Submitted:
24 May 2024
Posted:
27 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Neuronal Cell Culture
2.3. Ca2+ Imaging
2.4. Data Analysis
2.5. Quantification and Statistical Analysis
3. Results
3.1. Nx1α is the Prominent Nx Variant in Cultured Primary Hippocampal Neurons
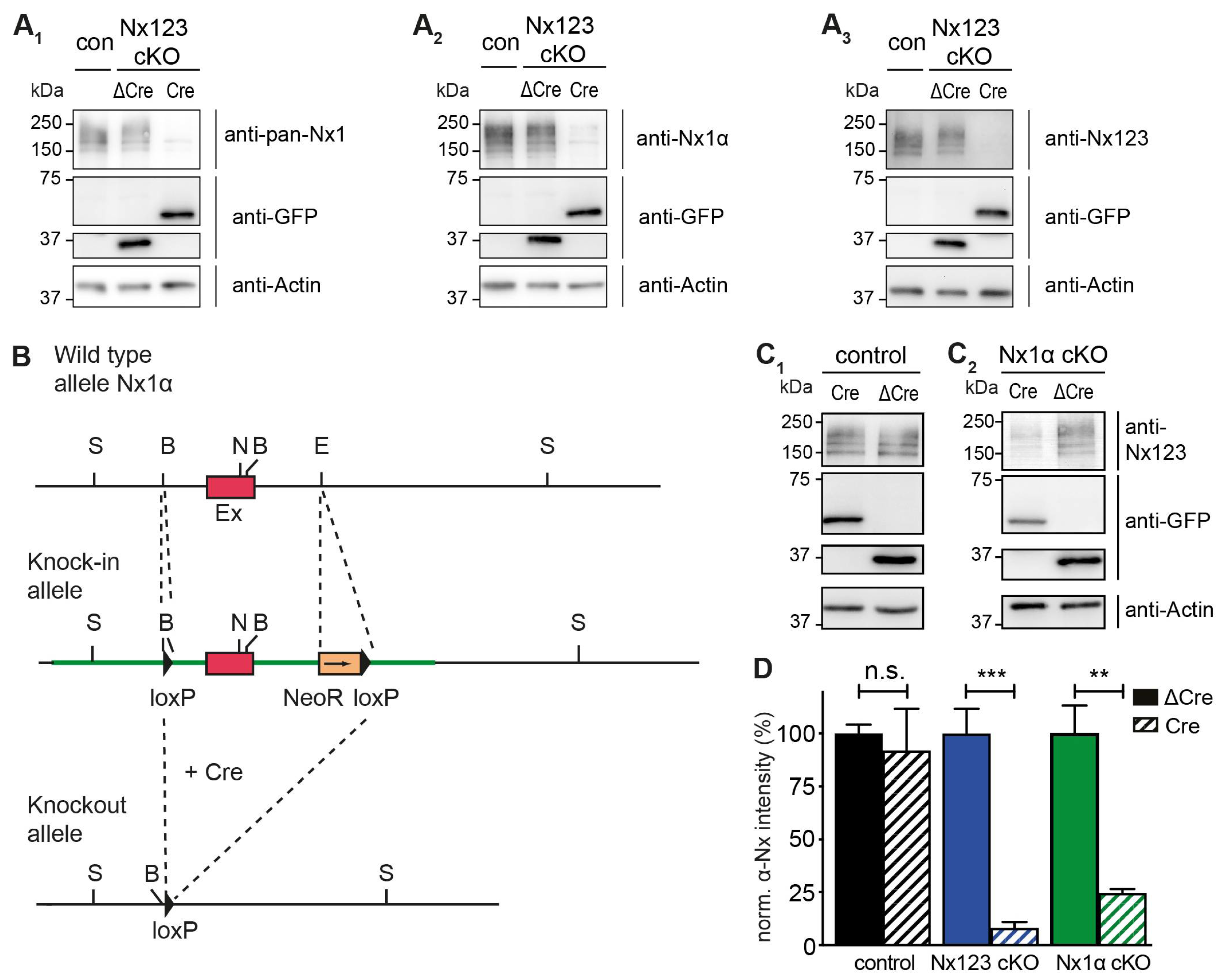
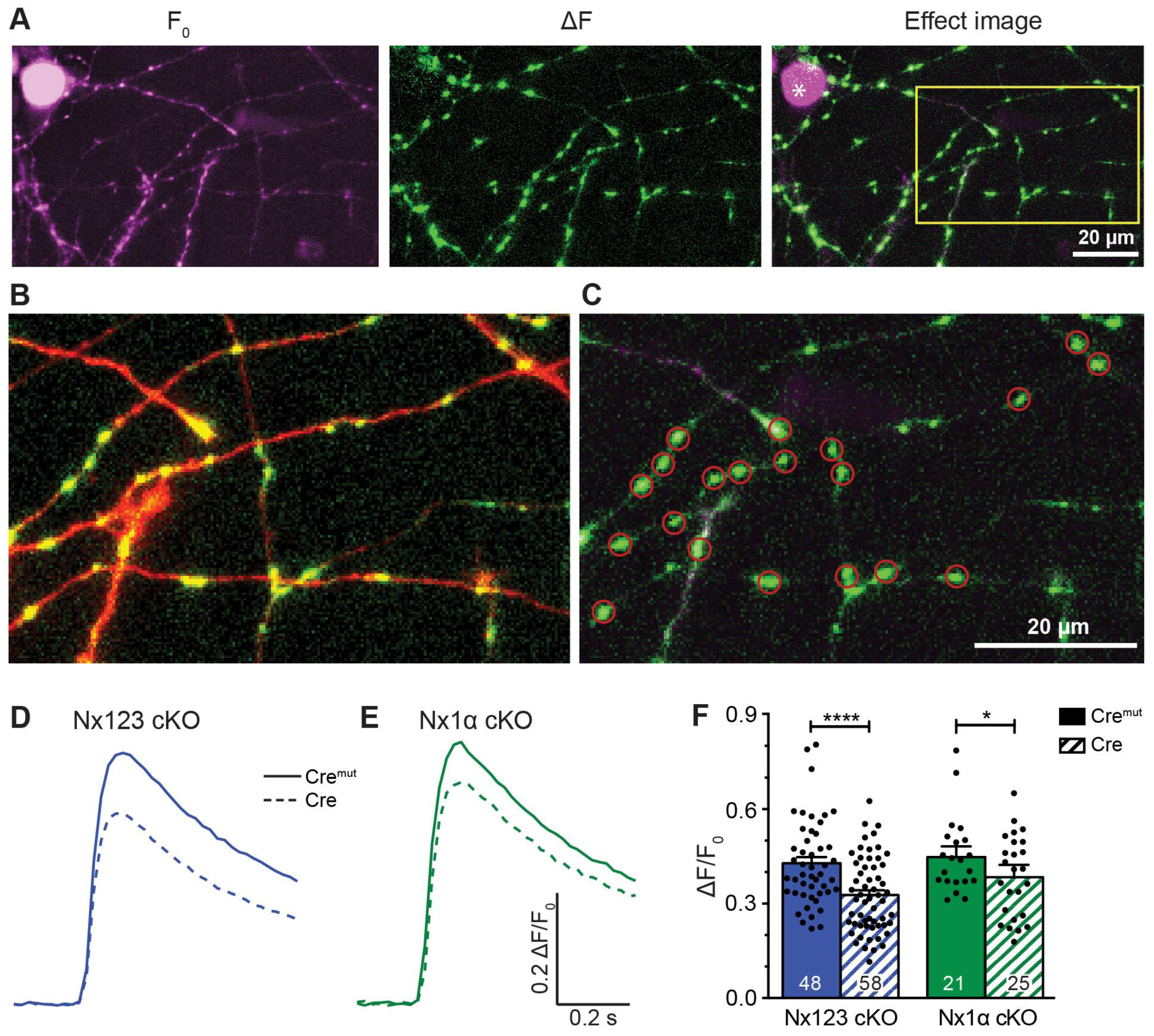
3.2. Deleting the Single Nx1α Variant is Sufficient to Reduce the Total Presynaptic Ca2+ Influx
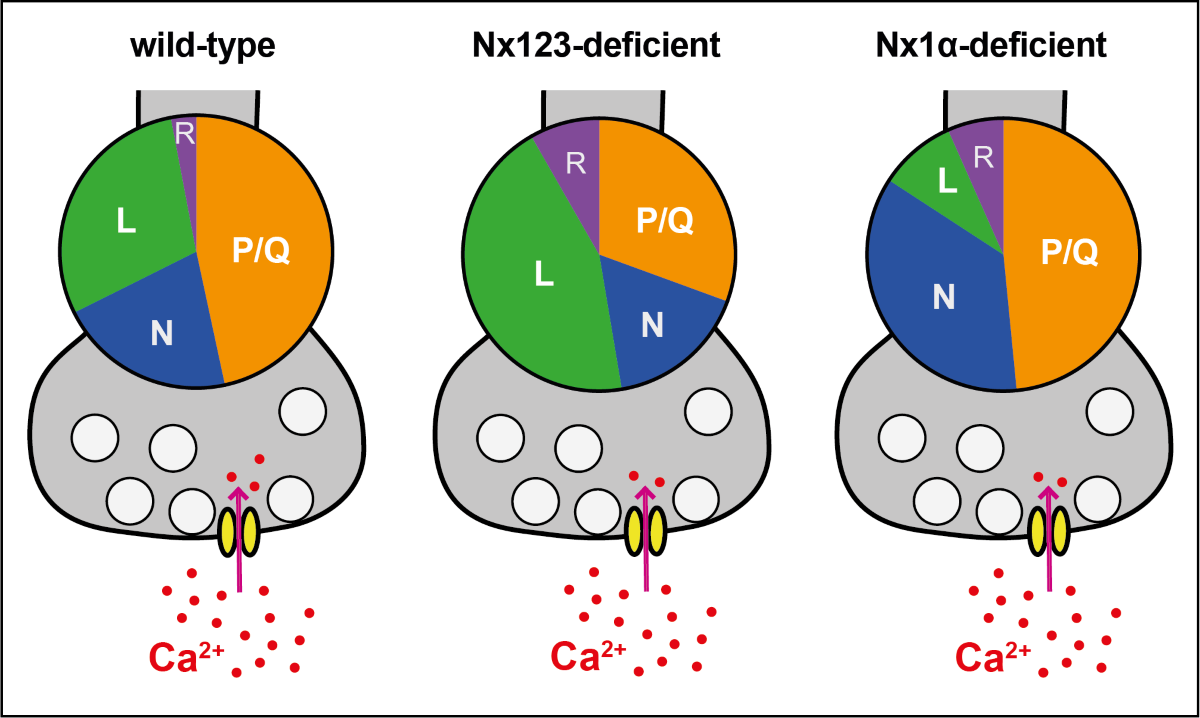
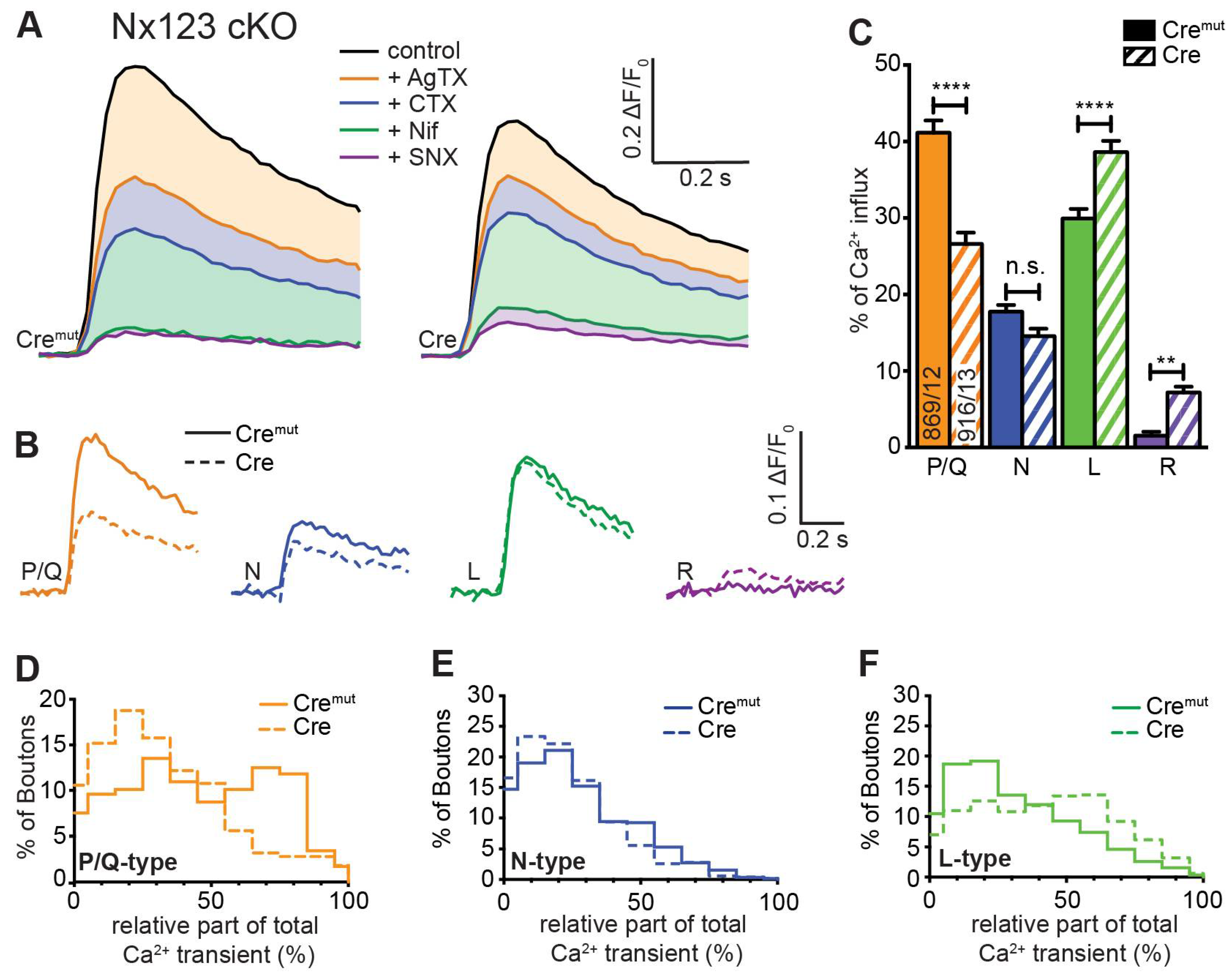
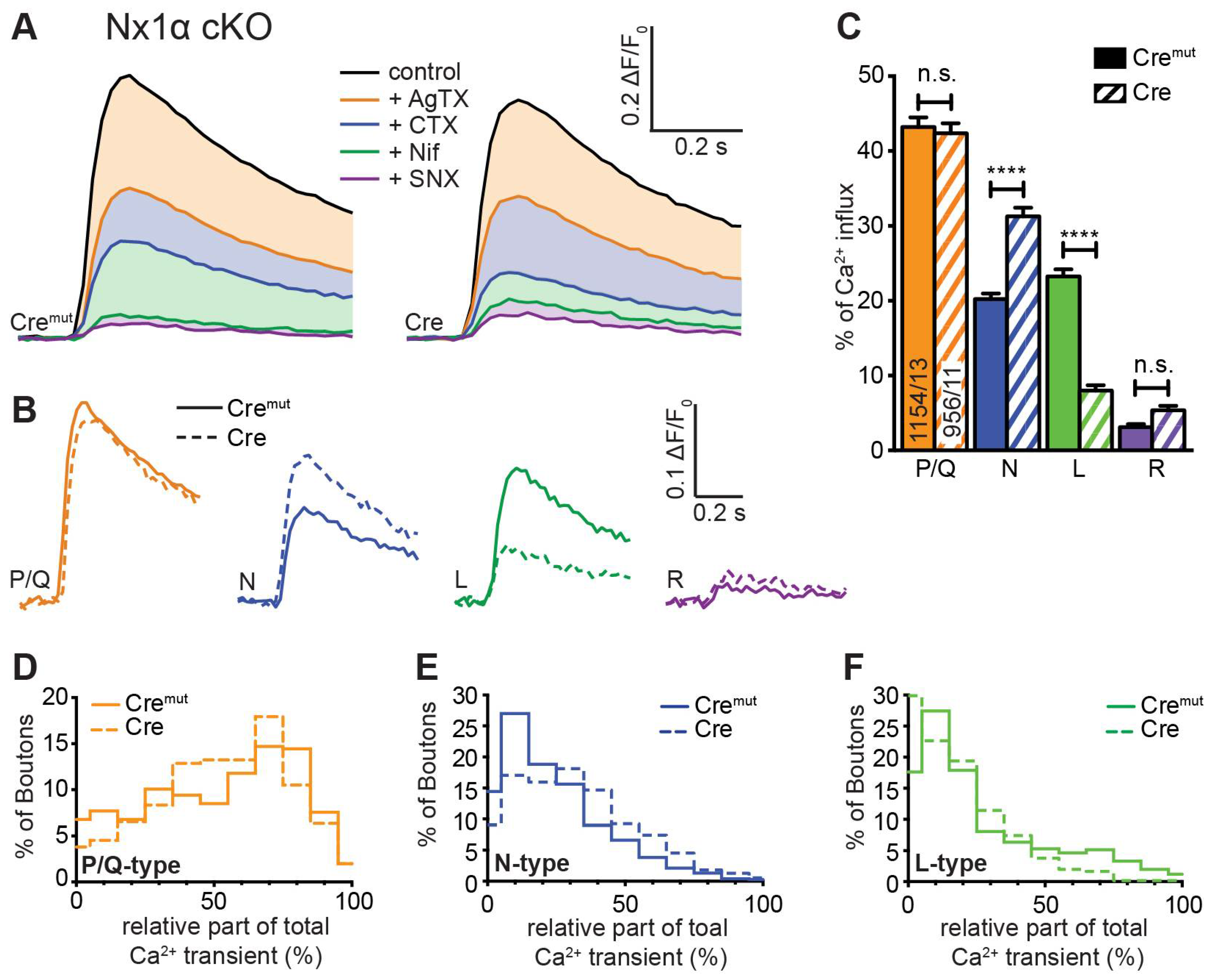
3.3. Deletion of All Nx Predominantly Reduced Ca2+ Influx through P/Q-Type VGCC
3.4. Deletion of the Single Nx1α Variant Altered the Pattern of VGCC Subtype Contribution to Presynaptic Ca2+ Influx
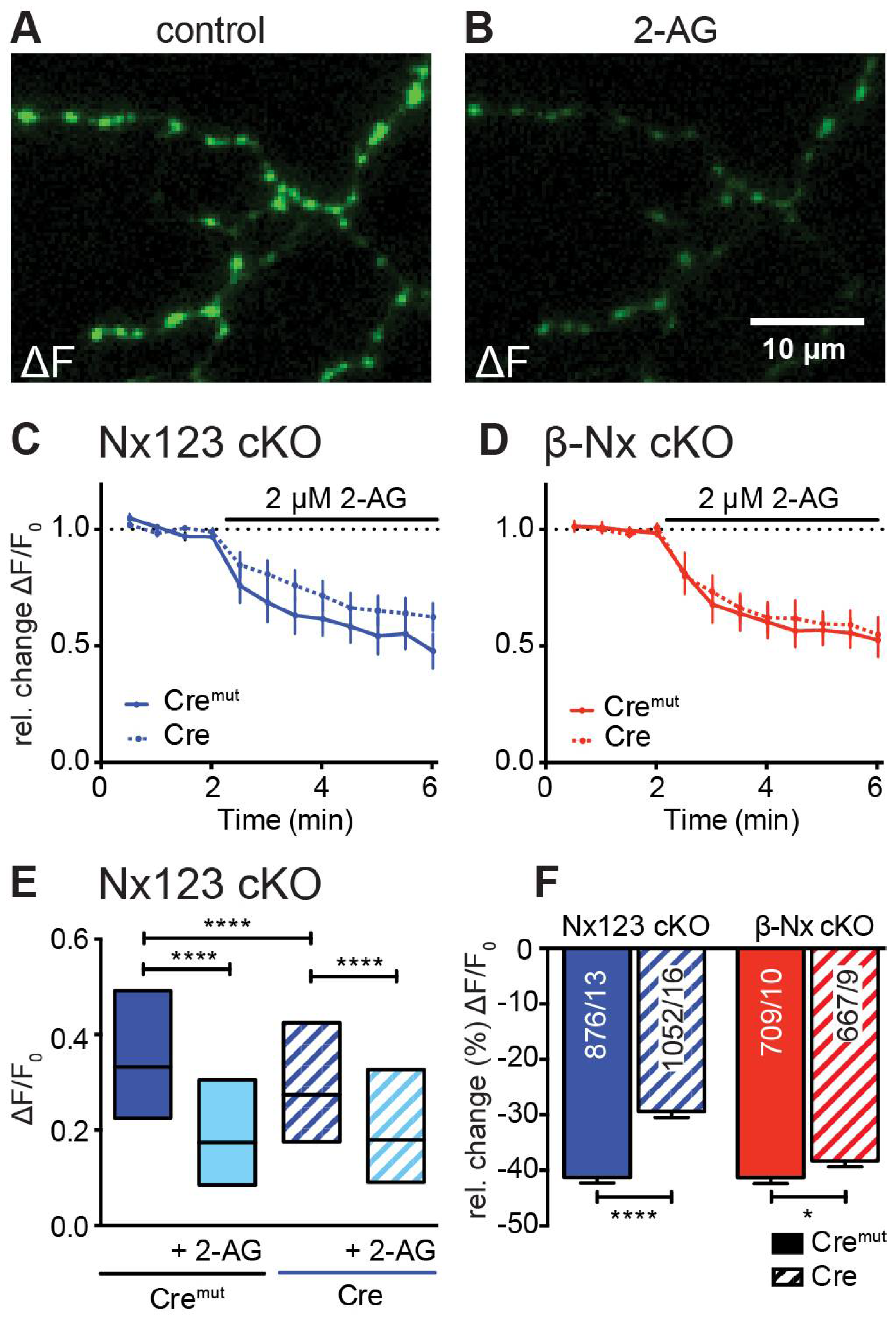
3.5. Deletions of Nx also Affect the Endocannabinoid Receptor-Dependent Modulation of Presynaptic Ca2+ Influx
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catterall, W. A. Structure and Regulation of Voltage-Gated Ca2+-Channels. Annu Rev Cell Dev Biol 2000, 16, 521–55. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, A. C.; Lee, A. Presynaptic calcium channels: specialized control of synaptic neurotransmitter release. Nat Rev Neurosci 2020, 21, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; He, L.; Zheng, H.; Xue, L.; Luo, F.; Shin, W.; Sun, T.; Kuner, T.; Yue, D. T.; Wu, L. G. Calcium-channel number critically influences synaptic strength and plasticity at the active zone. Nat Neurosci 2012, 15, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Harada, H.; Kamasawa, N.; Matsui, K.; Rothman, J. S.; Shigemoto, R.; Silver, R. A.; DiGregorio, D. A.; Takahashi, T. Nanoscale distribution of presynaptic Ca(2+) channels and its impact on vesicular release during development. Neuron 2015, 85, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bischofberger, J.; Jonas, P. Differential gating and recruitment of P/Q-, N-, and R-type Ca2+ channels in hippocampal mossy fiber boutons. J Neurosci 2007, 27, 13420–9. [Google Scholar] [CrossRef]
- Cao, Y. Q.; Tsien, R. W. Different relationship of N- and P/Q-type Ca2+ channels to channel-interacting slots in controlling neurotransmission at cultured hippocampal synapses. J Neurosci 2010, 30, 4536–46. [Google Scholar] [CrossRef]
- Chen, J. J.; Kaufmann, W. A.; Chen, C.; Arai, I.; Kim, O.; Shigemoto, R.; Jonas, P. Developmental transformation of Ca2+ channel-vesicle nanotopography at a central GABAergic synapse. Neuron 2024, 112, 755–771. [Google Scholar] [CrossRef]
- Helton, T. D.; Xu, W.; Lipscombe, D. Neuronal L-type calcium channels open quickly and are inhibited slowly. J Neurosci 2005, 25, 10247–51. [Google Scholar] [CrossRef]
- Brockhaus, J.; Bruggen, B.; Missler, M. Imaging and Analysis of Presynaptic Calcium Influx in Cultured Neurons Using synGCaMP6f. Front Synaptic Neurosci 2019, 11, 12. [Google Scholar] [CrossRef]
- Dunlap, K.; Luebke, J. I.; Turner, T. J. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995, 18, 89–98. [Google Scholar] [CrossRef]
- Nanou, E.; Catterall, W. A. Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease. Neuron 2018, 98, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Mochida, S. Presynaptic Calcium Channels. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, C.; Allen, N. J.; Susman, M. W.; O'Rourke, N. A.; Park, C. Y.; Ozkan, E.; Chakraborty, C.; Mulinyawe, S. B.; Annis, D. S.; Huberman, A. D.; Green, E. M.; Lawler, J.; Dolmetsch, R.; Garcia, K. C.; Smith, S. J.; Luo, Z. D.; Rosenthal, A.; Mosher, D. F.; Barres, B. A. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009, 139, 380–92. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yu, Y. P.; Zhou, C. Y.; Li, K. W.; Wang, D.; Chang, E.; Kim, D. S.; Vo, B.; Zhang, X.; Gong, N.; Sharp, K.; Steward, O.; Vitko, I.; Perez-Reyes, E.; Eroglu, C.; Barres, B.; Zaucke, F.; Feng, G.; Luo, Z. D. Central Mechanisms Mediating Thrombospondin-4-induced Pain States. J Biol Chem 2016, 291, 13335–48. [Google Scholar] [CrossRef] [PubMed]
- Tong, X. J.; Lopez-Soto, E. J.; Li, L.; Liu, H.; Nedelcu, D.; Lipscombe, D.; Hu, Z.; Kaplan, J. M. Retrograde Synaptic Inhibition Is Mediated by alpha-Neurexin Binding to the alpha2delta Subunits of N-Type Calcium Channels. Neuron 2017, 95, 326–340 e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fehlhaber, K. E.; Sarria, I.; Cao, Y.; Ingram, N. T.; Guerrero-Given, D.; Throesch, B.; Baldwin, K.; Kamasawa, N.; Ohtsuka, T.; Sampath, A. P.; Martemyanov, K. A. The Auxiliary Calcium Channel Subunit alpha2delta4 Is Required for Axonal Elaboration, Synaptic Transmission, and Wiring of Rod Photoreceptors. Neuron 2017, 93, 1359–1374 e6. [Google Scholar] [CrossRef] [PubMed]
- Brockhaus, J.; Schreitmuller, M.; Repetto, D.; Klatt, O.; Reissner, C.; Elmslie, K.; Heine, M.; Missler, M. alpha-Neurexins Together with alpha2delta-1 Auxiliary Subunits Regulate Ca(2+) Influx through Cav2.1 Channels. J Neurosci 2018, 38, 8277–8294. [Google Scholar] [CrossRef]
- Dahimene, S.; Page, K. M.; Kadurin, I.; Ferron, L.; Ho, D. Y.; Powell, G. T.; Pratt, W. S.; Wilson, S. W.; Dolphin, A. C. The alpha(2)delta-like Protein Cachd1 Increases N-type Calcium Currents and Cell Surface Expression and Competes with alpha(2)delta-1. Cell Rep 2018, 25, 1610–1621 e5. [Google Scholar] [CrossRef] [PubMed]
- Risher, W. C.; Kim, N.; Koh, S.; Choi, J. E.; Mitev, P.; Spence, E. F.; Pilaz, L. J.; Wang, D.; Feng, G.; Silver, D. L.; Soderling, S. H.; Yin, H. H.; Eroglu, C. Thrombospondin receptor alpha2delta-1 promotes synaptogenesis and spinogenesis via postsynaptic Rac1. J Cell Biol 2018, 217, 3747–3765. [Google Scholar] [CrossRef]
- Geisler, S.; Schopf, C. L.; Stanika, R.; Kalb, M.; Campiglio, M.; Repetto, D.; Traxler, L.; Missler, M.; Obermair, G. J. Presynaptic alpha(2)delta-2 Calcium Channel Subunits Regulate Postsynaptic GABA(A) Receptor Abundance and Axonal Wiring. J Neurosci 2019, 39, 2581–2605. [Google Scholar] [CrossRef]
- Reissner, C.; Runkel, F.; Missler, M. Neurexins. Genome Biol 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T. C. Synaptic Neurexin Complexes: A Molecular Code for the Logic of Neural Circuits. Cell 2017, 171, 745–769. [Google Scholar] [CrossRef]
- Missler, M.; Zhang, W.; Rohlmann, A.; Kattenstroth, G.; Hammer, R. E.; Gottmann, K.; Südhof, T. C. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 2003, 423, 939–948. [Google Scholar] [CrossRef]
- Sterky, F. H.; Trotter, J. H.; Lee, S. J.; Recktenwald, C. V.; Du, X.; Zhou, B.; Zhou, P.; Schwenk, J.; Fakler, B.; Sudhof, T. C. Carbonic anhydrase-related protein CA10 is an evolutionarily conserved pan-neurexin ligand. Proc Natl Acad Sci U S A 2017, 114, E1253–E1262. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, D.; Nguyen, T. M.; Russo, G.; Heber, S.; Patrignani, A.; Ahrne, E.; Scheiffele, P. Targeted combinatorial alternative splicing generates brain region-specific repertoires of neurexins. Neuron 2014, 84, 386–98. [Google Scholar] [CrossRef] [PubMed]
- Treutlein, B.; Gokce, O.; Quake, S. R.; Sudhof, T. C. Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing. Proc Natl Acad Sci U S A 2014, 111, E1291–9. [Google Scholar] [CrossRef]
- Fairless, R.; Masius, H.; Rohlmann, A.; Heupel, K.; Ahmad, M.; Reissner, C.; Dresbach, T.; Missler, M. Polarized targeting of neurexins to synapses is regulated by their C-terminal sequences. J Neurosci 2008, 28, 12969–81. [Google Scholar] [CrossRef]
- Neupert, C.; Schneider, R.; Klatt, O.; Reissner, C.; Repetto, D.; Biermann, B.; Niesmann, K.; Missler, M.; Heine, M. Regulated Dynamic Trafficking of Neurexins Inside and Outside of Synaptic Terminals. J Neurosci 2015, 35, 13629–47. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, H.; Peixoto, R. T.; Pines, M. K.; Ge, Y.; Oku, S.; Siddiqui, T. J.; Xie, Y.; Wu, W.; Archer-Hartmann, S.; Yoshida, K.; Tanaka, K. F.; Aricescu, A. R.; Azadi, P.; Gordon, M. D.; Sabatini, B. L.; Wong, R. O. L.; Craig, A. M. Heparan Sulfate Organizes Neuronal Synapses through Neurexin Partnerships. Cell 2018, 174, 1450–1464 e23. [Google Scholar] [CrossRef]
- Trotter, J. H.; Hao, J.; Maxeiner, S.; Tsetsenis, T.; Liu, Z.; Zhuang, X.; Sudhof, T. C. Synaptic neurexin-1 assembles into dynamically regulated active zone nanoclusters. J Cell Biol 2019, 218, 2677–2698. [Google Scholar] [CrossRef]
- Ichtchenko, K.; Hata, Y.; Nguyen, T.; Ullrich, B.; Missler, M.; Moomaw, C.; Südhof, T. C. , Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell 1995, 81, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Boucard, A. A.; Chubykin, A. A.; Comoletti, D.; Taylor, P.; Sudhof, T. C. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron 2005, 48, 229–36. [Google Scholar] [CrossRef]
- Reissner, C.; Klose, M.; Fairless, R.; Missler, M. Mutational analysis of the neurexin/neuroligin complex reveals essential and regulatory components. Proc Natl Acad Sci U S A 2008, 105, 15124–9. [Google Scholar] [CrossRef]
- de Wit, J.; Sylwestrak, E.; O'Sullivan, M. L.; Otto, S.; Tiglio, K.; Savas, J. N.; Yates, J. R., 3rd; Comoletti, D.; Taylor, P.; Ghosh, A. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron 2009, 64, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Fuccillo, M. V.; Malenka, R. C.; Sudhof, T. C. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron 2009, 64, 791–8. [Google Scholar] [CrossRef]
- Siddiqui, T. J.; Pancaroglu, R.; Kang, Y.; Rooyakkers, A.; Craig, A. M. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J Neurosci 2010, 30, 7495–506. [Google Scholar] [CrossRef]
- Sugita, S.; Saito, F.; Tang, J.; Satz, J.; Campbell, K.; Sudhof, T. C. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol 2001, 154, 435–45. [Google Scholar] [CrossRef]
- Reissner, C.; Stahn, J.; Breuer, D.; Klose, M.; Pohlentz, G.; Mormann, M.; Missler, M. Dystroglycan binding to alpha-neurexin competes with neurexophilin-1 and neuroligin in the brain. J Biol Chem 2014, 289, 27585–603. [Google Scholar] [CrossRef] [PubMed]
- Boucard, A. A.; Ko, J.; Sudhof, T. C. High affinity neurexin binding to cell adhesion G-protein-coupled receptor CIRL1/latrophilin-1 produces an intercellular adhesion complex. J Biol Chem 2012, 287, 9399–413. [Google Scholar] [CrossRef]
- Uemura, T.; Lee, S. J.; Yasumura, M.; Takeuchi, T.; Yoshida, T.; Ra, M.; Taguchi, R.; Sakimura, K.; Mishina, M. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell 2010, 141, 1068–79. [Google Scholar] [CrossRef]
- Matsuda, K.; Yuzaki, M. Cbln family proteins promote synapse formation by regulating distinct neurexin signaling pathways in various brain regions. Eur J Neurosci 2011, 33, 1447–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Rohlmann, A.; Sargsyan, V.; Aramuni, G.; Hammer, R. E.; Sudhof, T. C.; Missler, M. Extracellular domains of alpha-neurexins participate in regulating synaptic transmission by selectively affecting N- and P/Q-type Ca2+ channels. J Neurosci 2005, 25, 4330–42. [Google Scholar] [CrossRef] [PubMed]
- Dudanova, I.; Sedej, S.; Ahmad, M.; Masius, H.; Sargsyan, V.; Zhang, W.; Riedel, D.; Angenstein, F.; Schild, D.; Rupnik, M.; Missler, M. Important contribution of alpha-neurexins to Ca2+-triggered exocytosis of secretory granules. J Neurosci 2006, 26, 10599–613. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. R.; Aoto, J.; Tabuchi, K.; Foldy, C.; Covy, J.; Yee, A. X.; Wu, D.; Lee, S. J.; Chen, L.; Malenka, R. C.; Sudhof, T. C. beta-Neurexins Control Neural Circuits by Regulating Synaptic Endocannabinoid Signaling. Cell 2015, 162, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Klatt, O.; Repetto, D.; Brockhaus, J.; Reissner, C.; El Khallouqi, A.; Rohlmann, A.; Heine, M.; Missler, M. Endogenous beta-neurexins on axons and within synapses show regulated dynamic behavior. Cell Rep 2021, 35, 109266. [Google Scholar] [CrossRef]
- Chen, L. Y.; Jiang, M.; Zhang, B.; Gokce, O.; Sudhof, T. C. Conditional Deletion of All Neurexins Defines Diversity of Essential Synaptic Organizer Functions for Neurexins. Neuron 2017, 94, 611–625 e4. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Sclip, A.; Jiang, M.; Sudhof, T. C. , Neurexins cluster Ca(2+) channels within the presynaptic active zone. EMBO J 2020, 39, e103208. [Google Scholar] [CrossRef] [PubMed]
- Aoto, J.; Martinelli, D. C.; Malenka, R. C.; Tabuchi, K.; Sudhof, T. C. Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell 2013, 154, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Sun, Y.; Mohar, B.; Hulse, B. K.; Kerlin, A. M.; Hasseman, J. P.; Tsegaye, G.; Tsang, A.; Wong, A.; Patel, R.; Macklin, J. J.; Chen, Y.; Konnerth, A.; Jayaraman, V.; Looger, L. L.; Schreiter, E. R.; Svoboda, K.; Kim, D. S. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat Methods 2019, 16, 649–657. [Google Scholar] [CrossRef]
- Geppert, M.; Khvotchev, M.; Krasnoperov, V.; Goda, Y.; Missler, M.; Hammer, R. E.; Ichtchenko, K.; Petrenko, A. G.; Sudhof, T. C. Neurexin I alpha is a major alpha-latrotoxin receptor that cooperates in alpha-latrotoxin action. J Biol Chem 1998, 273, 1705–10. [Google Scholar] [CrossRef]
- Eroshenko, N.; Church, G. M. Mutants of Cre recombinase with improved accuracy. Nat Commun 2013, 4, 2509. [Google Scholar] [CrossRef] [PubMed]
- de Jong, A. P.; Schmitz, S. K.; Toonen, R. F.; Verhage, M. Dendritic position is a major determinant of presynaptic strength. J Cell Biol 2012, 197, 327–37. [Google Scholar] [CrossRef] [PubMed]
- Docs, K.; Meszar, Z.; Gonda, S.; Kiss-Szikszai, A.; Hollo, K.; Antal, M.; Hegyi, Z. The Ratio of 2-AG to Its Isomer 1-AG as an Intrinsic Fine Tuning Mechanism of CB1 Receptor Activation. Front Cell Neurosci 2017, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, S.; Kakazu, Y.; Koh, J. Y.; Harata, N. C. Evaluation of the effectiveness of Gaussian filtering in distinguishing punctate synaptic signals from background noise during image analysis. J Neurosci Methods 2014, 223, 92–113. [Google Scholar] [CrossRef] [PubMed]
- Motulsky, H. J.; Brown, R. E. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 2006, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Etherton, M. R.; Blaiss, C. A.; Powell, C. M.; Südhof, T. C. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci U S A 2009, 106, 17998–8003. [Google Scholar] [CrossRef] [PubMed]
- Grayton, H. M.; Missler, M.; Collier, D. A.; Fernandes, C. Altered social behaviours in neurexin 1alpha knockout mice resemble core symptoms in neurodevelopmental disorders. PLoS One 2013, 8, e67114. [Google Scholar] [CrossRef] [PubMed]
- Dachtler, J.; Ivorra, J. L.; Rowland, T. E.; Lever, C.; Rodgers, R. J.; Clapcote, S. J. Heterozygous deletion of alpha-neurexin I or alpha-neurexin II results in behaviors relevant to autism and schizophrenia. Behav Neurosci 2015, 129, 765–76. [Google Scholar] [CrossRef] [PubMed]
- Tottene, A.; Volsen, S.; Pietrobon, D. alpha(1E) subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. J Neurosci 2000, 20, 171–178. [Google Scholar] [CrossRef]
- Metz, A. E.; Jarsky, T.; Martina, M.; Spruston, N. R-type calcium channels contribute to afterdepolarization and bursting in hippocampal CA1 pyramidal neurons. J Neurosci 2005, 25, 5763–73. [Google Scholar] [CrossRef]
- Twitchell, W.; Brown, S.; Mackie, K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol 1997, 78, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R. I.; Nicoll, R. A. Endocannabinoid signaling in the brain. Science 2002, 296, 678–82. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Waku, K. 2-Arachidonoylglycerol and the cannabinoid receptors. Chem Phys Lipids 2000, 108, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Diana, M. A.; Marty, A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br J Pharmacol 2004, 142, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kreitzer AC, R. W. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 2001, 29, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Diana, M. A.; Levenes, C.; Mackie, K.; Marty, A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci 2002, 22, 200–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Linden, D. J. Neuromodulation at single presynaptic boutons of cerebellar parallel fibers is determined by bouton size and basal action potential-evoked Ca transient amplitude. J Neurosci 2009, 29, 15586–94. [Google Scholar] [CrossRef] [PubMed]
- Blankman, J. L.; Simon, G. M.; Cravatt, B. F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol 2007, 14, 1347–56. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Sclip, A.; Merrill, S.; Sudhof, T. C. Neurexins regulate presynaptic GABA(B)-receptors at central synapses. Nat Commun 2021, 12, 2380. [Google Scholar] [CrossRef]
- Heine, M.; Thoumine, O.; Mondin, M.; Tessier, B.; Giannone, G.; Choquet, D. Activity-independent and subunit-specific recruitment of functional AMPA receptors at neurexin/neuroligin contacts. Proc Natl Acad Sci U S A 2008, 105, 20947–52. [Google Scholar] [CrossRef]
- Aoto, J.; Foldy, C.; Ilcus, S. M.; Tabuchi, K.; Sudhof, T. C. Distinct circuit-dependent functions of presynaptic neurexin-3 at GABAergic and glutamatergic synapses. Nat Neurosci 2015, 18, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhang, X.; Dobie, F.; Wu, H.; Craig, A. M. , Induction of GABAergic postsynaptic differentiation by alpha-neurexins. J Biol Chem 2008, 283, 2323–34. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




