1. Introduction
Immunology research has experienced significant growth worldwide, catalyzing breakthroughs in understanding immune mechanisms, disease pathogenesis, and therapeutic interventions [
1]. This comprehensive literature review navigates through a diverse array of immunological investigations, spanning from innovative applications of bacterial proteins as antibody reagents [
2] to the intricate realms of clinical immunology and disease management [
3]. With a particular emphasis on pivotal research domains such as Engineering Chimeric Proteins for Immunodiagnosis [
4], Vaccine Development [
5], and Clinical Studies targeting diseases like salmonellosis [
6]and HIV [
7].
Additionally, the emergence of Extended-spectrum Beta-lactamases (ESBLs) poses a significant threat to healthcare systems worldwide, necessitating concerted efforts in understanding and combating antimicrobial resistance [
8]. The introduction outlines current research endeavors aimed at addressing critical priorities in global healthcare, including antimicrobial resistance [
9] and the development of advanced immunological techniques [
10] .
Significant advancements in immunological techniques and blood banking practices have not only enhanced diagnostic and therapeutic capacities [
11] but also improved patient care standards and safety protocols globally [
12]. Furthermore, the introduction explores the intricate journey of navigating clinical immunology, encompassing a spectrum of immune-mediated disorders and complex diseases such as Chronic Granulomatous Disease [
13], Transient Hypogammaglobulinemia of Infancy [
14], Neuropsychiatric Lupus Erythematosus [
15], Severe Combined Immunodeficiency [
16], Multiple Myeloma [
17], and Adult T Cell Leukemia/Lymphoma [
18]. Additionally, investigations concerning IgM antibodies in egg whites [
19] and tuberculosis research [
20]shed light on the comprehensive nature of immunological and microbiological research globally.
This introduction sets the stage for a comprehensive exploration of the diverse facets of immunology and microbiology, reflecting the pivotal role of these disciplines in advancing scientific knowledge and clinical care paradigms on a global scale. The subsequent sections delve deeper into specific research areas, highlighting their implications for global healthcare and emphasising the collaborative efforts aimed at improving patient outcomes worldwide. As the field of immunology continues to evolve, fueled by ongoing research and innovation, its impact on global health remains profound. By fostering collaboration and innovation, immunology research continues to play a pivotal role in addressing pressing healthcare challenges and improving the lives of individuals worldwide.
2. Unveiling the Potential of Bacterial Proteins as Antibody Reagents
Bacterial immunoglobulin-binding proteins (IBPs) represent a diverse class of molecules that have garnered significant attention due to their unique ability to interact with immunoglobulins. Among the well-studied IBPs are protein A (SpA) from Staphylococcus aureus [
21], protein G (SpG) from Streptococci [
22], and protein L (SpL) derived from Peptostreptococcus magnus [
23]. These proteins, typically displayed on the cell wall of microorganisms, play crucial roles in bacterial evasion strategies against the host immune system. One of the primary functions of IBPs is their involvement in modulating immune responses. They achieve this by various means, including the activation of the complement system through the classical pathway and the inhibition of phagocytosis. By interfering with these processes, IBPs contribute to the survival and persistence of bacteria within the host environment. Moreover, IBPs have been shown to induce polyclonal activation of B-lymphocytes, further complicating the host's immune response [
24].
One remarkable feature of IBPs is their broad specificity in binding to immunoglobulins [
25]. They can interact with a wide range of mammalian and non-mammalian immunoglobulins, including IgG [
24]. Importantly, the binding of IBPs to immunoglobulins does not interfere with the antigen-binding sites, allowing for their use as powerful tools in immunological assays and diagnostic tests. The utility of IBPs in serological diagnostics cannot be overstated. Proteins such as SpA and SpG have been extensively employed in various serological assays for the diagnosis of infectious diseases. For instance, they have been utilized in the detection of pathogens like Borrelia burgdorferi, the causative agent of Lyme disease, in both humans and animals [
26]. Their high affinity for immunoglobulins and specificity make them invaluable assets in accurately identifying infectious agents [
27].
Beyond diagnostic applications, IBPs hold immense potential in biomedical research, therapy, biotechnology, and industrial processes. Their ability to selectively bind to immunoglobulins has paved the way for the development of novel therapeutic strategies, including targeted drug delivery systems and immunotherapies. Furthermore, IBPs are being explored for their utility in various biotechnological processes, such as antibody purification and protein engineering [
24].
The Split Trehalase Immunoglobulin Assay (STIGA) utilizing bacterial immunoglobulin binding proteins (bIBPs) emerges as a versatile diagnostic tool for assessing IgG concentrations in diverse non-domestic animal species. It offers a streamlined approach, sidestepping the need for species-specific reagents. Results revealed activation across numerous species, barring birds. Notably, incorporation of Protein G, A, and L significantly enhanced detection rates, with Protein G demonstrating the highest efficacy. Interestingly, assays combining two bIBPs showed diminished performance compared to single-bIBP fusion. STIGA presents a promising avenue for facile and comprehensive assessment of total IgG levels in serum samples, transcending species boundaries for potential widespread application [
4]
Bacterial immunoglobulin-binding proteins play multifaceted roles in bacterial pathogenesis, immune evasion, and host-pathogen interactions. Their broad specificity and diverse applications make them indispensable tools in immunodiagnostics and offer promising avenues for advancing biomedical research and therapeutic interventions [
28,
29] .
3. Engineering Chimeric Proteins: Pioneering Immunodiagnosis
Chimeric proteins are hybrid molecules composed of segments or domains from different bacterial proteins, often engineered for specific purposes. These proteins combine the desirable characteristics of their parent proteins, making them versatile tools in various fields, including biotechnology, medicine, and research. In the context of immunoglobulin-binding proteins (IBPs), chimeric proteins can be created by combining domains from different IBPs or other proteins to enhance their binding specificity, stability, or other properties. For example, chimeric IBPs might incorporate elements from protein A, protein G, and protein L to broaden their range of target immunoglobulins or improve their performance in diagnostic assays [
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60].
The design of chimeric IBPs allows for customization according to specific applications. Researchers can tailor these proteins to optimize their performance in diagnostic tests, therapeutic interventions, or research experiments. Chimeric IBPs have the potential to improve the accuracy and efficiency of immunodetection assays, enhance the efficacy of immunotherapies, and facilitate the purification of immunoglobulins for various applications [
24].
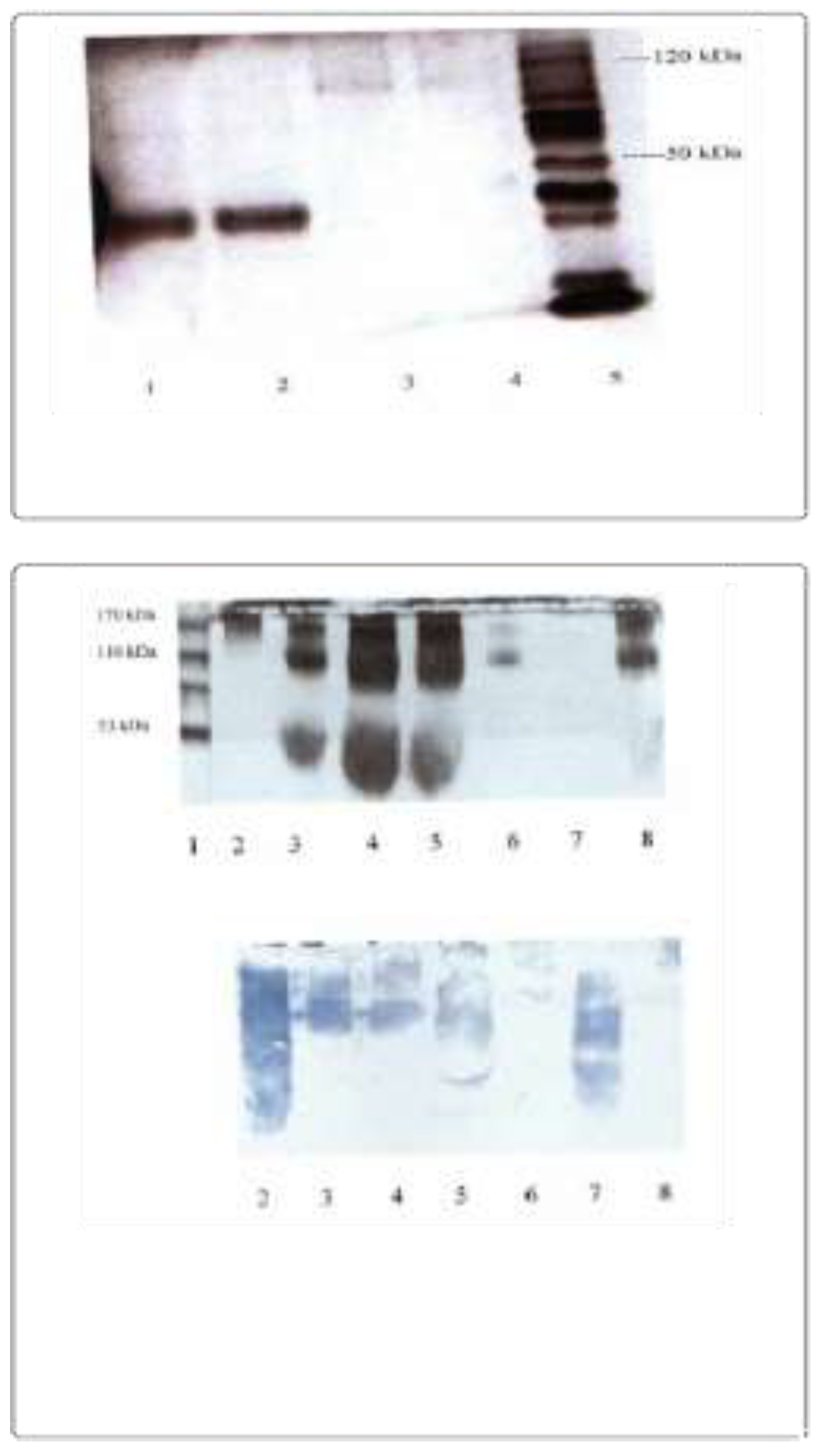
The objective of this study was to validate the feasibility of employing hybrid immunoglobulin-binding reagents in Enzyme-Linked Immunosorbent Assays (ELISAs) for the detection of IgG/IgY and specific antibodies against Salmonella spp. across various animal species, utilizing a universal diagnostic ELISA. Hybrid immunoglobulin-binding bacterial proteins (IBP) were developed, including recombinant proteins LA, LG, and AG, to enhance their binding affinity towards a broader spectrum of immunoglobulins, thus facilitating the binding, detection, and purification of immunoglobulins and their fragments. Conjugates, namely SpLA-LG-peroxidase and SpLAG-anti-IgY-peroxidase, were synthesized using the periodate method and proved to be effective reagents. Their binding affinity to immunoglobulins surpassed that of previously reported hybrid IgG-binding proteins such as SpAG, SpLA, and SpLG. IgY fractions were isolated from the egg yolks of various avian species using the chloroform-polyethylene glycol (PEG) method. An ELISA for detecting anti-Salmonella spp. antibodies was conducted with modifications to identify the presence of antibodies in humans, laying hens, geese, quails, and pigeons. Salmonella, a motile, flagellated, rod-shaped zoonotic pathogen belonging to the Enterobacteriaceae family, poses significant health risks, including typhoid fever and food-borne illnesses, with potentially fatal consequences and adverse impacts on both individuals and economies worldwide. The poultry industry is particularly susceptible to the detrimental effects of this pathogen. The authors concluded that universal ELISAs were effective and reproducible in detecting immunoglobulins from both avian and mammalian species. However, ELISA utilizing the SpLAG-anti-IgY-HRP conjugate (lane 3 and 4) exclusively reacted with the entire spectrum of animal antibodies (
Figure 1a). This conjugate was subsequently utilized to standardize a universal ELISA for determining anti-Salmonella antibodies, facilitating serological assessments in both mammalian and avian species [
61].
Figure 1 shows chimeric conjugates. Overall, chimeric proteins represent a promising avenue for advancing the capabilities of immunoglobulin-binding proteins and expanding their utility in diverse scientific and medical settings.
4. Illuminating Pathways in Vaccine Development and Clinical Studies
4.1. A Vaccine for Salmonellosis
The aim of this study was to evaluate the efficacy and safety of two different vaccines in protecting layer hen chickens against Salmonella infection. The first vaccine tested was a DNA vaccine containing Salmonella genomic DNA encapsulated in liposomes as a vector. The second vaccine was a live attenuated Salmonella vaccine composed of five attenuated Salmonella serovars, which were attenuated using indigenous plant extracts such as garlic and onion [
62] .
The results of the study revealed that both vaccines demonstrated a high capacity for protection, effectively preventing Salmonella infection following challenge with a wild-type strain of Salmonella Typhimurium. Furthermore, eggs produced by hyper-immunized chickens inhibited the growth of Salmonella species in vitro. ELISA analysis confirmed the production of specific antibodies targeting the lipopolysaccharide (LPS) of S. Typhimurium in vaccinated chickens [
62,
63].
Post-mortem examinations conducted as part of the study confirmed the presence of salmonellosis in the control group, while no signs of infection were observed in chickens immunized with either the DNA vaccine or the live attenuated vaccine. This study emphasises the efficacy of both prophylactic DNA and live attenuated vaccines (LAV) in preventing poultry salmonellosis. The findings suggest that these vaccines could serve as valuable tools for controlling Salmonella infections in poultry populations, thereby enhancing food safety and reducing the risk of transmission to humans through consumption of poultry products [
62,
63,
64]
4.2. An HIV Experimental Vaccines
The study investigated the potential of immunizing brown Leghorn layer hens with human immunodeficiency virus 1 (HIV-1) viral peptides to induce a robust immune response measurable in chicken eggs. Immunization involved intramuscular administration of KLH-conjugated HIV peptide candidate vaccines to nine healthy hens. Results showed that the vaccines effectively elicited strong anti-HIV immune responses in the hens. Subsequent feeding of chicks with hyperimmune eggs led to the production of anti-anti-idiotypic antibodies capable of neutralizing the original HIV antigen. Inhibition assays demonstrated significant inhibition of avian anti-HIV antibodies binding to immobilized HIV peptides in the serum samples of tested chicks, indicating the presence of anti-anti-idiotypic antibodies. This inhibition was absent in the sera of chicks not fed with hyperimmune eggs. The findings suggest that feeding chicks with hyperimmune eggs could stimulate the production of anti-anti-idiotypic antibodies, potentially offering avenues for immunotherapy against HIV infections, albeit further studies and clinical trials are warranted to validate these findings in humans [
65,
66].
Table 1,
Table 2 and
Table 3 reflect the results.
The study investigated the efficacy of immunizing brown Leghorn layer hens with HIV-1 viral peptides to induce an immune response detectable in chicken eggs. Hens were immunized with KLH-conjugated HIV peptide vaccines, and the resulting anti-HIV antibody response was assessed using ELISA. Chicks fed with hyperimmune eggs exhibited the production of anti-anti-idiotypic antibodies that effectively neutralized the original HIV antigen, as confirmed by inhibition assays. These findings align with previous research supporting the potential of anti-idiotypic vaccines against HIV/AIDS. Notably, the study identified highly immunogenic peptide motifs within the HIV envelope glycoproteins gp120 and gp41. These peptides elicited robust immune responses and demonstrated promise as vaccine candidates. The results suggest a potential avenue for HIV immunotherapy, indicating the importance of exploring novel approaches to combat HIV infections. However, further research and clinical trials are needed to validate these findings in humans. The study emphasises the significance of investigating alternative immunotherapeutic strategies and highlights the potential of hyperimmune egg-derived antibodies as a treatment modality. Overall, the findings contribute to the understanding of HIV immunology and emphasize the importance of continued research into effective HIV treatment options [
65,
67,
68] .
5. Microbiological Insights and Antimicrobial Resistance Surveillance
5.1. Extended-Spectrum Beta-Lactamases (ESBLs) Present a Significant Public Health Challenge Globally
ESBLs are enzymes produced by bacteria, particularly within the family Enterobacteriaceae, conferring resistance to various beta-lactam antibiotics. They are encoded by genes located on mobile genetic elements, facilitating their transfer between different bacterial strains and species. ESBLs challenge antibiotic efficacy and complicate treatment strategies due to their ability to hydrolyze a wide range of beta-lactam antibiotics [
69,
70] .
ESBLs are enzymes produced by certain bacteria, primarily within the family Enterobacteriaceae, which confer resistance to a wide range of beta-lactam antibiotics. These enzymes are encoded by genes located on mobile genetic elements, facilitating their transfer between different bacterial strains and species [
70,
71] .
ESBLs are classified into different types, including SHV, TEM, and CTX-M, each with distinct biochemical characteristics and resistance profiles. Detection methods, such as PCR and sequencing, are crucial for accurately identifying ESBL-producing organisms, aiding in surveillance and infection control efforts [
72,
73] .
Global epidemiological studies underscore the pervasive presence of ESBLs, reflecting a concerning burden of antimicrobial resistance. Investigations reveal dissemination across diverse reservoirs, spanning clinical settings to environmental niches. The interconnectedness between human and non-human sources accentuates the complexity of ESBL transmission, necessitating heightened surveillance and intervention strategies [
72,
74] .
Assessments in WHO Regions highlight the substantial burden of antimicrobial resistance associated with ESBLs. Estimates of deaths and disability-adjusted life-years attributable to bacterial AMR emphasize the critical impact on public health outcomes, particularly in lower respiratory and bloodstream infections. Employing a "one health" approach, epidemiological studies elucidate the multifaceted nature of ESBL epidemiology, emphasising the importance of understanding environmental factors contributing to antimicrobial resistance. Extended-spectrum beta-lactamases (ESBLs) pose a significant global public health challenge, necessitating comprehensive surveillance, research, and collaboration efforts to combat antimicrobial resistance effectively. These enzymes, primarily found in Enterobacteriaceae, confer resistance to a broad array of beta-lactam antibiotics, facilitated by mobile genetic elements' transfer between bacterial strains and species [
72,
75,
76] .
Global epidemiological studies have shed light on the widespread presence and impact of ESBLs. Investigations across diverse geographical regions have revealed alarming rates of ESBL prevalence in both clinical and environmental settings. Such studies underscore the urgency for comprehensive surveillance and intervention strategies to mitigate the proliferation of ESBL-producing bacteria [
77]. The global burden of antimicrobial resistance associated with ESBLs is substantial, leading to increased mortality and morbidity rates. Studies have highlighted the significant impact of ESBL-mediated resistance on public health outcomes, particularly in the context of lower respiratory tract infections and bloodstream infections. Such findings underscore the critical need for collaborative efforts to address the multifaceted challenges posed by ESBLs [
78,
79,
80] .
In summary, ESBLs represent a pressing global public health concern, necessitating comprehensive research efforts and collaborative initiatives to combat antimicrobial resistance effectively. By leveraging insights from epidemiological studies and employing innovative strategies, stakeholders can work towards preserving the efficacy of antibiotics and safeguarding public health.
5.2. Nosocomial Infections In Trinidad and Tobago
Nosocomial infections, or hospital-acquired infections, are prevalent issues, particularly in intensive care units and medical wards. In Trinidad and Tobago, data on nosocomial infections are scarce. Between 1992 and 1995, 7158 nosocomial infections were reported among 72,532 patients, with an incidence rate of 10.0 per 100 admissions. Europe exhibits varying incidence rates, ranging from 1% across all infection types to as high as 23.6% in pediatric intensive care units. In the United States, the Center for Disease Control and Prevention estimated approximately 1.7 million nosocomial infections annually, resulting in 99,000 deaths. This literature review provides insights into nosocomial infections affecting different body systems, including the skin, soft tissues, urinary tract, respiratory tract, bloodstream, and central nervous system. Risk factors, antibiotic resistance patterns, and management strategies for these infections are also discussed. Overall, the review highlights the significant burden of nosocomial infections and the importance of implementing effective preventive measures and management protocols to mitigate their impact on patient outcomes and healthcare systems [
81] .
6. Evolution of Immunological Techniques and Advancements in Blood Banking
Justiz-Vaillant A et al (2001) introduce a groundbreaking method for detecting red blood cell antibodies using bacterial antiglobulin. Published in the West Indian Medical Journal in 2001, the assay utilizes Staphylococcal protein A (SpA) and Streptococcal protein G (SpG) as bacterial antiglobulin reagents. This innovative approach represents a significant advancement in transfusion medicine, offering improved sensitivity and specificity in detecting antibodies that could lead to transfusion reactions [
82].
In their 2017 study published in ISBT Science, Charles et al. conducted a follow-up survey in Trinidad and Tobago to delve into the dynamics of knowledge, attitudes, and practices surrounding blood donation. With a sample size ranging from 349 to 356 participants, the study aimed to track changes over time in the community's perception and behavior towards blood donation. The study emphasises the importance of ongoing education, blood safety, and encourage regular blood donation [
83].
7. Navigating Clinical Immunology: From Bench to Bedside Management
7.1. Severe Combined Immunodeficiency Disorders
Severe Combined Immunodeficiency (SCID), is a group of rare, life-threatening disorders characterized by a profound impairment in the immune system. SCID is classified into different types based on the genetic mutations underlying the condition, each presenting unique challenges in diagnosis and management. The microbiological associations of SCID, including susceptibility to various pathogens due to compromised immunity, are discussed, shedding light on the infectious risks faced by individuals with this condition. Additionally, the paper explores treatment strategies for SCID, which typically involve hematopoietic stem cell transplantation (HSCT) or gene therapy to restore immune function. The publication provides valuable insights into the classification, microbiological aspects, and therapeutic options for SCID, contributing to the understanding and management of this severe immunodeficiency disorder [
84,
85,
86,
87] .
In addition to the provided information, Severe Combined Immunodeficiency (SCID) is characterized by a primary inherited immunodeficiency that typically manifests before three months of age and can lead to fatal outcomes. The condition arises from a deficiency in both T and B cell function, resulting in susceptibility to opportunistic infections caused by various pathogens such as bacteria, viruses, fungi, and protozoa. SCID presents in autosomal, X-linked, and sporadic forms, with early signs including recurrent infections and lymphopenia. Prompt immunological investigation is crucial upon suspicion of SCID, allowing for timely diagnosis and intervention. Stem cell transplantation stands as the primary treatment modality, aiming to restore immune function. This review aims to provide a comprehensive understanding of the microorganisms associated with SCID and their management. It delineates SCID as a syndrome and outlines the diverse range of microorganisms affecting affected children, along with approaches for investigation and treatment [
84].
7.2. Transient Hypogammaglobulinemia of the Infancy
Transient hypogammaglobulinemia of infancy (THI) is a primary immunodeficiency characterized by a temporary decline in serum immunoglobulin G (IgG) levels, typically occurring in infants aged 5 to 24 months. Preterm infants are especially vulnerable due to insufficient transfer of IgG across the placenta. This systematic review aimed to assess diagnostic criteria for THI. Sixteen studies out of 215 identified articles were eligible, with bias assessed across six domains. Thirty-one percent of the studies had low bias risk, 25% had high risk, and 44% were unclear. THI diagnosis is confirmed only after IgG levels normalize, indicating it's not benign, necessitating monitoring for recurrent infections. Diagnostic criteria should consider vaccine and isohaemagglutinin responses to distinguish THI from other infant immunological disorders [
88,
89,
90] .
7.3. Chronic Granulomatous Disease (CGD)
Chronic granulomatous disease (CGD) is a primary immunodeficiency resulting from mutations in genes encoding the subunits of the NADPH oxidase enzyme complex, impairing the phagocytic function of the innate immune system. This review aims to comprehensively address the pathogens associated with CGD and its management. Patients, typically children, with CGD face recurrent life-threatening infections and potential infectious or inflammatory complications. Management strategies involve antibacterial prophylaxis with trimethoprim-sulfamethoxazole, antifungal prophylaxis, typically with itraconazole, and interferon gamma immunotherapy to reduce infection risk. Hematopoietic stem cell transplantation (HCT) is the preferred treatment for CGD, offering successful outcomes [
91,
92,
93,
94] .
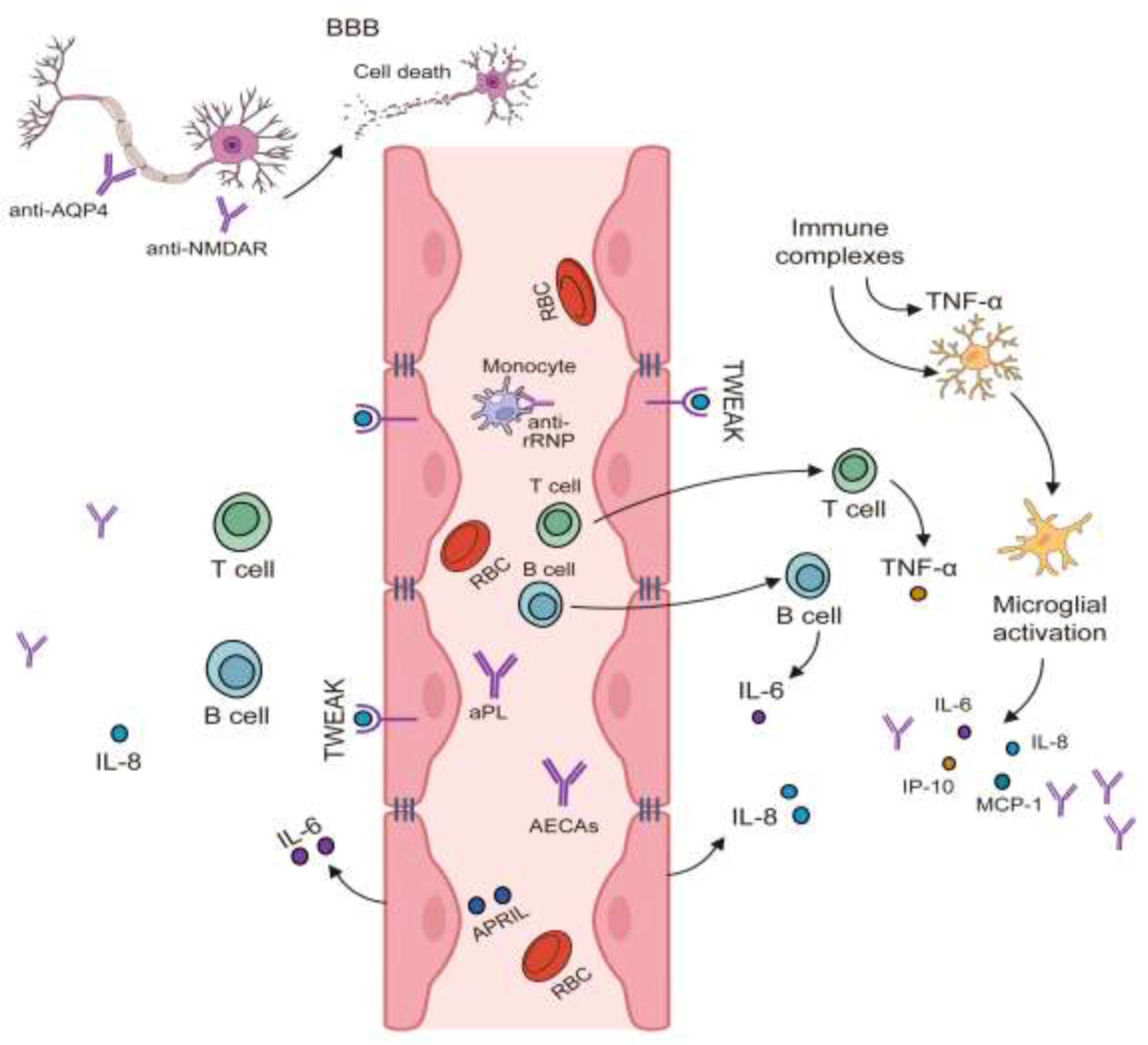
7.4. Neuropsychiatric Systemic Lupus Erythematosus (NSLE)
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease affecting various organs, including the nervous system. Its etiology involves environmental, genetic, and immunological factors leading to autoantibody production against self-antigens. Failure in self-tolerance mechanisms in T and B cells contributes to tissue damage. Diagnosis remains challenging despite available criteria. Neuropsychiatric manifestations, termed neuropsychiatric SLE (NPSLE), lack definitive pathological causes. Treatment focuses on symptomatic management, including antipsychotics, antidepressants, and anxiolytics for psychiatric symptoms, antiepileptic drugs for seizures, and immunosuppressants like corticosteroids for inflammation. Non-pharmacological interventions are also employed [
95,
96,
97,
98,
99,
100]
NPSLE is a multifaceted condition involving genetic factors, cytokines, immune cells, and environmental influences. Certain alleles of the HLA genes and the TREX1 gene are associated with increased NPSLE risk. Cytokines, crucial in immune signaling, contribute to inflammation and neurological symptoms. Elevated levels of cytokines like IFN-y, IL-17F, IL-21, IL-18, GM-CSF, and VEGF are observed in NPSLE patients, further highlighting the role of cytokines in its pathogenesis [
95,
96,
102].
In summary, while there are some overlaps in the types of microorganisms that affect SCID, CGD, and THI patients, each immunodeficiency disorder presents unique vulnerabilities to specific pathogens due to the nature of the immune system dysfunction involved.
Table 4 shows microbial pathogens associated with infections in Severe Combined Immunodeficiency (SCID), Chronic Granulomatous Disease (CGD), and Transient Hypogammaglobulinemia of Infancy (THI).
8. Advancements in Cancer Research: Insights and Innovations
Research and treatment of cancer, including multiple myeloma and adult T cell leukemia/lymphoma, in many regions holds significant importance due to several reasons. Firstly, these regions face unique challenges in healthcare infrastructure, access to specialized treatments, and epidemiological patterns of diseases [
103,
104,
105,
106].
Secondly, studying cancer contributes to the global understanding of cancer epidemiology and treatment. Different regions may have distinct genetic predispositions, environmental exposures, and lifestyle factors that influence cancer development and progression. Furthermore, addressing cancer globally is crucial for improving healthcare equity and reducing disparities. Access to cancer screening, diagnosis, and treatment may be limited in these regions compared to more developed healthcare systems. By investing in research and treatment initiatives, policymakers and healthcare providers can work towards reducing the burden of cancer and improving outcomes for affected individuals worldwide [
107,
108].
Overall, focusing on cancer research and treatment is essential for addressing healthcare needs, contributing to global cancer knowledge, and promoting health equity and access to care.
9. Egg Antibody Technology
The study investigates IgM antibodies in egg whites, a topic with limited information. Antibodies against bacterial antigens were prepared, using ELISA for detection and affinity chromatography for purification. Large quantities of anti-protein A antibodies were produced, confirmed by affinity chromatography from day 9 post-immunization egg whites. Samples with anti-SpA antibodies showed agglutination inhibition, while negative samples showed agglutination. This suggests successful production of IgM anti-protein A antibodies in egg whites, with inhibition of bacterial growth observed in vitro. Protein A-affinity chromatography aided in antibody characterization, highlighting its potential in antibody production and research [
109]
This study delves into the intricacies of experimental vaccine development methodology, particularly focusing on immunogenicity outcomes. It highlights the novel concept of idiotypic-antiidiotypic interactions as a mechanism for manipulating antibody responses against bacterial and viral proteins. Three experimental vaccines targeting HIV, Salmonella, and Staphylococcus aureus were explored methodologically [
103,
110]. The author suggests that immunized chicken eggs could serve as a unique medium for producing IgY antibodies against these pathogens. This research has significant implications for epidemiology globally, especially in regions where infectious diseases pose substantial health challenges. By elucidating vaccine development processes and potential applications, this study lays the groundwork for future clinical trials aimed at combating important infectious microorganisms affecting human populations. Other authors have reported production of IgY antibodies with similar results [
64,
111,
112,
113,
114].
10. Confronting Tuberculosis: A Global Perspectives
A retrospective study assessed HIV-1 prevalence among pulmonary tuberculosis patients. Among 406 TB patients, 11.6% were HIV-1 positive, mostly males aged 30-39. HIV-TB coinfected patients had a mortality rate of 23.4%, significantly higher than HIV-negative TB patients (3.9%). Standard quadruple drug therapy was effective, with no observed M. tuberculosis resistance. Jamaica's HIV-TB prevalence mirrors other developing nations, yet the elevated mortality emphasises the urgency for early HIV diagnosis and prompt initiation of highly active antiretroviral therapy [
115].
Molecular typing techniques have revolutionized the study of mycobacterial diseases, challenging traditional views and offering crucial insights. Since the 1990s, these methods have diversified, forming the cornerstone of molecular epidemiology in mycobacteriology. They aid in tracing infection sources, quantifying transmission, distinguishing reinfection from relapse, and tracking strain distribution and evolution. Moreover, they shed light on genetic mechanisms governing traits like virulence, transmissibility, and drug resistance. As genotyping unveils mycobacterial biology, it holds significant promise in combating these diseases [
116].
Mycobacterium tuberculosis, the ancient cause of tuberculosis, adeptly navigates human hosts by manipulating immune responses, achieving a delicate balance between host immunity and bacterial persistence. Recent findings highlight diverse host cell responses and dynamic bacterial niches during infection. M. tuberculosis employs various effectors to influence macrophage functions and inflammation, yet understanding of bacterial virulence factors across different infection stages and cellular reservoirs remains incomplete. This review explores
Mycobacterium tuberculosis’ immune evasion and provocation strategies throughout its infection cycle, emphasising the necessity for deeper molecular insights. Such insights are crucial for developing innovative host-targeted therapies, disease markers, and effective vaccines against tuberculosis [
117].
Mycobacterium tuberculosis adeptly counters host antimicrobial responses, employing diverse strategies to endure stress and persist within host tissues. Understanding these adaptive mechanisms is vital for managing disease progression effectively. Our review examines
Mycobacterium tuberculosis ' ability to sense and respond to host-associated stressors, shedding light on its phenotypic adaptation. Additionally, we emphasize the use of animal models to replicate human responses, uncovering the interplay between host environments and bacterial strategies. This comprehensive approach enhances our understanding of infection recalcitrance and informs strategies for effective disease management [
118].
This retrospective cohort study found a notably higher prevalence of QuantiFERON-TB Gold positivity among uveitis patients compared to the general US population (14.4% vs. 5%, p<.001). Positive QFT-G results were significantly associated with foreign birth or recent travel and granulomatous uveitis. However, characteristic signs of TB uveitis common in endemic regions were not observed. The study highlights the need for further investigation into the implications of elevated QFT-G positivity among uveitis patients, suggesting potential implications for TB screening and management in this population [
119].
We review two decades of research on
Mycobacterium tuberculosis drug tolerance, a challenge in tuberculosis (TB) treatment. M. tuberculosis exhibits reduced growth, metabolic shifts, and increased efflux pump activity under drug pressure, particularly affecting lipid metabolism and redox balance. Enhanced lipid synthesis thickens the cell wall, reducing drug sensitivity. Drug-specific responses include reprogramming mycolic acid biosynthesis (isoniazid), upregulating rpoB (rifampicin), and activating ATP synthesis and transcription factors (bedaquiline). Understanding these responses is crucial for developing drugs that prevent tolerance, potentially shortening TB treatment. This insight could lead to innovative treatment strategies targeting M. tuberculosis's adaptive mechanisms, improving TB management [
120].
Tuberculosis (TB) remains a leading global cause of death, largely due to latent TB infection (LTBI) contributing to new cases. Differential diagnosis challenges between LTBI and active TB (aTB) hinder TB control efforts. While traditional tests like the tuberculin skin test (TST) lack specificity, newer assays like interferon-gamma release assays offer improved sensitivity but still can't differentiate LTBI from aTB. This review explores the concept of LTBI, its immunological mechanisms, and the limitations of current diagnostic methods. It discusses emerging approaches, including biomarkers, omics technologies, and microbiota analysis, aiming to enhance LTBI differential diagnosis and advance TB management [
121].
Mycobacterium tuberculosis, the culprit behind a globally devastating infectious disease, employs strategies to evade the host immune response, entering a dormant state for extended periods. This review focuses on elucidating the local immune response provoked by
Mycobacterium tuberculosis within the lungs of active tuberculosis patients, utilizing data from untouched cells in bronchoalveolar lavage fluid (BALF) or exhaled breath condensate (EBC) samples. Predominant resident cells include macrophages and lymphocytes, fostering neutrophil recruitment. The response is marked by inflammation and oxidative stress, primarily orchestrated by macrophages and T lymphocytes. Elevated levels of cytokines, chemokines, and matrix metalloproteinases in the alveolar microenvironment contrast with healthy counterparts. Despite chronic infection, production of interferon (IFN)-γ, IL-17, and specific immunoglobulins (Ig) A and G indicate an induced adaptive immune response. The contributions of epithelial cells, antigen processing, tissue-resident memory T cells (Trm), and in situ vaccination efficacy warrant further investigation [
122,
123].
Tuberculosis persists as a significant health concern in the Caribbean, demanding ongoing attention. Factors such as socio-economic disparities, limited healthcare access, and HIV co-infection contribute to its prevalence. Strategies focus on early detection, effective treatment, and public health interventions to curb transmission. Collaboration with regional and international partners remains pivotal in combating tuberculosis across the West Indies [124-129].
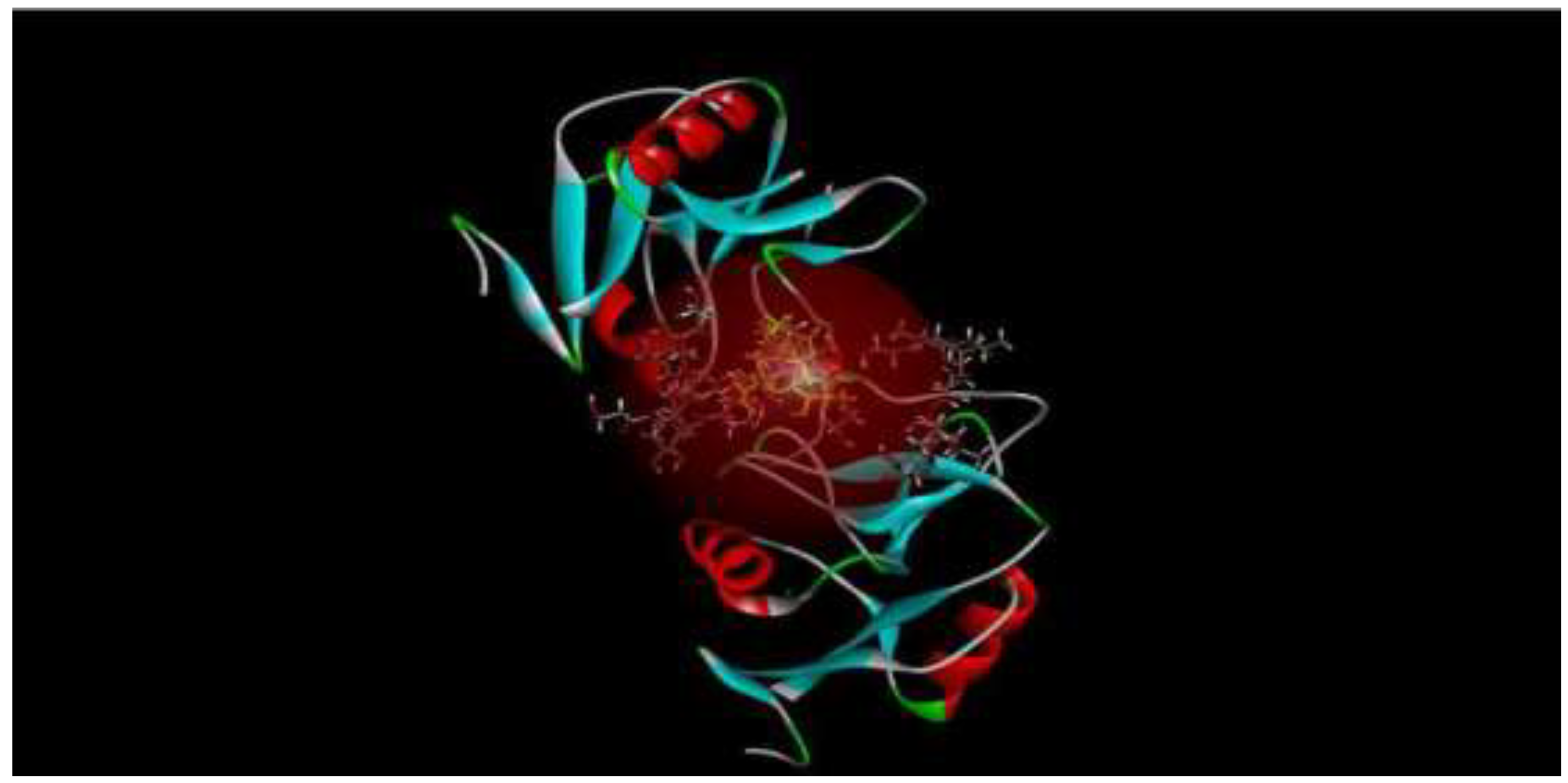
Mincle, a predominant C-type lectin in macrophages, aids in the macrophage response against mycobacteria. Docking software like Discovery Studio and Molegro Virtual Studios predict ligand-receptor binding positions, while ChemDraw facilitates ligand preparation due to time-intensive protein preparation. Mincle's unique role in mycobacterial-induced inflammatory signaling, notably in tuberculosis, is crucial. However, Molegro studio's limitation in displaying metallic atom interactions is noted. Macrophage detection of trehalose dimycolate (TDM) and trehalose dibenzenate (TDB) triggers CARD9 signaling, prompting pro-inflammatory cytokine and chemokine production. This research holds promise for therapeutic applications, particularly in tuberculosis research [
123,
130].
Figure 3 shows a diagram emphasising the five most feasible poses in which TDB, the derivative analog of TDM, binds to mincle [
130].
11. Conclusion
The exploration of bacterial immunoglobulin-binding proteins (IBPs) and the advent of chimeric proteins mark significant advancements in immunodiagnosis and therapy, offering novel approaches to combat infectious diseases and improve patient care. These versatile proteins, including SpA, SpG, and SpL, have revolutionized serological diagnostics, providing precise identification of infectious agents like bacteria, viruses and fungi. By leveraging IBPs' unique properties, researchers have developed next-generation immunodiagnostic tools characterized by enhanced specificity, stability, and diagnostic accuracy. Chimeric IBPs, engineered to optimize binding kinetics and broaden target specificity, hold immense promise in early disease detection and precision medicine. In the global pursuit of preventive healthcare, vaccine research remains a cornerstone of immunological endeavors. Clinical studies targeting prevalent infectious diseases such as HIV and tuberculosis offer invaluable insights into vaccine efficacy, immunogenicity, and safety profiles. By unraveling the complexities of host-pathogen interactions and immune responses, vaccine research not only addresses regional healthcare challenges but also strengthens global initiatives aimed at combating infectious diseases and enhancing population health on a worldwide scale. Microbiological studies globally have shed light on the intricate dynamics between microbial pathogens and antimicrobial agents, guiding stewardship initiatives and treatment protocols. The rise of extended-spectrum beta-lactamases (ESBLs) and other resistant traits underscores the urgent need for international collaborations in antimicrobial resistance surveillance to address multidrug-resistant pathogens on a global scale. The tireless drive for innovation in immunological techniques has revolutionized disease diagnosis, blood banking practices, and transfusion medicine worldwide. From rapid point-of-care assays for HIV detection to advanced screening protocols in blood banking facilities, researchers have improved diagnostic precision, enhanced blood safety, and mitigated transfusion-related risks, upholding public health standards globally. Clinical immunology research globally encompasses a wide spectrum of immune-mediated disorders, offering tailored management strategies to restore immune balance and enhance patient outcomes. The synergy between basic science discoveries and clinical insights emphasise the translational impact of immunology research in alleviating disease burden and improving the quality of life for affected individuals on a global scale. Research on cancer has provided valuable insights into various aspects of cancer management, contributing to the understanding of preventive measures, lifestyle interventions, and economic evaluations in market access decisions for cancer therapies. Furthermore, innovative approaches such as egg antibody technology and molecular typing techniques have offered promising avenues for infectious disease management and mycobacterial disease study, respectively. Understanding Mycobacterium tuberculosis' adaptive mechanisms and drug tolerance is crucial for effective disease management and the development of therapies and vaccines. In conclusion, the collective efforts of researchers worldwide in exploring immunological techniques and advancing disease research have paved the way for transformative improvements in healthcare delivery, disease prevention, and patient care on a global scale. Collaborative endeavors across disciplines and borders will continue to drive innovation and address the evolving challenges of infectious diseases, cancer, and other immune-mediated disorders, ultimately leading to improved health outcomes and well-being for populations worldwide.
Figure 4.
Overview of the literature review.
Figure 4.
Overview of the literature review.
Author Contributions
The manuscript was conceptualized by A.J.-V. and D.G., and planning and discussion were conducted by all authors. D.G. and A.J.-V. wrote the initial draft of the manuscript. All authors investigated, reviewed and edited the final manuscript. All authors have read and agreed to the final version of the manuscript.
Funding
This study did not receive any external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset supporting the findings of this study is included within the manuscript and its referenced sources, ensuring comprehensive access to the relevant data for further examination and analysis.
Acknowledgments
The authors sincerely thank the West Indian Immunology Society (WIIS) for their invaluable assistance. We also extend our gratitude to Drs. Kenneth Charles, Bijet Pandit, Shalini Pooransingh, Odette Arozarena-Barbosa and Mr. Reinand Thompson from The University of the West Indies, St. Augustine, and Eric Williams Medical Science Complex. Additionally, we offer a special thanks to the late Professor Monica Smikle from the Microbiology Department at the University Hospital of the West Indies, Mona Campus, Jamaica, West Indies. Professor Smikle's legacy resonates within her institution, the region, and internationally.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Thammavongsa, V.; Schneewind, O.; Missiakas, D. Recurrent Infections and Immune Evasion Strategies of Staphylococcus Aureus. Curr. Opin. Microbiol. 2012, 15, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, R.; Fekrvand, S.; Shahkarami, S.; Azizi, G.; Moazzami, B.; Abolhassani, H.; Aghamohammadi, A. The Hyper IgM Syndromes: Epidemiology, Pathogenesis, Clinical Manifestations, Diagnosis and Management. Clin. Immunol. 2019, 198, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Drikic, M.; Olsen, S.; De Buck, J. Detecting Total Immunoglobulins in Diverse Animal Species with a Novel Split Enzymatic Assay. BMC Vet. Res. 2019, 15, 374. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cavalcanti, T.Y.V.; Pereira, M.R.; de Paula, S.O.; Franca, R.F. de O. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Milligan, R.; Paul, M.; Richardson, M.; Neuberger, A. Vaccines for Preventing Typhoid Fever. Cochrane Database Syst. Rev. 2018, 5, CD001261. [Google Scholar] [CrossRef] [PubMed]
- Kumi Smith, M.; Jewell, B.L.; Hallett, T.B.; Cohen, M.S. Treatment of HIV for the Prevention of Transmission in Discordant Couples and at the Population Level. Adv. Exp. Med. Biol. 2018, 1075, 125–162. [Google Scholar] [PubMed]
- Husna, A.; Rahman, M.M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, M.T.; Alam, J.; Ashour, H.M. Extended-Spectrum β-Lactamases (ESBL): Challenges and Opportunities. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Pieper, D.H. Tackling Threats and Future Problems of Multidrug-Resistant Bacteria. Curr. Top. Microbiol. Immunol. 2016, 398, 3–33. [Google Scholar]
- Ferrer, E.; Lares, M.; Viettri, M.; Medina, M. [Comparison between immunological and molecular techniques for the diagnosis of Chagas disease]. Enferm. Infecc. Microbiol. Clin. 2013, 31, 277–282. [Google Scholar] [CrossRef]
- Storch, E.K.; Custer, B.S.; Jacobs, M.R.; Menitove, J.E.; Mintz, P.D. Review of Current Transfusion Therapy and Blood Banking Practices. Blood Rev. 2019, 38, 100593. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Każarnowicz, A.; Karaseva, N.; Sánchez, A.; De Boer, R.; Andric, Z.; Reck, M.; Atagi, S.; Lee, J.-S.; Garassino, M.; et al. Safety and Patient-Reported Outcomes of Atezolizumab, Carboplatin, and Etoposide in Extensive-Stage Small-Cell Lung Cancer (IMpower133): A Randomized Phase I/III Trial. Ann. Oncol. 2020, 31, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Gennery, A.R. Progress in Treating Chronic Granulomatous Disease. Br. J. Haematol. 2021, 192, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Emsen, A.; Uçaryılmaz, H.; Güler, T.; Artaç, H. Regulatory T and B Cells in Transient Hypogammaglobulinemia of Infancy. Turk. J. Pediatr. 2022, 64, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Nagafuchi, Y.; Fujio, K. Clinical and Immunological Biomarkers for Systemic Lupus Erythematosus. Biomolecules 2021, 11. [Google Scholar] [CrossRef]
- Chan, S.-W.B.; Zhong, Y.; Lim, S.C.J.; Poh, S.; Teh, K.L.; Soh, J.Y.; Chong, C.Y.; Thoon, K.C.; Seng, M.; Tan, E.S.; et al. Implementation of Universal Newborn Screening for Severe Combined Immunodeficiency in Singapore While Continuing Routine Bacille-Calmette-Guerin Vaccination Given at Birth. Front. Immunol. 2021, 12, 794221. [Google Scholar] [CrossRef]
- Iraqi, M.; Edri, A.; Greenshpan, Y.; Goldstein, O.; Ofir, N.; Bolel, P.; Abu Ahmad, M.; Zektser, M.; Campbell, K.S.; Rouvio, O.; et al. Blocking the PCNA/NKp44 Checkpoint to Stimulate NK Cell Responses to Multiple Myeloma. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Utsunomiya, A. Progress in Allogeneic Hematopoietic Cell Transplantation in Adult T-Cell Leukemia-Lymphoma. Front. Microbiol. 2019, 10, 2235. [Google Scholar] [CrossRef]
- Hamal, K.R.; Burgess, S.C.; Pevzner, I.Y.; Erf, G.F. Maternal Antibody Transfer from Dams to Their Egg Yolks, Egg Whites, and Chicks in Meat Lines of Chickens. Poult. Sci. 2006, 85, 1364–1372. [Google Scholar] [CrossRef]
- Bussi, C.; Gutierrez, M.G. Mycobacterium Tuberculosis Infection of Host Cells in Space and Time. FEMS Microbiol. Rev. 2019, 43, 341–361. [Google Scholar] [CrossRef]
- Forsgren, A.; Sjöquist, J. “Protein A” from S. Aureus. I. Pseudo-Immune Reaction with Human Gamma-Globulin. J. Immunol. 1966, 97, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Kronvall, G. A Surface Component in Group A, C, and G Streptococci with Non-Immune Reactivity for Immunoglobulin G. J. Immunol. 1973, 111, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Björck, L. Protein L. A Novel Bacterial Cell Wall Protein with Affinity for Ig L Chains. J. Immunol. 1988, 140, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Justiz-Vaillant, A.A.; Akpaka, P.E.; McFarlane-Anderson, N.; Smikle, M.F. Comparison of Techniques of Detecting Immunoglobulin-Binding Protein Reactivity to Immunoglobulin Produced by Different Avian and Mammalian Species. West Indian Med. J. 2013, 62, 12–20. [Google Scholar]
- De Château, M.; Nilson, B.H.; Erntell, M.; Myhre, E.; Magnusson, C.G.; Akerström, B.; Björck, L. On the Interaction between Protein L and Immunoglobulins of Various Mammalian Species. Scand. J. Immunol. 1993, 37, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Stöbel, K.; Schönberg, A.; Staak, C. A New Non-Species Dependent ELISA for Detection of Antibodies to Borrelia Burgdorferi S. L. in Zoo Animals. Int. J. Med. Microbiol. 2002, 291 Suppl 33, 88–99. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Wei, M.X.; Zhou, Z.Y.; Yu, J.Y.; Shi, X.Q. Prevalence of Antibodies to Toxoplasma Gondii in the Sera of Rare Wildlife in the Shanghai Zoological Garden, People’s Republic of China. Parasitol. Int. 2000, 49, 171–174. [Google Scholar] [CrossRef]
- Genovese, A.; Bouvet, J.P.; Florio, G.; Lamparter-Schummert, B.; Björck, L.; Marone, G. Bacterial Immunoglobulin Superantigen Proteins A and L Activate Human Heart Mast Cells by Interacting with Immunoglobulin E. Infect. Immun. 2000, 68, 5517–5524. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, L.M.; Soulika, A.M.; Silverman, G.J.; Lambris, J.D.; Levinson, A.I. Complement Activation by a B Cell Superantigen. J. Immunol. 1996, 157, 1200–1206. [Google Scholar] [CrossRef]
- A Justiz-Vaillant, A. Direct ELISA for Investigating the Binding of Chemically-Made Protein-LAG to Immunoglobulins. v1. v1. protocols.io 2020.
- A Justiz-Vaillant, A. Direct ELISA for Investigating the Binding of Chemically-Made Protein-LAG-Anti-IgY-Peroxidase to Both Avian and Mammalian Immunoglobulins. v1. v1. protocols.io 2020.
- A Justiz-Vaillant, A. Direct ELISA for Investigating the Binding of Peroxidase-Labeled Anti-Chicken IgY Conjugate with Avian Immunoglobulins v1. protocols.io 2020.
- A Justiz-Vaillant, A. Direct ELISA for Investigating the Binding of Recombinant or Chemically-Made Protein-AG to Immunoglobulins. v1. v1. protocols.io 2020.
- A Justiz-Vaillant, A. Direct ELISA for Investigating the Binding of Recombinant or Chemically-Made Protein-LG to Immunoglobulins. v1. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; F. Smikle, M. Direct ELISA for Investigating the Binding of Recombinant Protein-LA to Immunoglobulins. v1. protocols.io 2020.
- A Justiz-Vaillant, A. Universal Sandwich ELISA for Investigating the Binding of Avian and Mammalian Immunoglobulins to Streptococcal Protein-G (SpG) Using a Peroxidase-Labeled Protein LAG Conjugate (SpLAG-HRP). v1. protocols.io 2020.
- A Justiz-Vaillant, A. Universal Immunoblot Analysis for Investigating Protein-AG (SpAG)-Binding to Avian and Mammalian Immunoglobulins. v1. protocols.io 2020.
- A Justiz-Vaillant, A. Universal Immunoblot Analysis for Investigating Protein-LAG (SpLAG)-Binding to Mammalian and Avian Immunoglobulins. v1. protocols.io 2020.
- A Justiz-Vaillant, A. Immunoblot Analyses for Investigating SpLA Binding to Purified Mammalian and Avian Immunoglobulins. v1. protocols.io 2020.
- A Justiz-Vaillant, A. Universal Sandwich ELISA for Investigating the Binding of Protein-LAG (SpLAG) to Avian Immunoglobulins Using Anti-IgY-Peroxidase as Conjugate. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; F. Smikle, M. Sandwich ELISA for Investigating the Binding of Protein-LG (SpLG) to Avian Immunoglobulins Using Anti-IgY-Peroxidase as Conjugate. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; F. Smikle, M. Universal Sandwich ELISA for Investigating the Binding of Protein-LA (SpLA) to Avian Immunoglobulins Using a Peroxidase-Labeled -Anti-IgY Conjugate. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; F. Smikle, M. Universal Sandwich ELISA for Investigating the Binding of Protein-AG (SpAG) to Avian Immunoglobulins Using a Peroxidase-Labeled -Anti-IgY Conjugate. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; McFarlane-Anderson, N. Universal Sandwich Enzyme Linked Immunosorbent Assay for Investigating Protein-LG (SpLG) Interactions with Immunoglobulins Using a SpA-HRP Conjugate. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; McFarlane-Anderson, N. Universal Sandwich Enzyme Linked Immunosorbent Assay for Investigating Protein-LA (SpLA) Interactions with Immunoglobulins Using a SpG-HRP Conjugate. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; McFarlane-Anderson, N. Universal Sandwich Enzyme Linked Immunosorbent Assay for Investigating Protein-AG (SpAG) Interactions with Immunoglobulins Using a SpL-HRP Conjugate. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; McFarlane-Anderson, N. Enzyme Linked Immunosorbent Assay for Investigating the Binding of Protein-AG (SpAG) to Immunoglobulins. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; McFarlane-Anderson, N. Universal Sandwich Enzyme Linked Immunosorbent Assay for Investigating Staphylococcal Protein-A (SpA) Interactions with Immunoglobulins Using a SpLG-HRP Conjugate. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; McFarlane-Anderson, N. Universal Sandwich Enzyme Linked Immunosorbent Assay for Investigating Streptococcal Protein-G (SpG) Interactions with Immunoglobulins Using a SpLA-HRP Conjugate. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; McFarlane-Anderson, N. Universal Sandwich Enzyme Linked Immunosorbent Assay for Investigating Immunoglobulin-Binding Protein (IBP) Interactions Using a Conjugate SpAG-HRP. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; McFarlane-Anderson, N. Enzyme Linked Immunosorbent Assay for Investigating the Binding of Chemically Prepared Protein-LAG-Anti-IgY (SpLAG-Anti-IgY) to Avian and Mammalian Immunoglobulins. v1. protocols.io 2020.
- (9) (PDF) Enzyme Linked Immunosorbent Assay for Investigating the Binding of Chemically Prepared Protein-LAG (SpLAG) to Immunoglobulins. v1 Available online: https://www.researchgate.net/publication/363669178_Enzyme_linked_immunosorbent_assay_for_investigating_the_binding_of_chemically_prepared_protein-LAG_SpLAG_to_immunoglobulins_v1/fulltext/636003f512cbac6a3e11828a/Enzyme-linked-immunosorbent-assay-for-investigating-the-binding-of-chemically-prepared-protein-LAG-SpLAG-to-immunoglobulins-v1.pdf (accessed on 14 April 2024).
- A Justiz-Vaillant, A.; McFarlane-Anderson, N. Enzyme Linked Immunosorbent Assay for Investigating the Binding of Protein-LG (SpLG) to Immunoglobulins. v1. protocols.io 2020.
- A Justiz-Vaillant, A.; McFarlane-Anderson, N. Enzyme Linked Immunosorbent Assay for Investigating the Binding of Protein-LA (SpLA) to Immunoglobulins. v1. protocols.io 2020.
- A Justiz-Vaillant, A. Preparation of Horseradish Peroxidase (HRP) Conjugated to Chicken Anti-IgY v1. protocols.io 2020.
- A Justiz-Vaillant, A. Protocol of Preparation of a Protein-LAG Conjugated to Horseradish Peroxidase by the Periodate Method. v1. protocols.io 2020.
- A Justiz-Vaillant, A. Preparation of a Protein-AG Conjugated to Horseradish Peroxidase by the Periodate Method. v1. protocols.io 2020.
- A Justiz-Vaillant, A. Preparation of a Protein-LG Conjugated to Horseradish Peroxidase by the Periodate Method. v1. protocols.io 2020.
- A Justiz-Vaillant, A. Preparation of a Protein-LA Conjugated to Horseradish Peroxidase by the Periodate Method. v1. protocols.io 2020.
- A Justiz-Vaillant, A. Preparation of Horseradish Peroxidase (HRP) Conjugated Streptococcal Protein-G by the Periodate Method. v1. protocols.io 2020.
- Justiz-Vaillant, A.A.; Ferrer-Cosme, B.; Curtello, S. Universal Enzyme-Linked Immunosorbent Assays (ELISA) and Utility in the Detection of Antibodies against Salmonella Spp. in Several Animal Species. bioRxiv, 3347. [Google Scholar] [CrossRef]
- Curtello, S.; Vaillant, A.A.J.; Asemota, H.; Smikle, M.P.; Akpaka, P.E. A DNA Vaccine versus Attenuated Vaccine to Protect against Salmonella Infection in Chickens. JAMMR 2014, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Justiz Vaillant, A.A.; Grella, M.J. Vaccine (Vaccination); StatPearls Publishing, 2023;
- Vega, C.G.; Bok, M.; Vlasova, A.N.; Chattha, K.S.; Fernández, F.M.; Wigdorovitz, A.; Parreño, V.G.; Saif, L.J. IgY Antibodies Protect against Human Rotavirus Induced Diarrhea in the Neonatal Gnotobiotic Piglet Disease Model. PLoS One 2012, 7, e42788. [Google Scholar] [CrossRef] [PubMed]
- Justiz Vaillant, A.; Cosme, B.; Smikle, MF.; Pérez, O. Eggs from Hens Immunized with Specific KLH-Conjugated HIV Peptide Candidate Vaccines to Chicks Induces Specific Anti-HIV gp120 and gp41 Antibodies That Neutralize the Original HIV Antigens. Vaccine Res. 2020, 7, 92–96. [Google Scholar]
- Justiz Vaillant, A.A.; Gulick, P.G. HIV and AIDS Syndrome. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2022. [Google Scholar]
- Justiz Vaillant, A. A protocol and detailed methodological study on immunogenicity of various experimental vaccines. 2021, 7, 0–0.
- Justiz Vaillant, A.; Akpaka, P.E. Immunogenicity Studies of Various Experimental Vaccines in Chickens. bioRxiv 2020, 2020.08.31.276154. [CrossRef]
- Paterson David, L.; Bonomo Robert, A. Extended-Spectrum β-Lactamases: A Clinical Update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Courvalin, P.; Dantas, G.; Davies, J.; Eisenstein, B.; Huovinen, P.; Jacoby, G.A.; Kishony, R.; Kreiswirth, B.N.; Kutter, E.; et al. Tackling Antibiotic Resistance. Nat. Rev. Microbiol. 2011, 9, 894–896. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-Spectrum Beta-Lactamases: A Clinical Update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Pitout, J.D.D.; Laupland, K.B. Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae: An Emerging Public-Health Concern. Lancet Infect. Dis. 2008, 8, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; Alcalá, J.C.; Cisneros, J.M.; Grill, F.; Oliver, A.; Horcajada, J.P.; Tórtola, T.; Mirelis, B.; Navarro, G.; Cuenca, M.; et al. Community Infections Caused by Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli. Arch. Intern. Med. 2008, 168, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Carrer, A. [Carbapenemases in enterobacteriaceae]. Arch. Pediatr. 2010, 17 Suppl 4, S154–S162. [Google Scholar] [CrossRef]
- World Health Organization Antimicrobial Resistance in the WHO African Region: Biannual Report 2020; 2020.
- Sulis, G.; Sayood, S.; Gandra, S. Antimicrobial Resistance in Low- and Middle-Income Countries: Current Status and Future Directions. Expert Rev. Anti. Infect. Ther. 2022, 20, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.E.; Fey, P.D. Extended Spectrum Beta-Lactamase (ESBL)-Producing Enterobacteriaceae: Considerations for Diagnosis, Prevention and Drug Treatment. Drugs 2003, 63, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Global Action Plan on Antimicrobial Resistance. Microbe Wash. DC 2015, 10, 354–355.
- Akpaka, P.E.; Vaillant, A.; Wilson, C.; Jayaratne, P. Extended Spectrum Beta-Lactamase (ESBL) Produced by Gram-Negative Bacteria in Trinidad and Tobago. Int. J. Microbiol. 2021, 2021, 5582755. [Google Scholar] [CrossRef] [PubMed]
- Elliott, C.; Justiz-Vaillant, A. Nosocomial Infections: A 360-Degree Review. International Biological and Biomedical Journal 2018, 4, 0–0. [Google Scholar]
- Justiz Vaillant, A.; Mcfarlane-Anderson, N. Detection of Red Blood Cell Antibodies Using Bacterial Antiglobulin: A New Assay. West Indian Med. J. 2001, 50 (Suppl. 5). P-16.
- Charles, K.S.; Chisholm, K.; Gabourel, K.; Philip, K.; Ramdath, S.; Abdul-Hakeem, H.; Vaillant, A.; Pooransingh, S.; Legall, G.; Chantry, A. A Follow-up Survey of Knowledge, Attitudes and Practices Surrounding Blood Donation in Trinidad and Tobago. ISBT Sci. Ser. 2017, 12, 349–356. [Google Scholar] [CrossRef]
- Justiz-Vaillant, A.A.; Gopaul, D.; Akpaka, P.E.; Soodeen, S.; Arozarena Fundora, R. Severe Combined Immunodeficiency-Classification, Microbiology Association and Treatment. Microorganisms 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- McCusker, C.; Upton, J.; Warrington, R. Primary Immunodeficiency. Allergy Asthma Clin. Immunol. 2018, 14, 61. [Google Scholar] [CrossRef]
- Basheer, F.; Lee, E.; Liongue, C.; Ward, A.C. Zebrafish Model of Severe Combined Immunodeficiency (SCID) Due to JAK3 Mutation. Biomolecules 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumrah, R.; Vignesh, P.; Patra, P.; Singh, A.; Anjani, G.; Saini, P.; Sharma, M.; Kaur, A.; Rawat, A. Genetics of Severe Combined Immunodeficiency. Genes Dis 2020, 7, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Justiz-Vaillant, A.A.; Hoyte, T.; Davis, N.; Deonarinesingh, C.; De Silva, A.; Dhanpaul, D.; Dookhoo, C.; Doorpat, J.; Dopson, A.; Durgapersad, J.; et al. A Systematic Review of the Clinical Diagnosis of Transient Hypogammaglobulinemia of Infancy. Children 2023, 10. [Google Scholar] [CrossRef]
- Justiz Vaillant, A.A.; Wilson, A.M. Transient Hypogammaglobulinemia of Infancy; StatPearls Publishing, 2023;
- Moschese, V.; Graziani, S.; Avanzini, M.A.; Carsetti, R.; Marconi, M.; La Rocca, M.; Chini, L.; Pignata, C.; Soresina, A.R.; Consolini, R.; et al. A Prospective Study on Children with Initial Diagnosis of Transient Hypogammaglobulinemia of Infancy: Results from the Italian Primary Immunodeficiency Network. Int. J. Immunopathol. Pharmacol. 2008, 21, 343–352. [Google Scholar] [CrossRef]
- Justiz-Vaillant, A.A.; Williams-Persad, A.F.-A.; Arozarena-Fundora, R.; Gopaul, D.; Soodeen, S.; Asin-Milan, O.; Thompson, R.; Unakal, C.; Akpaka, P.E. Chronic Granulomatous Disease (CGD): Commonly Associated Pathogens, Diagnosis and Treatment. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- Mortaz, E.; Azempour, E.; Mansouri, D.; Tabarsi, P.; Ghazi, M.; Koenderman, L.; Roos, D.; Adcock, I.M. Common Infections and Target Organs Associated with Chronic Granulomatous Disease in Iran. Int. Arch. Allergy Immunol. 2019, 179, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Roos, D. Chronic Granulomatous Disease. Br. Med. Bull. 2016, 118, 50–63. [Google Scholar] [CrossRef]
- Grammatikos, A.; Gennery, A.R. Inflammatory Complications in Chronic Granulomatous Disease. J. Clin. Med. Res. 2024, 13. [Google Scholar] [CrossRef]
- Justiz-Vaillant, A.A.; Gopaul, D.; Soodeen, S.; Arozarena-Fundora, R.; Barbosa, O.A.; Unakal, C.; Thompson, R.; Pandit, B.; Umakanthan, S.; Akpaka, P.E. Neuropsychiatric Systemic Lupus Erythematosus: Molecules Involved in Its Imunopathogenesis, Clinical Features, and Treatment. Molecules 2024, 29. [Google Scholar] [CrossRef] [PubMed]
- Manca, E. Autoantibodies in Neuropsychiatric Systemic Lupus Erythematosus (NPSLE): Can They Be Used as Biomarkers for the Differential Diagnosis of This Disease? Clin. Rev. Allergy Immunol. 2022, 63, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Stock, A.D.; Putterman, C. Neuropsychiatric Lupus: New Mechanistic Insights and Future Treatment Directions. Nat. Rev. Rheumatol. 2019, 15, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.D.; Wen, J.; Putterman, C. Neuropsychiatric Lupus, the Blood Brain Barrier, and the TWEAK/Fn14 Pathway. Front. Immunol. 2013, 4, 484. [Google Scholar] [CrossRef]
- Gasparotto, M.; Gatto, M.; Binda, V.; Doria, A.; Moroni, G. Lupus Nephritis: Clinical Presentations and Outcomes in the 21st Century. Rheumatology 2020, 59, v39–v51. [Google Scholar] [CrossRef]
- Shin, J.I.; Lee, K.H.; Park, S.; Yang, J.W.; Kim, H.J.; Song, K.; Lee, S.; Na, H.; Jang, Y.J.; Nam, J.Y.; et al. Systemic Lupus Erythematosus and Lung Involvement: A Comprehensive Review. J. Clin. Med. Res. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Z.; Zhang, S.; Wu, Y.; Zhang, L.; Zhao, J.; Wang, Q.; Tian, X.; Li, M.; Zeng, X. Progress in the Pathogenesis and Treatment of Neuropsychiatric Systemic Lupus Erythematosus. J. Clin. Med. Res. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Justiz Vaillant, A.; Akpaka, P. Cytokines (IL-17, IL-23 and IL-33) in Systemic Lupus Erythematosus in Trinidad and Tobago. International Biological and Biomedical Journal 2021. [Google Scholar]
- Justiz Vaillant, A. Insights in the Management of Human T Cell-Lymphotropic Virus-1 Associated Adult-T Cell Leukaemia/lymphoma (ATL). International Biological and Biomedical Journal 2020, 6, 0–0. [Google Scholar]
- Justiz Vaillant, A. Multiple Myeloma Update. International Biological and Biomedical Journal 2018, 4, 136–141. [Google Scholar]
- Justiz-Vaillant, A.A.; Fundora, R.A.; Thompson, R.; Gopaul, D. Modifiable Cancer Risk Factors. IRJO 2023, 74–84. [Google Scholar]
- View of What Role, If Any, Should Economic Evaluation Play in Market Access Decisions of Pharmaceutical Treatments for Cancer Patients with Short Life Expectancy? Available online: https://ihrjournal.com/ihrj/article/view/421/1029 (accessed on 15 April 2024).
- Justiz-Vaillant, A.; Gardiner, L.; Mohammed, M.; Surajbally, M.; Maharaj, L.; Ramsingh, L.; Simon, M.; Seegobin, M.; Niles, M.; Vuma, S. Narrative Literature Review on Risk Factors Involved in Breast Cancer, Brain Cancer, Colon Rectal Cancer, Gynecological Malignancy, Lung Cancer, and Prostate Cancer. Preprints 2021.
- Justiz Vaillant, A. Risk factors for liver cancer worldwide. International Biological and Biomedical Journal 2021, 7, 0–0. [Google Scholar]
- Vaillant, A.J.; Ferrer-Cosme, B.; Vuma, S. Production of Antibodies in Egg Whites of Chickens. CJAST 2021, 17–22. [Google Scholar] [CrossRef]
- Justiz Vaillant, A.A.; Sabir, S.; Jan, A. Physiology, Immune Response. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2022. [Google Scholar]
- Mohammadi, M.; Zangooei, M.; Abbasi, E.; Ebrahimi Fana, S.; Aminian, M. Production of Anti-Tetanus Toxin IgY and Study of Its Protective Effects in a Mouse Model. J. Immunoassay Immunochem. 2023, 44, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Bentes, G.A.; Lanzarini, N.M.; Guimarães, J.R.; Heinemann, M.B.; Volotão, E. de M.; da Silva, A.D.S.; Heneine, L.G.D.; de Oliveira, J.M.; Pinto, M.A. Production and Evaluation of Chicken Egg Yolk Immunoglobulin (IgY) against Human and Simian Rotaviruses. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Bok, M.; Chacana, P.; Saif, L.; Fernandez, F.; Parreño, V. Egg Yolk IgY: Protection against Rotavirus Induced Diarrhea and Modulatory Effect on the Systemic and Mucosal Antibody Responses in Newborn Calves. Vet. Immunol. Immunopathol. 2011, 142, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Diraviyam, T.; Zhao, B.; Wang, Y.; Schade, R.; Michael, A.; Zhang, X. Effect of Chicken Egg Yolk Antibodies (IgY) against Diarrhea in Domesticated Animals: A Systematic Review and Meta-Analysis. PLoS One 2014, 9, e97716. [Google Scholar] [CrossRef] [PubMed]
- Akpaka, P.E.; Tulloch-Reid, M.; Justiz-Vaillant, A.; Smikle, M.F. Prevalence of Human Immunodeficiency Virus Infection in Patients with Pulmonary Tuberculosis at the National Chest Hospital in Jamaica. Rev. Panam. Salud Publica 2006, 19, 38–43. [Google Scholar] [CrossRef]
- Jagielski, T.; Minias, A.; van Ingen, J.; Rastogi, N.; Brzostek, A.; Żaczek, A.; Dziadek, J. Methodological and Clinical Aspects of the Molecular Epidemiology of Mycobacterium Tuberculosis and Other Mycobacteria. Clin. Microbiol. Rev. 2016, 29, 239–290. [Google Scholar] [CrossRef]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune Evasion and Provocation by Mycobacterium Tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. [Google Scholar] [CrossRef] [PubMed]
- Parbhoo, T.; Mouton, J.M.; Sampson, S.L. Phenotypic Adaptation of Mycobacterium Tuberculosis to Host-Associated Stressors That Induce Persister Formation. Front. Cell. Infect. Microbiol. 2022, 12, 956607. [Google Scholar] [CrossRef]
- Yakin, M.; Kesav, N.; Cheng, S.K.; Caplash, S.; Gangaputra, S.; Sen, H.N. The Association between QuantiFERON-TB Gold Test and Clinical Manifestations of Uveitis in the United States. Am. J. Ophthalmol. 2021, 230, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Goossens, S.N.; Sampson, S.L.; Van Rie, A. Mechanisms of Drug-Induced Tolerance in Mycobacterium Tuberculosis. Clin. Microbiol. Rev. 2020, 34. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Wu, X. Differential Diagnosis of Latent Tuberculosis Infection and Active Tuberculosis: A Key to a Successful Tuberculosis Control Strategy. Front. Microbiol. 2021, 12, 745592. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.T.; Guzmán-Beltrán, S.; Bobadilla, K.; Santos-Mendoza, T.; Flores-Valdez, M.A.; Gutiérrez-González, L.H.; González, Y. Human Pulmonary Tuberculosis: Understanding the Immune Response in the Bronchoalveolar System. Biomolecules 2022, 12. [Google Scholar] [CrossRef]
- Justiz Vaillant, A.A.; Qurie, A. Interleukin; StatPearls Publishing, 2022.
- Baboolal, S.; Millet, J.; Akpaka, P.E.; Ramoutar, D.; Rastogi, N. First Insight into Mycobacterium Tuberculosis Epidemiology and Genetic Diversity in Trinidad and Tobago. J. Clin. Microbiol. 2009, 47, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.; Baboolal, S.; Akpaka, P.E.; Ramoutar, D.; Rastogi, N. Phylogeographical and Molecular Characterization of an Emerging Mycobacterium Tuberculosis Clone in Trinidad and Tobago. Infect. Genet. Evol. 2009, 9, 1336–1344. [Google Scholar] [CrossRef]
- Baboolal, S.; Ramoutar, D.; Akpaka, P.E. Comparison of the QuantiFERON®-TB Gold Assay and Tuberculin Skin Test to Detect Latent Tuberculosis Infection among Target Groups in Trinidad & Tobago. Rev. Panam. Salud Publica 2010, 28, 36–42. [Google Scholar]
- Montane Jaime, L.K.; Akpaka, P.E.; Vuma, S.; Justiz-Vaillant, A.A. A Healthy Patient with Positive Mantoux Test but Negative Quantiferon Gold Assay and No Evidence of Risk Factors - to Treat or Not to Treat? IDCases 2019, 18, e00658. [Google Scholar] [CrossRef]
- Millet, J.; Baboolal, S.; Streit, E.; Akpaka, P.E.; Rastogi, N. A First Assessment of Mycobacterium Tuberculosis Genetic Diversity and Drug-Resistance Patterns in Twelve Caribbean Territories. Biomed Res. Int. 2014, 2014, 718496. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Baboolal, S.; Akpaka, P.E.; Millet, J.; Rastogi, N. Finer Characterization of Mycobacterium Tuberculosis Using Spoligotyping and 15-Loci MIRU-VNTRs Reveals Phylogeographical Specificities of Isolates Circulating in Guyana and Suriname. Infect. Genet. Evol. 2015, 30, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Soodeen, S.; Justiz-Vaillant, A.; Jalsa, N. It Is Possible Molecular Docking of Carbohydrates to a Mycobacterium Tuberculosis Molecule? Preprints 2023.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).