1. Introduction
Childhood trauma, particularly complex forms involving chronic interpersonal adversities such as parental negligence, has been linked to multiple domains of impairment in adolescence [
1], as well as mental health issues [
2,
3,
4,
5]. Electroencephalography (EEG) studies have suggested alterations in neural activity, including alpha oscillation abnormalities, in individuals with trauma-related disorders [
2,
6].
A certain topography of alpha oscillatory dysregulation in post-traumatic conditions would be of interest to both researchers and clinicians. The temporal lobes are believed to play a significant role in regulating emotions[
7,
8], given their functional connection to the emotion regulation pathways of structures, such as the insular cortex, amygdala, and hippocampus[
9]. Therefore, they may be good candidates for exploring EEG markers for fear regulation disorders. Although there have been some findings on the dysregulation of neural connectivity in these areas [
10,
11], the relationship between EEG oscillations, such as alpha power in temporal locations, and psychological trauma is still poorly understood.
The impact of complex childhood trauma (CCT) can be profound, particularly in children and adolescents. This can manifest as a range of cognitive and emotional difficulties, risky behaviors, and mental health challenges. Often, clinical trauma symptomatology is [
12] indistinguishable from other stress and emotion regulation disorders, making targeted treatment challenging. Also, children with clinically significant trauma symptoms in foster care are 46% more likely to experience placement instability, highlighting the need for early trauma assessment and intervention [
13]. Therefore, having reliable neurophysiological patterns detectable as traces of CCT exposure could be important because complex trauma survivors often receive inaccurate diagnoses, such as borderline personality disorder (BPD), leading to stigma and ineffective treatment. Adolescents who reported multiple or chronic trauma events were more likely to exhibit symptoms associated with developmental trauma disorder (DTD), highlighting the need for improved diagnosis and treatment of complex trauma in youth [
14]. Proper identification of CCT or the diagnosis of CPTSD can lead to improved self-understanding, reduced stigma, and effective treatment choices.
The spectral distribution of EEG at the scalp level, which can be obtained through qEEG analysis, has been suggested as a neurophenotype. It has been proven to be stable in repeated measurements [
15] and is associated with neural system functions that produce specific behaviors [
16].
Here, we propose temporal alpha power as a neuromarker, as it indicates neural oscillatory activity in regions associated with emotion regulation and memory consolidation [
17,
18]. Previous studies have suggested alterations in EEG patterns among individuals with PTSD, including increased alpha activity, particularly in the right temporal lobe ([
11,
19,
20].
Alpha oscillations are typically associated with a state of cortical idling or inhibition, reflecting decreased neuronal activity in the regions in which they occur. In the right temporal or temporo-occipital sites, excessive alpha oscillations may indicate an overactive inhibitory mechanism, potentially leading to disruptions in sensory processing, attentional control, and emotional regulation, depending on which large-scale network is involved. Functionally, excess inhibition could manifest as difficulties in recognizing and interpreting visual or auditory stimuli, impairments in attentional focus or sustained attention, and challenges in regulating emotional responses to environmental stimuli.
The relationship between affective system dysregulation, neurotransmitters, and abnormalities in alpha oscillations has been explored in several studies, most of which are related to GABAergic neurotransmission, which is essential for generating alpha oscillations. Research suggests that GABAergic dysfunction may contribute to abnormalities in alpha oscillations observed in conditions such as anxiety disorders and post-traumatic stress disorder (PTSD) [
21,
22]
In brain physiology, excessive power in alpha oscillations in the temporo-occipital regions can arise because of several factors related to underlying neural dynamics and connectivity. The thalamocortical dysrhythmia model of neuropsychiatric disorders [
23] refers to a disruption in the normal oscillatory interactions between the thalamus and cortex, leading to excessive synchronized oscillations, including alpha rhythms. In this model, abnormal thalamic inputs to the cortex result in increased alpha power, particularly in regions receiving projections from the thalamus such as the temporo-occipital cortex. These states have been conceptualized as oscillopathies [
24] and may reflect malfunctioning networks. It has also been suggested that reduced neural excitability in the temporo-occipital region can lead to heightened synchronization and excessive alpha power [
25]. Additionally, long-term neuroplastic changes in response to injury, environmental factors, or pathological conditions can reshape the neural circuitry and promote aberrant oscillatory patterns [
26].
Temporal Alpha and Fear Conditioning and Learnig
Evidence suggests a relationship between excessive alpha power in the temporal region and fear-related processes, including fear conditioning and fear learning circuits [
20,
27].
Fear conditioning is a type of associative learning in which a neutral stimulus is associated with a fearful event, leading to a conditioned fear response. This process involves complex neural circuits, including regions of the temporal lobe such as the amygdala, which play a crucial role in processing and encoding emotional memories. [
28]. Proposed neurobiological correlates of hypervigilance in PTSD may result from heightened salience processing [
29], but may also impair self-awareness and autobiographical memory[
30]. According to a meta-analysis conducted by Koch et al. in 2016, individuals with Posttraumatic Stress Disorder (PTSD) show hyperactivity in the ventral anterior cingulate cortex and parahippocampus/amygdala, as well as hypoactivity in the (posterior) insula and middle frontal gyrus. Additionally, functional connectivity studies indicate that PTSD patients exhibit enhanced connectivity in the salience network (SN), but decreased connectivity in the default mode network (DMN) [
8,
30] decreased within-DMN connectivity and disrupted DMN-SN and DMN-CEN coupling was found in adolescents with PTSD in a fMRI study [
31].
Few studies have reported changes in EEG patterns [
32], including increased alpha power, in individuals with PTSD [
33] or during fear conditioning tasks [
27,
33]. These changes may indicate dysregulation of fear-learning circuits. Studies using resting-state MEG have identified abnormal neural activation in multiple cortical areas and medial temporal structures related to emotional processing in veterans with PTSD[
8]. However, the specific role of excessive temporal alpha power in fear conditioning circuits is not fully understood. Alterations in alpha power could reflect diverse underlying neural processes related to emotional regulation [
34], attentional biases [
35], or other cognitive functions [
36] implicated in PTSD and fear-related disorders. According to Heller’s neuropsychological model, separate neural systems underlie the valence and arousal dimensions of emotions, with a parietotemporal system modulating arousal in the right hemisphere. [
37]. Despite indirect or anecdotal references pointing to increased alpha activity, particularly in the right temporal lobe in traumatized individuals, there is a lack of scientific research linking alterations in EEG temporal alpha patterns to PTSD. Clinicians applying neuroregulatory techniques through individualized protocols often refer to the “T6-alpha” as a biomarker for psychological trauma in their clinical communications; therefore, this potential marker requires systematic validation through scientific investigation.
To our knowledge, this is the first study to examine temporal alpha power in EEG as a potential marker of CCT in adolescents. Analyzing electric oscillations on the surface of the brain’s temporal lobes can aid in comprehending the neural mechanisms underlying trauma-related psychopathology, considering its association with fear circuitry, learning, and conditioning.
Research Aim:
This study aimed to investigate the impact of complex childhood trauma exposure on temporal alpha power, assessed via resting-state EEG, in an institutionalized adolescent population.
We examined temporal alpha power in adolescents with a history of complex childhood trauma and compared it with that in healthy controls. Furthermore, we explored the association between temporal alpha power and self-reported symptoms of trauma-related psychopathology, including PTSD subscales, dissociation, and depression, in adolescents.
We hypothesized that adolescents exposed to CCT would exhibit alterations in temporal alpha power, characterized by either increased or decreased activity, compared to neurotypical matched controls. We preregistered our hypothesis and analytic approach at:
https://osf.io/5ya3v/?view_only=41270f74556745c399c0307579cb66d7
2. Materials and Methods
2.1. Participants/Data:
The data for this study were collected as part of a larger project concerning EEG neuromarkers of trauma in adolescents (author et al., manuscript under preparation). This project was reviewed and approved by institutions legally responsible for the participants (the National and Local Child Protection Agencies) and institutions involved in the study implementation (local university and psychiatric hospital). The Full data collection protocol, which includes inclusion/exclusion criteria and EEG data collection and processing procedures was preregistered (prior to collecting any data) and can be found at:
https://osf.io/m3fdv/?view_only=cc6d743714504749a85cc093c85039cc.
The target population was adolescents between 12 and 17 years of age, living in residential housing for a minimum of three consecutive months, with a history of one or more traumatic childhood experiences, without any suspected neurological pathologies such as epilepsy or cerebral palsy, and no changes in medication/psychotherapy 3 months prior to data collection. All necessary measures were implemented to ensure participant confidentiality and data protection.
The trauma group in the current study was constituted based on data from 26 adolescents (8 males, 18 females) with a history of neglect or abuse who had been placed in the foster care system in [County]. Informed consent was obtained from all participants and their legal guardians prior to study participation, and a compensation of 20 [local currency] (~4 USD) was offered to each participant. Participants were screened for trauma exposure, trauma-related symptoms, and psychiatric comorbidities (depression, alexithymia, and dissociation) (for descriptive statistics and details about screening instruments, see
Table 1).
The control group was constituted based on EEG data that is available on an open repository, the Child Mind Institute Biobank - Multimodal Resource for Studying Information Processing in the Developing Brain (MIPDB) [
38]
Table 1.
Descriptive Statistics for the Trauma and Control Groups.
Table 1.
Descriptive Statistics for the Trauma and Control Groups.
| |
Group |
| |
Trauma |
|
Control |
| |
M |
SD |
|
M |
SD |
| Age |
15 |
1.73 |
|
14.21 |
1.50 |
| IQ |
82.85 |
1.73 |
|
97.04 |
1.50 |
| PTSD (screening)a
|
17.5 |
9.5 |
|
Not available/Not applicable |
| PTSD (assessment)b
|
11.5 |
5.5 |
|
| CPTSD b
|
20 |
8.8 |
|
| DSO b
|
8.5 |
4.9 |
|
| Alexithymiac
|
15.4 |
8.5 |
|
| Dissociationd
|
2.2 |
2.2 |
|
| Depressione
|
10.8 |
8.3 |
|
| Years in the protection system |
9.6 |
4.8 |
|
| |
Yes (n) |
No (n) |
|
| PTSD (criteria met) |
13 |
12 |
|
| CPTSD (criteria met) |
6 |
19 |
|
| n |
26 |
28 |
2.2. Procedure:
The EEG data was collected using a 19-channel Mitsar-EEG-BT Lite QEEG edition system along with a 21-electrode EEG cap (Medcap) featuring AgCl electrodes placed according to the 10-20 system, as described in the Preregistration for Data Collection (
https://osf.io/s2h58/?view_only=6bacd53d6dee4d7c9b9c45e0b48978bb). The participants completed a resting-state EEG recording while seated comfortably with their eyes open and then closed for a designated duration (5 min for each condition). The EEG data was then processed and analyzed to extract the temporal alpha power within the frequency range of 8-12 Hz using the EEGLab GUI [
44]. The preprocessing pipeline involved bandpass filtering (0.1 – 40 Hz), bad channel removal, visual inspection for artifact removal, resampling to 250 Hz, re-referencing to average, and ICA decomposition for ocular and muscle artifact correction (IClabel plugin). Epoching and Frequency Analysis were performed using the EEGLAB Spectral Analysis plugin (eegstats ver. 1.2) to compute the power spectral density (PSD) of the EEG data at temporal locations. This step involves applying a Fast Fourier Transform (FFT) spectral analysis technique to estimate the power spectrum.
2.3. Data Analysis:
For each resting-state condition (eyes-open, eyes closed), we used a 2 between (group: trauma vs. control) × 2 within (location: left vs. right) mixed analysis of variance to examine our preregistered hypothesis (DV: temporal alpha power). We did not preregister planned contrasts, therefore we ran pairwise comparisons instead and applied a false discovery rate correction [
45]. However, of theoretical interest are the comparisons between groups for each location (left, right), which we report here. The same analyses were run with participant age added as covariate. For exploratory purposes, for the trauma group, we also computed the Pearson correlation coefficient between the temporal alpha power for each condition (EO, EC) and each location (left, right) and participants’ phenotype scores (trauma-related symptoms and psychiatric comorbidities).
3. Results
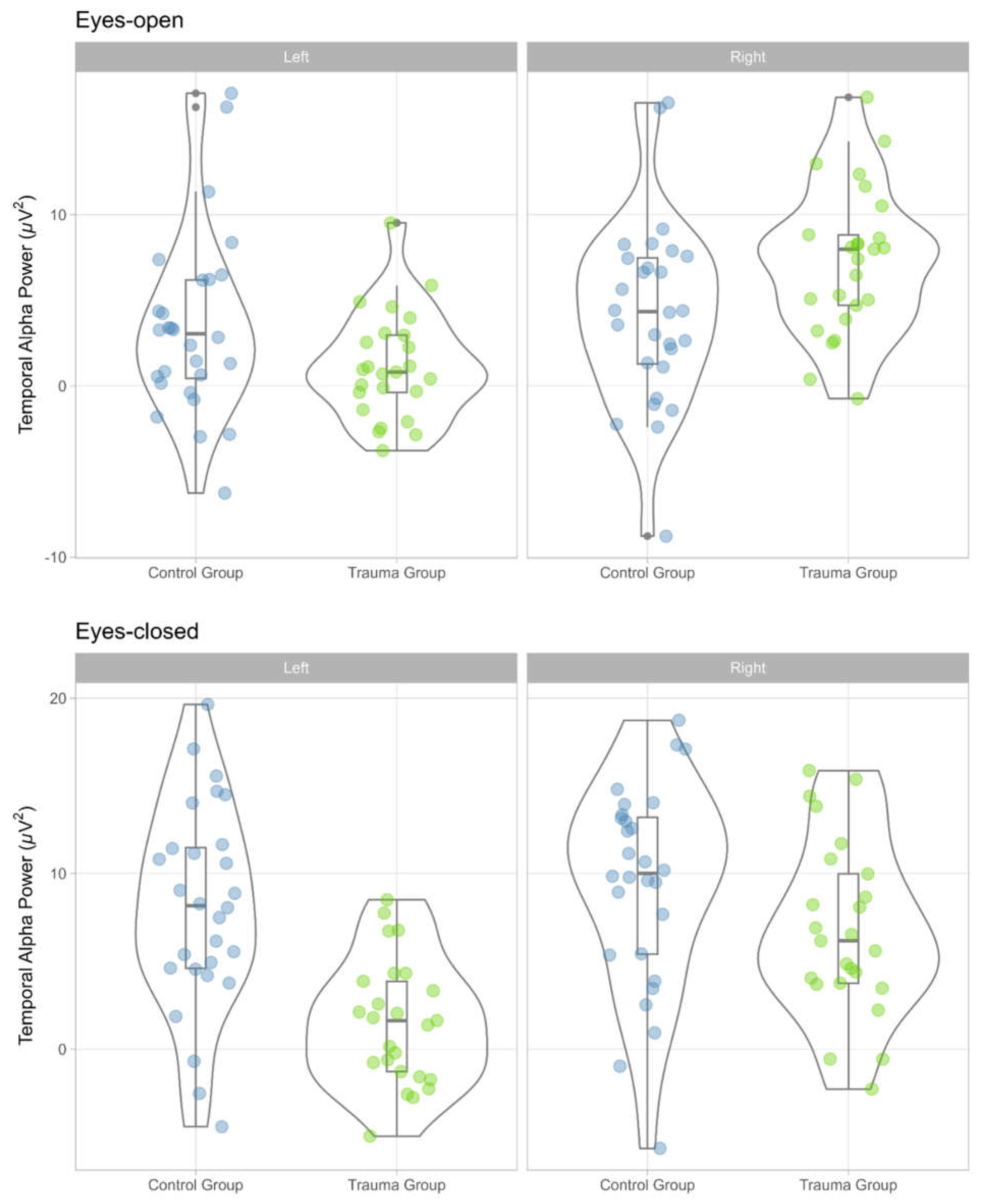
The distribution of temporal alpha power values for the trauma group and the control group, by condition (eyes-open, eyes-closed), location (left, right) is displayed in
Figure 1.
As expected, we found significant differences in temporal alpha power between the trauma group and the control group. Specifically, in the eyes-open condition, there was significant interaction between group and temporal alpha location (left, right), F (1, 51) = 32.94, p < .001, η²p = .39, 90% CI (.22, .53), η²G =.08. Pairwise comparisons showed that right temporal alpha power was higher for the trauma group, M = 7.31, SE = 0.98, relative to the control group, M = 4.28, SE = 0.93, t (51) = 2.25, p = .043, Cohen’s d = 0.91, 95% CI (-0.20, 2.02). There was no statistically significant difference in the case of left temporal alpha power between the trauma group, M = 1.15, SE = 0.88, relative to the control group, M = 3.44, SE = 0.83, t (51) = -1.89, p = .078, Cohen’s d = -0.69, 95% CI (-1.68, 0.31).
In the eyes-closed condition, there was a statistically significant difference in temporal alpha power between the two groups, both as main effect, F (1, 51) = 12.34, p < .001, η²p = .20, 90% CI (.06, .35), η²G =.17, and as interaction with location F (1, 51) = 12.38, p < .001, η²p = .195, 90% CI (.06, .35), η²G =.04. For the main effect of group, we found that temporal alpha was lower for the trauma group, M = 4.25, SE = 0.96, t (50) = 3.28, p = .002, Cohen’s d = -1.13, 95% CI (-1.83, -0.44). In interaction with location, left temporal alpha was significantly reduced in the trauma group, M = 1.50, SE = 0.94, relative to the control group, M = 8.11, SE = 0.94 t (51) = -4.77, p < .001, Cohen’s d = -1.70, 95% CI (-2.68, -0.72). There was no statistically significant difference in the case of right temporal alpha power between the trauma group, M = 6.10, SE = 1.09, relative to the control group, M = 9.19, SE = 1.03, t (51) = -1.44, p = .194, Cohen’s d = -0.56, 95% CI (-1.68, 0.31).
Adding age as a covariate did not change main findings. Moreover, there were no statistically significant correlations between temporal alpha and phenotype scores in the trauma group. Full results output for all analyses reported can be found on the Open Science Framework.
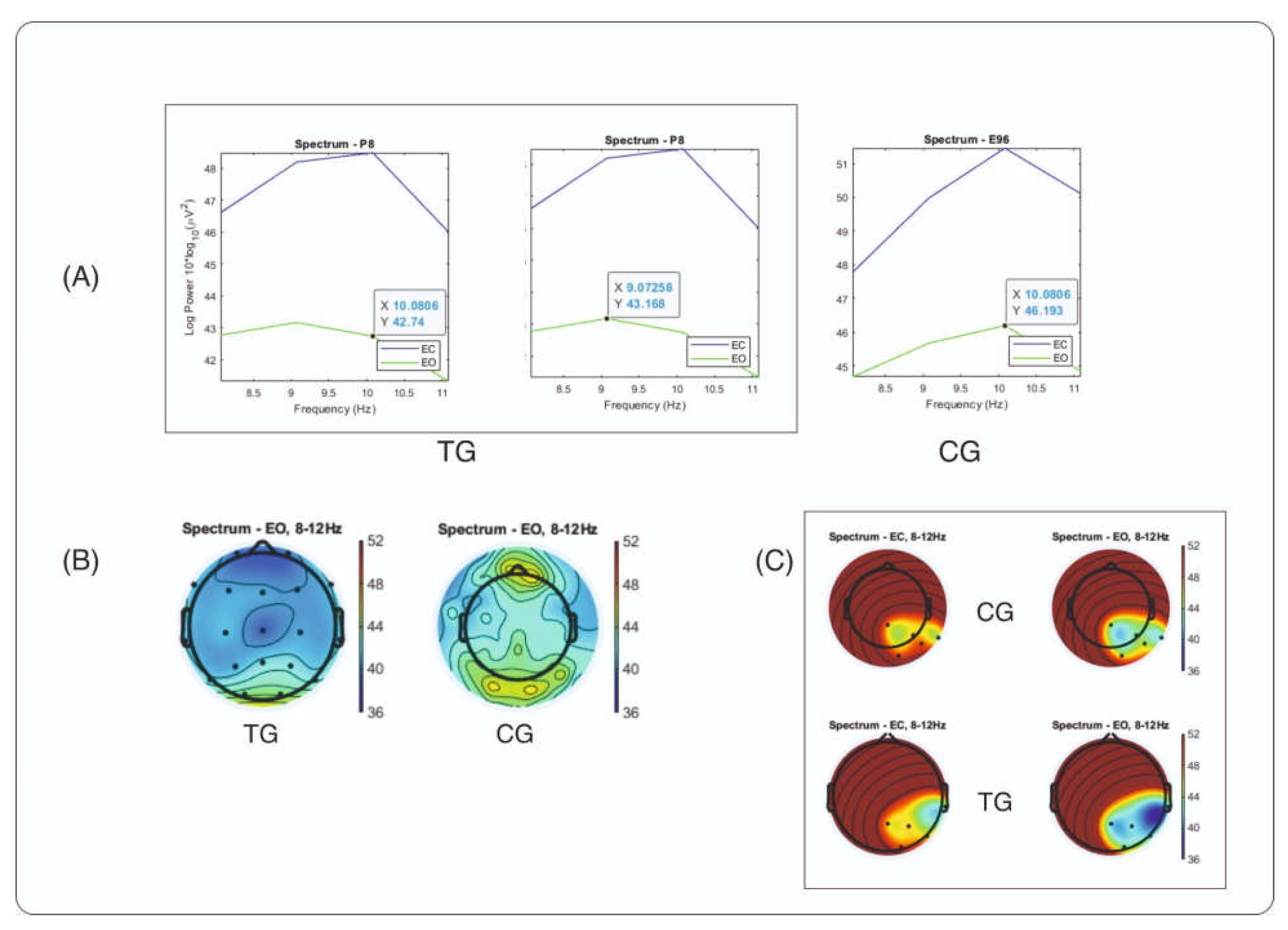
The results that distinguish the TG (trauma group) from the CG (control group) in temporal alpha in the EO condition are presented as spectral graphs and maps in
Figure 2. The RMS power of the right temporal channel is plotted for both groups (
Figure 2, A). The graph shows a two-peak alpha wave in the TG, which could possibly explain the higher power spectral density (PSD) of right temporal alpha that we observed in our data compared to the CG. In addition, the topography of alpha distribution across scalp is presented for two groups: TG and CG (
Figure 2, B), indicating that there is no visible excess of temporal alpha in the TG group compared to the CG group in the same power range. One possible explanation for this result could be that the topography map shows the absolute power of the brain waves across the scalp, which may be generally lower in the TG group (Author et. al, manuscript under preparation). When a specific region of interest consisting of five electrode sites (T6 and its neighboring sites T4, P4, Pz, and O2) was selected for averaging power, a noticeable difference was observed between the groups (
Figure 2, C). In this region, the TG shows a more widespread alpha area, which may be responsible for the higher PSD alpha values calculated in the right temporal site. Further spectral maps of absolute and relative EEG power and topographical maps compared with those of matched controls from the HBI Database are available in the
Supplementary Materials.
4. Discussion
As hypothesized, we found significant differences in temporal alpha power between the trauma and control groups, with dissociation depending on the resting-state condition (eyes-open, eyes-closed). Particularly, we found that in the eyes-open condition, right, but not left, temporal alpha was significantly higher in adolescents with trauma, relative to healthy controls. In the eyes-closed condition, left, but not right, temporal alpha was significantly lower in adolescents with trauma, relative to healthy controls. Our findings suggest that childhood trauma exposure may have a measurable impact on alpha oscillatory patterns, specifically in the right temporal region.
While our findings contribute to the growing body of literature on neurobiological markers of CCT, several limitations should be acknowledged. First, the sample size was relatively small, which may limit the generalizability of the findings. Future research with larger, more diverse samples is needed to confirm and extend our findings. Also the self-report measures may be subject to biases and inaccuracies, particularly in retrospective reporting of traumatic experiences.
Nevertheless, the current study is part of a larger effort by many researchers of deeper understanding of the neuropsychological aftermath of CCT in developmental samples. Research suggests that current diagnostic criteria for PTSD / C-PTSD may not adequately identify how adolescents express (C)-PTSD symptoms in response to childhood and adolescent complex trauma [
46,
47]. A developmental trauma framework was proposed to offer a broader perspective on the mental health of adolescents affected by CCT [
48,
49]. This framework highlights the importance of considering symptoms as reactions to trauma rather than as separate mental health issues. The complex trauma framework [
50] provides a strength-based, survival-driven reframe of trauma, considering an individual’s behaviors, interpersonal difficulties, identity and self-image, and psychiatric diagnoses as adaptive strategies to survive overwhelming experiences in a hostile world [
51]However, while some adolescents with CCT may show high internalizing symptoms or dissociation scales in response to trauma, others may appear less affected and score lower or “normal” on trauma or psychopathology assessments. This raises the question of how we should measure CCT and raises the importance of validating screening neuromarkers such as the one presented here.
The present study focused on temporal alpha power as a potential biomarker of CCT exposure in adolescents. Future research should explore other neural markers of CCT, especially the temporal dynamics of brain oscillations as well as event-related potentials, and examine their collective utility in predicting trauma-related psychopathology. Furthermore, integrating peripheral physiological measures and electroencephalography (EEG) with comprehensive clinical assessments may provide a more nuanced understanding of the proposed neuromarkers, especially those linked to locations associated with fear response and regulation, such as the temporal region.
In conclusion, our study highlights the importance of considering neural markers, such as temporal alpha power, in understanding the long-term consequences of CCT exposure and in the early detection of CCT exposure in developmental samples. Thus, more effective interventions to mitigate the impact on the affected individuals may be developed.
Findings from this study may contribute to a better understanding of the neurobiological impact of complex childhood trauma in adolescent brains, while the identification of neural markers, such as temporal alpha power, may inform the development of targeted interventions for trauma-related psychopathology in adolescents. Potential clinical implications may include the use of EEG-based biomarkers for early identification and intervention in populations affected by CCT, such as personalized qEEG-guided neurofeedback protocol development.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
Conceptualization, GMM and AMZ; methodology, GMM and AMZ; formal analysis, GMM.; investigation, GMM and CIB.; resources, CIB.; data curation, GMM; writing—original draft preparation, GMM and AMZ.; writing—review and editing, GMM, CIB, and AMZ.; funding acquisition, GMM. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Trauma Research Foundation, grant contract number 5465/17.11.2023, and by [xxx] through the research grant LBUS-IRG-2023.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the [xxx].
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Acknowledgments
We want to acknowledge the contribution of our colleague xxx for the data analysis.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish results.
References
- Cook, A.; Spinazzola, J.; Ford, J.; Lanktree, C.; Blaustein, M.; Cloitre, M.; DeRosa, R.; Hubbard, R.; Kagan, R.; Liautaud, J.; et al. Complex trauma in children and adolescents. Psychiatr. Ann. 2005, 35, 390–398. [Google Scholar] [CrossRef]
- Meiers, G.; Nooner, K.; De Bellis, M.D.; Debnath, R.; Tang, A. Alpha EEG asymmetry, childhood maltreatment, and problem behaviors: A pilot home-based study. Child Abuse Negl. 2020, 101, 104358. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Koenen, K.C.; Hill, E.D.; Petukhova, M.; Sampson, N.A.; Zaslavsky, A.M.; Kessler, R.C. Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 815–830.e14. [Google Scholar] [CrossRef]
- Kessler, R.C.; McLaughlin, K.A.; Green, J.G.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Aguilar-Gaxiola, S.; Alhamzawi, A.O.; Alonso, J.; Angermeyer, M.; et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry 2010, 197, 378–385. [Google Scholar] [CrossRef]
- Sheridan, M.A.; McLaughlin, K.A. Neurodevelopmental mechanisms linking ACEs with psychopathology. In Adverse Childhood Experiences; Elsevier, 2020; pp. 265–285 ISBN 9780128160657.
- Nooner, K.B.; Meiers, G.; Treadwell, T.; Butler, L.B. Changes in electroencephalography alpha associated with childhood neglect and adolescent alcohol use. Child Maltreat. 2023, 28, 297–306. [Google Scholar] [CrossRef]
- Nicholson, A.A.; Rabellino, D.; Densmore, M.; Frewen, P.A.; Paret, C.; Kluetsch, R.; Schmahl, C.; Théberge, J.; Neufeld, R.W.J.; McKinnon, M.C.; et al. The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real-time fMRI neurofeedback. Hum. Brain Mapp. 2017, 38, 541–560. [Google Scholar] [CrossRef] [PubMed]
- Badura-Brack, A.S.; Heinrichs-Graham, E.; McDermott, T.J.; Becker, K.M.; Ryan, T.J.; Khanna, M.M.; Wilson, T.W. Resting-State Neurophysiological Abnormalities in Posttraumatic Stress Disorder: A Magnetoencephalography Study. Front. Hum. Neurosci. 2017, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.A.; Peverill, M.; Gold, A.L.; Alves, S.; Sheridan, M.A. Child maltreatment and neural systems underlying emotion regulation. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Herringa, R.J.; Birn, R.M.; Ruttle, P.L.; Burghy, C.A.; Stodola, D.E.; Davidson, R.J.; Essex, M.J. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci USA 2013, 110, 19119–19124. [Google Scholar] [CrossRef]
- Li, L.; Pan, N.; Zhang, L.; Lui, S.; Huang, X.; Xu, X.; Wang, S.; Lei, D.; Li, L.; et al. Hippocampal subfield alterations in pediatric patients with post-traumatic stress disorder. Soc. Cogn. Affect. Neurosci. 2021, 16, 334–344. [Google Scholar] [CrossRef]
- Howells, F.; Stein, D.; Russell, V. Childhood Trauma is Associated with Altered Cortical Arousal: Insights from an EEG Study. Frontiers in Integrative Neuroscience 2012, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.L.; Palmer, A.N.; Akin, B.A.; Dunkerley, S.; Brook, J. Investigating the Relationship between Trauma Symptoms and Placement Instability. Child Abuse Negl. 2020, 108, 104660. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.K.; Borntrager, C.F.; Rostad, W. Measuring trauma: considerations for assessing complex and non-PTSD Criterion A childhood trauma. J. Trauma Dissociation 2014, 15, 184–203. [Google Scholar] [CrossRef] [PubMed]
- Maltez, J.; Hyllienmark, L.; Nikulin, V.V.; Brismar, T. Time course and variability of power in different frequency bands of EEG during resting conditions. Neurophysiol. Clin. 2004, 34, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kropotov, J.D. Quantitative EEG, Event-Related Potentials and Neurotherapy; Elsevier, 2009; ISBN 9780123745125.
- Klimesch, W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci (Regul Ed) 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Tuladhar, A.M.; ter Huurne, N.; Schoffelen, J.-M.; Maris, E.; Oostenveld, R.; Jensen, O. Parieto-occipital sources account for the increase in alpha activity with working memory load. Hum. Brain Mapp. 2007, 28, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.H.; Griffiths, K.; Felmingham, K.L.; Shankman, S.A.; Drinkenburg, W.; Arns, M.; Clark, C.R.; Bryant, R.A. Disorder specificity despite comorbidity: resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder. Biol. Psychol. 2010, 85, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Metzger, L.J.; Paige, S.R.; Carson, M.A.; Lasko, N.B.; Paulus, L.A.; Pitman, R.K.; Orr, S.P. PTSD arousal and depression symptoms associated with increased right-sided parietal EEG asymmetry. J. Abnorm. Psychol. 2004, 113, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, F.; Yang, L.; Tuolihong, L.; Wang, X.; Du, Z.; Zhang, Y.; Yin, X.; Li, Y.; Lu, K.; et al. Involvement of the GABAergic system in PTSD and its therapeutic significance. Front. Mol. Neurosci. 2023, 16, 1052288. [Google Scholar] [CrossRef]
- Fang, Q.; Li, Z.; Huang, G.-D.; Zhang, H.-H.; Chen, Y.-Y.; Zhang, L.-B.; Ding, Z.-B.; Shi, J.; Lu, L.; Yang, J.-L. Traumatic stress produces distinct activations of gabaergic and glutamatergic neurons in amygdala. Front. Neurosci. 2018, 12, 387. [Google Scholar] [CrossRef]
- Schulman, J.J.; Cancro, R.; Lowe, S.; Lu, F.; Walton, K.D.; Llinás, R.R. Imaging of thalamocortical dysrhythmia in neuropsychiatry. Front. Hum. Neurosci. 2011, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Logothetis, N.; Singer, W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron 2013, 80, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Iemi, L.; Gwilliams, L.; Samaha, J.; Auksztulewicz, R.; Cycowicz, Y.M.; King, J.-R.; Nikulin, V.V.; Thesen, T.; Doyle, W.; Devinsky, O.; et al. Ongoing neural oscillations influence behavior and sensory representations by suppressing neuronal excitability. Neuroimage 2022, 247, 118746. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Feurra, M.; Pellegrino, G.; Brittain, J.-S. Investigating and modulating physiological and pathological brain oscillations: the role of oscillatory activity in neural plasticity. Neural Plast. 2019, 2019, 9403195. [Google Scholar] [CrossRef]
- Bierwirth, P.; Antov, M.I.; Stockhorst, U. Oscillatory and non-oscillatory brain activity reflects fear expression in an immediate and delayed fear extinction task. Psychophysiology 2023, 60, e14283. [Google Scholar] [CrossRef]
- Etkin, A.; Wager, T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 2007, 164, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Lanius, R.A.; Frewen, P.A.; Tursich, M.; Jetly, R.; McKinnon, M.C. Restoring large-scale brain networks in PTSD and related disorders: a proposal for neuroscientifically-informed treatment interventions. Eur. J. Psychotraumatol. 2015, 6, 27313. [Google Scholar] [CrossRef]
- Koch, S.B.J.; van Zuiden, M.; Nawijn, L.; Frijling, J.L.; Veltman, D.J.; Olff, M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress. Anxiety 2016, 33, 592–605. [Google Scholar] [CrossRef]
- Viard, A.; Mutlu, J.; Chanraud, S.; Guenolé, F.; Egler, P.-J.; Gérardin, P.; Baleyte, J.-M.; Dayan, J.; Eustache, F.; Guillery-Girard, B. Altered default mode network connectivity in adolescents with post-traumatic stress disorder. Neuroimage Clin. 2019, 22, 101731. [Google Scholar] [CrossRef]
- Ribas, V.R.; Ribas, R.G.; Nóbrega, J. de A.; da Nóbrega, M.V.; Espécie, J.A. de A.; Calafange, M.T.; Calafange, C. de O.M.; Martins, H.A. de L. Pattern of anxiety, insecurity, fear, panic and/or phobia observed by quantitative electroencephalography (QEEG). Dement. Neuropsychol. 2018, 12, 264–271. [CrossRef]
- Newson, J.J.; Thiagarajan, T.C. EEG frequency bands in psychiatric disorders: A review of resting state studies. Front. Hum. Neurosci. 2018, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hua, Y.; Xiu, L.; Oei, T.P.; Hu, P. Resting state frontal alpha asymmetry predicts emotion regulation difficulties in impulse control. Pers. Individ. Dif. 2020, 159, 109870. [Google Scholar] [CrossRef]
- Im, S.; Fitzpatrick, S.; Hien, D.A.; Lopez-Castro, T.; Pawlak, A.; Melara, R.D. Frontal Alpha Asymmetry in Children with Trauma Exposure. Clin. EEG Neurosci. 2022, 53, 418–425. [Google Scholar] [CrossRef]
- López-Castro, T.; Martin, L.; Nickley, S.; Saraiya, T.C.; Melara, R.D. Frontal alpha asymmetry in posttraumatic stress disorder: group differences among individuals with and without PTSD during an inhibitory control task. Clin. EEG Neurosci. 2023, 54, 472–482. [Google Scholar] [CrossRef]
- Heller, W. Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology 1993, 7, 476–489. [Google Scholar] [CrossRef]
- Langer, N.; Ho, E.J.; Alexander, L.M.; Xu, H.Y.; Jozanovic, R.K.; Henin, S.; Petroni, A.; Cohen, S.; Marcelle, E.T.; Parra, L.C.; et al. A resource for assessing information processing in the developing brain using EEG and eye tracking. Sci. Data 2017, 4, 170040. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, A.M.; Brymer, M.J.; Kim, S.; Briggs, E.C.; Ippen, C.G.; Ostrowski, S.A.; Gully, K.J.; Pynoos, R.S. Psychometric properties of the UCLA PTSD reaction index: part I. J. Trauma. Stress 2013, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Haselgruber, A.; Sölva, K.; Lueger-Schuster, B. Validation of ICD-11 PTSD and complex PTSD in foster children using the International Trauma Questionnaire. Acta Psychiatr. Scand. 2020, 141, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Way, I.F.; Applegate, B.; Cai, X.; Franck, L.K.; Black-Pond, C.; Yelsma, P.; Roberts, E.; Hyter, Y.; Muliett, M. Children’s Alexithymia Measure (CAM): A New Instrument for Screening Difficulties with Emotional Expression. J. Child Adolesc. Trauma 2010, 3, 303–318. [Google Scholar] [CrossRef]
- Putnam, F.W.; Helmers, K.; Trickett, P.K. Development, reliability, and validity of a child dissociation scale. Child Abuse Negl. 1993, 17, 731–741. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II. American Psychological Association (APA) 1996. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Unable to find information for 13888810.
- Kolko, D.J.; Hurlburt, M.S.; Zhang, J.; Barth, R.P.; Leslie, L.K.; Burns, B.J. Posttraumatic stress symptoms in children and adolescents referred for child welfare investigation. A national sample of in-home and out-of-home care. Child Maltreat. 2010, 15, 48–63. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, B.A. Developmental Trauma Disorder: Toward a rational diagnosis for children with complex trauma histories. Psychiatr. Ann. 2005, 35, 401–408. [Google Scholar] [CrossRef]
- Spinazzola, J.; van der Kolk, B.; Ford, J.D. Developmental trauma disorder: A legacy of attachment trauma in victimized children. J. Trauma. Stress 2021, 34, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.D. Why We Need a Developmentally Appropriate Trauma Diagnosis for Children: a 10-Year Update on Developmental Trauma Disorder. J. Child Adolesc. Trauma 2021. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Koenen, K.C.; Ambler, A.; Arseneault, L.; Caspi, A.; Fisher, H.L.; Moffitt, T.E.; Danese, A. Unravelling the contribution of complex trauma to psychopathology and cognitive deficits: a cohort study. Br. J. Psychiatry Suppl. 2021, 219, 448–455. [Google Scholar] [CrossRef]
- Rayburn, A.D.; McWey, L.M.; Cui, M. The interrelationships between trauma and internalizing symptom trajectories among adolescents in foster care. Child. Youth Serv. Rev. 2016, 61, 332–336. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).