Submitted:
27 April 2024
Posted:
30 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
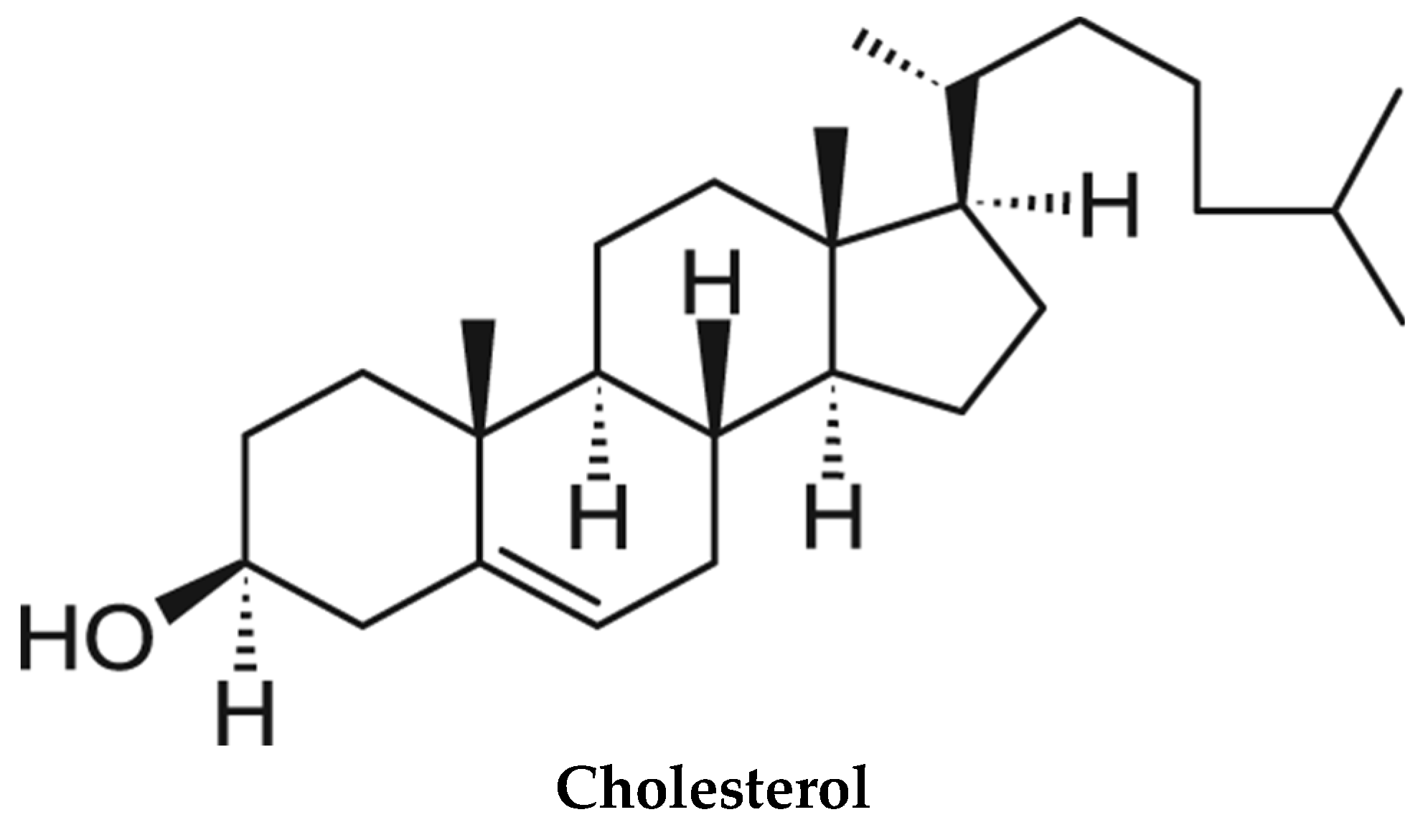
2. Lipid Phases, and Their Significance
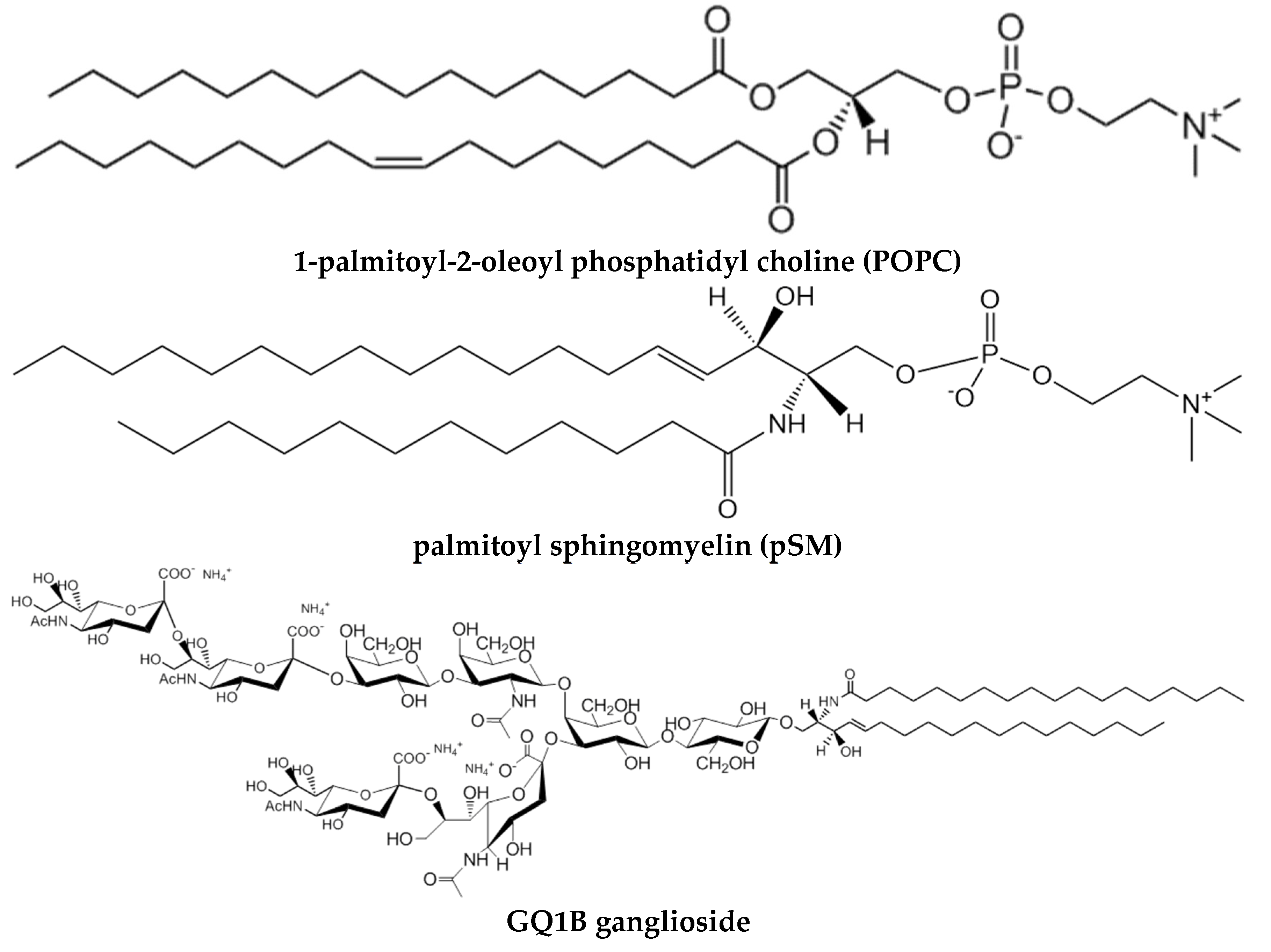
3. Model Membranes: Monolayers and Bilayers
3.1. Monolayers
3.2. Bilayers
4. Biophysical Methods in the Study of Aβ Peptide Interaction with Membranes
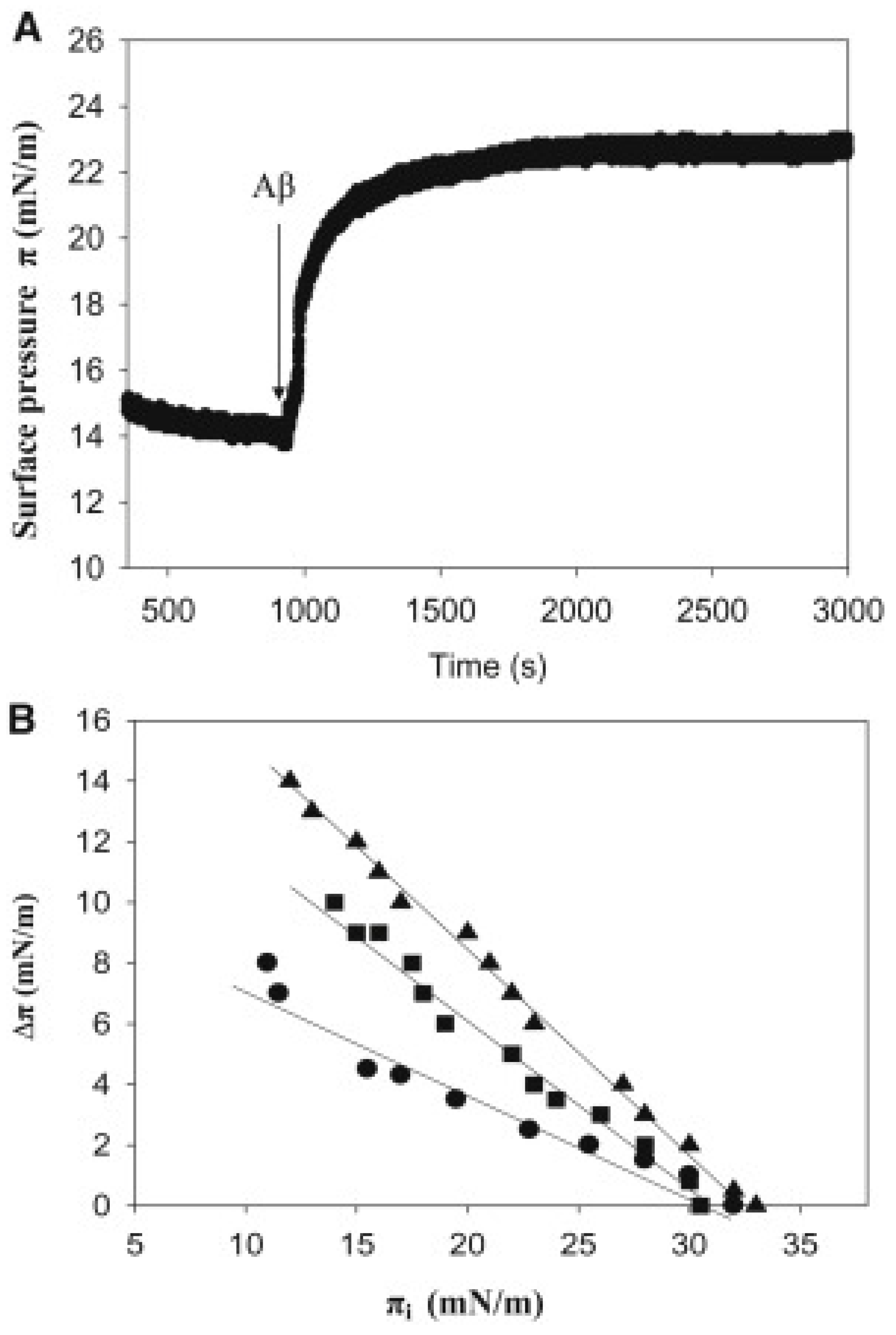
4.1. The Langmuir Balance and Langmuir Monolayers
4.2. Sucrose Gradient Ultracentrifugation
4.3. Calorimetric Methods
4.4. Thioflavin T (ThT) Fluorescence Assays
4.5. Computational Methods
4.6. Miscellaneous Techniques
5. A Review of Selected Results
5.1. Monolayer Studies
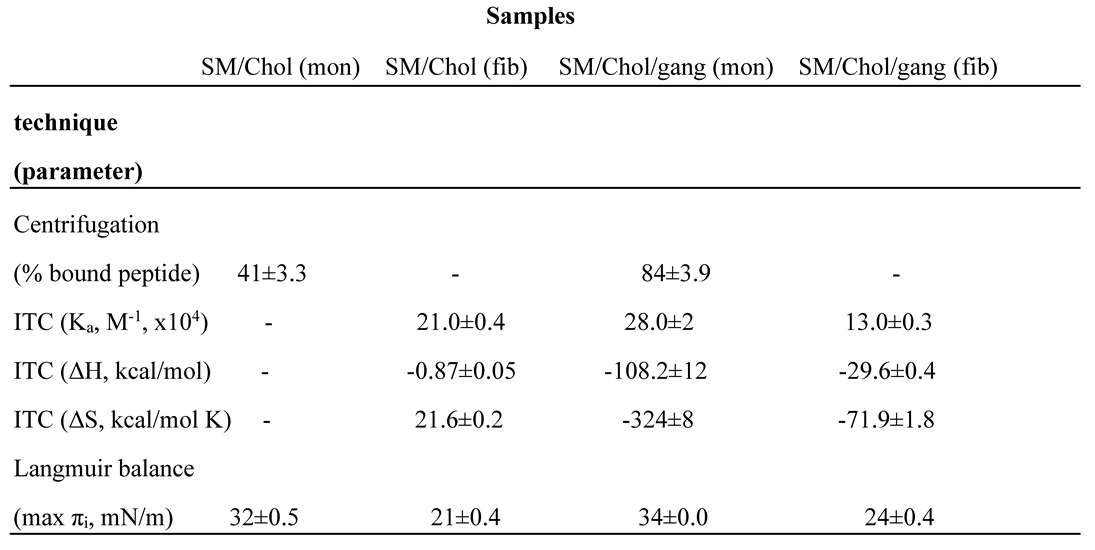
5.2. Isothermal Calorimetric Studies
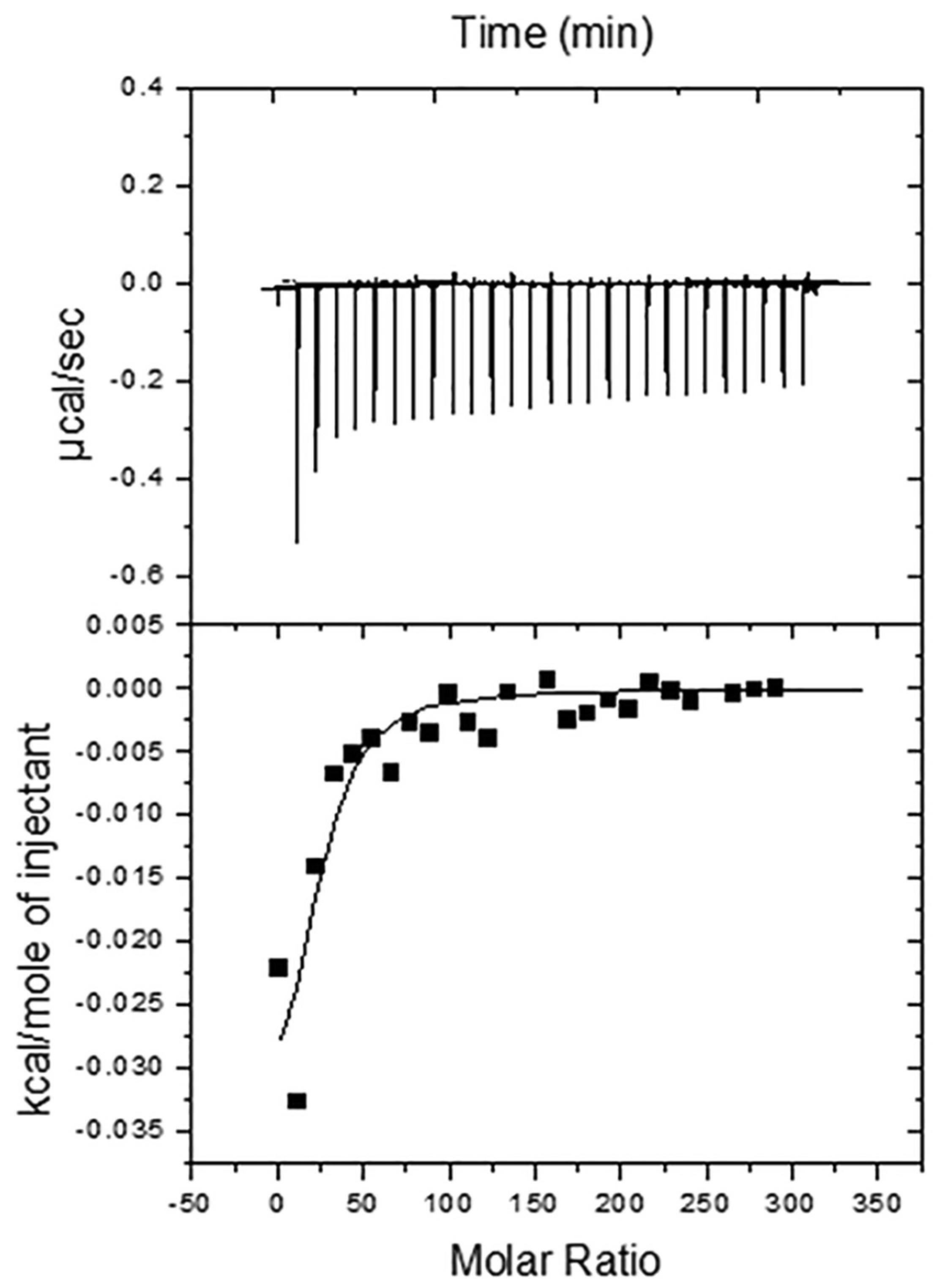
5.3. Molecular Dynamics (MD) Studies
6. An Effort in Understanding
6.1. The Validity of Models
6.2. Aβ Peptide Generation, Membrane Binding, Adsorption, and Insertion
6.2. Ordered and Disordered Bilayers
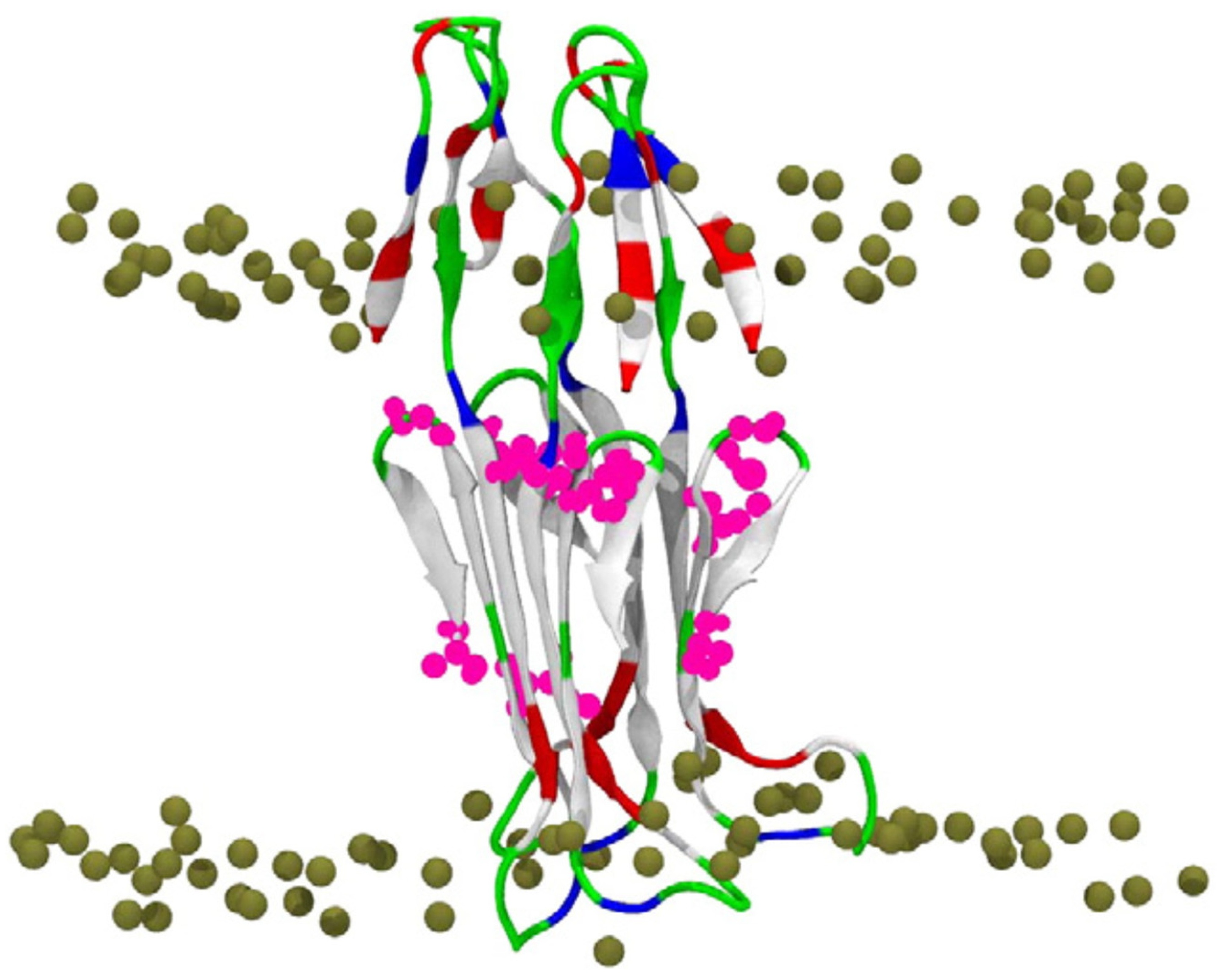
6.3. Electrostatic Forces
6.4. The Role of Specific Lipids
6.5. Aggregation State
6.6. Data Integration
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cummings, J.L. Alzheimer disease, JAMA 2002, 287, 2335-2338. [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [CrossRef]
- Small, D.H.; Mok, S.S.; Bornstein, J.C. Alzheimer's disease and Abeta toxicity: from top to bottom, Nat. Rev. Neurosci. 2001, 2, 595–598. [CrossRef]
- Yin, Y.I.; Bassit, B.; Zhu, L.; Yang, X.; Wang, C.; Li, Y.M.J. {gamma}-Secretase substrate concentration modulates the Abeta42/Abeta40 ratio: implications for Alzheimer disease, Biol. Chem. 2007, 282, 23639–23644. [CrossRef]
- Karisetti, B.C.; Bhatnagar, A.; Armour, E.M.; Beaver, M.; Zhang, H.; Elefant, F. Amyloid-β peptide impact on synaptic function and neuroepigenetic gene control reveal new therapeutic strategies for Alzheimer’s disease, Front. Mol. Neurosci. 2020, 13, 577622. [CrossRef]
- Matsuzaki, K. Physicochemical interactions of amyloid beta-peptide with lipid bilayers, Biochim. Biophys. Acta 2007, 1768, 1935–1942. [CrossRef]
- Terzi, E.; Hölzemann, G.; Seelig, J. Self-association of beta-amyloid peptide (1-40) in solution and binding to lipid membranes, J. Mol. Biol. 1995, 252, 633–642. [CrossRef]
- Ahyayauch, H.; Raab, M.; J.V. Busto, J.V.; Andraka, N.; Arrondo, J.L.; Masserini, M.; Tvaroska, I.; Goñi, F.M. Binding of β-amyloid (1-42) peptide to negatively charged phospholipid membranes in the liquid-ordered state: modeling and experimental studies, Biophys. J. 2012, 103, 453–463. [CrossRef]
- Ahyayauch, H.; de la Arada, I.; Masserini, M.E.; Arrondo, J.L.R.; Goñi, F.M.; Alonso, A. The binding of aβ42 peptide monomers to sphingomyelin/ cholesterol/ ganglioside bilayers assayed by density gradient ultracentrifugation. Int. J. Mol. Sci. 2020, 21, 1674. [CrossRef]
- Ahyayauch, H.; García-Arribas, A.B.; Masserini, M.E.; Pantano, S.; Goñi, F.M.; Alonso, A. β-Amyloid (1-42) peptide adsorbs but does not insert into ganglioside-containing phospholipid membranes in the liquid-disordered state: modelling and experimental studies. Int. J. Biol. Macromol. 2020, 164, 2651-2658. [CrossRef]
- Ahyayauch, H.; Masserini, M.; Goñi, F.M.; Alonso, A. The interaction of Aβ42 peptide in monomer, oligomer or fibril forms with sphingomyelin/cholesterol/ ganglioside bilayers. Int. J. Biol. Macromol. 2021, 168, 611-619. [CrossRef]
- Ahyayauch, H.; Masserini, M.E.; Goñi, F.M.; Alonso, A. The influence of lipid electric charge on the binding of Aβ-amyloid (1–42) amyloid peptide to bilayers in the liquid-ordered state. Biomolecules, 2024, 14, 298. [CrossRef]
- Small, D.M. The Physical Chemistry of Lipids. From Alkanes to Phospholipids, Plenum Press, New York, 1986, 43–87.
- Luzzati, V.; Tardieu, A.; Taupin, D. A pattern-recognition approach to the phase problem: application to the X-ray diffraction study of biological membranes and model systems, J. Mol. Biol. 1972, 64, 269–286. [CrossRef]
- Quinn, P.J.; Wolf, C. An X-ray diffraction study of model membrane raft structures, FEBS J. 2010, 277, 4685–4698. [CrossRef]
- Caffrey, M. The study of lipid phase transition kinetics by time-resolved X-ray diffraction, Annu. Rev. Biophys. Biophys. Chem. 1989, 18, 159–186. [CrossRef]
- Cullis, P.R.; de Kruijff, B. Lipid polymorphism and the functional roles of lipids in biological membranes, Biochim. Biophys. Acta 1979, 559, 399–420. [CrossRef]
- Ladbrooke, B.D.; Williams, R.M.; Chapman, D. Studies on lecithin–cholesterol–water interactions by differential scanning calorimetry and X-ray diffraction, Biochim. Biophys. Acta 1968, 150, 333–340. [CrossRef]
- McMullen, T.P.; Wong, B.C.; Tham, E.L.; Lewis, R.N.; McElhaney, R.N. Differential scanning calorimetric study of the interaction of cholesterol with the major lipids of the Acholeplasma laidlawii B membrane, Biochemistry 1996, 35, 16789–16798. [CrossRef]
- Goñi, F.M. The basic structure and dynamics of cell membranes: An update of the Singer–Nicolson model. Biochim. Biophys. Acta, 2014, 1838, 1467-1476. [CrossRef]
- Ipsen, J.H.; Karlström, G.; Mouritsen, O.G.; Wennerström, H.; Zuckermann, M.J. Phase equilibria in the phosphatidylcholine–cholesterol system, Biochim. Biophys. Acta 1987, 905, 162–172. [CrossRef]
- Sackmann, E. A charge-decoration technique for studying the heterogeneity of coexistent monolayer phases by electron microscopy. Nature, 1985, 313, 299–301. [CrossRef]
- McConnell, H.M. Structures and transitions in lipid monolayers at the air-water interface. Annu. Rev. Phys. Chem 1991, 42, 171–195. [CrossRef]
- McConnell, H.M.; Vrljic M. Liquid-liquid immiscibility in membranes. Ann. Rev. Biophys. Biomol. Struct 2003, 32, 469–492. [CrossRef]
- Brown, R.E.; Brockman, H.L. Using monomolecular films to characterize lipid lateral interactions. In: McIntosh, T.J., editor. Methods Molecular Biology. 398. Humana Press Inc.; Totowa, NJ, 2007. pp. 41-58. [CrossRef]
- Slotte, J.P. Lateral domain formation in mixed monolayers containing cholesterol and dipalmitoylphosphatidylcholine or N-palmitoylsphingomyelin. Biochim. Biophys. Acta 1995, 1235, 419–427. [CrossRef]
- Nag, K.; Pao, J.S.; Harbottle, R.R.; Possmayer, F.; Petersen, N.O.; Bagatolli, L.A. Segregation of saturated chain lipids in pulmonary surfactant films and bilayers. Biophys. J. 2002, 82, 2041–2051. [CrossRef]
- Veatch, S.L.; Keller, S.L. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 2002, 8 9, 268101–268104. [CrossRef]
- Stottrup, B.L.; Keller, S.L. Phase behaviour of lipid monolayers containing DPPC and cholesterol analogs. Biophys. J. 2006, 90, 3176–3183. [CrossRef]
- Phillips, M.C. The physical state of phospholipids and cholesterol in monolayers, bilayers, and membranes. Prog. Surf. Membr. Sci. 1972, 5, 139–221. [CrossRef]
- Dietrich, C.; Bagatolli, L.A.; Volovyk, Z.N.; Thompson, N.L.; Levi, M.; Jacobson, K.; Gratton, E. Lipid rafts reconstituted in model membranes. Biophys. J. 2001, 80, 1417–1428. [CrossRef]
- Dietrich, C.; Volovyk, Z.N.; Levi, M.; Thompson, N.L.; Jacobson, K. Partitioning of Thy-1, GM1, and cross-linked phospholipid analogs into lipid rafts reconstituted in supported model membrane monolayers. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 10642–10647. [CrossRef]
- Yuan, C.; Johnston, L.J. Atomic force microscopy studies of ganglioside GM1 domains in phosphatidylcholine and phosphatidylcholine/cholesterol bilayers. Biophys. J. 2001, 81, 1059–1069. [CrossRef]
- Yuan, C.; Furlong, J.; Burgos, P.; Johnston, L.J. The size of lipid rafts: an atomic force microscopy study of ganglioside GM1 domains in sphingomyelin/DOPC/cholesterol membranes. Biophys. J. 2002, 82, 2526–2535. [CrossRef]
- Lawrence, J.C.; Saslowsky, D.E.; Edwardson, J.M.; Henderson, R.M. Real-time analysis of the effects of cholesterol on lipid raft behavior using atomic force microscopy. Biophys. J. 2003, 84, 1827–1832. [CrossRef]
- McQuaw, C.M.; Zheng, L.; Ewing, A.G.; Winograd, N. Localization of sphingomyelin in cholesterol domains by imaging mass spectrometry. Langmuir 2007, 23, 5645–5650. [CrossRef]
- McQuaw, C.M.; Sostarecz, A.G.; Zheng, L.; Ewing, A.G.; Winograd, N. Lateral heterogeneity of dipalmitoylphosphatidylethanolamine-cholesterol Langmuir-Blodgett films investigated with imaging time-of-flight secondary ion mass spectrometry and atomic force microscopy. Langmuir 2005, 21, 807–813. [CrossRef]
- Zheng, L.; McQuaw, C.M.; Ewing, A.G.; Winograd, N. Sphingomyelin/phosphatidylcholine and cholesterol interactions studied by imaging mass spectrometry. J. Am. Chem. Soc. 2007, 129,15730–15731. [CrossRef]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta 2000, 1469, 159–195. [CrossRef]
- Chapman, D.; Gómez-Fernández, J.C.; Goñi, F.M. Intrinsic protein–lipid interactions. Physical and biochemical evidence. FEBS Lett. 1979, 98, 211–223. [CrossRef]
- Maltseva, E.; Brezesinski, G. Adsorption of amyloid b (1–40) peptide to phosphatidylethanolamine monolayers. ChemPhysChem. 2004, 5, 1185–1190. [CrossRef]
- Maltseva, E.; Kerth, A.; Brezesinski, G. Adsorption of amyloid b (1–40) peptide at phospholipid monolayers. ChemBioChem. 2005, 6, 1817–1824. [CrossRef]
- Alvarez, A.B.; Caruso, B.; Rodríguez, P.E.A.; Petersen, S.B.; Fidelio, G.D. Aβ-amyloid fibrils are self-triggered by the interfacial lipid environment and low peptide content. Langmuir. 2020, 36, 8056-8065. [CrossRef]
- Alvarez, A.B.; Rodríguez, P.E.A.; Fidelio, G.D. Gangliosides smelt nanostructured amyloid Aβ(1-40) fibrils in a membrane lipid environment. Biochim. Biophys. Acta Biomembr. 2022,1864,183749. [CrossRef]
- Gobbi, M.; Re, F.; Canovi, M.; Beeg, M.; Gregori, M.; Sesana, S.; Sonnino, S.; Brogioli, D.; Musicanti, C.; Gasco, P.; et al. Lipid-based nanoparticles with high binding affinity for amyloid-beta1-42 peptide. Biomaterials. 2010, 31, 6519–6529. [CrossRef]
- Arnulphi, C.; Sot, J.; García-Pacios, M.; Arrondo, J.L.; Alonso, A.; Goñi, F.M. Triton X-100 partitioning into sphingomyelin bilayers at subsolubilizing detergent concentrations: effect of lipid phase and a comparison with dipalmitoylphosphatidylcholine. Biophys. J. 2007, 93, 3504–3514. [CrossRef]
- Seelig, J. Titration calorimetry of lipid-peptide interactions, Biochim. Biophys. Acta 1997, 1331, 103–116. [CrossRef]
- Heerklotz, H.; Lantzsch, G.; Binder, H.; Klose, G.; Blume, A. Thermodynamic characterization of dilute aqueous lipid/detergent mixtures of POPC and C12EO8 by means of isothermal titration calorimetry. J. Phys. Chem. 1996, 100, 6764–6774. [CrossRef]
- Martinez-Senac, M.M.; Villalain, J.; Gomez-Fernandez, J.C. Structure of the Alzheimer b-amyloid peptide (25-35) and its interaction with negatively charged phospholipid vesicles. Eur. J. Biochem. 1999, 265, 744-753. [CrossRef]
- Ferraretto, A.; Pitto, M.; Palestini, P.; Masserini, M. Lipid domains in the membrane: thermotropic properties. Biochemistry 1997, 36, 9232–9236. [CrossRef]
- Nilsson, M. R. Techniques to study amyloid fibril formation in vitro. Methods 2004, 34, 151–160. [CrossRef]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta. 2010, 1804, 1405–1412. [CrossRef]
- Hossain, S.; Hashimoto, M.; Katakura, M.; Miwa, K.; Shimada, T.; Shido, O. Mechanism of docosahexaenoic acid-induced inhibition of in vitro Abeta1-42 fibrillation and Abeta1-42-induced toxicity in SH-S5Y5 cells. J. Neurochem. 2009, 111, 568-579. [CrossRef]
- Crescenzi, O.; Tomaselli, S.; Guerrini, R.; Salvadori, S.; D’Ursi, A.M.; Temussi, P.A.; Picone, D. Solution structure of the Alzheimer amyloid beta-peptide (1-42) in an apolar microenvironment. Similarity with a virus fusion domain. Eur. J. Biochem. 2002, 269, 5642–5648. [CrossRef]
- Herrera, F.E.; Pantano, S. Salt induced asymmetry in membrane simulations by partial restriction of ionic motion. J. Chem. Phys. 2009, 130, 195105. [CrossRef]
- Schüttelkopf, A.W.; van Aalten, D.M.F. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 1355–1363. [CrossRef]
- Oostenbrink, C.; Villa, A.; Mark, A.E.; Van Gunsteren, W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: the GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 2004, 25, 1656–1676. [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. E. J. Chem. Theory Comput. 2008, 4, 435–447. [CrossRef]
- Tieleman, D. P.; Berendsen, H.J.C. Molecular dynamics simulations of fully hydrated dipalmitoylphosphatidylcholine bilayer with different macroscopic boundary conditions and parameters. J. Chem. Phys. 1996, 105, 4871–4880. [CrossRef]
- Scott, W.; Hunenberger, P.; van Gunsteren, W. The GROMOS biomolecular simulation program package. J. Phys. Chem. 1999, 103, 3596–3607. [CrossRef]
- Berger, O.; Edholm, O.; Jähnig, F. Molecular dynamics simulations of a fluid bilayer of dipalmitoylphosphatidylcholine at full hydration, constant pressure, and constant temperature. Biophys. J. 1997, 72, 2002–2013. [CrossRef]
- Lemkul, J. A.; Bevan, D.R. A comparative molecular dynamics analysis of the amyloid b-peptide in a lipid bilayer. Arch. Biochem. Biophys. 2008, 470, 54–63. [CrossRef]
- Lemkul, J. A.; Bevan, D.R. Perturbation of membranes by the amyloid b-peptide—a molecular dynamics study. FEBS J. 2009, 276, 3060–3075. [CrossRef]
- Darré, L.; Machado, M.R.; Dans, P.D.; Herrera, F.E.; Pantano, S. Another coarse grain model for aqueous solvation: what for? J. Chem. Theory Comput. 2010, 6, 3793–3807. [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Ewald: an N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [CrossRef]
- Terzi, E.; Hölzemann, G.; Seelig, J. Interaction of Alzheimer beta-amyloid peptide(1-40) with lipid membranes. Biochemistry 1997,36, 14845-14852. [CrossRef]
- Terzi, E.; Hölzemann, G.; Seelig, J. Reversible random coil-beta-sheet transition of the Alzheimer beta-amyloid fragment (25-35). Biochemistry. 1994, 33, 1345-1350. [CrossRef]
- Terzi, E.; Hölzemann, G.; Seelig, J. Alzheimer beta-amyloid peptide 25-35: electrostatic interactions with phospholipid membranes. Biochemistry. 1994, 33, 7434-7441. [CrossRef]
- Kirkitadze, M.D.; Condron, M.M.; Teplow, D.B. Identification and characterization of key kinetic intermediates in amyloid beta-protein fibrillogenesis. J. Mol. Biol. 2001, 312,1103-1119. [CrossRef]
- Chang, H-W.; Ma, H-I.; Wu, Y.-S.; … ; Chan, J.C.C. Site specific NMR characterization of abeta-40 oligomers cross seeded by abeta-42 oligomers. Chem Sci. 2022, 13, 8526–8535. [CrossRef]
- Matsuzaki, K.; Kato, K.; Yanagisawa, K. Abeta polymerization through interaction with membrane gangliosides. Biochim. Biophys. Acta 2010 1801, 868-877. [CrossRef]
- Vitiello, G.; Di Marino, S.; D’Ursi, A.M.; D’Errico, G. Omega-3 fatty acids regulate the interaction of the Alzheimer’s A(25–35) peptide with lipid membranes. Langmuir 2013, 29, 14239–14245. [CrossRef]
- Chi, E.Y.; Frey, S.L.; Lee, K.Y.C. Ganglioside GM1-mediated amyloid-beta fibrillogenesis and membrane disruption. Biochemistry 2007, 46, 1913–1924. [CrossRef]
- Lin, M. S.; Chen, X.B.; …; Chen, W.Y. Dynamic fluorescence imaging analysis to investigate the cholesterol recruitment in lipid monolayer during the interaction between b-amyloid (1-40) and lipid monolayers. Colloids Surf. B Biointerfaces. 2009, 74, 59–66. [CrossRef]
- Thakur, G.; Pao, C.; Leblanc, R.M. Surface chemistry of lipid raft and amyloid Ab (1-40) Langmuir monolayer. Colloids Surf. B Biointerfaces. 2011, 87, 369–377. [CrossRef]
- Sánchez-Magraner, L.; Cortajarena, A.L.; …; Ostolaza, H. Membrane insertion of Escherichia coli alpha-hemolysin is independent from membrane lysis. J. Biol. Chem. 2006, 281, 5461–5467. [CrossRef]
- Alvarez, A.B.; Rodríguez, P.E.A.; Fidelio, G.D.; Caruso, B. Aβ amyloid fibers drastically alter the topography and mechanical properties of lipid membranes. Langmuir. 2023, 39, 18923-18934. [CrossRef]
- Alvarez, A.B.; Rodríguez, P.E.A.; Fidelio, G.D. Interfacial Aβ fibril formation is modulated by the disorder-order state of the lipids: The concept of the physical environment as amyloid inductor in biomembranes. Biochim. Biophys. Acta Biomembr. 2024, 1866, 184234. [CrossRef]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The amyloid-beta oligomer hypothesis: beginning of the third decade. J. Alzheimers Dis. 2018, 64, S567-S610. [CrossRef]
- Xu, Y.; Shen, J.; Luo, X.; …; Jiang, H. Conformational transition of amyloid β-peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 5403–5407. [CrossRef]
- Lemkul, J.A.; Bevan, D.R. A comparative molecular dynamics analysis of the amyloid beta-peptide in a lipid bilayer. Arch. Biochem. Biophys. 2008, 470, 54-63. [CrossRef]
- Davis, C.H.; Berkowitz, M.L. Interaction between amyloid-β (1–42) peptide and phospholipid bilayers: a molecular dynamics study. Biophys. J. 2009, 96, 785-797. [CrossRef]
- Manna M.; Mukhopadhyay, C. Binding, conformational transition and dimerization of amyloid-β peptide on GM1-containing ternary membrane: insights from molecular dynamics simulation. PLoS ONE 2013, 8, e71308. [CrossRef]
- Poojari, C.; Kukol, A.; Strodel, B. How the amyloid-β peptide and membranes affect each other: an extensive simulation study. Biochim. Biophys. Acta 2013, 1828, 327-339. [CrossRef]
- Brown, A.M.; Bevan, D.R. Molecular dynamics simulations of amyloid β-peptide (1-42): tetramer formation and membrane interactions. Biophys. J. 2016, 111, 937–949. [CrossRef]
- Tachi, Y.; Itoh, S.G.; Okumura, H. Molecular dynamics simulations of amyloid-β peptides in heterogeneous environments. Biophys. Physicobiol. 2022, 19, e190010. [CrossRef]
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 1991, 26, 3156–3168. [CrossRef]
- Torres, M.; Price, S.L.; …; Escribá, P.V. Membrane lipid modifications and therapeutic effects mediated by hydroxydocosahexaenoic acid on Alzheimer's disease. Biochim. Biophys. Acta 2014, 1838, 1680-1692. [CrossRef]
- Ntarakas, N.; Ermilova, I.; Lyubartsev, AP. Effect of lipid saturation on amyloid-beta peptide partitioning and aggregation in neuronal membranes: molecular dynamics simulations. Eur. Biophys. J. 2019, 48, 813–824. [CrossRef]
- Matthes, D.; de Groot, B.L. Molecular dynamics simulations reveal the importance of amyloid-beta oligomer β-sheet edge conformations in membrane permeabilization. J. Biol. Chem. 2023, 299, 103034. [CrossRef]
- Hollingsworth, S.A.; Dror, R.O. Molecular dynamics simulation for all. Neuron. 2018, 99, 1129-1143. [CrossRef]
- Wiatrak, B.; Piasny, J.; Kuźniarski, A.; Gąsiorowski, K. Interactions of amyloid-β with membrane proteins. Int. J. Mol. Sci. 2021, 22, 6075. [CrossRef]
- Chang, C.C.; Edwald, E.; Veatch, S.; Steel, D.G.; Gafni, A. Interactions of amyloid-β peptides on lipid bilayer studied by single molecule imaging and tracking. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1616-1624. [CrossRef]
- Kalvodova, L.; Kahya, N.; Simons, K. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J. Biol. Chem. 2005, 280, 36815–36823. [CrossRef]
- Ehehalt, R.; Keller, P.; Simons, K. Amyloidogenic processing of the Alzheimer b-amyloid precursor protein depends on lipid rafts. J. Cell Biol. 2003, 160, 113–123. [CrossRef]
- Park, I. H.; Hwang, E.M.; Mook-Jung, I. Lovastatin enhances Ab production and senile plaque deposition in female Tg2576 mice. Neurobiol. Aging 2003, 24, 637–643. [CrossRef]
- Abad-Rodriguez, J.; Ledesma, M.D.; Dotti, C.G. Neuronal membrane cholesterol loss enhances amyloid peptide generation. J. Cell Biol. 2004, 167, 953–960. [CrossRef]
- Riddell, D. R.; Christie, G.; Dingwall, C. Compartmentalization of b-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr. Biol. 2001, 11, 1288–1293. [CrossRef]
- Chauhan, A.; Ray, I.; Chauhan, V.P. Interaction of amyloid beta-protein with anionic phospholipids: possible involvement of Lys28 and C-terminus aliphatic amino acids. Neurochem. Res. 2000, 25, 423-9. [CrossRef]
- Goñi, F.M. "Rafts": A nickname for putative transient nanodomains. Chem. Phys. Lipids 2019, 218, 34-39. [CrossRef]
- Goñi, F.M. Sphingomyelin: What is it good for? Biochem. Biophys. Res. Commun. 2022, 633, 23-25. [CrossRef]
- Bukiya, A.N.; Dopico, A.M. (eds.) Cholesterol. From chemistry and biophysics to the clinic. Academic Press, London, 2022.
- Bode, D.C.; Baker, M.D.; Viles, J.H. Ion channel formation by amyloid-β42 oligomers but not amyloid-β40 in cellular membranes. J. Biol. Chem. 2017, 292, 1404 –1413. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).