Submitted:
29 April 2024
Posted:
30 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
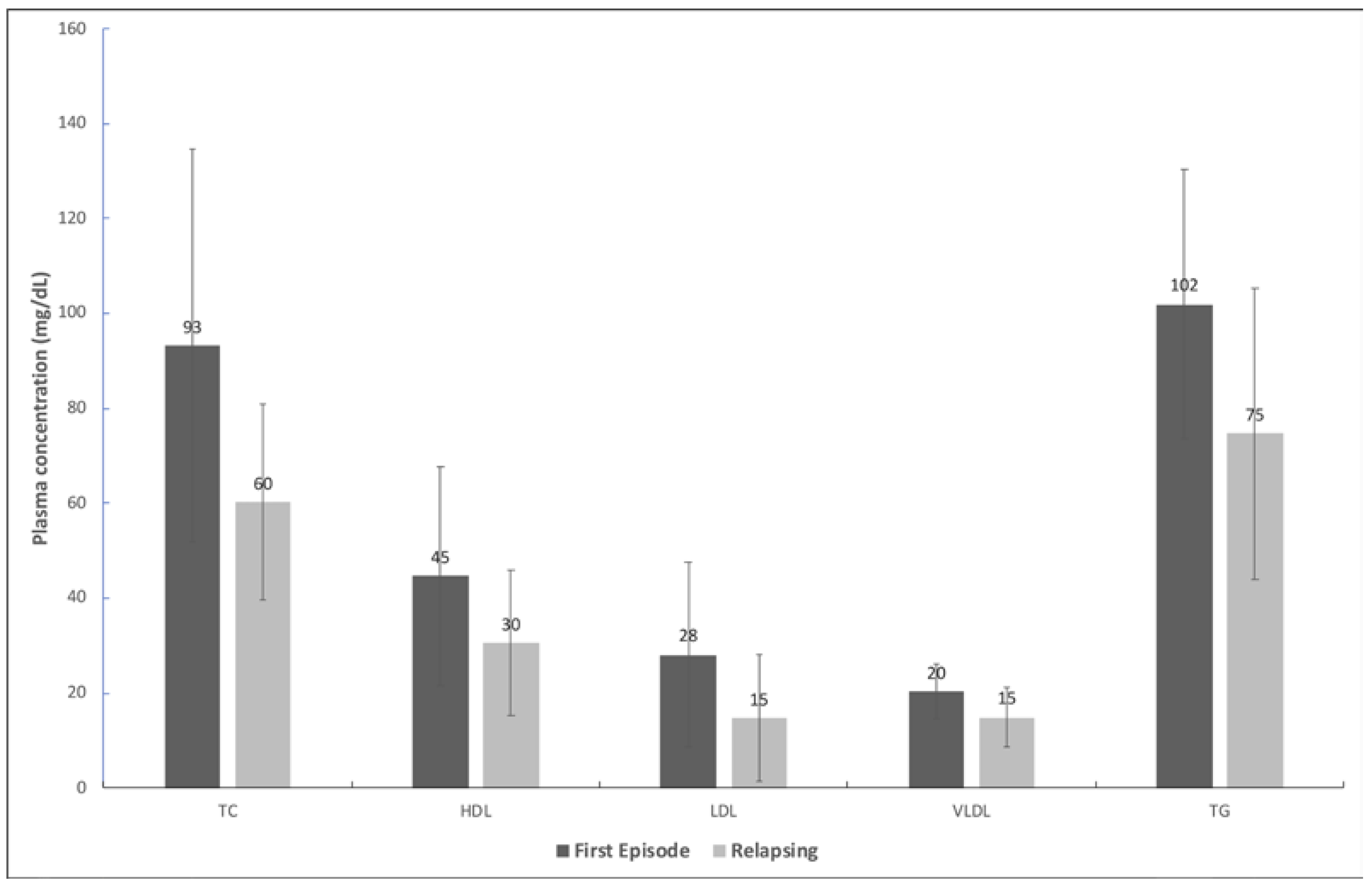
3. Results
4. Discussion
Authors’ contribution
Institutional Review Board Statement
Informed Consent Statement
Financial support
Conflicts of Interest
Acknowledgments
References
- Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet 2018; 392: 951–970.
- Costa DL, Rocha RL, Carvalho RMA; et al. Serum cytokines associated with severity and complications of kala-azar. Pathog Glob Health 2013; 107: 78–87. [CrossRef]
- Wasunna KMM, Raynes JGG, Were JBOB; et al. Acute phase protein concentrations predict parasite clearance rate during therapy for visceral leishmaniasis. Trans R Soc Trop Med Hyg 1995; 89: 678–681. [CrossRef]
- Alvar J, Vélez ID, Bern C; et al. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012; 7: e35671. [CrossRef]
- Reinaldo LGC, Araújo-Júnior RJC, Diniz TM; et al. Splenectomy in Patients with Visceral Leishmaniasis Resistant to Conventional Therapy and Secondary Prophylaxis: A Retrospective Cohort. Am J Trop Med Hyg; in press. Epub ahead of print 20 June 2022. [CrossRef]
- Cota GF, de Sousa MR, Rabello A. Predictors of Visceral Leishmaniasis Relapse in HIV-Infected Patients: A Systematic Review. PLoS Negl Trop Dis 2011; 5: e1153. [CrossRef]
- World Health Organization & Cochrane Response. WHO guideline for the treatment of visceral leishmaniasis in HIV coinfected patients in East Africa and South-East Asia: Web annex A: A systematic review on the treatment of visceral leishmaniasis in HIV-Leishmania coinfected persons in East Africa and So. https://apps.who.int/iris/handle/10665/354546. Licença: CC BY-NC-SA 3.0 IGO. (2022).
- Doitsh G, Greene WC. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe 2016; 19: 280–291. [CrossRef]
- Srivastava P, Prajapati VK, Rai M; et al. Unusual Case of Resistance to Amphotericin B in Visceral Leishmaniasis in a Region in India Where Leishmaniasis Is Not Endemic. J Clin Microbiol 2011; 49: 3088–3091. [CrossRef]
- Kip AE, Blesson S, Alves F; et al. Low antileishmanial drug exposure in HIV-positive visceral leishmaniasis patients on antiretrovirals: An Ethiopian cohort study. J Antimicrob Chemother 2021; 76: 1258–1268. [CrossRef]
- Wasan KM, Kennedy AL, Cassidy SM; et al. Pharmacokinetics, Distribution in Serum Lipoproteins and Tissues, and Renal Toxicities of Amphotericin B and Amphotericin B Lipid Complex in a Hypercholesterolemic Rabbit Model: Single-Dose Studies. Antimicrob Agents Chemother 1998; 42: 3146–3152. [CrossRef]
- Zauli-Nascimento RC, Miguel DC, Yokoyama-Yasunaka JKU; et al. In vitro sensitivity of Leishmania ( Viannia ) braziliensis and Leishmania ( Leishmania ) amazonensis Brazilian isolates to meglumine antimoniate and amphotericin B. Trop Med Int Heal. Epub ahead of print October 2009. [CrossRef]
- Rastrojo A, García-Hernández R, Vargas P; et al. Genomic and transcriptomic alterations in Leishmania donovani lines experimentally resistant to antileishmanial drugs. Int J Parasitol Drugs Drug Resist 2018; 8: 246–264. [CrossRef]
- Morizot G, Jouffroy R, Faye A; et al. Antimony to Cure Visceral Leishmaniasis Unresponsive to Liposomal Amphotericin B. PLoS Negl Trop Dis 2016; 10: e0004304. [CrossRef]
- Ponte-Sucre A, Gamarro F, Dujardin J-C; et al. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl Trop Dis 2017; 11: e0006052. [CrossRef]
- Lachaud L, Bourgeois N, Plourde M; et al. Parasite Susceptibility to Amphotericin B in Failures of Treatment for Visceral Leishmaniasis in Patients Coinfected with HIV Type 1 and Leishmania infantum. Clin Infect Dis 2009; 48: e16–e22. [CrossRef]
- Durand R, Paul M, Pratlong F; et al. Leishmania infantum : Lack of Parasite Resistance to Amphotericin B in a Clinically Resistant Visceral Leishmaniasis. Antimicrob Agents Chemother 1998; 42: 2141–2143. [CrossRef]
- Cota GF, de Sousa MR, de Assis TSM; et al. Exploring prognosis in chronic relapsing visceral leishmaniasis among HIV-infected patients: Circulating Leishmania DNA. Acta Trop 2017; 172: 186–191.
- Costa DLDL, Rocha RLRLRL, Chaves E de BF; et al. Predicting death from kala-azar: Construction, development, and validation of a score set and accompanying software. Rev Soc Bras Med Trop 2016; 49: 728–740.
- Takele Y, Mulaw T, Adem E; et al. Immunological factors, but not clinical features, predict visceral leishmaniasis relapse in patients co-infected with HIV. Cell Reports Med 2022; 3: 100487. [CrossRef]
- Silva-Freitas ML, Cota GF, Machado-de-Assis TS; et al. Immune Activation and Bacterial Translocation: A Link between Impaired Immune Recovery and Frequent Visceral Leishmaniasis Relapses in HIV-Infected Patients. PLoS ONE 2016; 11: e0167512. [CrossRef]
- Gautam S, Kumar R, Singh N; et al. CD8 T Cell Exhaustion in Human Visceral Leishmaniasis. J Infect Dis 2014; 209: 290–299. [CrossRef]
- Silva-Freitas ML, Corrêa-Castro G, Cota GF; et al. Impaired Thymic Output Can Be Related to the Low Immune Reconstitution and T Cell Repertoire Disturbances in Relapsing Visceral Leishmaniasis Associated HIV/AIDS Patients. Front Immunol; 11. Epub ahead of print 20 May 2020. [CrossRef]
- Francois B, Jeannet R, Daix T; et al. Interleukin-7 restores lymphocytes in septic shock: The IRIS-7 randomized clinical trial. JCI Insight; 3. Epub ahead of print 8 March 2018. [CrossRef]
- Szwarcwald CL, Malta DC, Pereira CA; et al. Valores de referência para exames laboratoriais de colesterol, hemoglobina glicosilada e creatinina da população adulta brasileira. Rev Bras Epidemiol; 22. Epub ahead of print 2019. [CrossRef]
- Lal CS, Kumar A, Kumar S; et al. Hypocholesterolemia and increased triglyceride in pediatric visceral leishmaniasis. Clin Chim Acta 2007; 382: 151–153. [CrossRef]
- Grunfeld C, Pang M, Doerrler W; et al. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1992; 74: 1045–1052. [CrossRef]
- Souza SJ, Luzia LA, Santos SS; et al. Lipid profile of HIV-infected patients in relation to antiretroviral therapy: A review. Rev Assoc Med Bras 2013; 59: 186–198. [CrossRef]
- Wallet MA, Buford TW, Joseph A-M; et al. Increased inflammation but similar physical composition and function in older-aged, HIV-1 infected subjects. BMC Immunol 2015; 16: 43. [CrossRef]
- Harhay MOMO, Olliaro PLPL, Vaillant M; et al. Who Is a Typical Patient with Visceral Leishmaniasis? Characterizing the Demographic and Nutritional Profile of Patients in Brazil, East Africa, and South Asia. Am J Trop Med Hyg 2011; 84: 543–550. [CrossRef]
- Grinspoon S, Mulligan K. Weight Loss and Wasting in Patients Infected with Human Immunodeficiency Virus. Clin Infect Dis 2003; 36: S69–S78. [CrossRef]
- Carpentier YA, Scruel O. Changes in the concentration and composition of plasma lipoproteins during the acute phase response. Curr Opin Clin Nutr Metab Care 2002; 5: 153–158. [CrossRef]
- Feingold KR, Grunfeld C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. Epub ahead of print 2000. DOI: 26561701.
- Veiga GRS, Ferreira HS, Sawaya AL; et al. Dyslipidaemia and Undernutrition in Children from Impoverished Areas of Maceió, State of Alagoas, Brazil. Int J Environ Res Public Health 2010; 7: 4139–4151. [CrossRef]
- Verma DGK, Yadav DYS, Yadav DRK; et al. Study of lipid profile levels in malnourished and healthy children: A case control study acquired pneumonia in children. Pediatr Rev Int J Pediatr Res 2018; 5: 156–161.
- Zhang Z, Pereira S, Luo M; et al. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017; 9: 829. [CrossRef]
- Stellaard F. From Dietary Cholesterol to Blood Cholesterol, Physiological Lipid Fluxes, and Cholesterol Homeostasis. Nutrients 2022; 14: 1643. [CrossRef]
- Olsson AG, Angelin B, Assmann G; et al. Can cholesterol be too low? Possible risks of extremely low levels. J Intern Med 2017; 281: 534–553. [CrossRef]
- Wu Z, Huang Z, Lichtenstein AH; et al. The risk of ischemic stroke and hemorrhagic stroke in Chinese adults with low-density lipoprotein cholesterol concentrations < 70 mg/dL. BMC Med 2021; 19: 142. [CrossRef]
- Hofmaenner DA, Arina P, Kleyman A; et al. Association Between Hypocholesterolemia and Mortality in Critically Ill Patients With Sepsis: A Systematic Review and Meta-Analysis. Crit Care Explor 2023; 5: e0860. [CrossRef]
| Patient ID | Mean EC50¹ | Standard error | No. of experiments | No. of previous VL episodes |
| (µM) | (µM) | |||
| 55672 | 0.042 | 0.007 | 5 | 2 |
| 57682 | 0.037 | 0.006 | 5 | 2 |
| 69052 | 0.034 | 0.007 | 4 | 2 |
| 70532 | 0.024 | 0.008 | 5 | 2 |
| 56093 | 0.026 | 0.006 | 4 | 0 |
| MHOM/BR/2005/NLC4 | 0.051 | 0.005 | 3 | - |
| Characteristics | First episode n (%) |
Relapsing n (%) |
p-value |
|---|---|---|---|
| Sex | |||
| Male | 6 (100.0) | 20 (87.0) | |
| Female | 0 (0.0) | 3 (13.0) | 0.350 |
| Age-group | |||
| Up to 40 years old | 4 (66.7) | 8 (34.8) | |
| More than 40 years | 2 (33.3) | 15 (65.2) | 0.158 |
| Symptoms and signs | |||
| Weight loss | 6 (100.0) | 17 (73.4) | 0.160 |
| Pallor | 5 (83.3) | 17 (73.4) | 0.631 |
| Fever | 6 (100.0) | 13 (56.5) | 0.046 |
| Asthenia/weakness | 4 (66.7) | 14 (60.9) | 0.794 |
| Splenomegaly | 3 (50.0) | 13 (56.5) | 0.775 |
| Hepatomegaly | 2 (33.3) | 9 (39.1) | 0.794 |
| Jaundice | 2 (33.3) | 8 (34.8) | 0.947 |
| HAART* | 6/6 (100.0) | 15/23 (0.65) | 0.43 |
| Death | 0 (0.0) | 4 (17.4) | 0.55 |
| Laboratory data | Course of VL | p-valuec | |||
| First episode (N=6) | Relapsing (N=23) | ||||
| na | Mean (95% CIb) | na | Mean (95% CI) | ||
| Hemoglobin (g/dL) | 5 | 8.0 (3.6; 12.5) |
19 | 7.8 (7.2; 8.4) |
0.522 |
| Leukocytes (per mm³) | 5 | 4.643 (1,060; 4; 8.223) |
21 | 2.397 (1,713; 3.083) |
0.013 |
| Neutrophils (cells/mm³) | 5 | 2,410 (-272; 5092.9) |
19 | 1,409 (889; 1930) |
0.076 |
| Lymphocytes (cells /mm³) | 5 | 1,654 (-88; 3,396) |
19 | 660 (457; 864) |
0.005 |
| Platelets (number/mm³) |
6 | 151,952 (151,952;184,813) |
21 | 190,217 (89.298; 291,135) |
0.297 |
| Albumin (g/dL) | 1 | 1.8 | 10 | 2.7 (1.9; 3.6) |
- |
| Globulin (g/dL) | 1 | 9.9 | 9 | 6.0 (4.8;7.2) |
- |
| CD4+ (cells/mm³) | 5 | 152 (-32; 332) |
15 | 142 (89; 196) |
0.877 |
| CD8+ (cells/mm³) | 5 | 955 (-197.4; 2,106.55) |
14 | 606 (423; 789) |
0.222 |
| Lipid profile | Outcome |
p-value |
|
| Survival (na=25) Mean (95% CIb) |
Death (n=4) Mean (95% CI) |
||
| TCc (mg/dL) | 68.3 (56.1; 80.5) | 60.7 (20.8; 100.7) | 0.633 |
| LDLd (mg/dL) | 20.4 (14.2; 26.7) | 3.6 (-3.8; 10.9) | 0.038 |
| HDLe (mg/dL) | 32.7 (25.3; 40.2) | 37.3 (8.6; 65.9) | 0.644 |
| VLDLf (mg/dL) | 15.2 (12.7; 17.6) | 20.0 (5.7; 34.2) | 0.170 |
| TGg (mg/dL) | 75.8 (63.7; 88.0) | 99.8 (28.4; 171.1) | 0.170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





