Submitted:
05 May 2024
Posted:
06 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results & Discussion
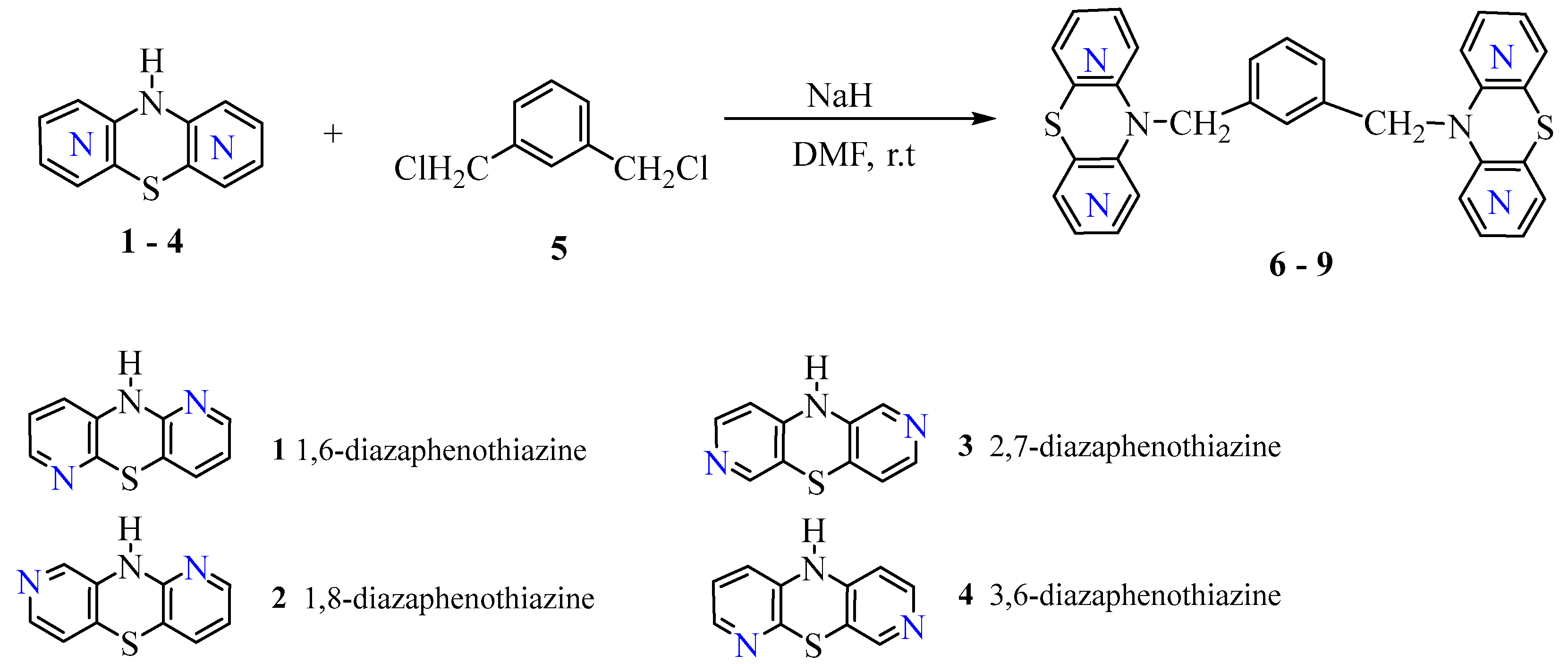
2.1. Chemistry Part
2.2. Structural Identification Using Spectroscopic Methods
2.3. In Silico Target Prediction
2.4. Biological Part – Analysis of Cytotoxicity towards Cancer and Normal Cells
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for Synthesis of Compounds (6-9)
3.2. In Silico Target Prediction
3.3. Biological Evaluation
3.3.1. Cell Line and Culture
3.3.2. MTT Cell Viability Assay
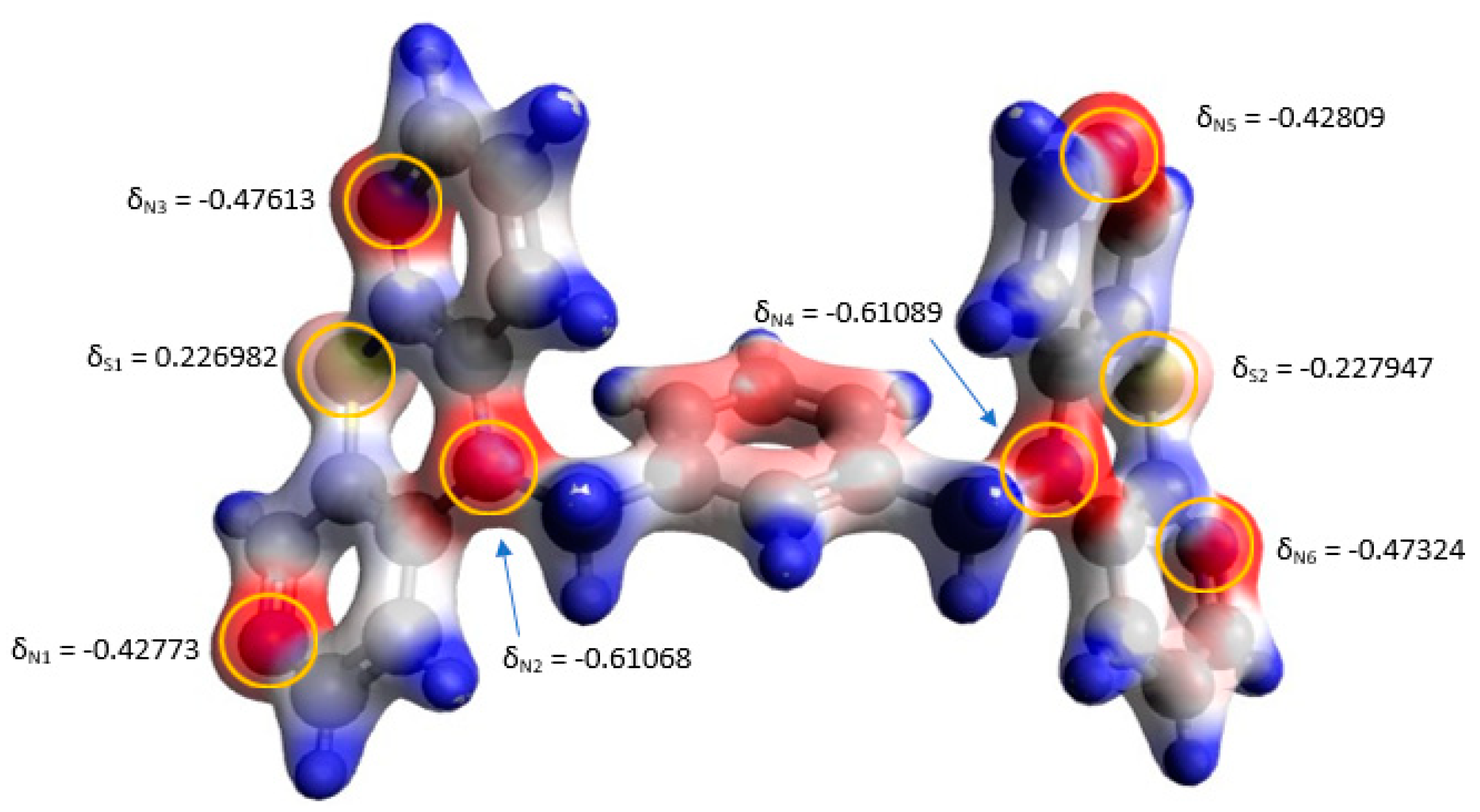
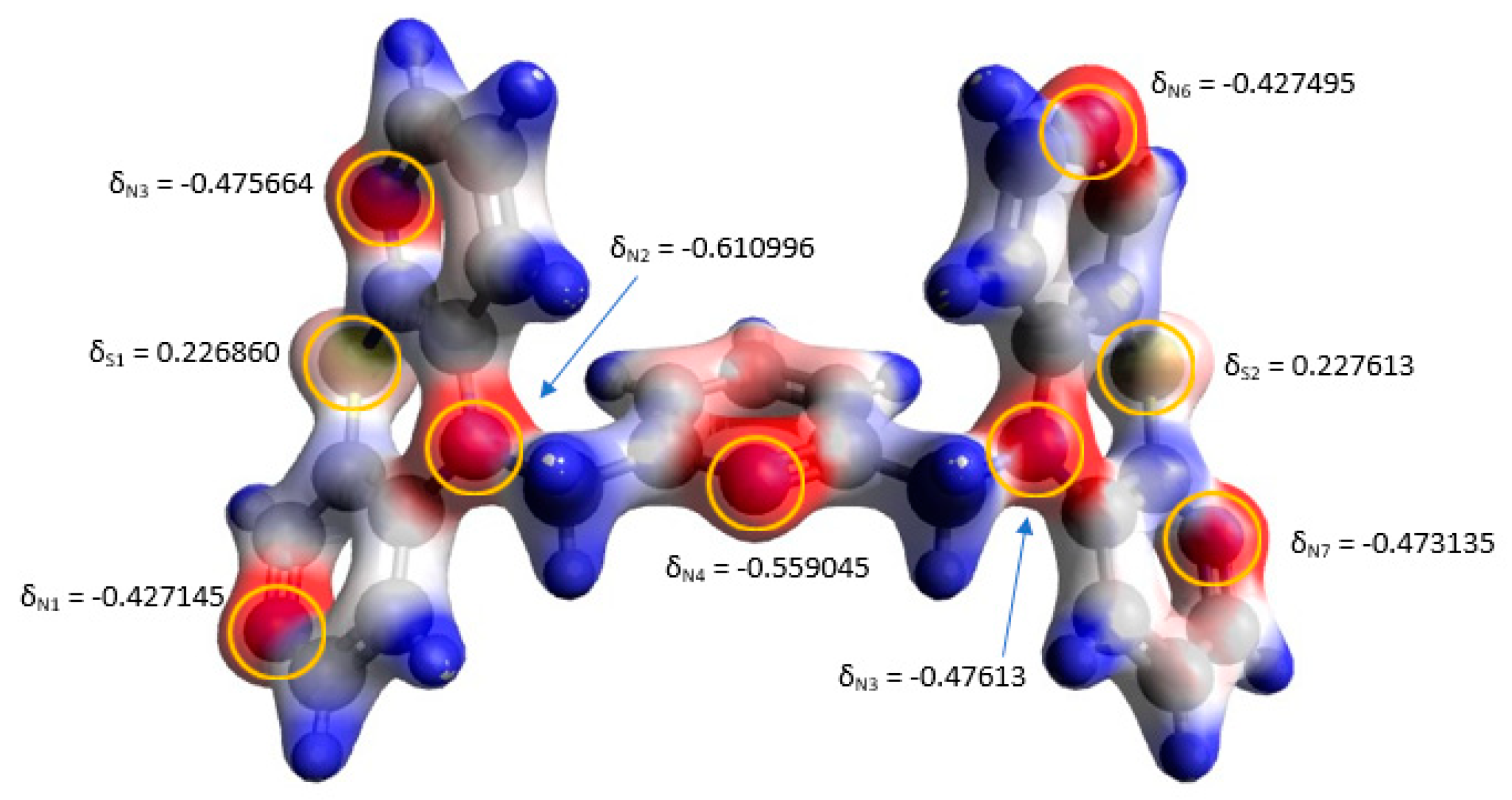
3.4. Quantum Mechanical Calculations
3.5. The Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Muñoz, F.; Alamo, C.; Cuenca, E.; Shen, W.W.; Clervoy, P.; Rubio, G. History of the Discovery and Clinical Introduction of Chlorpromazine. Annals of Clinical Psychiatry 2005, 17, 113–135. [Google Scholar] [CrossRef]
- Jaszczyszyn, A.; Gąsiorowski, K.; Świątek, P.; Malinka, W.; Cieślik-Boczula, K.; Petrus, J.; Czarnik-Matusewicz, B. Chemical structure of phenothiazines and their biological activity. Pharmacological Reports 2012, 64, 16–23. [Google Scholar] [CrossRef]
- Otręba, M.; Stojko, J.; Rzepecka-Stojko, A. Phenothiazine derivatives and their impact on the necroptosis and necrosis processes. A review Toxicology 2023, 492, 1–9. [Google Scholar] [CrossRef]
- Kumar, A.; Vigato, C.; Boschi, D.; Lolli, M.L.; Kumar, D. Phenothiazines as anti-cancer agents: SAR overview and synthetic strategies. European Journal of Medicinal Chemistry 2023, 254, 115337. [Google Scholar] [CrossRef]
- Swoboda, D.; Nycz, J.E.; Karaush-Karmazin, N.; Minaev, B.; Książek, M.; Kusz, J.; Podsiadły, R. Synthesis and Spectroscopic Characterization of Selected Phenothiazines and Phenazines Rationalized Based on DFT Calculation. Molecules 2022, 27, 7519–1. [Google Scholar] [CrossRef]
- Li, J.J. Heterocyclic Chemistry in Drug Discovery; Wiley: Hoboken, 2013; Volume 52, pp. 1–16. [Google Scholar] [CrossRef]
- Otręba, M.; Stojko, J.; Rzepecka-Stojko, A. The role of phenothiazine derivatives in autophagy regulation: A systematic review. Journal of Applied Toxicology 2023, 43, 474–489. [Google Scholar] [CrossRef]
- Lopes, R.M.; Souza, A.C.; Otręba, M.; Rzepecka-Stojko, A.; Tersariol, I.; Rodrigues, T. Targeting autophagy by antipsychotic phenothiazines: potential drug repurposing for cancer therapy. Biochemical Pharmacology 2024, 222, 116075. [Google Scholar] [CrossRef]
- Xu, F.; Xi, H.; Liao, M.; Zhang, Y.; Ma, H.; Wu, M.; Xue, Q.; Sun, H.; Zhang, Y.; Xia, Y. Repurposed antipsychotic chlorpromazine inhibits colorectal cancer and pulmonary metastasis by inducing G2/M cell cycle arrest, apoptosis, and autophagy. Cancer Chemotherapy and Pharmacology 2022, 89, 331–346. [Google Scholar] [CrossRef]
- Li, A.; Chen, X.; Jing, Z.; Chen, J. Trifluoperazine induces cellular apoptosis by inhibiting autophagy and targeting NUPR1 in multiple myeloma. FEBS Open Bio 2020, 10, 2097–2106. [Google Scholar] [CrossRef]
- Posso, M.C.; Domingues, F.C.; Ferreira, S.; Silvestre, S. Development of Phenothiazine Hybrids with Potential Medicinal Interest: A Review. Molecules 2022, 27, 276. [Google Scholar] [CrossRef]
- Rineh, A.; Dolla, N.K.; Ball, A.R.; Magana, M.; Bremner, J.B.; Hamblin, M.R.; Tegos, G.P.; Kelso, M.J. Attaching the NorA Efflux Pump Inhibitor INF55 to Methylene Blue Enhances Antimicrobial Photodynamic Inactivation of Methicillin-Resistant Staphylococcus aureus in Vitro and in Vivo. ACS Infectious Diseases 2017, 3, 756–766. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Gotay, W.J.P.; Pereira, V.S.; de Oliveira, J.S.; Pereira-Neto, W.A.; Castelo-Branco, D.d.S.C.M.; de Aguiar Cordeiro, R.; Sidrim, J.J.C.; Rocha, M.F.G. Antifungal Activity of Promethazine and Chlorpromazine Against Planktonic Cells and Biofilms of Cryptococcus neoformans/Cryptococcus gattii Complex Species. Medical Mycology 2020, 58, 906–912. [Google Scholar] [CrossRef]
- Piccini, L.E.; Castilla, V.; Damonte, E.B. Inhibition of dengue virus infection by trifluoperazine. Archives of Virology 2022, 167, 2203–2212. [Google Scholar] [CrossRef]
- Simanjuntak, Y.; Liang, J.J.; Lee, Y.L.; Lin, Y.L. Repurposing of prochlorperazine for use against dengue virus infection. Journal of Infectious Diseases 2015, 211, 394–404. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Tucci, P.; Buttari, B.; Nwobodo, D.C.; Marini, P.; Saso, L. Phenothiazines: Nrf2 activation and antioxidant effects. Journal of Biochemical and Molecular Toxicology 2024, 38, 23661. [Google Scholar] [CrossRef]
- Takács, D.; Csonka, Á.; Horváth, Á.; Windt, T.; Gajdács, M.; Riedl, Z.; Hajós, G.; Amaral, L.; Molnár, J.; Spengler, G. Reversal of ABCB1-related Multidrug Resistance of Colonic Adenocarcinoma Cells by Phenothiazines. Anticancer Research 2015, 35, 3245–3251. [Google Scholar]
- Mosnaim, A.D.; Ranade, V.V.; Wolf, M.E.; Puente, J.; Valenzuela, M.A. Phenothiazine molecule provides the basic chemical structure for various classes of pharmacotherapeutic agents. American Journal of Therapeutics 2006, 13, 261–273. [Google Scholar] [CrossRef]
- Ohlow, M.; Moosmann, B. Phenothiazine: The seven lives of pharmacology’s first lead structure. Drug Discovery Today 2011, 16, 119–131. [Google Scholar] [CrossRef]
- Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Synthesis and properties of diaza-, triaza- and tetraazaphenothiazines. Journal of Heterocyclic Chemistry 2009, 46, 355–391. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Jeleń, M. Phenothiazines Modified with the Pyridine Ring as Promising Anticancer Agents. Life 2021, 11, 206. [Google Scholar] [CrossRef]
- Kumar, A.; Vigato, C.; Boschi, D.; Lolli, M.L.; Kumar, D. Phenothiazines as anti-cancer agents: SAR overview and synthetic strategies. European Journal of Medicinal Chemistry 2023, 254, 115337. [Google Scholar] [CrossRef]
- Mitchell, S.C. Phenothiazine: the parent molecule. Current Drug Targets 2006, 7, 1181–1189. [Google Scholar] [CrossRef]
- Gupta, R.R.; Kumar, M. Synthesis, properties and reactions of phenothiazines. Phenothiazine and 1,4-Benzothiazines—Chemical and Biological Aspect. Elsevier 1988, 108, 1–161. [Google Scholar]
- Zhang, J.; Chen, M.; Wenzhi, Z.; Okechukwu Nwabueze, P.; Morak-Młodawska, B.; Pluta, K.; Jeleń, M.; Md Akim, A.; Ang, K.P.; Ooi, K.K. 10H-3,6-Diazaphenothiazines Induce G2/M Phase Cell Cycle Arrest, Caspase-dependent Apoptosis and Inhibits Cell Invasion of A2780 Ovarian Carcinoma Cells through Regulation on NF-κB and [BIRC6-XIAP] Complexes. Drug Design, Development and Therapy 2017, 11, 3045–3063. [Google Scholar] [CrossRef]
- Skonieczna, M.; Kasprzycka, A.; Jeleń, M.; Morak-Młodawska, B. Tri- and Pentacyclic Azaphenothiazine as Pro-Apoptotic Agents in Lung Carcinoma with a Protective Potential to Healthy Cell Lines. Molecules 2022, 27, 5255. [Google Scholar] [CrossRef]
- Sochacka, J.; Pacholczyk, M.; Jeleń, M.; Morak-Młodawska, B.; Pluta, K. Interaction of new tri-, tetra-, and pentacyclic azaphenothiazine derivatives with calf thymus DNA: Spectroscopic and molecular docking studies. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy 2021, 262. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Zimecki, M.; Jeleń, M.; Artym, J.; Kocięba, M. Synthesis and selected immunological properties of 10-substituted 1,8-diazaphenothiazines. Medicinal Chemistry Research 2015, 24, 1408–1418. [Google Scholar] [CrossRef]
- Martula, E.; Morak-Młodawska, B.; Jeleń, M.; Okechukwu, P.; Balachandran, A.; Tehirunavukarasu, P.; Anamalay, K.; Ulaganathan, V. Synthesis and structural characterization of novel dimers of dipyridothiazine as promising antiproliferative agents. Molecules 2023, 28, 7662. [Google Scholar] [CrossRef]
- Kiemle, D.; Webster, F.X.; Silverstein, R.M.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th Edition; John Wiley & Sons Inc, 2014; ISBN 978-0-470-61637-6. [Google Scholar]
- Hermann, C.h.K.F.; Morrill, T.C.; Shriner, R.L.; Fuson, R.C. Systematic Identification of Organic Compounds, Ninth Edition; Wiley, 2023; ISBN 9781119799665. [Google Scholar]
- Way2Drug Understanding Chemical-Biological Interactions, Predictive Services. Available online: www.way2drug.com/passonline/index.php (accessed on 18 December 2023).
- Druzhilovskiy, D.S.; Rudik, A.V.; Filimonov, D.A.; Gloriozova, T.A.; Lagunin, A.A.; Dmitriev, A.V.; Pogodin, P.V.; Dubovskaya, V.I.; Ivanov, S.M.; Tarasova, O.A.; Bezhentsev, V.M.; Murtazalieva, K.A.; Semin, M.I.; Maiorov, I.S.; Gaur, A.S.; Sastry, G.N.; Poroikov, V.V. Computational platform Way2Drug: from the prediction of biological activity to drug repurposing. Russian Chemical Bulletin 2017, 66, 1832–1841. [Google Scholar] [CrossRef]
- Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, 2019.
- Avogadro: an open-source molecular builder and visualization tool. Version 1.2.0. Available online: http://avogadro.cc/ (accessed on 12 March 2024).
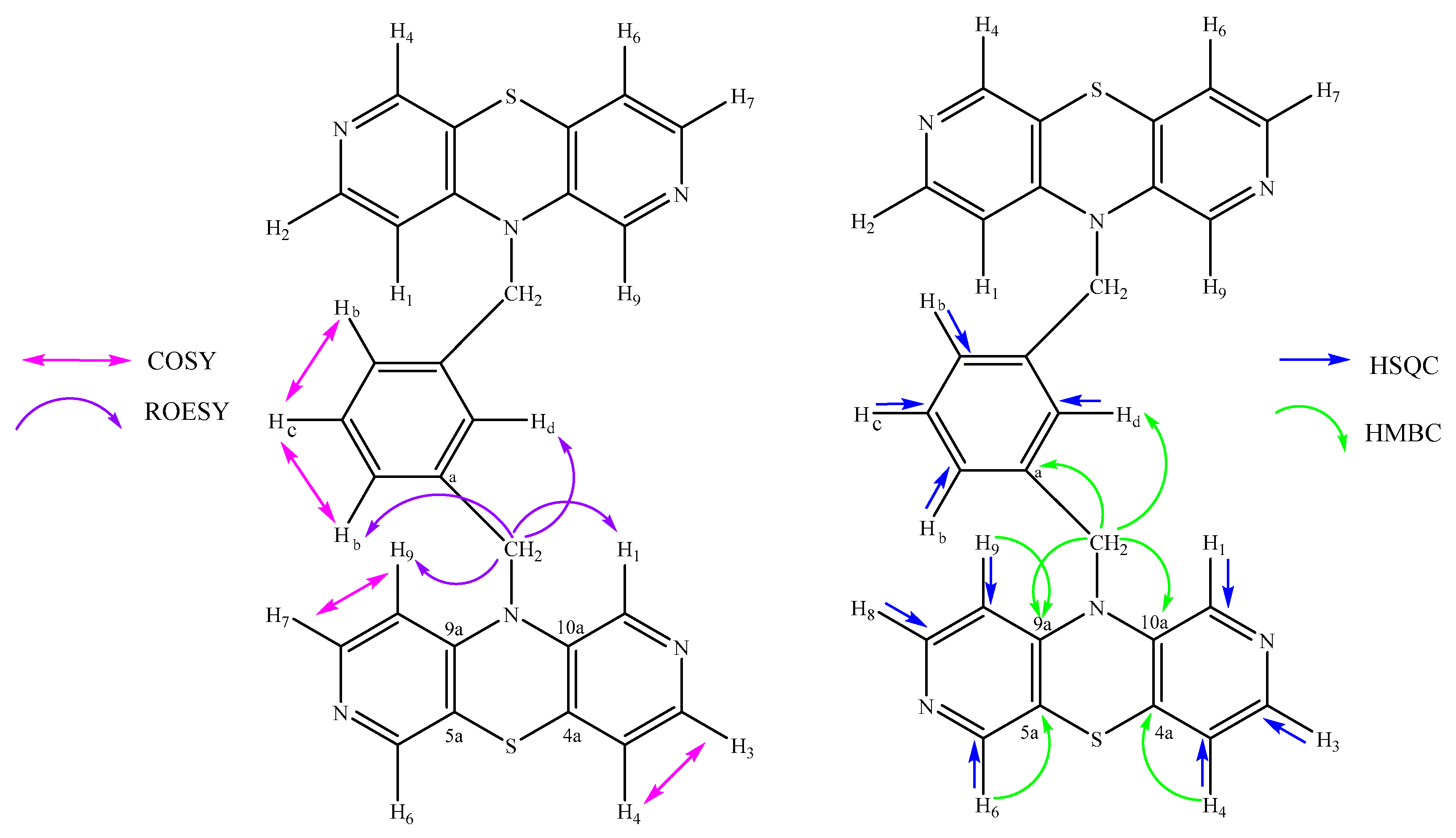
| 1H NMR (ppm) | ROESY | COSY | 13C NMR (ppm) | HSQC | HMBC | |
|---|---|---|---|---|---|---|
| CH2 5.06 H9 6.35 Hd 6.94 H4 6.97 Hb 7.25 Hc 7.45 H1 7.70 H8 7.98 H6 8.01 H3 8.10 |
5.06-7.70 5.06-6.35 5.06-7.25 5.06-6.94 6.35-8.01 7.70-6.97 6.97-8.10 |
5.06-6.94 5.06-7.25 7.25-7.45 6.35-7.96 6.97-8.10 |
C CH2 51.21 C9 109.49 C9a 117.19 Cd 121.74 C4 123.39 Cb 126.06 Cc 130.79 C10a 133.35 Ca 134.46 C1 135.56 C4a 137.22 C6 143.44 C3 145.05 C8 147.14 C5a 151.47 |
5.06-51.21 H9 6.35-109.53 Hd 6.94-121.74 H4 6.97-123.39 Hb 7.25-126.06 Hc 7.45-130.79 H1 7.70-135.56 H8 7.98-147.14 H6 8.01-143.44 H3 8.10-145.05 |
Hc 7.45-Ca 134.46 CH2 5.06-C5a 151.47 CH2 5.06- C4a 137.22 H3 8.10- C10a 133.35 H8 7.98- C9a 117.19 |
|
| No. | Probability of Activity Spectrum | ||||
| 6 | (28%) Histone deacetylase stimulant | (77%) Glycosylphosphatidylinositol phospholipase D inhibitor | (47%) Transcription factor inhibitor | (33%) Alzheimer’s disease treatment | (46%) Antiallergic |
| 7 | (29%) Mitochondrial processing peptidase inhibitor | (83%) Glycosylphosphatidylinositol phospholipase D inhibitor | (18%) Antipsychotic | (30%) Cytochrome P450 inhibitor | (10%) MAP3K8 inhibitor |
| 8 | (75%) Neurodegenerative diseases treatment | (84%) Glycosylphosphatidylinositol phospholipase D inhibitor | (65%) Histone deacetylase stimulant | (42%) Alzheimer’s disease treatment | (41%) Cytochrome P450 inhibitor |
| 9 | (76%) Histone deacetylase stimulant | (66%) Antiallergic | (60%) Neurodegenerative diseases treatment | (58%) Alzheimer’s disease treatment | (59%) Antiasthmatic |
| No. | Probability of Cytotoxicity towards Cancer Cell Lines | ||||
| 6 | Breast cancer MDA-MB-468 (62%) | Melanoma UACC-257(51%) |
Non-small cell lung carcinoma NCI-H322M (49%) | Colon carcinoma HCT-116 (42%) | Pancreatic carcinoma YAPC (43%) |
| 7 | Breast cancer MDA-MB-468 (59%) | Melanoma UACC-257 (55%) |
Renal carcinoma 786 (45%) | Colon carcinoma HCT-116 (39%) | Pancreatic carcinoma YAPC (44%) |
| 8 | Breast cancer MDA-MB-468 (39%) | Melanoma UACC-257 (54%) |
Renal carcinoma 786-0 (49%) | Colon carcinoma HCT-116 (55%) | Ovarian adenocarcinoma OVCAR-4 (43%) |
| 9 | Breast cancer MDA-MB-468 (44%) | Melanoma UACC-257 (49%) |
Renal carcinoma 786-0 (44%) | Colon carcinoma HCT-116 (54%) | Ovarian adenocarcinoma OVCAR-4 (37%) |
| No. | Cancer Cells Normal Cells | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SW480 | SW620 | MDA-MB-231 | A-549 | LN-229 | HaCaT | ||||||
| IC50 | SI | IC50 | SI | IC50 | SI | IC50 | SI | IC50 | SI | IC50 | |
| 6 | 92.6 ± 5.31 | 1.08 | >100 | 1 | 83.2 ± 3.38 | 1.20 | 81.5 ± 5.71 | 1.22 | >100 | 1 | >100 |
| 7 | >100 | 0.93 | >100 | 0.93 | 67.3 ± 4.13 | 1.38 | >100 | 0.93 | >100 | 0.93 | 92.7 ± 5.00 |
| 8 | >100 | 1 | >100 | 1 | >100 | 1 | >100 | 1 | >100 | 1 | >100 |
| 9 | >100 | 1 | >100 | 1 | 96.5 ± 1.37 | 1.03 | >100 | 1 | >100 | 1 | >100 |
| Doxorubicin | 0.7 ± 0.1 | 0.4 | 0.3 ± 0.1 | 1.0 | 1.6 ± 0.23 | 0.19 | 0.2 ± 0.09 | 1.5 | 1.1 ± 0.12 | 0.27 | 0.3 ± 0.1 |
| Cisplatin | 10.4 ± 0.9 | 0.6 | 6.7 ± 1.1 | 0.9 | 7.8 ± 0.98 | 0.81 | 3.2 ± 1.24 | 5.08 | 2.6 ± 0.15 | 2.42 | 6.3 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





