Submitted:
03 May 2024
Posted:
06 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction:
2. Materials and Methods:
3. Results and Discussion:
3.1. Diamond Film Morphology - SEM
3.2. Film Surface Characterisation - EDS
3.3. Diamond Film Quality – Raman Spectroscopy
3.4. Chemistry of the Deposited Film - FTIR
3.5. Nitrogen Addition Enhances GaN Etching – XRD & Electrical Property
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amano, H.; Baines, Y.; Beam, E.; Borga, M.; Bouchet, T.; Chalker, P.R.; Charles, M.; Chen, K.J.; Chowdhury, N.; Chu, R.; et al. The 2018 GaN power electronics roadmap. J. Phys. D: Appl. Phys. 2018, 51, 163001. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Sun, M.; Piedra, D.; Chowdhury, N.; Palacios, T. Materials and processing issues in vertical GaN power electronics. Mater. Sci. Semicond. Process. 2018, 78, 75–84. [Google Scholar] [CrossRef]
- Lee, W.S.; Lee, K.W.; Lee, S.H.; Cho, K.; Cho, S. A GaN/Diamond HEMTs with 23 W/mm for Next Generation High Power RF Application, 2019 IEEE MTT-S International Microwave Symposium (IMS), Boston, MA, USA, (2019) 1395-1398. [CrossRef]
- Cuenca, J.A.; Smith, M.D.; Field, D.E.; Massabuau, F.C.-P.; Mandal, S.; Pomeroy, J.; Wallis, D.J.; Oliver, R.A.; Thayne, I.; Kuball, M.; et al. Thermal stress modelling of diamond on GaN/III-Nitride membranes. Carbon 2021, 174, 647–661. [Google Scholar] [CrossRef]
- Mahrokh, M.; Yu, H.; Guo, Y. Thermal Modeling of GaN HEMT Devices With Diamond Heat-Spreader. IEEE J. Electron Devices Soc. 2020, 8, 986–991. [Google Scholar] [CrossRef]
- Izsák, T.; Vanko, G.; Držík, M.; Kasemann, S.; Zehetner, J.; Vojs, M.; Zaťko, B. ; Potocký,.; Kromka, A. Direct Deposition of CVD Diamond Layers on Top of GaN Membranes, Proceedings 2020, 56, 35. [CrossRef]
- Mandal, S.; Thomas, E.L.H.; Middleton, C.; Gines, L.; Griffiths, J.T.; Kappers, M.J.; Oliver, R.A.; Wallis, D.J.; Goff, L.E.; Lynch, S.A.; et al. Surface Zeta Potential and Diamond Seeding on Gallium Nitride Films. ACS Omega 2017, 2, 7275–7280. [Google Scholar] [CrossRef]
- Smith, E.J.W.; Piracha, A.H.; Field, D.; Pomeroy, J.W.; Mackenzie, G.R.; Abdallah, Z.; Massabuau, F.C.-. .-P.; Hinz, A.M.; Wallis, D.J.; Oliver, R.A.; et al. Mixed-size diamond seeding for low-thermal-barrier growth of CVD diamond onto GaN and AlN. Carbon 2020, 167, 620–626. [Google Scholar] [CrossRef]
- May, P.; Tsai, H.; Wang, W.; Smith, J. Deposition of CVD diamond onto GaN. Diam. Relat. Mater. 2006, 15, 526–530. [Google Scholar] [CrossRef]
- Daniel Francis, Martin Kuball, 14 - GaN-on-diamond materials and device technology: A review, Editor(s): Marko J. Tadjer, Travis J. Anderson, In Woodhead Publishing Series in Electronic and Optical Materials, Thermal Management of Gallium Nitride Electronics, Woodhead Publishing, 2022, Pages 295-331, ISBN 9780128210840. [CrossRef]
- Zhan, T.; Xu, M.; Cao, Z.; Zheng, C.; Kurita, H.; Narita, F.; Wu, Y.-J.; Xu, Y.; Wang, H.; Song, M.; et al. Effects of Thermal Boundary Resistance on Thermal Management of Gallium-Nitride-Based Semiconductor Devices: A Review. Micromachines 2023, 14, 2076. [Google Scholar] [CrossRef]
- Mendes, J.C.; Liehr, M.; Li, C. Diamond/GaN HEMTs: Where from and Where to? Materials 2022, 15, 415. [Google Scholar] [CrossRef]
- Wort, C.J.; Balmer, R.S. Diamond as an electronic material. Mater. Today 2008, 11, 22–28. [Google Scholar] [CrossRef]
- Kohn, E.; Denisenko, A. Concepts for diamond electronics. Thin Solid Films 2007, 515, 4333–4339. [Google Scholar] [CrossRef]
- Markham, M.; Dodson, J.; Scarsbrook, G.; Twitchen, D.; Balasubramanian, G.; Jelezko, F.; Wrachtrup, J. CVD diamond for spintronics. Diam. Relat. Mater. 2011, 20, 134–139. [Google Scholar] [CrossRef]
- Ueda, K.; Soumiya, T.; Asano, H. Ferromagnetic Schottky junctions using diamond semiconductors. Diam. Relat. Mater. 2012, 25, 159–162. [Google Scholar] [CrossRef]
- Ueda, K.; Kawamoto, K.; Asano, H. High-temperature and high-voltage characteristics of Cu/diamond Schottky diodes. Diam. Relat. Mater. 2015, 57, 28–31. [Google Scholar] [CrossRef]
- Isberg, J.; Ferro, G.; Siffert, P. Diamond Electronic Devices. AIP Conf. Proc. 2010, 1292, 123. [Google Scholar] [CrossRef]
- Liu, D.; Sun, H.; Pomeroy, J.W.; Francis, D.; Faili, F.; Twitchen, D.J.; Kuball, M. GaN-on-diamond electronic device reliability: Mechanical and thermo-mechanical integrity. Appl. Phys. Lett. 2015, 107, 251902. [Google Scholar] [CrossRef]
- Rouzbahani, R.; Sankaran, K.J.; Pobedinskas, P.; Haenen, K. Advances in n-Type Chemical Vapor Deposition Diamond Growth: Morphology and Dopant Control, Rozita Rouzbahani, Kamatchi Jothiramalingam Sankaran, Paulius Pobedinskas, and Ken Haenen. Accounts Mater. Res. 2024. [Google Scholar] [CrossRef]
- Truscott, B.S.; Kelly, M.W.; Potter, K.J.; Ashfold, M.N.R.; Mankelevich, Y.A. Microwave Plasma-Activated Chemical Vapor Deposition of Nitrogen-Doped Diamond. II: CH4/N2/H2 Plasmas. J. Phys. Chem. A 2016, 120, 8537–8549. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Sankaran, K.J.; Korneychuk, S.; Verbeeck, J.; Haenen, K.; Jiang, X.; Yang, N. High-performance supercabatteries using graphite@diamond nano-needle capacitor electrodes and redox electrolytes. Nanoscale 2019, 11, 17939–17946. [Google Scholar] [CrossRef]
- Li, M.-J.; Lu, X.-Y.; Sun, B.-R.; Li, C.-Y.; Li, B.; Jin, Z.-S. Nitrogen-doped diamond film and its doping behavior. Carbon 2007, 45, 2325. [Google Scholar] [CrossRef]
- Tang, C.; Fernandes, A.; Costa, F.; Pinto, J. Effect of microwave power and nitrogen addition on the formation of {100} faceted diamond from microcrystalline to nanocrystalline. Vacuum 2011, 85, 1130–1134. [Google Scholar] [CrossRef]
- Cuenca, J.A.; Sankaran, K.J.; Pobedinskas, P.; Panda, K.; Lin, I.-N.; Porch, A.; Haenen, K.; Williams, O.A. Microwave cavity perturbation of nitrogen doped nano-crystalline diamond films. Carbon 2018, 145, 740–750. [Google Scholar] [CrossRef]
- Smith, M.D.; Cuenca, J.A.; Field, D.E.; Fu, Y.-C.; Yuan, C.; Massabuau, F.; Mandal, S.; Pomeroy, J.W.; Oliver, R.A.; Uren, M.J.; et al. GaN-on-diamond technology platform: Bonding-free membrane manufacturing process. AIP Adv. 2020, 10, 035306. [Google Scholar] [CrossRef]
- Janssen, W.; Turner, S.; Sakr, G.; Jomard, F.; Barjon, J.; Degutis, G.; Lu, Y.-G.; D'Haen, J.; Hardy, A.; Van Bael, M.; et al. Substitutional phosphorus incorporation in nanocrystalline CVD diamond thin films. Phys. Status solidi (RRL) – Rapid Res. Lett. 2014, 8, 705–709. [Google Scholar] [CrossRef]
- Lloret, F.; Sankaran, K.J.; Millan-Barba, J.; Desta, D.; Rouzbahani, R.; Pobedinskas, P.; Gutierrez, M.; Boyen, H.-G.; Haenen, K. Improved Field Electron Emission Properties of Phosphorus and Nitrogen Co-Doped Nanocrystalline Diamond Films. Nanomaterials 2020, 10, 1024. [Google Scholar] [CrossRef] [PubMed]
- K. Haenen, P. Pobedinskas, R. Ramaneti, Growing Diamond Layers, Patent no. EP3745446A1; Published on 02/12/2020,
- Williams, O.A.; Douhéret, O.; Daenen, M.; Haenen, K.; Ōsawa, E.; Takahashi, M. Enhanced diamond nucleation on monodispersed nanocrystalline diamond. Chem. Phys. Lett. 2007, 445, 255–258. [Google Scholar] [CrossRef]
- Sankaran, K.J.; Yeh, C.-J.; Hsieh, P.-Y.; Pobedinskas, P.; Kunuku, S.; Leou, K.-C.; Tai, N.-H.; Lin, I.-N.; Haenen, K. Origin of Conductive Nanocrystalline Diamond Nanoneedles for Optoelectronic Applications. ACS Appl. Mater. Interfaces 2019, 11, 25388–25398. [Google Scholar] [CrossRef]
- D. K. Schroder, Semiconductor Material and Device Characterization (3rd edition), Sec. 8.3 Hall effect and mobility, (2015) 466-479, John Wiley & Sons, Inc.
- L. J. van der Pauw, A method of measuring specific resistivity and Hall effect of discs of arbitrary shape, Phil. Res. Rep. 13 (1958) 1-9.
- L. J. van der Pauw, A method of measuring specific resistivity and Hall effect on lamella of arbitrary shape, Phil. Res. Rep. 20 (1958) 220-224.
- Mallik, A.K.; Bysakh, S.; Sreemany, M.; Roy, S.; Ghosh, J.; Roy, S.; Mendes, J.C.; Gracio, J.; Datta, S. Property mapping of polycrystalline diamond coatings over large area. J. Adv. Ceram. 2014, 3, 56–70. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Chaudhari, G.N.; Chinchamalatpure, V.R.; Ghosh, S.A. Structural and Electrical Characterization of GaN Thin Films on Si(100). Am. J. Anal. Chem. 2011, 02, 984–988. [Google Scholar] [CrossRef]
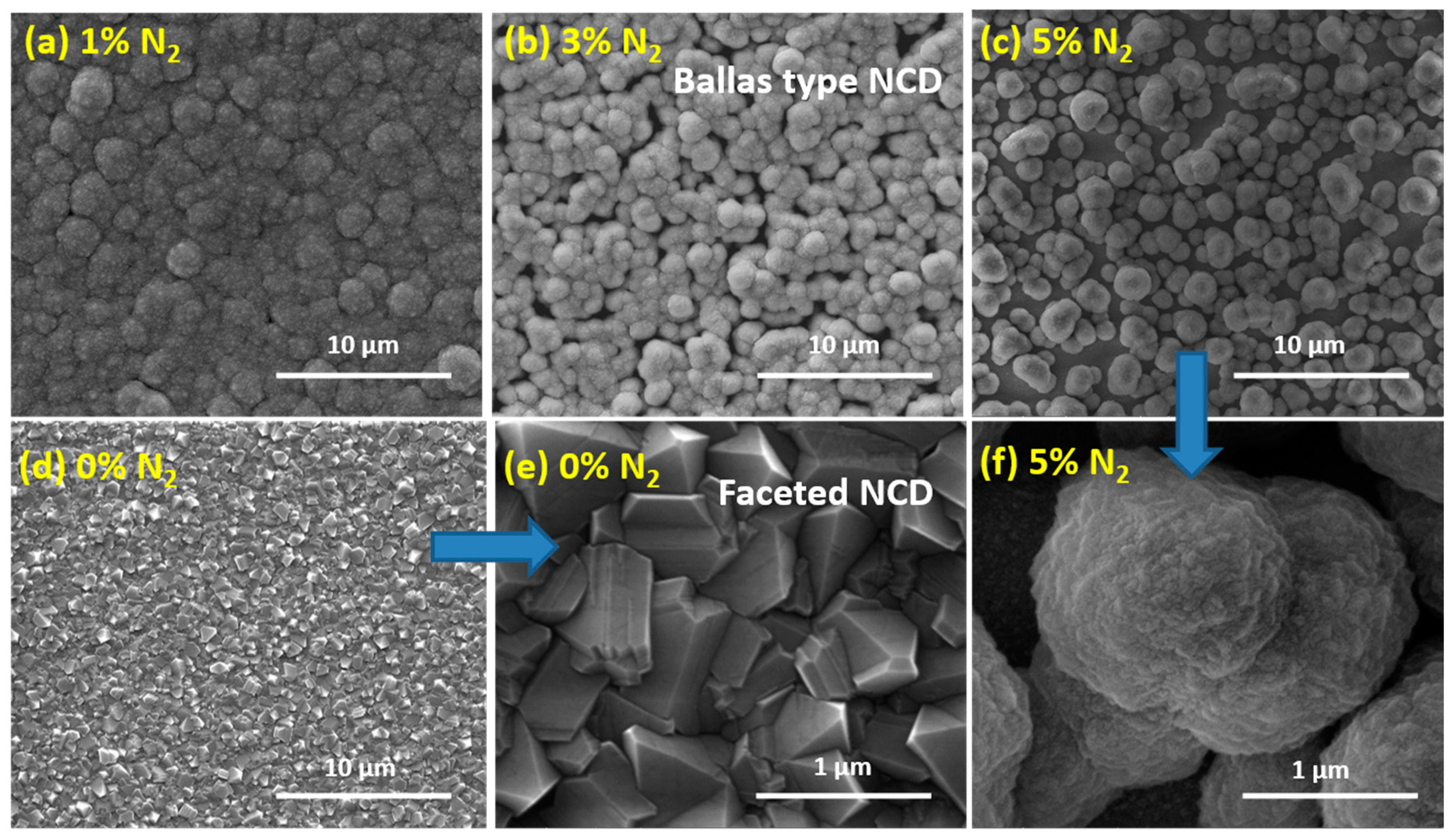
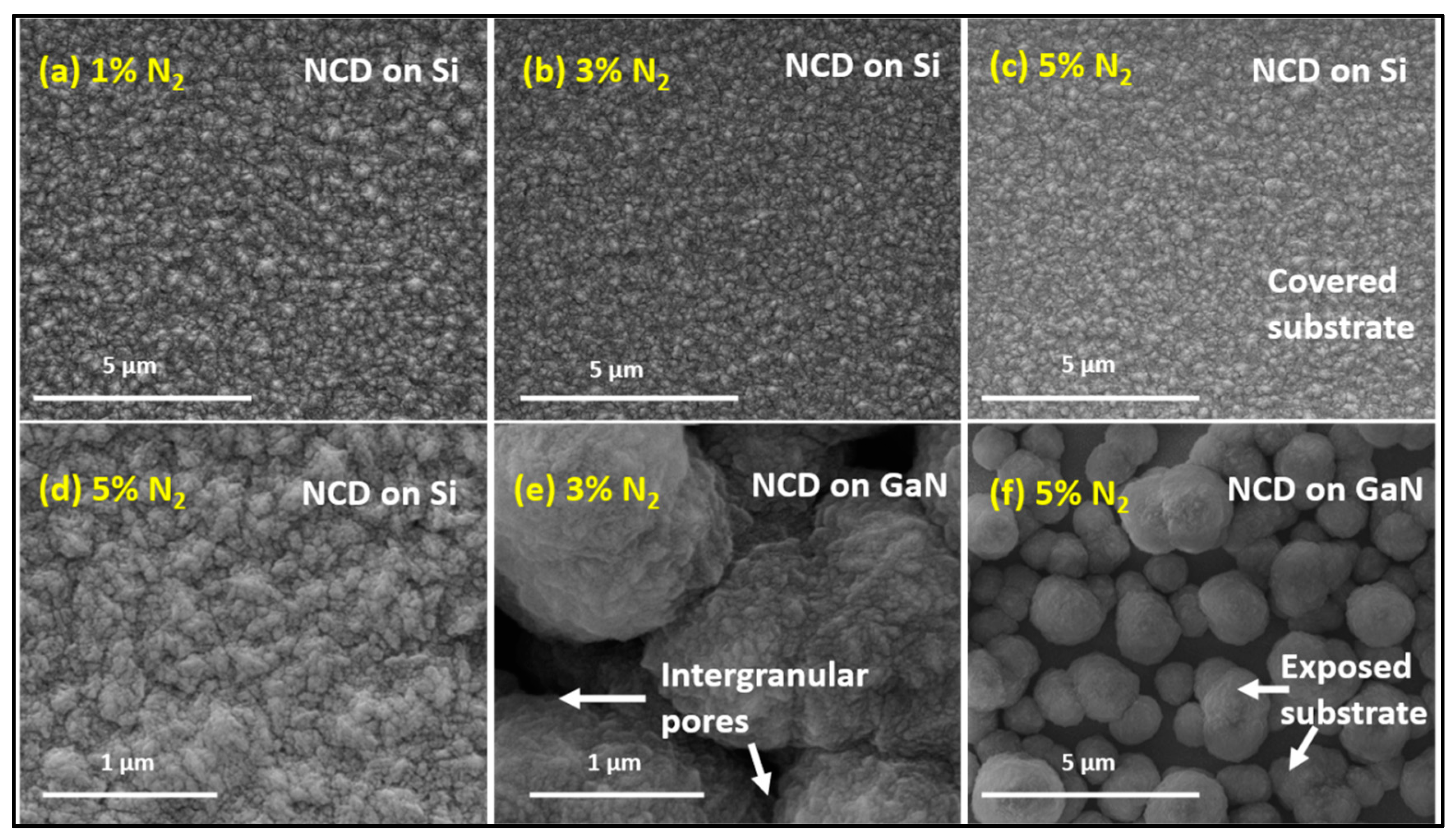
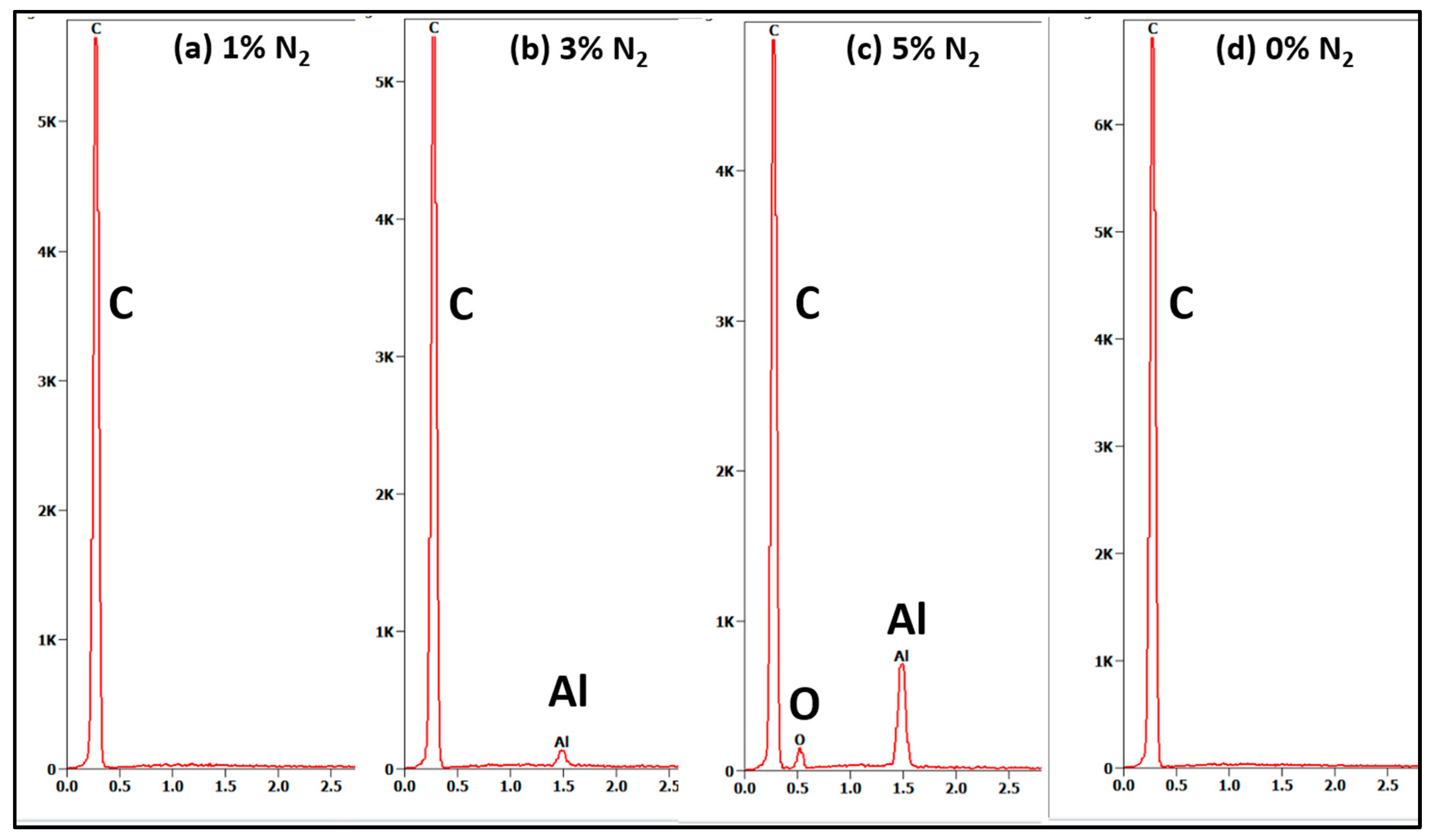
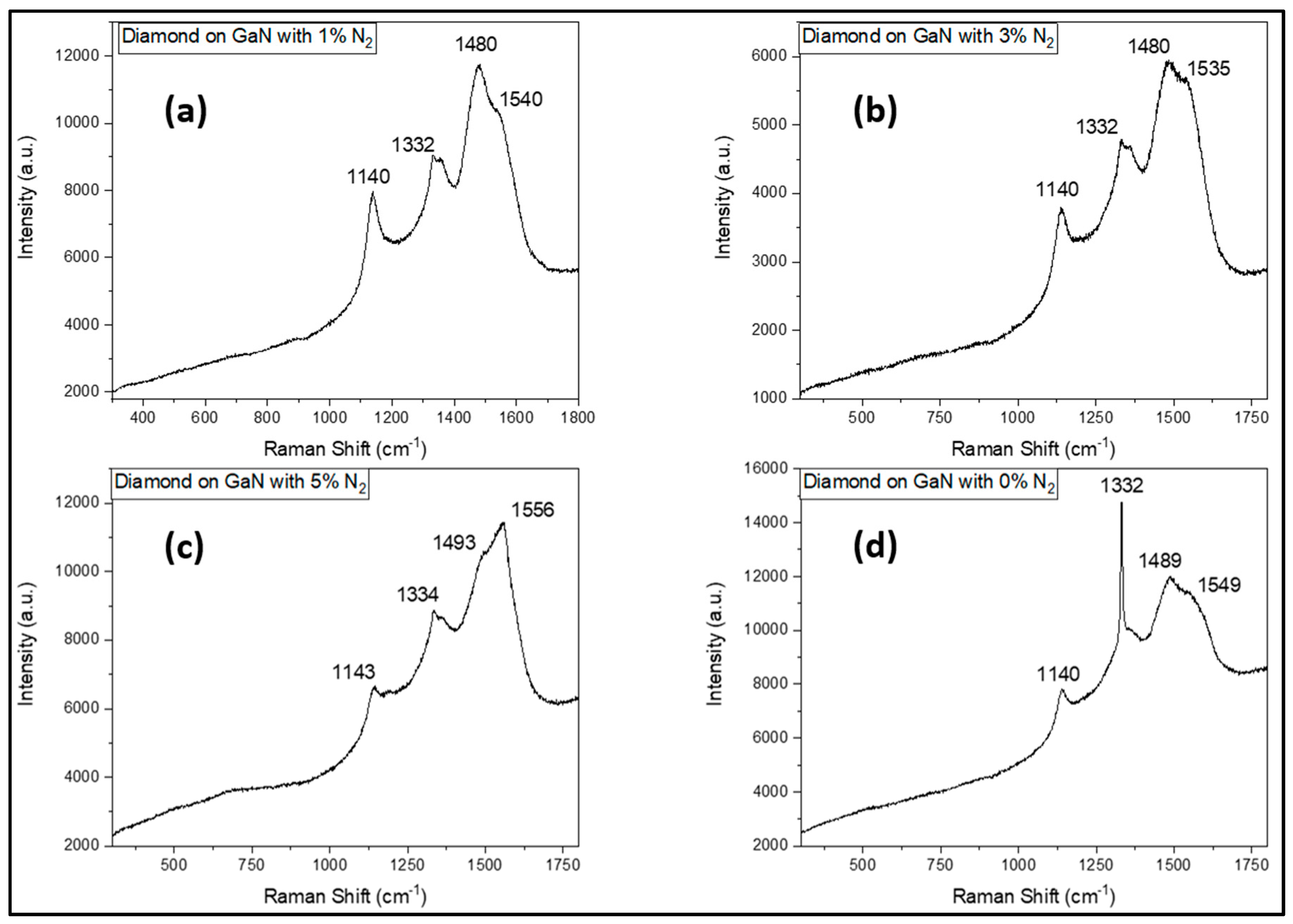
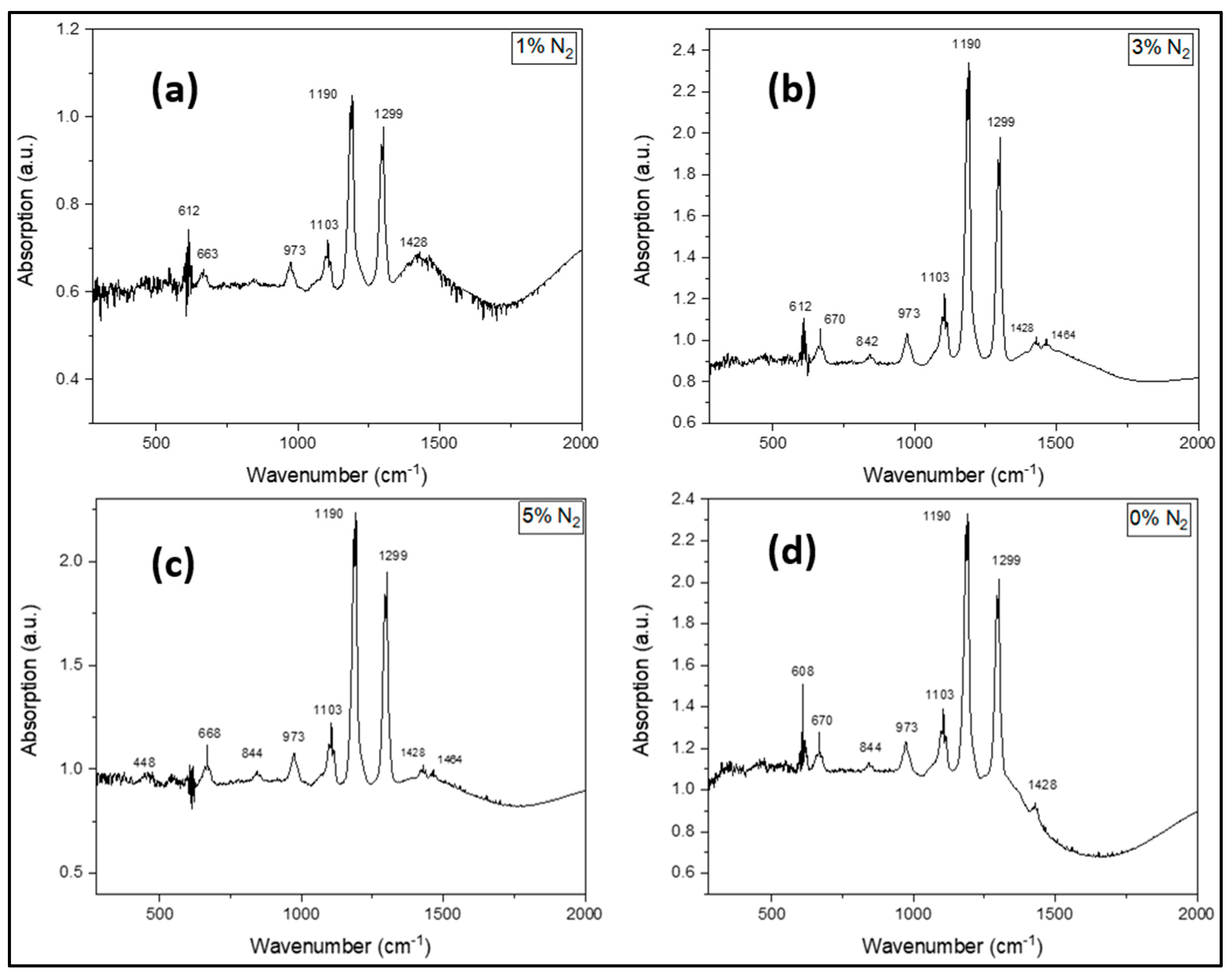
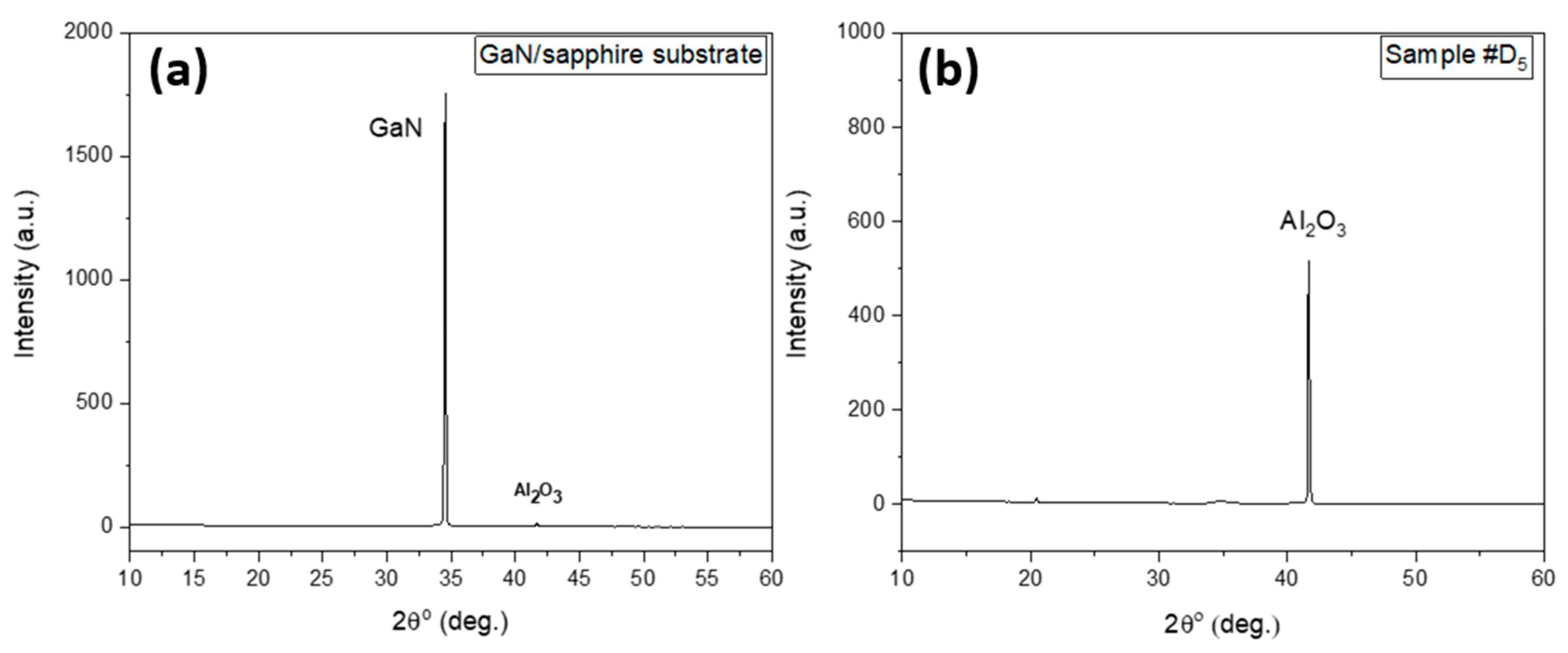
| Sample # | N2 (%) |
H2 (%) |
CH4 (%) |
CO2 (%) |
Tsubstrate (oC) |
Power (kW) |
|---|---|---|---|---|---|---|
| D0 | 0 | 94 | 6 | 0 | 600 | 3 |
| D1 | 1 | 93 | 6 | 0 | 600 | 3 |
| D3 | 3 | 91 | 6 | 0 | 600 | 3 |
| D5 | 5 | 89 | 6 | 0 | 600 | 3 |
| Sample # |
N2 (%) |
Al EDS peak intensity at 1.49 eV (counts) |
Ga-N FTIR peak intensity (a.u.) |
Diamond qualities (Raman peak ratio Isp3 / ID) (%) | Diamond average grain sizes (μm) |
|---|---|---|---|---|---|
| D0 | 0 | absent | 151 | 59 | 0.5 |
| D1 | 1 | absent | 74 | 50 | 2.0 |
| D3 | 3 | 105 | 110 | 50 | 0.9 |
| D5 | 5 | 680 | absent | 50 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





