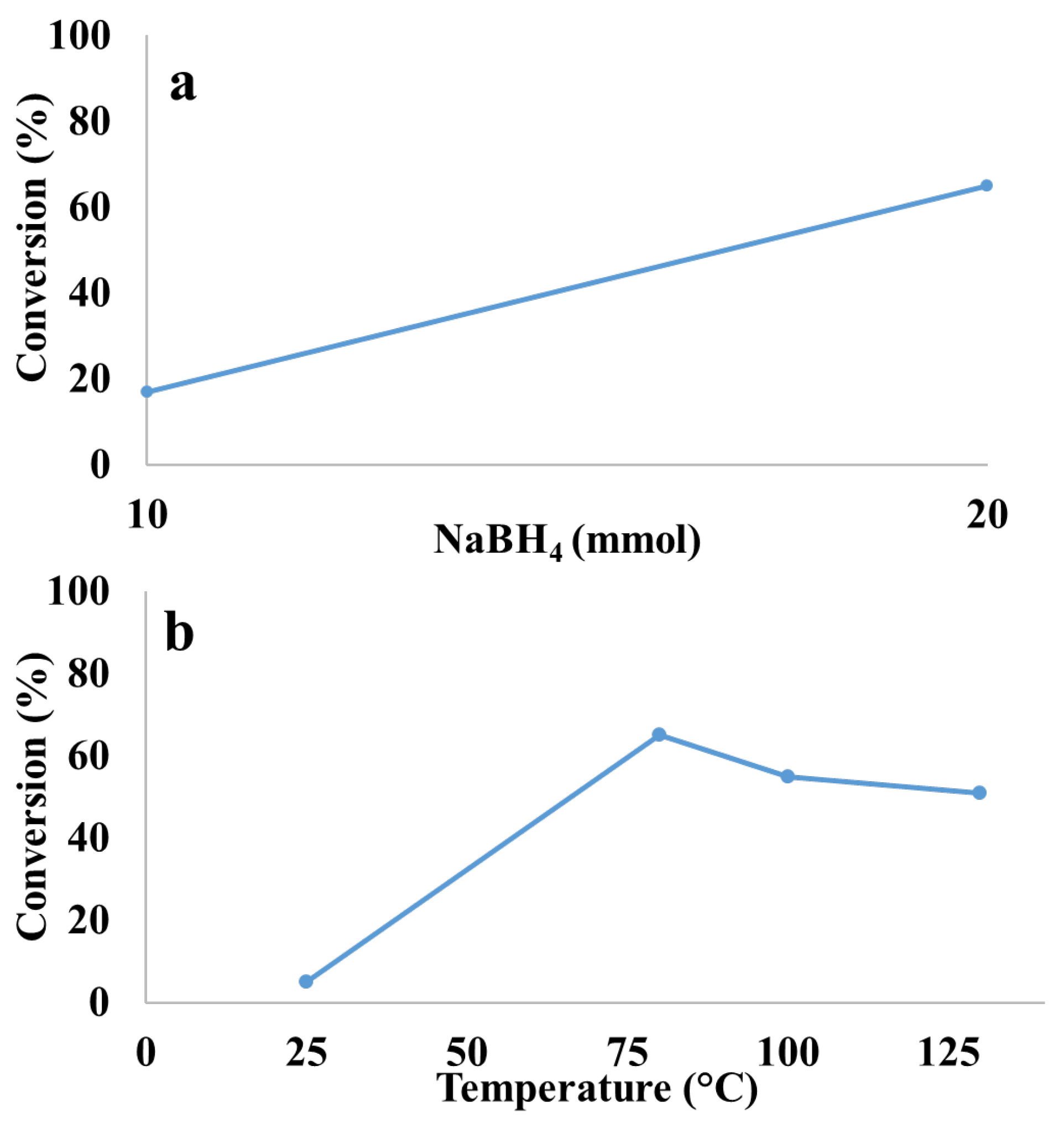
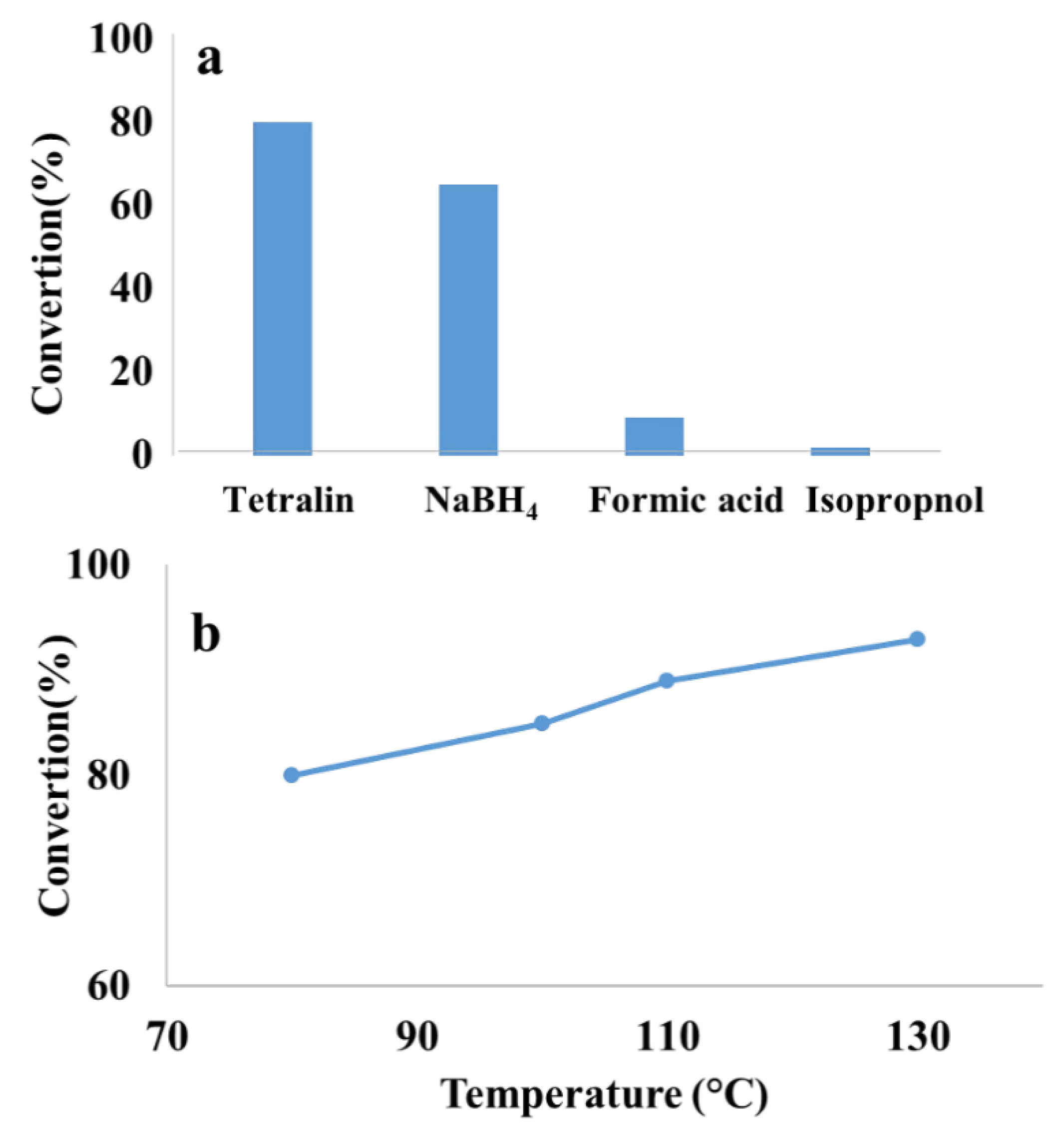
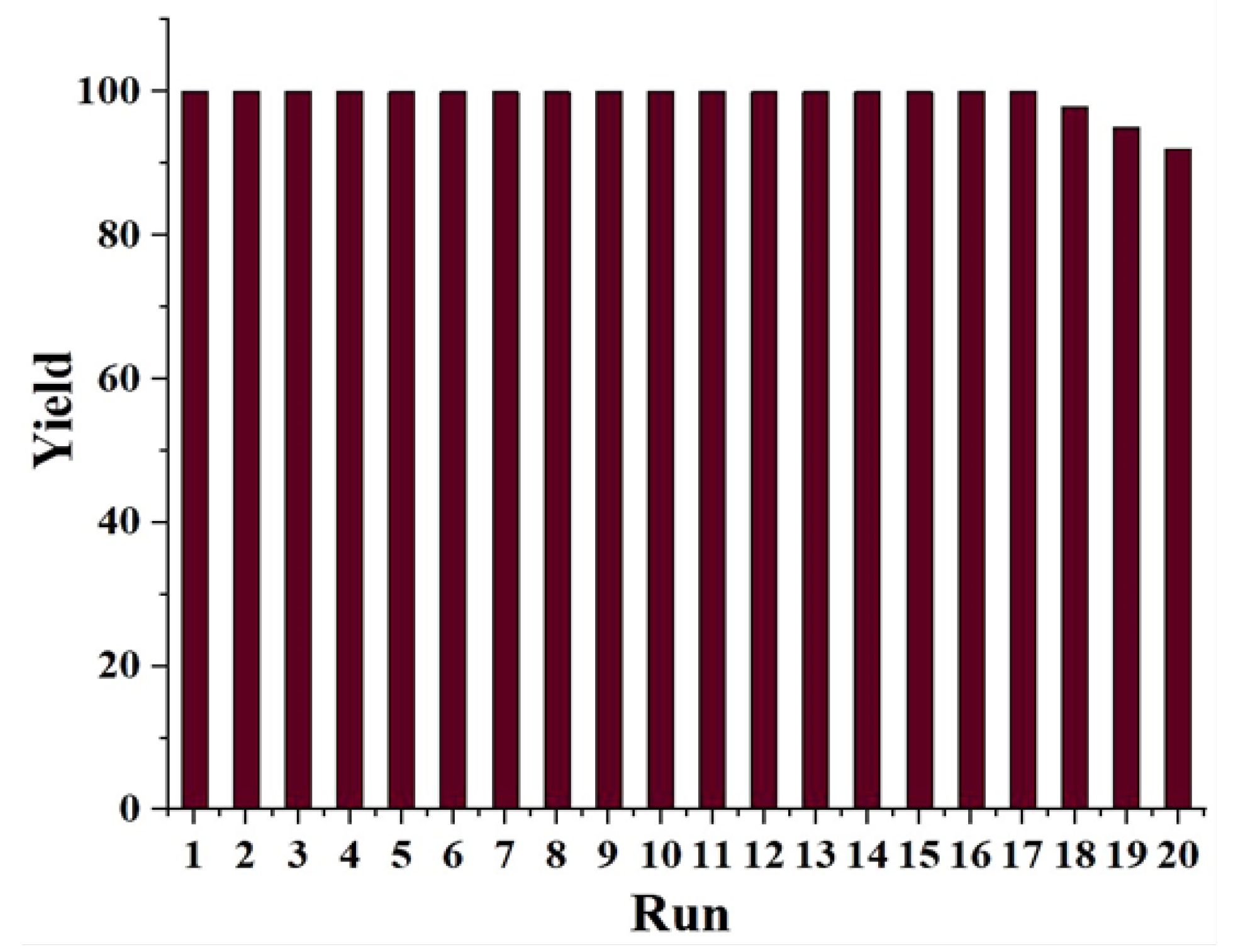
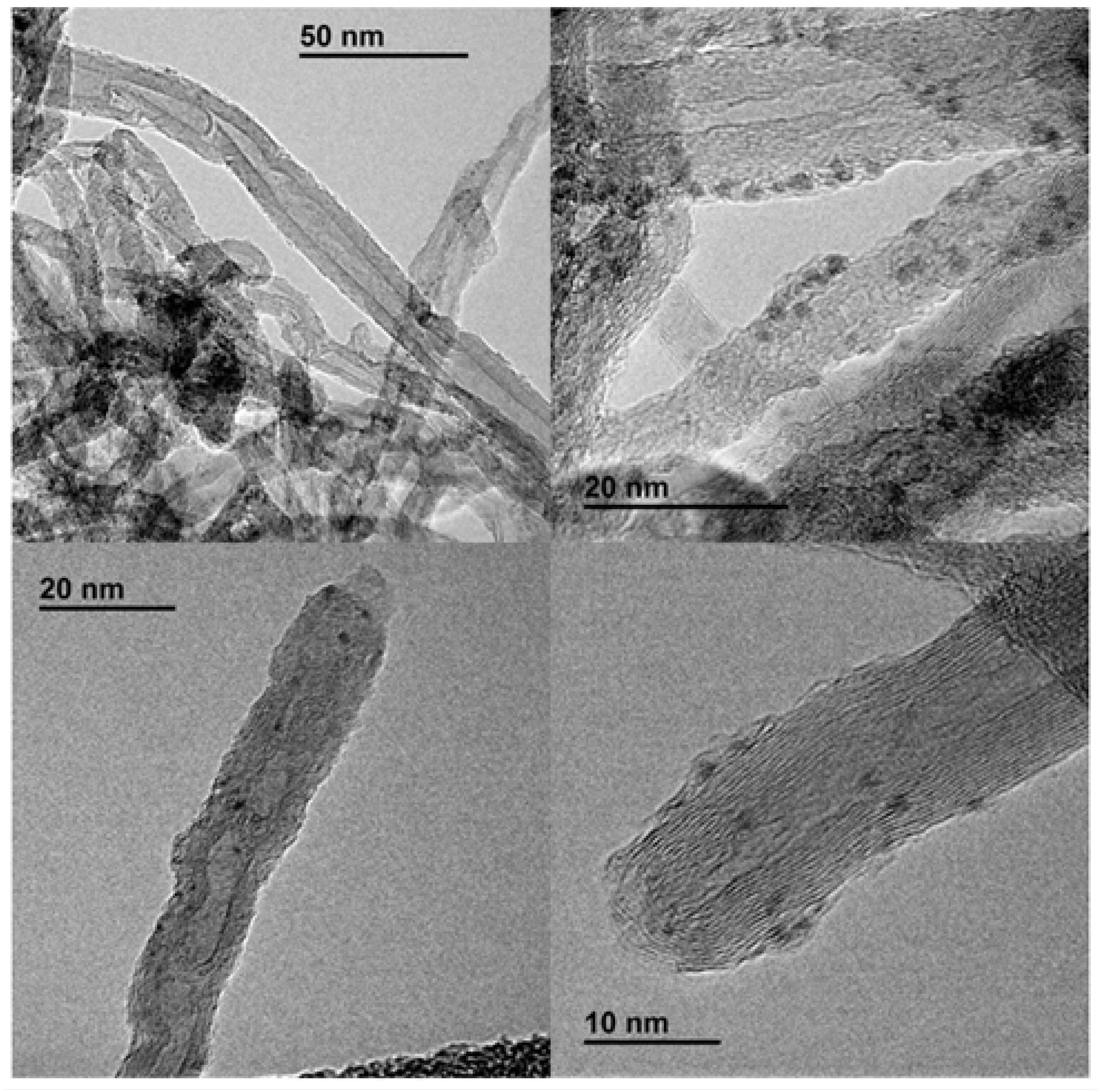
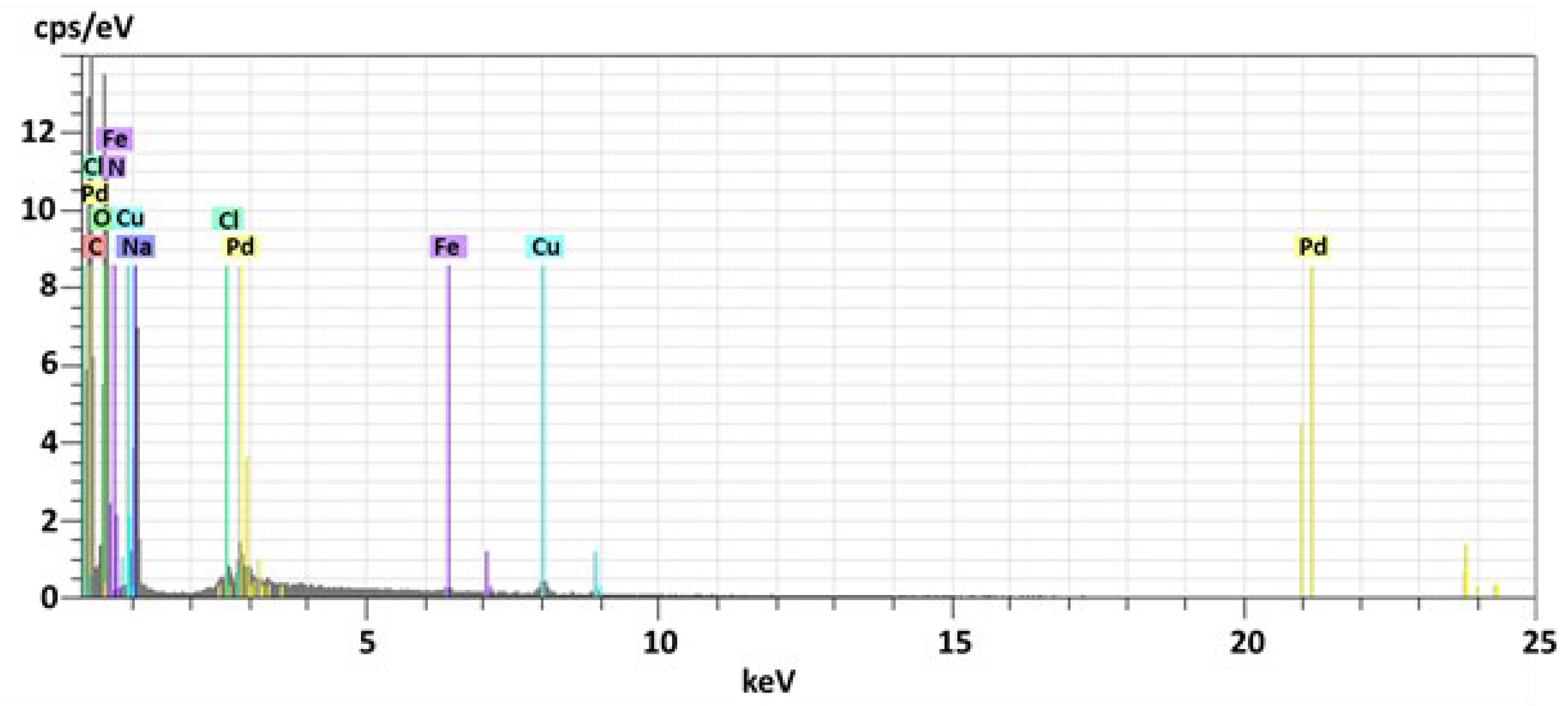
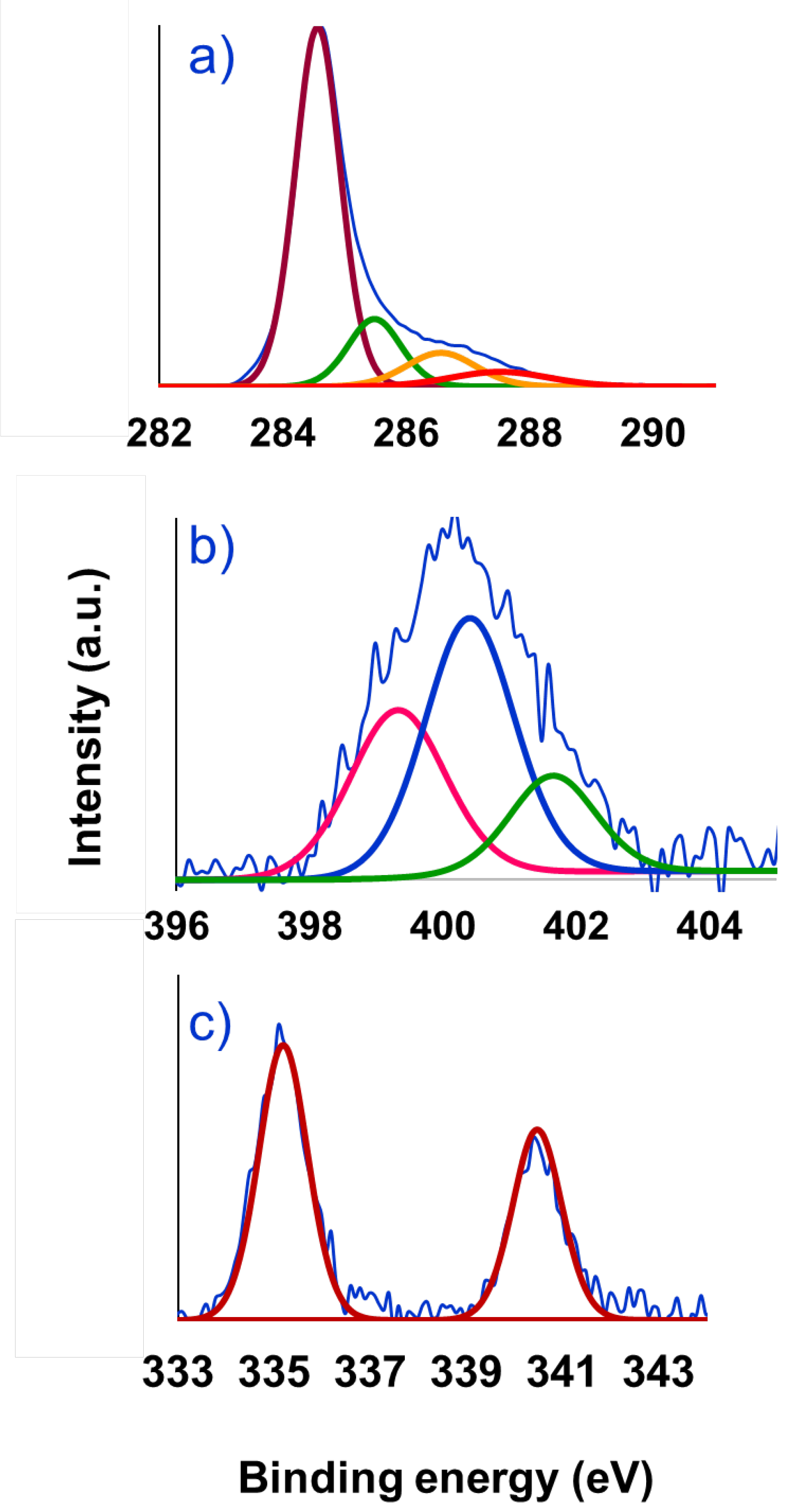
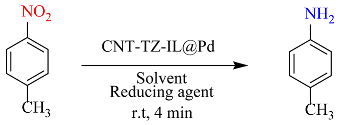
| Entry | Solvent | Reducing agent | Yield |
|---|---|---|---|
| 1 | EtOH | NaBH4 | 100 |
| 2 | H2O | NaBH4 | 99 |
| 3 | H2O: EtOH | NaBH4 | 100 |
| 4 | Ethyl acetate | NaBH4 | 10 |
| 5 | CH3CN | NaBH4 | 4 |
| 6 | DMSO | NaBH4 | 6 |
| 7 | DMF | NaBH4 | 63 |
| 8 | THF | NaBH4 | 5 |
| 9 | H2O | Formic acid | 3 |
| 10 | H2O | Ammonium format | 4 |
| 11 | H2O | Glycerol | 2 |
| 12 | H2O | Isopropyl alcohol | 7 |
| 13 | H2O | Hydrazine | 2 |
| 14 | H2O | NaBH4 | 100 |
| 15 | H2O | NaBH4 | 61 |
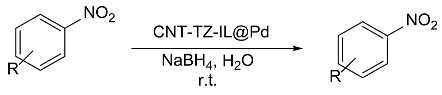
| Entry | ArNO2 | Time (min) | ArNH2 | Yield(%) |
|---|---|---|---|---|
| 1 | 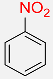 |
4 |  |
99 |
| 2 |  |
10 | 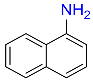 |
96 |
| 3 | 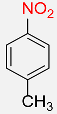 |
4 | 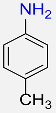 |
99 |
| 4 | 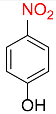 |
2 | 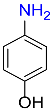 |
98 |
| 5 | 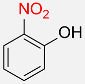 |
3 | 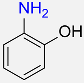 |
95 |
| 6 | 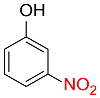 |
4 | 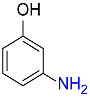 |
93 |
| 7 | 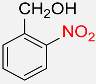 |
3 | 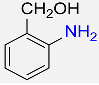 |
96 |
| 8 | 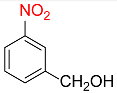 |
4 | 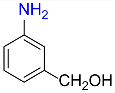 |
93 |
| 9 | 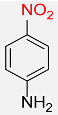 |
5 | 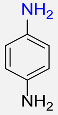 |
97 |
| 10 | 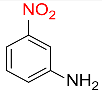 |
7 | 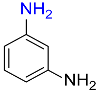 |
95 |
| 11 | 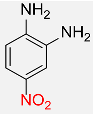 |
7 | 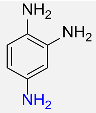 |
98 |
| 12 | 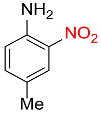 |
5 |  |
94 |
| 13 | 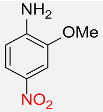 |
6 | 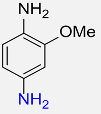 |
90 |
| 14 | 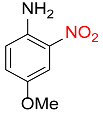 |
7 | 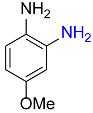 |
90 |
| 15 | 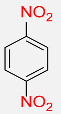 |
5 | 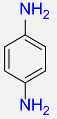 |
99b |
| 16 | 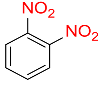 |
6 | 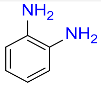 |
96b |
| 17 | 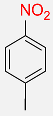 |
4 | 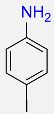 |
90c |
| 18 | 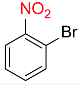 |
10 | 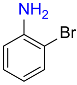 |
88 |
| 19 | 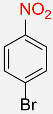 |
5 | 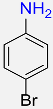 |
98 |
| 20 | 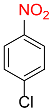 |
7 |  |
97 |
| 21 | 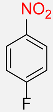 |
8 | 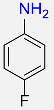 |
98 |
| 22 | 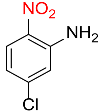 |
5 | 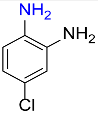 |
97 |
| 23 | 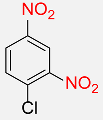 |
6 | 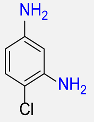 |
96b |
| 24 | 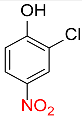 |
5 | 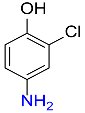 |
98 |
| 25 | 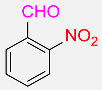 |
8 | 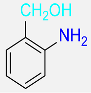 |
97b |
| 26 | 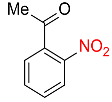 |
8 | 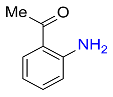 |
85 |
| 27 | 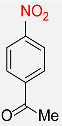 |
8 | 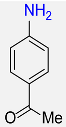 |
96 |
| 28 | 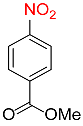 |
21 | 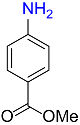 |
92 |
| 29 | 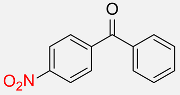 |
60 | 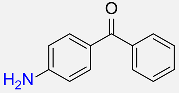 |
87f |
| 30 | 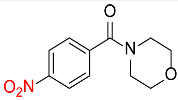 |
120 | 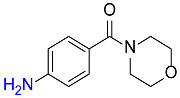 |
85c,f |
| 31 | 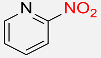 |
8 | 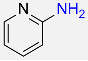 |
94 |
| 32 | 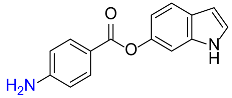 |
540 | 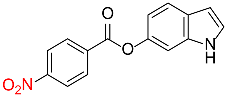 |
89d,f |
| 33 | 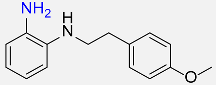 |
420 | 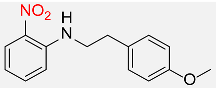 |
83d,f |
| 34 | 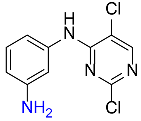 |
180 | 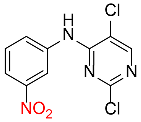 |
94d,f |
| 35 | 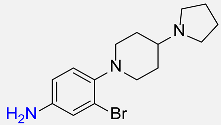 |
360 |  |
91d,f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





