Submitted:
25 April 2024
Posted:
26 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Methods:
2.2. Methods:
2.2.1. Synthesis and Characterization of Graphene Oxide Nanoparticle (GO):
2.2.2. Cell Culture Studies:
- (A)
- Toxicity study:
- (B)
- Cell damage analysis:
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| 18S | GATGGTAGTCGCCGTGCC | GCCTGCTGCCTTCCTTGG |
| CYP2E1 | AACTGTCCCCGGGACCTC | GCGCTCTGCACTGTGCTTT |
| EGR1 | AGCCCTACGAGCACCTGAC | GGGCAGTCGAGTGGTTTG |
| SREBP2 | CTCCATTGACTCTGAGCCAGGA | GAATCCGTGAGCGGTCTACCAT |
| Il6 | CATCCTCGACGGCATCTCAG | GCAGAAGAGAGCCAACCAAC |
| TNF | CTCTTCTGCCTGCTGCACTTG | ATGGGCTACAGCTTGTCACTC |
| Il10 | ACTGCTAACCGACTCCTTA | TAAGGAGTCGGTTAGCAGT |
| AMPK | AGGAAGAATCCTGTGACAAGCAC | CCGATCTCTGTGGAGTAGCAGT |
| NrF2 | GAGAGCCCAGTCTTCATTGC | TGCTCAATGTCCTGTTGCAT |
| HO1 | AAGCCGAGAATGCTGAGTTCA | CGGGTGTAGATATGGTACAAGGA |
| Occludin | CTCGAGAAAGTGCTGAGTGCCTGGAC | AAGCTTTCGGTGACCAATTCACCTGA |
| ZO-1 | TATTATGGCACATCAGCACG | TGGGCAAACAGACCAAGC |
| PPAR | GGCTTCATGACAAGGGAGTTTC | AACTCAAACTTGGGCTCCATAAAG |
2.3. Stastical Analysis
3. Results
3.1. Characterisation of the Nanoparticles:
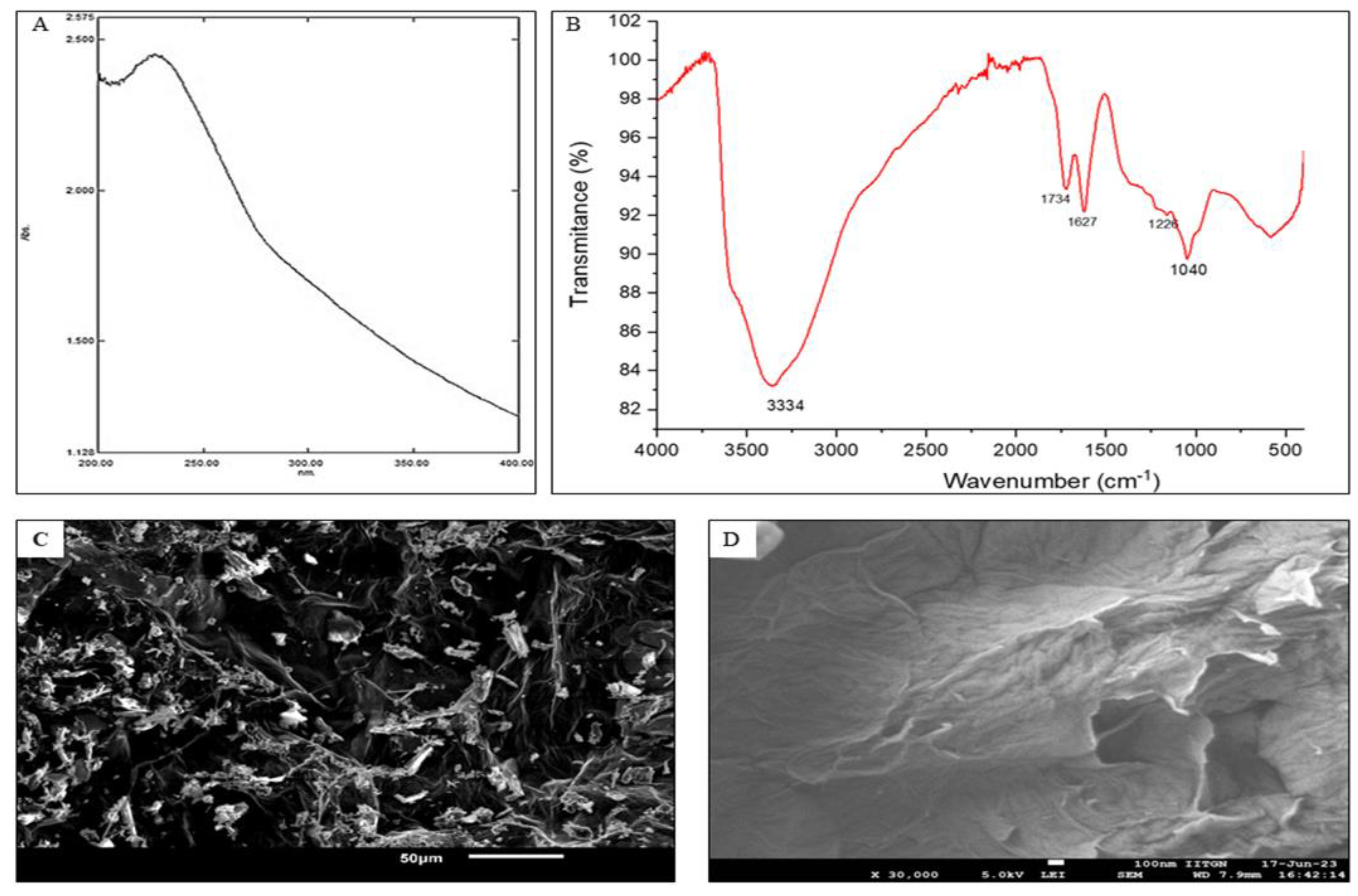
3.2. Activity in In Vitro Model:
3.2.1. Cell Viability Study:
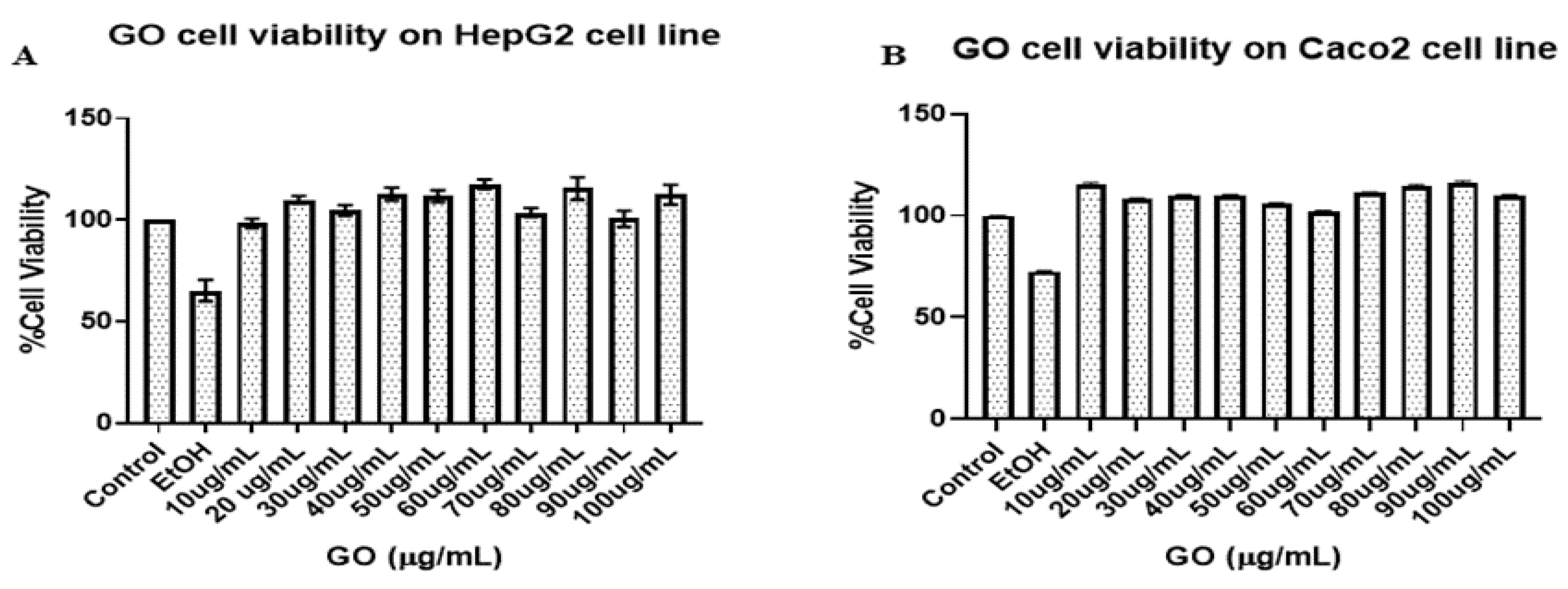
3.2.1. Cell damage study:
- A)
- AO-EtBr and DAPI staining:
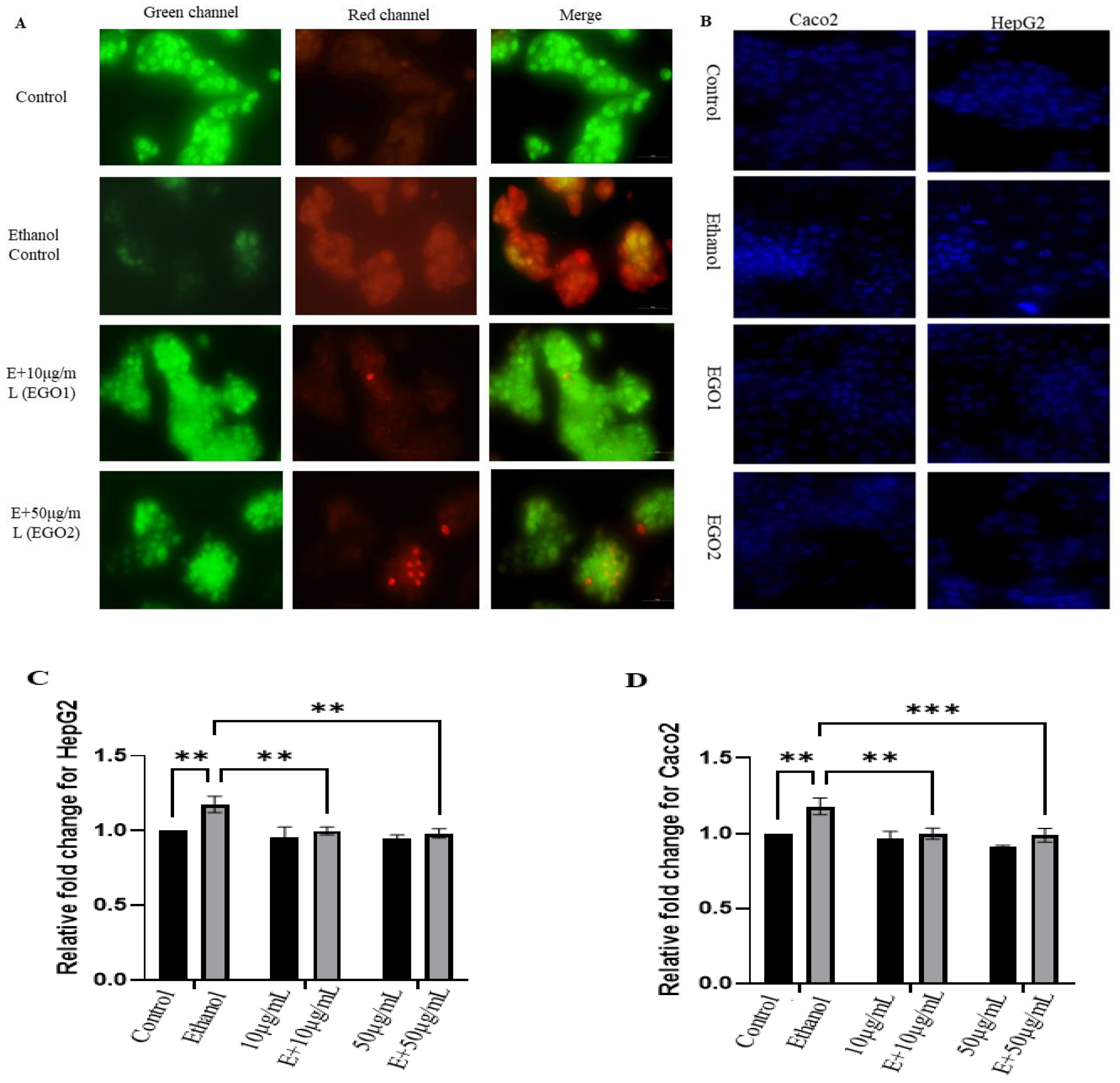
- (B)
- Lipid Accumulation Study:
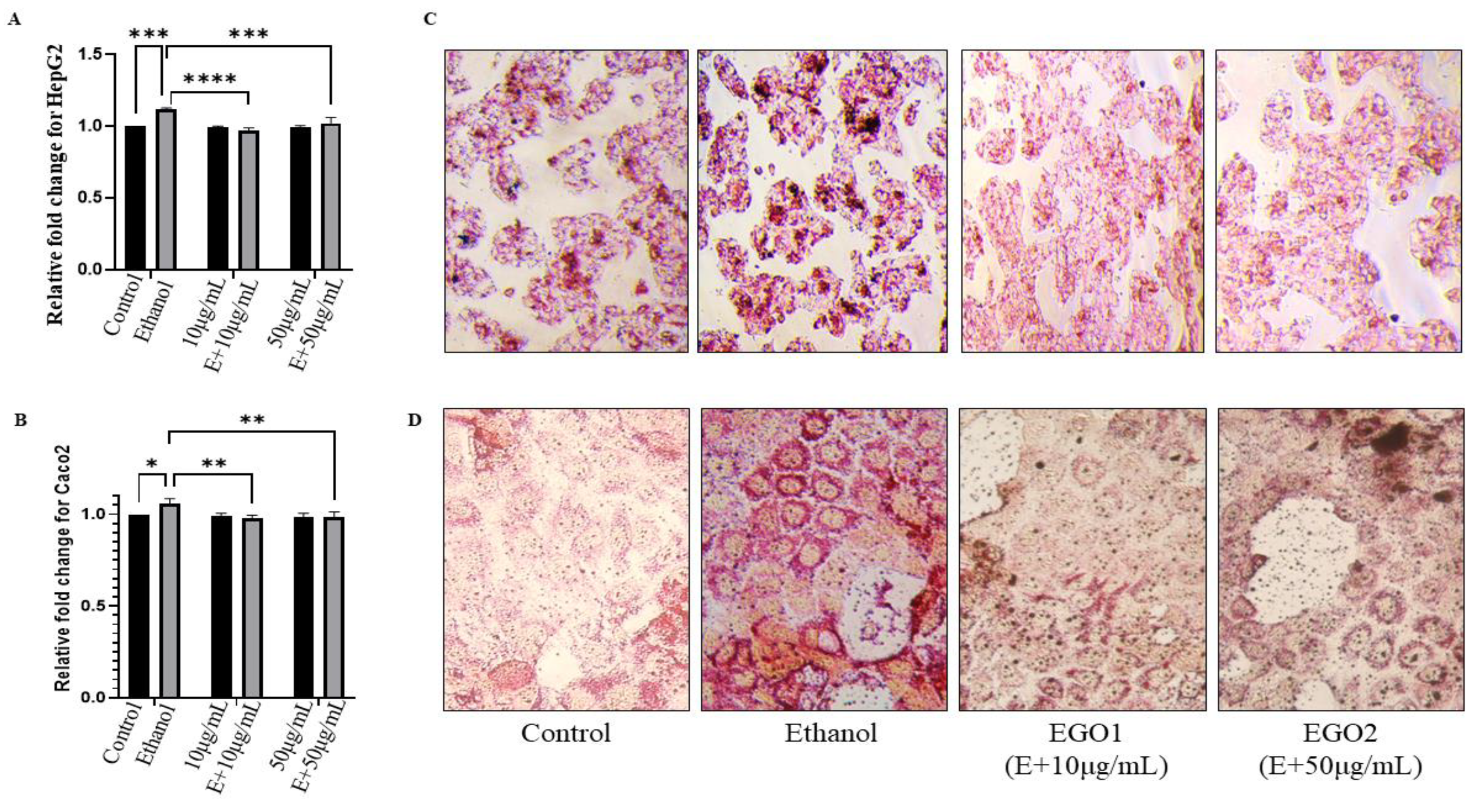
- (C)
- Reactive oxygen spices analysis:
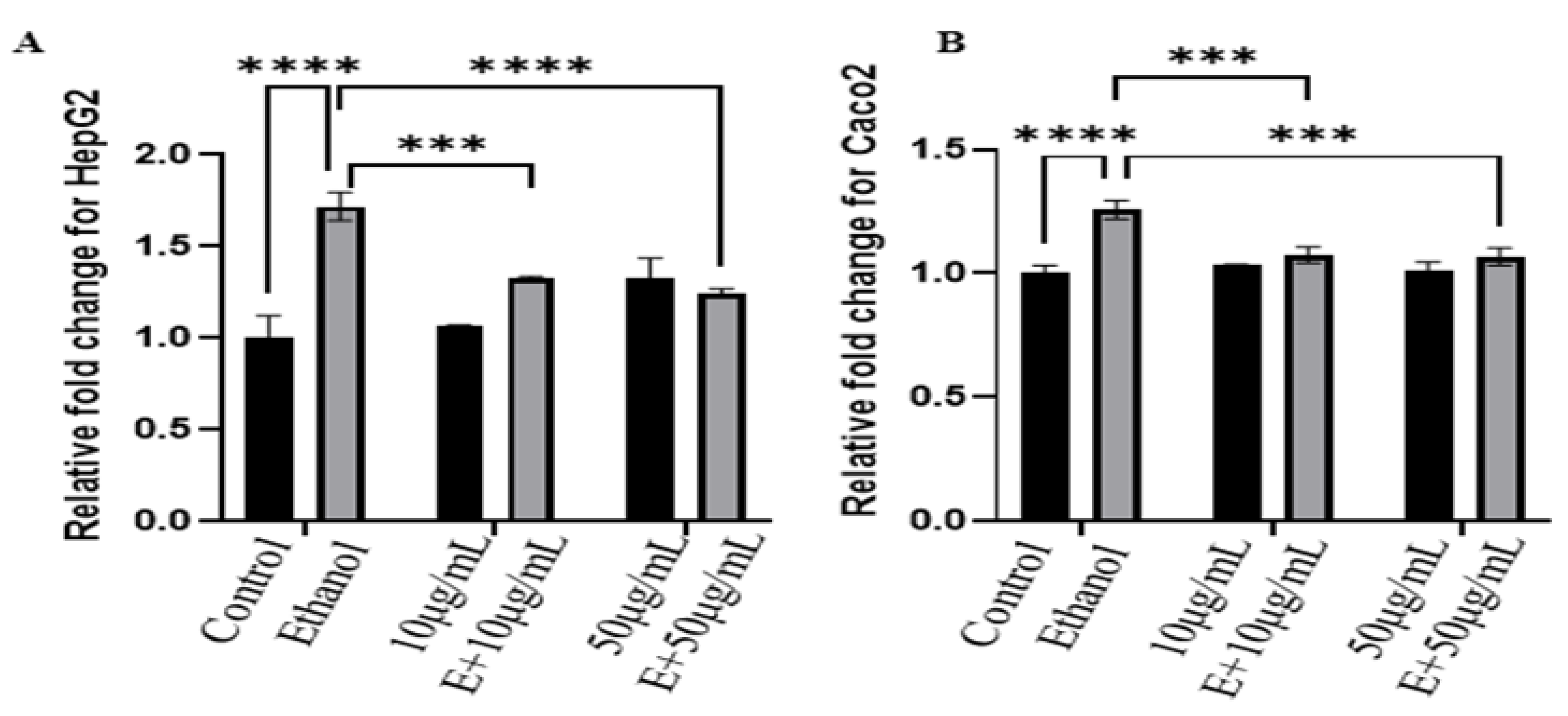
- (D)
- Gene Expression Analysis:
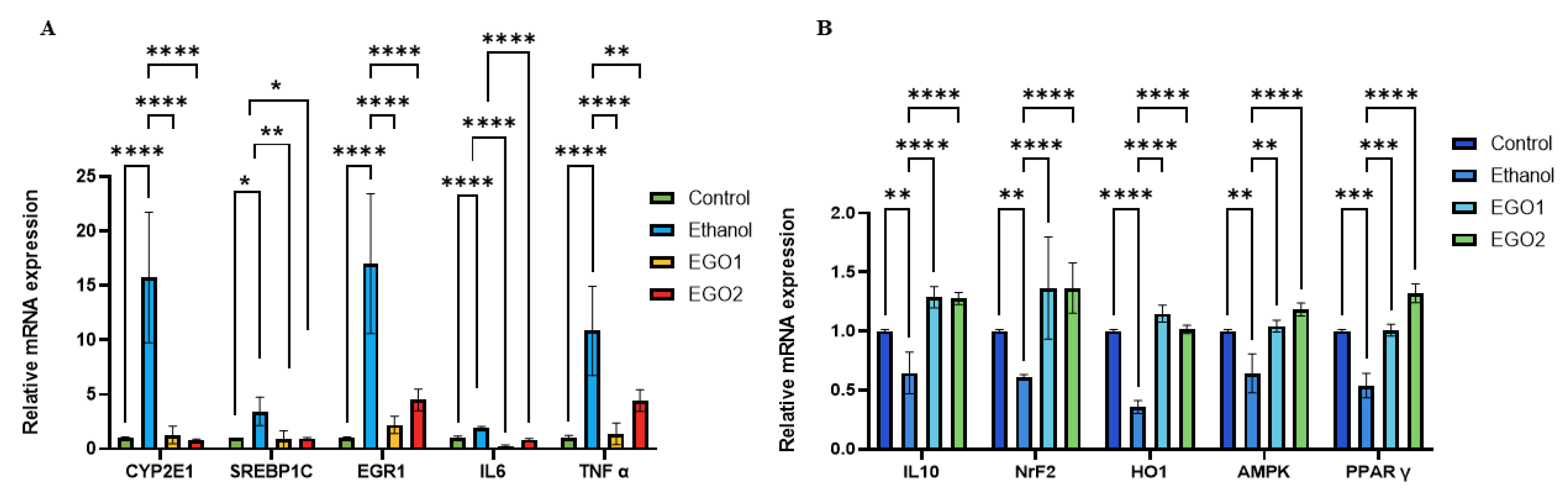

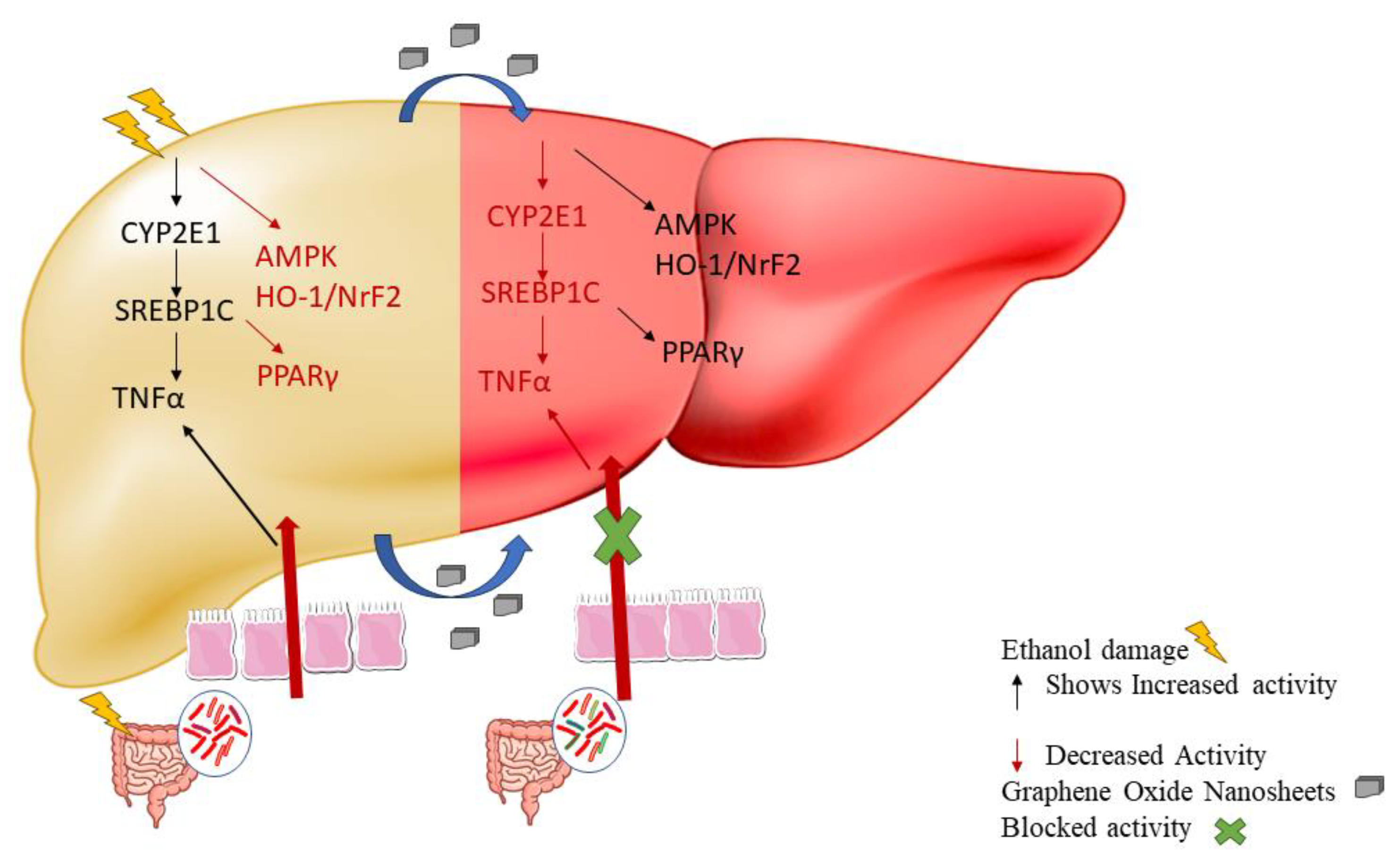
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niu X, Zhu L, Xu Y, Zhang M, Hao Y, Ma L, et al. Global prevalence, incidence, and outcomes of alcohol related liver diseases: a systematic review and meta-analysis. BMC Public Health. 2023 Dec 1;23(1). [CrossRef]
- Aghara H, Chadha P, Zala D, Mandal P. Stress mechanism involved in the progression of alcoholic liver disease and the therapeutic efficacy of nanoparticles. Front Immunol. 2023;14. [CrossRef]
- Zhu L, Wang Y, Pan CQ, Xing H. Gut microbiota in alcohol-related liver disease: pathophysiology and gut-brain cross talk. Vol. 14, Frontiers in Pharmacology. 2023. [CrossRef]
- Subramaniyan V, Chakravarthi S, Jegasothy R, Seng WY, Fuloria NK, Fuloria S, et al. Alcohol-associated liver disease: A review on its pathophysiology, diagnosis and drug therapy. Vol. 8, Toxicology Reports. 2021. [CrossRef]
- Yoon EL, Kim W. Current and future treatment for alcoholic-related liver diseases. Vol. 38, Journal of Gastroenterology and Hepatology (Australia). 2023. [CrossRef]
- Osna, N.A.; Donohue, T.M.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol research 2017, 38, 147–161. [Google Scholar]
- Zhao R, Zhu M, Zhou S, Feng W, Chen H. Rapamycin-Loaded mPEG-PLGA Nanoparticles Ameliorate Hepatic Steatosis and Liver Injury in Non-alcoholic Fatty Liver Disease. Front Chem. 2020;8. [CrossRef]
- Hu R, Liu S, Anwaier G, Wang Q, Shen W, Shen Q, et al. Formulation and intestinal absorption of naringenin loaded nanostructured lipid carrier and its inhibitory effects on nonalcoholic fatty liver disease. Nanomedicine [Internet]. 2021 Feb;32:102310. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1549963420301647. [CrossRef]
- Lai WF, Wong WT. Use of graphene-based materials as carriers of bioactive agents. Vol. 16, Asian Journal of Pharmaceutical Sciences. 2021. [CrossRef]
- Marcano DC, Kosynkin D V., Berlin JM, Sinitskii A, Sun Z, Slesarev A, et al. Improved synthesis of graphene oxide. ACS Nano. 2010;4(8). [CrossRef]
- Habte AT, Ayele DW, Hu M. Synthesis and Characterization of Reduced Graphene Oxide (rGO) Started from Graphene Oxide (GO) Using the Tour Method with Different Parameters. Advances in Materials Science and Engineering. 2019;2019. [CrossRef]
- Nasirzadeh N, Azari MR, Rasoulzadeh Y, Mohammadian Y. An assessment of the cytotoxic effects of graphene nanoparticles on the epithelial cells of the human lung. Toxicol Ind Health. 2019;35(1). [CrossRef]
- Cummings BS, Schnellmann RG. Measurement of Cell Death in Mammalian Cells. Curr Protoc Pharmacol. 2004 Jun;25(1). [CrossRef]
- Liu K, Liu P cheng, Liu R, Wu X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med Sci Monit Basic Res. 2015;21. [CrossRef]
- Kasibhatla S, Amarante-Mendes GP, Finucane D, Brunner T, Bossy-Wetzel E, Green DR. Acridine Orange/Ethidium Bromide (AO/EB) Staining to Detect Apoptosis. Cold Spring Harb Protoc. 2006;2006(3). [CrossRef]
- Rubin, E.; Rottenberg, H. Ethanol-induced injury and adaptation in biological membranes. Federation Proceedings 1982, 41, 2465–2471. [Google Scholar]
- Kema VH, Khan I, Jamal R, Vishwakarma SK, Lakki Reddy C, Parwani K, et al. Protective Effects of Diallyl Sulfide Against Ethanol-Induced Injury in Rat Adipose Tissue and Primary Human Adipocytes. Alcohol Clin Exp Res. 2017;41(6). [CrossRef]
- Patel F, Parwani K, Patel D, Mandal P. Metformin and Probiotics Interplay in Amelioration of Ethanol-Induced Oxidative Stress and Inflammatory Response in an in Vitro and in Vivo Model of Hepatic Injury. Mediators Inflamm. 2021;2021. [CrossRef]
- Karbowski M, Kurono C, Wozniak M, Ostrowski M, Teranishi M, Nishizawa Y, et al. Free radical-induced megamitochondria formation and apoptosis. Free Radic Biol Med. 1999;26(3–4). [CrossRef]
- Emiru TF, Ayele DW. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egyptian Journal of Basic and Applied Sciences. 2017;4(1). [CrossRef]
- Surekha G, Krishnaiah KV, Ravi N, Padma Suvarna R. FTIR, Raman and XRD analysis of graphene oxide films prepared by modified Hummers method. In: Journal of Physics: Conference Series. 2020. [CrossRef]
- Khalili, D. Graphene oxide: A promising carbocatalyst for the regioselective thiocyanation of aromatic amines, phenols, anisols and enolizable ketones by hydrogen peroxide/KSCN in water. New Journal of Chemistry. 2016;40(3). [CrossRef]
- Rhazouani A, Gamrani H, El Achaby M, Aziz K, Gebrati L, Uddin MS, et al. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of in Vitro and in Vivo Studies. Vol. 2021, BioMed Research International. 2021. [CrossRef]
- Rattana T, Chaiyakun S, Witit-Anun N, Nuntawong N, Chindaudom P, Oaew S, et al. Preparation and characterization of graphene oxide nanosheets. In: Procedia Engineering. 2012. [CrossRef]
- Oh, W.C.; Zhang, F.J. Preparation and characterization of graphene oxide reduced from a mild chemical method. Asian Journal of Chemistry 2011, 23, 875–879. [Google Scholar]
- Ribble D, Goldstein NB, Norris DA, Shellman YG. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5. [CrossRef]
- Wang QX, Ma X. Liver: a unique immune organ. Zhonghua Gan Zang Bing Za Zhi. 2021;29(6). [CrossRef]
- Kong LZ, Chandimali N, Han YH, Lee DH, Kim JS, Kim SU, et al. Pathogenesis, early diagnosis, and therapeutic management of alcoholic liver disease. Vol. 20, International Journal of Molecular Sciences. 2019. [CrossRef]
- Sarkar, D.; Jung, M.K.; Wang, H.J. Alcohol and the immune system. Alcohol Research: Current Reviews 2015, 37, 153. [Google Scholar]
- Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, et al. Alcoholic liver disease. Vol. 4, Nature Reviews Disease Primers. Nature Publishing Group; 2018. [CrossRef]
- Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Vol. 29, Seminars in Liver Disease. 2009. [CrossRef]
- Moslehi A, Hamidi-Zad Z. Role of SREBPs in liver diseases: A mini-review. Vol. 6, Journal of Clinical and Translational Hepatology. 2018. [CrossRef]
- Jung JH, Kim SE, Suk KT, Kim DJ. Gut microbiota-modulating agents in alcoholic liver disease: Links between host metabolism and gut microbiota. Vol. 9, Frontiers in Medicine. Frontiers Media S.A.; 2022. [CrossRef]
- Slevin E, Baiocchi L, Wu N, Ekser B, Sato K, Lin E, et al. Kupffer Cells: Inflammation Pathways and Cell-Cell Interactions in Alcohol-Associated Liver Disease. Vol. 190, American Journal of Pathology. 2020. [CrossRef]
- Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Spurnic AR, et al. Gut-liver axis, gut microbiota, and its modulation in the management of liver diseases: A review of the literature. Vol. 20, International Journal of Molecular Sciences. 2019. [CrossRef]
- Priyadarsini S, Mohanty S, Mukherjee S, Basu S, Mishra M. Graphene and graphene oxide as nanomaterials for medicine and biology application. Vol. 8, Journal of Nanostructure in Chemistry. 2018. [CrossRef]
- Di Mauro G, Amoriello R, Lozano N, Carnasciali A, Guasti D, Becucci M, et al. Graphene Oxide Nanosheets Reduce Astrocyte Reactivity to Inflammation and Ameliorate Experimental Autoimmune Encephalomyelitis. ACS Nano. 2023;17(3). [CrossRef]
- Aliyev E, Filiz V, Khan MM, Lee YJ, Abetz C, Abetz V. Structural characterization of graphene oxide: Surface functional groups and fractionated oxidative debris. Nanomaterials. 2019;9(8). [CrossRef]
- Bellier N, Baipaywad P, Ryu N, Lee JY, Park H. Recent biomedical advancements in graphene oxide- and reduced graphene oxide-based nanocomposite nanocarriers. Vol. 26, Biomaterials Research. 2022. [CrossRef]
- Kucki M, Diener L, Bohmer N, Hirsch C, Krug HF, Palermo V, et al. Uptake of label-free graphene oxide by Caco-2 cells is dependent on the cell differentiation status. J Nanobiotechnology. 2017;15(1). [CrossRef]
- Qi W, Xue Z, Yuan W, Wang H. Layer-by-layer assembled graphene oxide composite films for enhanced mechanical properties and fibroblast cell affinity. J Mater Chem B. 2014;2(3). [CrossRef]
- Cebadero-Domínguez O, Ferrández-Gómez B, Sánchez-Ballester S, Moreno J, Jos A, Cameán AM. In vitro toxicity evaluation of graphene oxide and reduced graphene oxide on Caco-2 cells. Toxicol Rep. 2022;9. [CrossRef]
- Ruiz ON, Fernando KAS, Wang B, Brown NA, Luo PG, McNamara ND, et al. Graphene oxide: a nonspecific enhancer of cellular growth. ACS Nano. 2011;5(10). [CrossRef]
- Qiu Y, Wang Z, Owens ACE, Kulaots I, Chen Y, Kane AB, et al. Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology. Nanoscale. 2014;6(20). [CrossRef]
- Patlolla AK, Randolph J, Kumari SA, Tchounwou PB. Toxicity evaluation of graphene oxidein kidneys of sprague-dawley rats. Int J Environ Res Public Health. 2016;13(4). [CrossRef]
- de Frutos S, Griera M, Lavín-López M del P, Martínez-Rovira M, Martínez-Rovira JA, Rodríguez-Puyol M, et al. A new graphene-based nanomaterial increases lipolysis and reduces body weight gain through integrin linked kinase (ILK). Biomater Sci. 2023;11(14). [CrossRef]
- Zhang Y, Yan T, Wang T, Liu X, Hamada K, Sun D, et al. Crosstalk between CYP2E1 and PPARα substrates and agonists modulate adipose browning and obesity. Acta Pharm Sin B. 2022. [CrossRef]
- Thomes PG, Osna NA, Davis JS, Donohue TM. Cellular steatosis in ethanol oxidizing-HepG2 cells is partially controlled by the transcription factor, early growth response-1. International Journal of Biochemistry and Cell Biology. 2013;45(2). [CrossRef]
- Castagnola V, Deleye L, Podestà A, Jaho E, Loiacono F, Debellis D, et al. Interactions of Graphene Oxide and Few-Layer Graphene with the Blood-Brain Barrier. Nano Lett. 2023;23(7). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





