Submitted:
08 May 2024
Posted:
08 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Search Strategy and Article Management Process
2.4. Study Selection
2.5. Study Quality Assessment
2.6. Data Extraction
- a)
- Authors and year of publication: The full citation details, including the year the study was published.
- b)
- Main aim: The primary objective or research question addressed by the study.
- c)
-
Methodology:
- c.1)
- Subjects: The participant characteristics, such as species (human, animal), population (athletes, healthy individuals), age, sex, etc.
- c.2)
- Study design: The type of study design (e.g. randomized controlled trial, observational, systematic review).
- c.3)
- CBD supplement: Details on the CBD intervention, including the formulation (oral, topical, etc.), dosage, and administration protocol (frequency, timing).
- d)
- Main results (CBD effects): The key findings and conclusions related to the effects of CBD, such as impact on recovery, performance, inflammation, pain, sleep, etc.
- e)
- Recommendations for future research: Any suggestions or directions for further study proposed by the authors.
3. Results
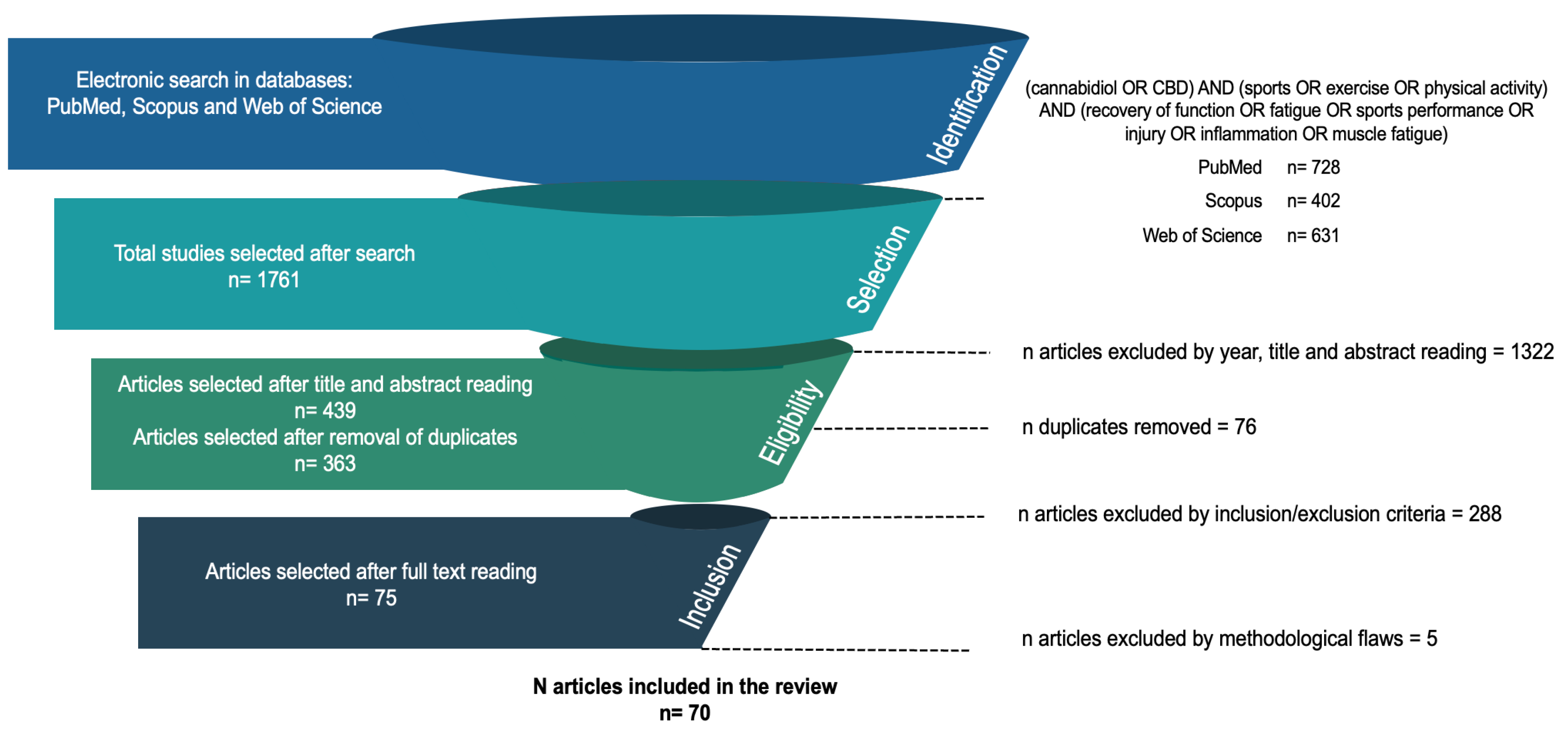
3.1. Search, Selection and Inclusion of Publications
3.2. Type of Study, Subjects and Clinical Evidence of CBD
3.3. Effects of CBD, doses and Administration Methods
| # | Authors | Aim | Methodology | Main results (CBD effects) |
Recommendations for future research |
|---|---|---|---|---|---|
| Preclinic research (animals) (n= 27) | |||||
| 1 | Zieba et al. (2019) [42] | Effect of CBD on anxiety-related behavior | n = 72 mice with acute administration of CBD at 5-20 mg/kg versus control | Better adaptation to the new anxiolytic environment | Clarify the relationship between CBD and pre-pulse inhibition. |
| 2 | Schleicher et al. (2019) [43] |
Effect of CBD on behavioral profile, motor performance, anxiety, and memory | Acute administration of CBD in mice at 20 mg/kg for six months versus control | Non-negative effects | Optimal treatment strategies. |
| 3 | Myers et al. (2019) [44] | Effects of CBD on cognition | n = 335 mice Acute CBD intake at 3-10 mg/kg, chronic at 5-20 mg/kg |
Anxiolytic | More suitable animal models. |
| 4 | Iannotti et al. (2019) [45] | Effect of cannabinoids (CBD) on inflammation, functional autophagy, and improvement of muscle function | Mice, muscular dystrophies Chronic intake of CBD at 60 mg/kg, three times per week for two weeks |
Improved structural and functional muscle enhancement and recovery | Replication and extension of findings. |
| 5 | Santiago et al. (2019) [46] | Effects of CBD on neuroplasticity, inflammation, and cognitive function | Diabetic rats Chronic CBD at 10 mg/kg, once a day for 30 days. |
Reduction of blood glucose levels, cognitive enhancer, reduction of neurodegeneration and inflammation, and attenuation of decreased levels of BDNF (neuroprotective) | Effects of low-high dose ratio of THC and CBD administration. |
| 6 | Casey et al. (2017) [47] | Effects of cannabinoids on neuropathic pain | Mice, chronic constriction injury 0.01-0.015 mg/g |
Potential in the treatment of neuropathic pain Anti-allodynic properties |
Confirm results. |
| 7 | Hayakawa et al. (2007) [48] | Effects of CBD as a neuroprotective agent. | Mouse, cerebral ischemia Dissolved at 1% |
Neuroprotection through an anti-inflammatory mechanism of the CB1 receptor | Confirm chronic effects of CBD administration on cerebral ischemia. |
| 8 | Mori et al. (2017) [49] | Effects of CBD on functional recovery after cerebral ischemic injury | Mice, bilateral common carotid artery occlusion Short-term 10 mg/kg CBD before and after the event |
Prevention of anxiety-like behavior, memory impairments, and despair-like behavior | Clarify the relationship between CBD and pre-pulse inhibition. |
| 9 | Murillo-Rodríguez et al. (2018) [50] | Effects of CBD on neurochemicals related to wakefulness | Rats, microdialysis surgeries Intravenous dose of 5-30 mg/kg |
Increases acetylcholine levels in a brain region related to wakefulness control. | Clarification of how CBD induces improvement in neurobiological processes involving acetylcholine. |
| 10 | García-Baños et al. (2021) [51] |
Effects of CBD demonstrating that Phytocannabinoid could attenuate alcohol-induced cognitive deficits | n = 6 rats. CBD (20 mg/kg ip). 10 ml alcohol or water | Counteract neuroinflammatory-induced cognitive impairments with PLAE treatment with CBD. | Further studies evaluating dose-dependent positive or adverse effects of CBD. |
| 11 | Mukhopadhyay et al. (2011) [52] | Effects of cannabidiol (CBD) on myocardial dysfunction, inflammation, oxidative/nutrient stress, cell death, and interrelated signaling pathways | Mice. Dose of 50 mg/kg dissolved in 100 ml citrate buffer pH 4.5 for 5 consecutive days. | Did not alter glucose levels. Improved myocardial dysfunction. | CBD as a potential therapeutic in the treatment of diabetic cardiovascular conditions and oxidative stress. |
| 12 | Gregorio et al. (2019) [53] | Determining whether acute CBD administration modulates DRN 5-HT neuronal activity and effect of repeated treatment with low doses of CBD on mechanical allodynia | Rats. Acute increasing intravenous doses of CBD (0.1-1.0 mg/kg). Repeated treatment with CBD (5 mg/kg/day, subcutaneously) | Repeated CBD treatment could prevent mechanical allodynia and anxiety-like behavior. | Repeating with low doses of CBD induces analgesia and reduces anxiety. |
| 13 | Bis-Humbert et al. (2021) [54] | Comparing the antidepressant-like response induced by cannabidiol | Rats. 3, 10, and 30 mg/kg | Decreased body weight. Improved despair-like behavior. Did not modulate anxiety-like behavior. | Support the idea that cannabidiol exerts antidepressant and anxiolytic effects. |
| 14 | Borys et al. (1979) [55] | Effects in mice of both acute and subacute CBD treatment on sleep time | Rats. 120 mg/kg | Significant increases in sleep. | Inhibitory action of CBD is still unknown. |
| 15 | Peres et al. (2016) [56] | CBD treatment would attenuate motor and cognitive impairments | Rats. 0.5 or 5 mg/kg | CBD improves motor and cognitive impairments. | Include CBD in the pharmacotherapy of Parkinson's disease. |
| 16 | Vuolo et al. (2015) [57] | Effects of CBD on inflammatory parameters (evaluated by cytokine levels) in an asthma model. | Rats. Dose of 5 mg/kg. | Significantly reduced cytokine levels, exhibiting anti-inflammatory effects. | Beneficial effect of CBD in an animal model of asthma. |
| 17 | Wheal et al. (2014) [58] | CBD effect decreases insulitis, inflammation, neuropathic pain, and myocardial dysfunction in preclinical models | Rats. Dose of 10 mg. | Relaxing | Improves the ability of arteries to relax through increased production of vasodilatory products. |
| 18 | Hammell et al. (2016) [59] | Cannabidiol (CBD) attenuates inflammation and pain without side effects | Rats. | Reduced joint inflammation. | Effective doses for reduce inflammation. |
| 19 | Belardo et al. (2019) [60] | Effects of CBD on neurological dysfunctions associated with TBI | Dose of 0.6 to 6.2 mg/day. | Restored behavioral alterations and partially normalized cortical biochemical changes. | CBD as a pharmacological tool to improve neurological dysfunctions caused by trauma. |
| 20 | Ceprián et al. (2017) [61] | Protective effect of CBD in a neonatal rat model of AIS. | Dissolved in hemp seed oil and tocopherol. CBD (30 μl, 10% oil). | Improved neurobehavioral function in terms of strength, hemiparesis, coordination, and sensorimotor performance. | CBD administration following middle cerebral artery occlusion (MCAO). |
| 21 | Costa et al. (2004a) [62] | Anti-inflammatory and anti-hyperalgesic effects of cannabidiol | Rats. Dose of 5 mg/kg intraperitoneally. | Anti-hyperalgesic effect. | Beneficial effect on two inflammation symptoms. |
| 22 | Costa et al. (2004b) [63] | Anti-hyperalgesic effect of CBD | Rats. Oral dose (5-40 mg/kg). | Anti-hyperalgesic effect. | The potential involvement of transient receptor potential vanilloid type 1 receptor. Could be a molecular target of anti-hyperalgesic action. |
| 23 | Costa et al. (2007) [64] | Therapeutic potential in neuropathic pain. | Rats. Oral dose of 10 mg kg-1 (5 ml kg-1). | Anti-inflammatory and immunomodulatory effects. | Therapeutic use for pain. |
| 24 | Ignatowska-Jankowska et al. (2011) [65] | Effects of repeated CBD administration on body weight gain in rats | Rats. at doses of 2.5 and 5 mg/kg/day | Ability to modulate weight gain. | Further investigation into the regulation of body weight. |
| 25 | Murillo-Rodríguez, et al. (2006) [66] | Effects of CBD on sleep | Rats. Doses of 10mg/5mg intracerebroventricularly | Modulates wakefulness. | Addressing vanilloid receptors. |
| 26 | Schiavon et al. (2014) [67] | Cannabidiol, one of the main non-psychoactive components | Mice. Doses of 3, 10, and 30 mg/kg | Protective effect of CBD on neuronal death. | The mechanisms underlying the neuroprotective effects of CBD. |
| 27 | Wang et al. (2017) [68] | Effects of CBD on alcohol-induced chronic and compulsive feeding-induced liver injury. | Mice. Doses: ethanol (5 g/kg body weight) and 5 or 10 mg/kg/day of CBD. | Antioxidant, cytoprotective, and anti-inflammatory properties. Attenuates chronic liver injury and ethanol-induced steatosis. | Therapeutic potential in liver diseases associated with inflammation, oxidative stress, metabolic dysregulation, and steatosis. |
| Clinical studies in humans (n=19) | |||||
| 28 | Zuardi et al. (1993a) [69] | Explore the effect of CBD and aspirin in stressful situations. | n = 40 (♂ = 18, ♀ = 22), CBD single healthy doce at 300 mg dissolved in corn oil (100 mg / ml) | Anxiolytic | Realize confirmatory analysis of results |
| 29 | Zuardi et al. (1993b) [70] | Explore the effects of CBD on plasma prolactin, growth hormone, and cortisol. | n = 11, healthy Single dose of CBD at 300 mg and 400 mg |
Sedative and anti-inflammatory | Realize confirmatory analysis of results |
| 30 | Martin et al. (2019) [71] | Long-term effects of CBD on cognitive function. | n = 27, epilepsy Annual CBD intake of 36,5 mg / kg / día |
No effects on cognitive function | Realize new studies with Randomized, placebo-controlled, and larger samples. |
| 31 | Allendorfer et al. (2019) [72] | Effects of CBD oral solution on attention. | n = 22 epilepsy 25 mg / kg / d for at least 2 weeks |
No effect on mood | Controlled trials and examination of long-term effects, follow-ups after treatment. |
| 32 | Santos et al. (2021) [73] | Reduce tremors in patients with ET. | n=19 ET patients. Oral dose of CBD (300 mg). | A single dose had no effect on upper limb tremors | Chronic treatment with CBD. |
| 33 | Kasper et al. (2020) [74] | Prevalence of CBD use in professional rugby league and union players. | n=517 rugby players. Survey. | CBD use showed improvement in recovery/pain (80%) and sleep (78%), and 68% of players reported a perceived benefit | Need to explore claims regarding pain and sleep. |
| 34 | Cochrane-Snyman et al. (2021) [75] | Effect of CBD oil on perceived muscle pain, inflammation, and strength performance. | n=13 men Dose: 150 mg. |
They did not support that supplementation with CBD oil would have an effect on muscle damage and inflammation after an ECC protocol. | Investigate broader ranges of CBD dosage and scheduling in trained and untrained men and women. |
| 35 | Neubauer et al. (2018) [76] | Evaluate the effectiveness of complementary therapy with CBD. | Patients with epilepsy. Dose of less than 8 mg/kg/day. | Less intense seizures, shorter seizure duration, shorter recovery time, and other positive side effects of CB treatment. | Potential benefits as adjunctive therapy. |
| 36 | Lopez et al. (2020) [77] | Effects of a CBD oil extract on stress resilience, perceived recovery, mood, affect, and body composition. | 65 men and women. Dose of 60 mg. | Improved HDL cholesterol, supported psychometric measures of sleep, stress response, and perceived vitality. | Supplementary use. |
| 37 | Hatchett et al. (2020) [78] | Determine the influence of cannabidiol oil in attenuating delayed onset muscle soreness. | N=23. Dose of 16.67 mg. | Muscle recovery | Investigate the role of CBD dosage level, nutrition, sleep, exercise type, and other factors on CBD's ability to attenuate exercise-induced muscle damage effects and aid in the recovery process. |
| 38 | Sahinovic et al. (2022) [79] | Effects of acute CBD treatment on physiological and psychological responses to aerobic exercise | n=9, trained males (57.4±4 ml/kg/min) CBD (300 mg) vs Placebo 1.5h before exercise |
CBD appeared to increase VO2, ratings of pleasure and blood lactate compared to placebo. | Larger studies are required to confirm and better understand these preliminary findings. |
| 39 | Alhamoruni et al. (2012) [80] | Determine if cannabinoids modulate increased permeability associated with inflammation in vitro. | Dose of 10 ng · mL -1. | Recovery of increased permeability | Locally produced endocannabinoids, acting through CB1 receptors, play a role in mediating changes in permeability with inflammation. |
| 40 | Arndt and de Wit (2017) [81] | Effects of CBD on responses to negative emotional stimuli as a model for its potential anxiety-reducing effects. | n=38. Oral CBD 300, 600, and 900 mg. |
CBD did not produce detectable subjective effects or alterations in mood or anxiety. | Further research on the behavioral and neural mechanisms of CBD and |
| 41 | Bergamaschi et al. (2011) [82] | Effects of a simulated public speaking test. | N=24 CBD dose of 600 mg |
Reduced anxiety, cognitive impairment, and discomfort in speech performance, and decreased anticipatory speech alertness. | More research to determine the precise mechanisms of action of CBD in different anxiety disorders. |
| 42 | Birnbaum et al. (2019) [83] | Evaluate the pharmacokinetics of an oral cannabidiol capsule with and without food. | n=8. Dose of 99% pure CBD capsules on an empty stomach as well as under fed conditions. | More precise pharmacokinetic parameters | Use of CBD capsules |
| 43 | Jadoon et al. (2017) [84] | Investigate if CBD reduces blood pressure in humans. | n=9. Dose of 600 mg. | Reduces resting blood pressure and attenuates blood pressure increase during stress | Research to establish if CBD has a role in the treatment of cardiovascular disorders. |
| 44 | Kraft et al. (2008) [85] | Effects of oral cannabis extract in two different human models of acute inflammatory pain and hyperalgesia. | n=18 women. Oral capsule administration. | Hyperalgesic | Future clinical studies in patients with chronic pain |
| 45 | Linares et al. (2018) [86] | Effect of a clinically anxiolytic dose of CBD on the sleep-wake cycle. | n=27. Dose of 300 mg. | Does not interfere with the sleep cycle | Address the effects of CBD on the sleep-wake cycle in patient populations |
| 46 | Masataka (2019) [87] | Evaluate the efficacy of CBD treatment for adolescents with social anxiety disorder. | n=17. Dose of 300 mg. | Reduced anxiety | Useful option for treating social anxiety. |
| 47 | Shannon and Opila-Lehman (2016) [88] | Evidence that CBD is effective as a safe alternative treatment to traditional psychiatric medications for reducing anxiety and insomnia. | n=1, 10-year-old girl. CBD supplements (25 mg) at bedtime, and 6 mg to 12 mg of sublingual CBD spray administered during the day |
Reduces anxiety and improves sleep | Study long-term effects. |
| 48 | Isenmann et al. (2021) [89] | Effect of CBD after resistance training on performance and muscle damage | n=21 CBD after exercise (60mg on 250 mL water) vs Placebo |
Small significant effects on muscle damage and recovery after 72 h in CBD group vs Placebo | More data are required for clearer statements concerning potential pro-regenerative effects of CBD |
| 49 | Crossland et al. (2022) [90] | Determine that CBD is effective to reduce inflammation and enhances performance for strenuous eccentric exercise | 27 female (18-26 years-old) Isolate CBD (5 mg/kg; 3 times -2h, 0h and +10h) vs Placebo |
No effect for inflammation, muscle damage and subjective fatigue | Study of varying CBD supplements to determine if other phytochemicals in cannabis plant prove effective for recovery |
| Reviews and meta-analysis (n=21) | |||||
| 50 | McCartney et al. (2020) [32] | Present preliminary preclinical laboratory animal data in humans, non-athletes. | Narrative review | Anti-inflammatory Neuroprotector Analgesic Anxyolitic |
Rigorous and controlled studies in humans |
| 51 | Burggren et al. (2019) [91] | Review the effects of CBD on brain structure, function, and cognition. | Literature review | Insufficient evidence | Short and long-term consequences Effect in older adults Efficacy and safety of existing products |
| 52 | Burstein (2015) [30] | Review the effects of CBD on inflammation. | Literature review | Anti-inflammatory Decrease of secondary inflammatory effects |
Human trials with clinical application Advantages of CBD over other cannabinoids Synthetic analogs with greater potency than CBD |
| 53 | Hill et al. (2012) [92] | Effects of phyto-cannabinoids (CBD) in preclinical models of central nervous system disease. | Literature review | Neuroprotective anticonvulsant (modulates immune cell activity and limits oxidative stress) |
Long-term, double-blind, placebo-controlled trials are needed. Participants with different affective disorders. |
| 54 | Booz (2011) [93] | Effects of CBD on inflammation and oxidative stress. | Narrative review | Anti-inflammatory Antioxidant |
More details are needed on how CBD targets inflammatory signaling. Test studies on the therapeutic utility of CBD. |
| 55 | Lorenzetti et al. (2016) [94] | Recent findings from human structural neuroimaging research. | Systematic review | CBD protects against these harmful effects in the hippocampus, prefrontal cortex, amygdala, and cerebellum. | Urgent development of consensus-based guidelines to quantify cannabis consumption and exposure in human studies. |
| 56 | Rojas-Valverde (2021) [33] | Explore the potential role of CBD in sports recovery. | Narrative review | CBD has anti-inflammatory, antioxidant, anxiolytic properties, and improves sleep quality. | Specific investigations are required to determine if cannabis can provide indirect benefits to athletes. |
| 57 | Kramer et al. (2020) [95] | Effects of chronic cannabis consumption on physiological parameters of sports performance. | Systematic review | It did not have a significant effect on athletic performance. Resting heart rate was the only physiological measure that differed. | Specific investigations are required to determine if cannabis can provide indirect benefits to athletes. |
| 58 | Singh and Neary (2020) [96] | Neuroprotective effects of cannabinoids, specifically the phytocannabinoid CBD, after a traumatic brain injury (TBI). | Systematic review | Enhancing neuroprotection by reducing inflammation. | Influence the blood-brain barrier, brain-derived neurotrophic factors, cognitive ability, brain vasculature, cardiovascular physiology, and neurogenesis. |
| 59 | Reillo (2019) [97] | Examine sport-related traumatic brain injury and the preventive and therapeutic use of cannabidiol among athletes. | Systematic review | Efficacy of preventive and therapeutic administration of cannabidiol (CBD) in head injuries. | Recommended as a preventive and therapeutic intervention in the treatment of traumatic brain injury. |
| 60 | Fine and Rosenfeld (2013) [98] | Link the endocannabinoid system and phytocannabinoids to their potentially therapeutic role in chronic pain management. | Systematic review | Analgesic for chronic pain. | Administered orally. |
| 61 | Sholler et al. (2020) [99] | Synthesize the efficacy of CBD as a therapeutic agent. | Systematic review | Efficacy of CBD as a therapeutic for various medical conditions, including epilepsy, anxiety, pain/inflammation, schizophrenia, and substance use disorders. | Rigorous and controlled evidence of the therapeutic efficacy of CBD is lacking. |
| 62 | Lowin et al. (2019) [100] | General effects of cannabinoids on inflammation. | Systematic review | Anti-inflammatory effects. | Targeting the right receptors in the right place. |
| 63 | Stanley et al. (2013) [101] | Establish whether the cardiovascular system is a potential therapeutic target for CBD. | Systematic review | CBD reduces the cardiovascular response to stress models. | More evidence of the positive role of CBD in the heart. |
| 64 | Bruni et al. (2018) [102] | Synergistic effect in pain treatment. | Systematic review | Therapeutic, anti-inflammatory. | Further assessment of nanotechnology systems. |
| 65 | Zurier and Burstein (2016) [103] | Ability to facilitate inflammation resolution. | Systematic review | Anti-inflammatory Reduction of fibrosis |
Cannabinoids becoming safe and effective anti-inflammatory medications. |
| 66 | Vuolo et al. (2019) [104] | CBD in respiratory pathways remodeling. | Systematic review | Anti-inflammatory Reduction of asthma |
More details on airway remodeling. |
| 67 | Crippa et al.(2011) [105] | Investigate generalized social anxiety disorder (GSAD) using functional neuroimaging. | Preliminary report | Reduction of anxiety | Effects on activity in the limbic and paralimbic areas of the brain. |
| 68 | Huestis et al. (2011) [106] | Support the status of cannabis regarding the Prohibited List. | Systematic review | Positive effect on performance | Further research on the development of tolerance after long-term frequent exposure. |
| 69 | Lattanzi et al. (2018) [107] | Efficacy and safety of CBD as an adjunctive treatment in patients with epilepsy. | Systematic review and metanalysis | Reduction of seizures frequency | Does not produce euphoric or intrusive side effects. |
| 70 | Ware et al. (2018) [108] | Identify and highlight challenges in interpreting information regarding elite athletic performance and identify important areas of research that need to be addressed. | Literature review | Promising effect for the treatment of chronic pain. | There is no evidence that cannabis consumption is a performance-enhancing drug. |
4. Discussion
4.1. Preclinic Research (Animals)
4.2. Noninflammatory and Antioxidant Effects in Humans
4.3. Pain and Discomfort in Humans
4.4. Sleep Quality
4.5. Cognitive and Psychological Effects
4.6. Study Limitations
5. Conclusions
5.1. Practical Applications and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rojas-Valverde, D.; Gutiérrez-Vargas, R.; Rodríguez-Montero, A.; Pereira, L.A.; Loturco, I.; Martín-Rodríguez, S. Reduced Muscle Contractile Function in Elite Young Soccer Players after a Short-Congested Fixture Period. Proc. Inst. Mech. Eng. Part P J. Sports Eng. Technol. 2018, 175433711881795. [CrossRef]
- Martínez-Guardado, I.; Rojas-Valverde, D.; Gutiérrez-Vargas, R.; Ugalde Ramírez, A.; Gutiérrez-Vargas, J.C.; Sánchez-Ureña, B. Intermittent Pneumatic Compression and Cold Water Immersion Effects on Physiological and Perceptual Recovery During Multi-Sports International Championship. J. Funct. Morphol. Kinesiol. 2020, 5, 45. [CrossRef]
- Ament, W.; Verkerke, G.J. Exercise and Fatigue. Sports Med. Auckl. NZ 2009, 39, 389–422. [CrossRef]
- Metsios, G.S.; Moe, R.H.; Kitas, G.D. Exercise and Inflammation. Best Pract. Res. Clin. Rheumatol. 2020, 101504. [CrossRef]
- Kreher, J.B.; Schwartz, J.B. Overtraining Syndrome: A Practical Guide. Sports Health Multidiscip. Approach 2012, 4, 128–138. [CrossRef]
- Haugen, T.; Seiler, S.; Sandbakk, Ø.; Tønnessen, E. The Training and Development of Elite Sprint Performance: An Integration of Scientific and Best Practice Literature. Sports Med. - Open 2019, 5, 44. [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [CrossRef]
- Merry, T.L.; Ristow, M. Do Antioxidant Supplements Interfere with Skeletal Muscle Adaptation to Exercise Training? J. Physiol. 2016, 594, 5135–5147. [CrossRef]
- Rojas-Valverde, D.; Montoya-Rodríguez, J.; Azofeifa-Mora, C.; Sanchez-Urena, B. Effectiveness of Beetroot Juice Derived Nitrates Supplementation on Fatigue Resistance during Repeated-Sprints: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2020, 1–12. [CrossRef]
- Campos, A.C.; Moreira, F.A.; Gomes, F.V.; Del Bel, E.A.; Guimarães, F.S. Multiple Mechanisms Involved in the Large-Spectrum Therapeutic Potential of Cannabidiol in Psychiatric Disorders. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3364–3378. [CrossRef]
- Lim, S.Y.; Sharan, S.; Woo, S. Model-Based Analysis of Cannabidiol Dose-Exposure Relationship and Bioavailability. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 291–300. [CrossRef]
- WHO Cannabidiol (CBD): World Health Organisation Expert Committe on Drug Dependence. Thyrty-Ninth Meet. 2017.
- Stout, S.M.; Cimino, N.M. Exogenous Cannabinoids as Substrates, Inhibitors, and Inducers of Human Drug Metabolizing Enzymes: A Systematic Review. Drug Metab. Rev. 2014, 46, 86–95. [CrossRef]
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [CrossRef]
- Millar, S.A.; Stone, N.L.; Bellman, Z.D.; Yates, A.S.; England, T.J.; O’Sullivan, S.E. A Systematic Review of Cannabidiol Dosing in Clinical Populations. Br. J. Clin. Pharmacol. 2019, 85, 1888–1900. [CrossRef]
- Cunha, J.M.; Carlini, E.A.; Pereira, A.E.; Ramos, O.L.; Pimentel, C.; Gagliardi, R.; Sanvito, W.L.; Lander, N.; Mechoulam, R. Chronic Administration of Cannabidiol to Healthy Volunteers and Epileptic Patients. Pharmacology 1980, 21, 175–185. [CrossRef]
- Zuardi, A.; Crippa, J.; Dursun, S.; Morais, S.; Vilela, J.; Sanches, R.; Hallak, J. Cannabidiol Was Ineffective for Manic Episode of Bipolar Affective Disorder. J. Psychopharmacol. Oxf. Engl. 2010, 24, 135–137. [CrossRef]
- Docter, S.; Khan, M.; Gohal, C.; Ravi, B.; Bhandari, M.; Gandhi, R.; Leroux, T. Cannabis Use and Sport: A Systematic Review. Sports Health 2020, 12, 189–199. [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2019, 5, 12–31. [CrossRef]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Nisio, M.D.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-Analysis. JAMA 2015, 313, 2456–2473. [CrossRef]
- Pinto, J.V.; Saraf, G.; Frysch, C.; Vigo, D.; Keramatian, K.; Chakrabarty, T.; Lam, R.W.; Kauer-Sant’Anna, M.; Yatham, L.N. Cannabidiol as a Treatment for Mood Disorders: A Systematic Review. Can. J. Psychiatry 2020, 65, 213–227. [CrossRef]
- Higgins, T.R.; Greene, D.A.; Baker, M.K. Effects of Cold Water Immersion and Contrast Water Therapy for Recovery From Team Sport: A Systematic Review and Meta-Analysis. J. Strength Cond. Res. 2017, 31, 1443–1460. [CrossRef]
- Rojas-Valverde, D.; Sánchez-Ureña, B.; Crowe, J.; Timón, R.; Olcina, G.J. Exertional Rhabdomyolysis and Acute Kidney Injury in Endurance Sports: A Systematic Review. Eur. J. Sport Sci. 2020, 0, 1–28. [CrossRef]
- McPartland, J.M.; Duncan, M.; Marzo, V.D.; Pertwee, R.G. Are Cannabidiol and Δ9-Tetrahydrocannabivarin Negative Modulators of the Endocannabinoid System? A Systematic Review. Br. J. Pharmacol. 2015, 172, 737–753. [CrossRef]
- Carek, P.J.; Laibstain, S.E.; Carek, S.M. Exercise for the Treatment of Depression and Anxiety. Int. J. Psychiatry Med. 2011, 41, 15–28. [CrossRef]
- Tantimonaco, M.; Ceci, R.; Sabatini, S.; Catani, M.V.; Rossi, A.; Gasperi, V.; Maccarrone, M. Physical Activity and the Endocannabinoid System: An Overview. Cell. Mol. Life Sci. 2014, 71, 2681–2698. [CrossRef]
- Cuñetti, L.; Manzo, L.; Peyraube, R.; Arnaiz, J.; Curi, L.; Orihuela, S. Chronic Pain Treatment With Cannabidiol in Kidney Transplant Patients in Uruguay. Transplant. Proc. 2018, 50, 461–464. [CrossRef]
- Shannon, S.; Lewis, N.; Lee, H.; Hughes, S. Cannabidiol in Anxiety and Sleep: A Large Case Series. Perm. J. 2019, 23. [CrossRef]
- Burstein, S. Cannabidiol (CBD) and Its Analogs: A Review of Their Effects on Inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [CrossRef]
- Zarrabi, A.J.; Frediani, J.K.; Levy, J.M. The State of Cannabis Research Legislation in 2020. N. Engl. J. Med. 2020, 382, 1876–1877. [CrossRef]
- McCartney, D.; Benson, M.J.; Desbrow, B.; Irwin, C.; Suraev, A.; McGregor, I.S. Cannabidiol and Sports Performance: A Narrative Review of Relevant Evidence and Recommendations for Future Research. Sports Med. - Open 2020, 6, 27. [CrossRef]
- Rojas-Valverde, D. Potential Role of Cannabidiol (CBD) on Sport Recovery: A Narrative Review. Front. Physiol. 2021, 12. [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906.
- Whittemore, R.; Knafl, K. The Integrative Review: Updated Methodology. J. Adv. Nurs. 2005, 52, 546–553. [CrossRef]
- A Step-by-Step Guide to Conducting an Integrative Review; Toronto, C.E., Remington, R., Eds.; Springer International Publishing: Cham, 2020; ISBN 978-3-030-37503-4.
- Souza, M.T. de; Silva, M.D. da; Carvalho, R. de Integrative Review: What Is It? How to Do It? Einstein São Paulo 2010, 8, 102–106.
- Medina, J.M.; McKeon, P.O.; Hertel, J. Rating the Levels of Evidence in Sports-Medicine Research. Int. J. Athl. Ther. Train. 2006, 11, 38–41. [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. BMC Vet. Res. 2020, 16, 242. [CrossRef]
- Crocker, T.F.; Lam, N.; Jordão, M.; Brundle, C.; Prescott, M.; Forster, A.; Ensor, J.; Gladman, J.; Clegg, A. Risk-of-Bias Assessment Using Cochrane’s Revised Tool for Randomized Trials (RoB 2) Was Useful but Challenging and Resource-Intensive: Observations from a Systematic Review. J. Clin. Epidemiol. 2023, 161, 39–45. [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [CrossRef]
- Zieba, J.; Sinclair, D.; Sebree, T.; Bonn-Miller, M.; Gutterman, D.; Siegel, S.; Karl, T. Cannabidiol (CBD) Reduces Anxiety-Related Behavior in Mice via an FMRP-Independent Mechanism. Pharmacol. Biochem. Behav. 2019, 181, 93–100. [CrossRef]
- Schleicher, E.M.; Ott, F.W.; Mueller, M.; Silcher, B.; Sichler, M.E.; Loew, M.J.; Wagner, J.M.; Bouter, Y. Prolonged Cannabidiol Treatment Lacks on Detrimental Effects on Memory, Motor Performance and Anxiety in C57BL/6J Mice. Front. Behav. Neurosci. 2019, 13, 94. [CrossRef]
- Myers, A.M.; Siegele, P.B.; Foss, J.D.; Tuma, R.F.; Ward, S.J. Single and Combined Effects of Plant-Derived and Synthetic Cannabinoids on Cognition and Cannabinoid-Associated Withdrawal Signs in Mice. Br. J. Pharmacol. 2019, 176, 1552–1567. [CrossRef]
- Iannotti, F.A.; Pagano, E.; Moriello, A.S.; Alvino, F.G.; Sorrentino, N.C.; D’Orsi, L.; Gazzerro, E.; Capasso, R.; De Leonibus, E.; De Petrocellis, L.; et al. Effects of Non-Euphoric Plant Cannabinoids on Muscle Quality and Performance of Dystrophic Mdx Mice. Br. J. Pharmacol. 2019, 176, 1568–1584. [CrossRef]
- Santiago, A.N.; Mori, M.A.; Guimaraes, F.S.; Milani, H.; Weffort de Oliveira, R.M. Effects of Cannabidiol on Diabetes Outcomes and Chronic Cerebral Hypoperfusion Comorbidities in Middle-Aged Rats. Neurotox. Res. 2019, 35, 463–474. [CrossRef]
- Casey, S.L.; Atwal, N.; Vaughan, C.W. Cannabis Constituent Synergy in a Mouse Neuropathic Pain Model. Pain 2017, 158, 2452–2460. [CrossRef]
- Hayakawa, K.; Mishima, K.; Nozako, M.; Hazekawa, M.; Irie, K.; Fujioka, M.; Orito, K.; Abe, K.; Hasebe, N.; Egashira, N.; et al. Delayed Treatment with Cannabidiol Has a Cerebroprotective Action via a Cannabinoid Receptor-Independent Myeloperoxidase-Inhibiting Mechanism. J. Neurochem. 2007, 102, 1488–1496. [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Cannabidiol Reduces Neuroinflammation and Promotes Neuroplasticity and Functional Recovery after Brain Ischemia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [CrossRef]
- Murillo-Rodríguez, E.; Arankowsky-Sandoval, G.; Rocha, N.B.; Peniche-Amante, R.; Veras, A.B.; Machado, S.; Budde, H. Systemic Injections of Cannabidiol Enhance Acetylcholine Levels from Basal Forebrain in Rats. Neurochem. Res. 2018, 43, 1511–1518. [CrossRef]
- García-Baos, A.; Puig-Reyne, X.; García-Algar, Ó.; Valverde, O. Cannabidiol Attenuates Cognitive Deficits and Neuroinflammation Induced by Early Alcohol Exposure in a Mice Model. Biomed. Pharmacother. Biomedecine Pharmacother. 2021, 141, 111813. [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Horváth, B. Cannabidiol Protects against Hepatic Ischemia/Reperfusion Injury by Attenuating Inflammatory Signaling and Response, Oxidative/Nitrative Stress, and Cell Death. Free Radic Biol Med 2011, 50.
- Gregorio, D.; McLaughlin, R.; Posa, L. Cannabidiol Modulates Serotonergic Transmission and Reverses Both Allodynia and Anxiety-like Behavior in a Model of Neuropathic Pain. Pain. 2019, 160.
- Bis-Humbert, C.; García-Cabrerizo, R.; García-Fuster, M.J. Antidepressant-like Effects of Cannabidiol in a Rat Model of Early-Life Stress with or without Adolescent Cocaine Exposure. Pharmacol. Rep. 2021. [CrossRef]
- Borys, H.K.; Ingall, G.B.; Karler, R. Development of Tolerance to the Prolongation of Hexobarbitone Sleeping Time Caused by Cannabidiol. Br. J. Pharmacol. 1979, 67, 93–101.
- Peres, F.F.; Levin, R.; Suiama, M.A.; Diana, M.C.; Gouvêa, D.A.; Almeida, V.; Santos, C.M.; Lungato, L.; Zuardi, A.W.; Hallak, J.E.C.; et al. Cannabidiol Prevents Motor and Cognitive Impairments Induced by Reserpine in Rats. Front. Pharmacol. 2016, 7, 343. [CrossRef]
- Vuolo, F.; Petronilho, F.; Sonai, B.; Ritter, C.; Hallak, J.E.C.; Zuardi, A.W.; Crippa, J.A.; Dal-Pizzol, F. Evaluation of Serum Cytokines Levels and the Role of Cannabidiol Treatment in Animal Model of Asthma. Mediators Inflamm. 2015, 2015, 538670. [CrossRef]
- Wheal, A.J.; Cipriano, M.; Fowler, C.J.; Randall, M.D.; O’Sullivan, S.E. Cannabidiol Improves Vasorelaxation in Zucker Diabetic Fatty Rats through Cyclooxygenase Activation. J. Pharmacol. Exp. Ther. 2014, 351, 457–466. [CrossRef]
- Hammell, D.C.; Zhang, L.P.; Ma, F.; Abshire, S.M.; McIlwrath, S.L.; Stinchcomb, A.L.; Westlund, K.N. Transdermal Cannabidiol Reduces Inflammation and Pain-Related Behaviours in a Rat Model of Arthritis. Eur. J. Pain Lond. Engl. 2016, 20, 936–948. [CrossRef]
- Belardo, C.; Iannotta, M.; Boccella, S.; Rubino, R.C.; Ricciardi, F.; Infantino, R.; Pieretti, G.; Stella, L.; Paino, S.; Marabese, I.; et al. Oral Cannabidiol Prevents Allodynia and Neurological Dysfunctions in a Mouse Model of Mild Traumatic Brain Injury. Front. Pharmacol. 2019, 10, 352. [CrossRef]
- Ceprián, M.; Jiménez-Sánchez, L.; Vargas, C.; Barata, L.; Hind, W.; Martínez-Orgado, J. Cannabidiol Reduces Brain Damage and Improves Functional Recovery in a Neonatal Rat Model of Arterial Ischemic Stroke. Neuropharmacology 2017, 116, 151–159. [CrossRef]
- Costa, B.; Colleoni, M.; Conti, S. Oral Anti-Inflammatory Activity of Cannabidiol, a Non-Psychoactive Constituent of Cannabis, in Acute Carrageenan-Induced Inflammation in the Rat Paw. Naunyn. Schmiedebergs Arch. Pharmacol. 2004, 369, 294–299. [CrossRef]
- Costa, B.; Giagnoni, G.; Franke, C.; Trovato, A.; Colleoni, M. Vanilloid TRPV1 Receptor Mediates the Antihyperalgesic Effect of the Nonpsychoactive Cannabinoid, Cannabidiol, in a Rat Model of Acute Inflammation. Br. J. Pharmacol. 2004, 143, 247–250. [CrossRef]
- Costa, B.; Trovato, A.; Comelli, F.; Giagnoni, G.; Colleoni, M. The Non-Psychoactive Cannabis Constituent Cannabidiol Is an Orally Effective Therapeutic Agent in Rat Chronic Inflammatory and Neuropathic Pain. Eur. J. Pharmacol. 2007, 556, 75–83. [CrossRef]
- Ignatowska-Jankowska, B.; Jankowski, M.M.; Swiergiel, A.H. Cannabidiol Decreases Body Weight Gain in Rats: Involvement of CB2 Receptors. Neurosci. Lett. 2011, 490, 82–84. [CrossRef]
- Murillo-Rodríguez, E.; Millán-Aldaco, D.; Palomero-Rivero, M.; Mechoulam, R.; Drucker-Colín, R. Cannabidiol, a Constituent of Cannabis Sativa, Modulates Sleep in Rats. FEBS Lett 2006, 580.
- Schiavon, A.P.; Soares, L.M.; Bonato, J.M.; Milani, H.; Guimarães, F.S.; Weffort de Oliveira, R.M. Protective Effects of Cannabidiol against Hippocampal Cell Death and Cognitive Impairment Induced by Bilateral Common Carotid Artery Occlusion in Mice. Neurotox. Res. 2014, 26, 307–316. [CrossRef]
- Wang, Y.; Mukhopadhyay, P.; Cao, Z.; Wang, H.; Feng, D.; Haskó, G.; Mechoulam, R.; Gao, B.; Pacher, P. Cannabidiol Attenuates Alcohol-Induced Liver Steatosis, Metabolic Dysregulation, Inflammation and Neutrophil-Mediated Injury. Sci. Rep. 2017, 7, 12064. [CrossRef]
- Zuardi, A.; Cosme, R.; Graeff, F.; Guimaraes, F. Effects of Ipsapirone and Cannabidiol on Human Experimental Anxiety. J. Psychopharmacol. (Oxf.) 1993, 7, 82–88. [CrossRef]
- Zuardi, A.W.; Guimarães, F.S.; Moreira, A.C. Effect of Cannabidiol on Plasma Prolactin, Growth Hormone and Cortisol in Human Volunteers. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 1993, 26, 213–217.
- Martin, R.C.; Gaston, T.E.; Thompson, M.; Ampah, S.B.; Cutter, G.; Bebin, E.M.; Szaflarski, J.P. Cognitive Functioning Following Long-Term Cannabidiol Use in Adults with Treatment-Resistant Epilepsy. Epilepsy Behav. 2019, 97, 105–110. [CrossRef]
- Allendorfer, J.B.; Nenert, R.; Bebin, E.M.; Gaston, T.E.; Grayson, L.E.; Hernando, K.A.; Houston, J.T.; Hansen, B.; Szaflarski, J.P. fMRI Study of Cannabidiol-Induced Changes in Attention Control in Treatment-Resistant Epilepsy. Epilepsy Behav. 2019, 96, 114–121. [CrossRef]
- Santos de Alencar, S.; Crippa, J.A.S.; Brito, M.C.M.; Pimentel, Â.V.; Cecilio Hallak, J.E.; Tumas, V. A Single Oral Dose of Cannabidiol Did Not Reduce Upper Limb Tremor in Patients with Essential Tremor. Parkinsonism Relat. Disord. 2021, 83, 37–40. [CrossRef]
- Kasper, A.M.; Sparks, S.A.; Hooks, M.; Skeer, M.; Webb, B.; Nia, H.; Morton, J.P.; Close, G.L. High Prevalence of Cannabidiol Use Within Male Professional Rugby Union and League Players: A Quest for Pain Relief and Enhanced Recovery. Int. J. Sport Nutr. Exerc. Metab. 2020, 1–8. [CrossRef]
- Cochrane-Snyman, K.C.; Cruz, C.; Morales, J.; Coles, M. The Effects of Cannabidiol Oil on Noninvasive Measures of Muscle Damage in Men. Med. Sci. Sports Exerc. 2021, 53, 1460–1472. [CrossRef]
- Neubauer, D.; Perković Benedik, M.; Osredkar, D. Cannabidiol for Treatment of Refractory Childhood Epilepsies: Experience from a Single Tertiary Epilepsy Center in Slovenia. Epilepsy Behav. EB 2018, 81, 79–85. [CrossRef]
- Lopez, H.L.; Cesareo, K.R.; Raub, B.; Kedia, A.W.; Sandrock, J.E.; Kerksick, C.M.; Ziegenfuss, T.N. Effects of Hemp Extract on Markers of Wellness, Stress Resilience, Recovery and Clinical Biomarkers of Safety in Overweight, But Otherwise Healthy Subjects. J. Diet. Suppl. 2020, 17, 561–586. [CrossRef]
- Hatchett, A.; Armstrong, K.; Hughes, B.; Parr, B. The Influence Cannabidiol on Delayed Onset of Muscle Soreness. Int. J. Phys. Educ. Sports Health 2020, 89–94.
- Sahinovic, A.; Irwin, C.; Doohan, P.T.; Kevin, R.C.; Cox, A.J.; Lau, N.S.; Desbrow, B.; Johnson, N.A.; Sabag, A.; Hislop, M.; et al. Effects of Cannabidiol on Exercise Physiology and Bioenergetics: A Randomised Controlled Pilot Trial. Sports Med. - Open 2022, 8, 27. [CrossRef]
- Alhamoruni, A.; Wright, K.L.; Larvin, M.; O’Sullivan, S.E. Cannabinoids Mediate Opposing Effects on Inflammation-Induced Intestinal Permeability. Br. J. Pharmacol. 2012, 165, 2598–2610. [CrossRef]
- Arndt, D.L.; de Wit, H. Cannabidiol Does Not Dampen Responses to Emotional Stimuli in Healthy Adults. Cannabis Cannabinoid Res. 2017, 2, 105–113. [CrossRef]
- Bergamaschi, M.M.; Queiroz, R.H.C.; Chagas, M.H.N.; de Oliveira, D.C.G.; De Martinis, B.S.; Kapczinski, F.; Quevedo, J.; Roesler, R.; Schröder, N.; Nardi, A.E.; et al. Cannabidiol Reduces the Anxiety Induced by Simulated Public Speaking in Treatment-Naïve Social Phobia Patients. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011, 36, 1219–1226. [CrossRef]
- Birnbaum, A.K.; Karanam, A.; Marino, S.E.; Barkley, C.M.; Remmel, R.P.; Roslawski, M.; Gramling-Aden, M.; Leppik, I.E. Food Effect on Pharmacokinetics of Cannabidiol Oral Capsules in Adult Patients with Refractory Epilepsy. Epilepsia 2019, 60, 1586–1592. [CrossRef]
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A Single Dose of Cannabidiol Reduces Blood Pressure in Healthy Volunteers in a Randomized Crossover Study. JCI Insight 2017, 2, e93760, 93760. [CrossRef]
- Kraft, B.; Frickey, N.A.; Kaufmann, R.M.; Reif, M.; Frey, R.; Gustorff, B.; Kress, H.G. Lack of Analgesia by Oral Standardized Cannabis Extract on Acute Inflammatory Pain and Hyperalgesia in Volunteers. Anesthesiology 2008, 109, 101–110. [CrossRef]
- Linares, I.M.P.; Guimaraes, F.S.; Eckeli, A.; Crippa, A.C.S.; Zuardi, A.W.; Souza, J.D.S.; Hallak, J.E.; Crippa, J.A.S. No Acute Effects of Cannabidiol on the Sleep-Wake Cycle of Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Front. Pharmacol. 2018, 9, 315. [CrossRef]
- Masataka, N. Anxiolytic Effects of Repeated Cannabidiol Treatment in Teenagers With Social Anxiety Disorders. Front. Psychol. 2019, 10, 2466. [CrossRef]
- Shannon, S.; Opila-Lehman, J. Effectiveness of Cannabidiol Oil for Pediatric Anxiety and Insomnia as Part of Posttraumatic Stress Disorder: A Case Report. Perm. J. 2016, 20, 16–005. [CrossRef]
- Isenmann, E.; Veit, S.; Starke, L.; Flenker, U.; Diel, P. Effects of Cannabidiol Supplementation on Skeletal Muscle Regeneration after Intensive Resistance Training. Nutrients 2021, 13, 3028. [CrossRef]
- Crossland, B.W.; Rigby, B.R.; Duplanty, A.A.; King, G.A.; Juma, S.; Levine, N.A.; Clark, C.E.; Ramirez, K.P.; Varone, N.L. Acute Supplementation with Cannabidiol Does Not Attenuate Inflammation or Improve Measures of Performance Following Strenuous Exercise. Healthcare 2022, 10, 1133. [CrossRef]
- Burggren, A.C.; Shirazi, A.; Ginder, N.; London, E.D. Cannabis Effects on Brain Structure, Function, and Cognition: Considerations for Medical Uses of Cannabis and Its Derivatives. Am. J. Drug Alcohol Abuse 2019, 45, 563–579. [CrossRef]
- Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Phytocannabinoids as Novel Therapeutic Agents in CNS Disorders. Pharmacol. Ther. 2012, 133, 79–97. [CrossRef]
- Booz, G.W. Cannabidiol as an Emergent Therapeutic Strategy for Lessening the Impact of Inflammation on Oxidative Stress. Free Radic. Biol. Med. 2011, 51, 1054–1061. [CrossRef]
- Lorenzetti, V.; Solowij, N.; Yücel, M. The Role of Cannabinoids in Neuroanatomic Alterations in Cannabis Users. Biol. Psychiatry 2016, 79, e17-31. [CrossRef]
- Kramer, A.; Sinclair, J.; Sharpe, L.; Sarris, J. Chronic Cannabis Consumption and Physical Exercise Performance in Healthy Adults: A Systematic Review. J. Cannabis Res. 2020, 2, 34. [CrossRef]
- Singh, J.; Neary, J.P. Neuroprotection Following Concussion: The Potential Role for Cannabidiol. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2020, 47, 289–300. [CrossRef]
- Reillo, M.R. Cannabidiol (CBD) in the Management of Sports-Related Traumatic Brain Injury: Research and Efficacy. Sports Inj. Med. 2019, 3. [CrossRef]
- Fine, P.G.; Rosenfeld, M.J. The Endocannabinoid System, Cannabinoids, and Pain. Rambam Maimonides Med. J. 2013, 4, e0022. [CrossRef]
- Sholler, D.J.; Schoene, L.; Spindle, T.R. Therapeutic Efficacy of Cannabidiol (CBD): A Review of the Evidence from Clinical Trials and Human Laboratory Studies. Curr. Addict. Rep. 2020, 7, 405–412. [CrossRef]
- Lowin, T.; Schneider, M.; Pongratz, G. Joints for Joints: Cannabinoids in the Treatment of Rheumatoid Arthritis. Curr. Opin. Rheumatol. 2019, 31, 271–278. [CrossRef]
- Stanley, C.P.; Hind, W.H.; O’Sullivan, S.E. Is the Cardiovascular System a Therapeutic Target for Cannabidiol? Br. J. Clin. Pharmacol. 2013, 75, 313–322. [CrossRef]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Mol. Basel Switz. 2018, 23, 2478. [CrossRef]
- Zurier, R.B.; Burstein, S.H. Cannabinoids, Inflammation, and Fibrosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 3682–3689. [CrossRef]
- Vuolo, F.; Abreu, S.C.; Michels, M.; Xisto, D.G.; Blanco, N.G.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Reis, C.; Bahl, M.; et al. Cannabidiol Reduces Airway Inflammation and Fibrosis in Experimental Allergic Asthma. Eur. J. Pharmacol. 2019, 843, 251–259. [CrossRef]
- Crippa, J.A.S.; Derenusson, G.N.; Ferrari, T.B.; Wichert-Ana, L.; Duran, F.L.S.; Martin-Santos, R.; Simões, M.V.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural Basis of Anxiolytic Effects of Cannabidiol (CBD) in Generalized Social Anxiety Disorder: A Preliminary Report. J. Psychopharmacol. Oxf. Engl. 2011, 25, 121–130. [CrossRef]
- Huestis, M.A.; Mazzoni, I.; Rabin, O. Cannabis in Sport: Anti-Doping Perspective. Sports Med. Auckl. NZ 2011, 41, 949–966. [CrossRef]
- Lattanzi, S.; Brigo, F.; Trinka, E.; Zaccara, G.; Cagnetti, C.; Del Giovane, C.; Silvestrini, M. Efficacy and Safety of Cannabidiol in Epilepsy: A Systematic Review and Meta-Analysis. Drugs 2018, 78, 1791–1804. [CrossRef]
- Ware, M.; Jensen, D.; Barrette, A.; Vernec, A.; Derman, W. Cannabis and the Health and Performance of the Elite Athlete. Clin J Sport Med 2018, 28.
- Armstrong, R.; Warren, G.; Warren, J. Mechanisms of Exercise-Induced Muscle-Fiber Injury. Sports Med. 1991, 12, 184–207. [CrossRef]
- Yeager, M.P.; Pioli, P.A.; Guyre, P.M. Cortisol Exerts Bi-Phasic Regulation of Inflammation in Humans. Dose-Response 2010, 9, 332–347. [CrossRef]
- Gamelin, F.-X.; Cuvelier, G.; Mendes, A.; Aucouturier, J.; Berthoin, S.; Di Marzo, V.; Heyman, E. Cannabidiol in Sport: Ergogenic or Else? Pharmacol. Res. 2020, 156, 104764. [CrossRef]
- Clarkson, P.; Nosaka, K.; Braun, B. Muscle Function After Exercise-Induced Muscle Damage and Rapid Adaptation. Med. Sci. Sports Exerc. 1992, 24, 512–520.
- Hainline, B.; Derman, W.; Vernec, A.; Budgett, R.; Deie, M.; Dvorak, J.; Harle, C.; Herring, S.A.; McNamee, M.; Meeuwisse, W.; et al. International Olympic Committee Consensus Statement on Pain Management in Elite Athletes. Br. J. Sports Med. 2017, 51, 1245–1258. [CrossRef]
- Rudroff, T.; Sosnoff, J. Cannabidiol to Improve Mobility in People with Multiple Sclerosis. Front. Neurol. 2018, 9. [CrossRef]
- Hill, K.P.; Palastro, M.D.; Johnson, B.; Ditre, J.W. Cannabis and Pain: A Clinical Review. Cannabis Cannabinoid Res. 2017, 2, 96–104. [CrossRef]
- Fox, J.L.; Scanlan, A.T.; Stanton, R.; Sargent, C. Insufficient Sleep in Young Athletes? Causes, Consequences, and Potential Treatments. Sports Med. 2020, 50, 461–470. [CrossRef]
- Verma, K.; Singh, D.; Srivastava, A. The Impact of Complementary and Alternative Medicine on Insomnia: A Systematic Review. Cureus 2022, 14, e28425. [CrossRef]
- Murillo-Rodríguez, E.; Budde, H.; Veras, A.B.; Rocha, N.B.; Telles-Correia, D.; Monteiro, D.; Cid, L.; Yamamoto, T.; Machado, S.; Torterolo, P. The Endocannabinoid System May Modulate Sleep Disorders in Aging. Curr. Neuropharmacol. 2020, 18, 97–108. [CrossRef]
| PICOS | Inclusion criteria | Exclusion criteria |
| Population | Studies involving humans (e.g. athletes and healthy individuals), as well as animal models (e.g. mice, rats). | Studies that not involve humans or animals. |
| Intervention/ Exposure |
Examining the use of CDB in any formulation (e.g. oral, topical) or dosage as primary intervention. | Not examining the use of CBD as primary intervention. |
| Comparator | Studies with a placebo or control group were included, as well as those without a comparator. | None. |
| Outcomes | Studies reporting outcomes related to sports/exercise recovery, physical performance, and/or physiological effects of CBD (e.g. inflammation, pain, sleep, cognition). | Studies that report only the psychoactive effects of CBD. |
| Study design | Scientific evidence classified as a first and second level were incorporated[38], that Included experimental (randomized trials, non-randomized trials), observational (cohort, case-control), and systematic reviews/meta-analyses. | Studies were non-empirical or lacked sufficient methodological details. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




