Submitted:
23 April 2024
Posted:
24 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
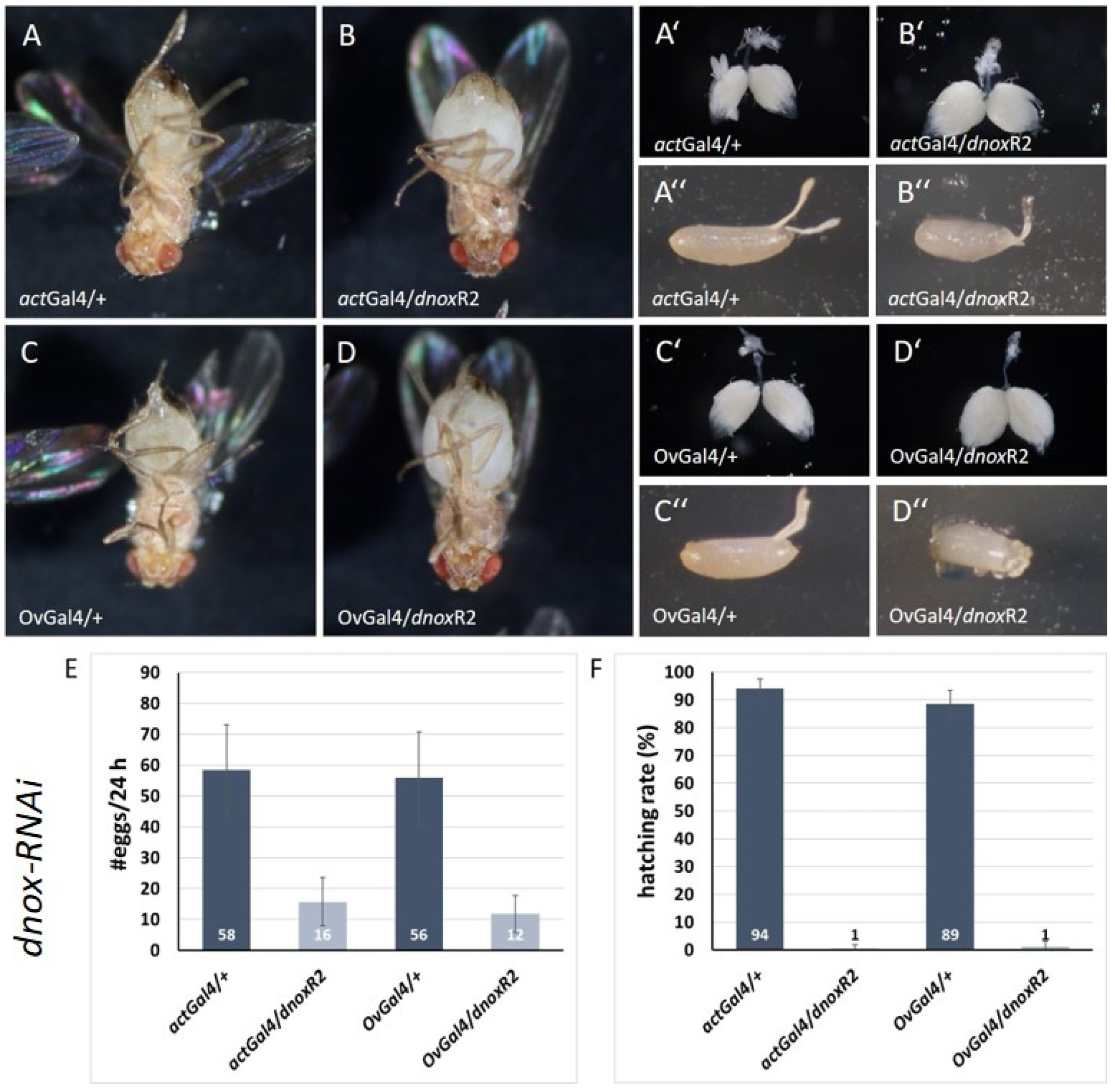
2. Results
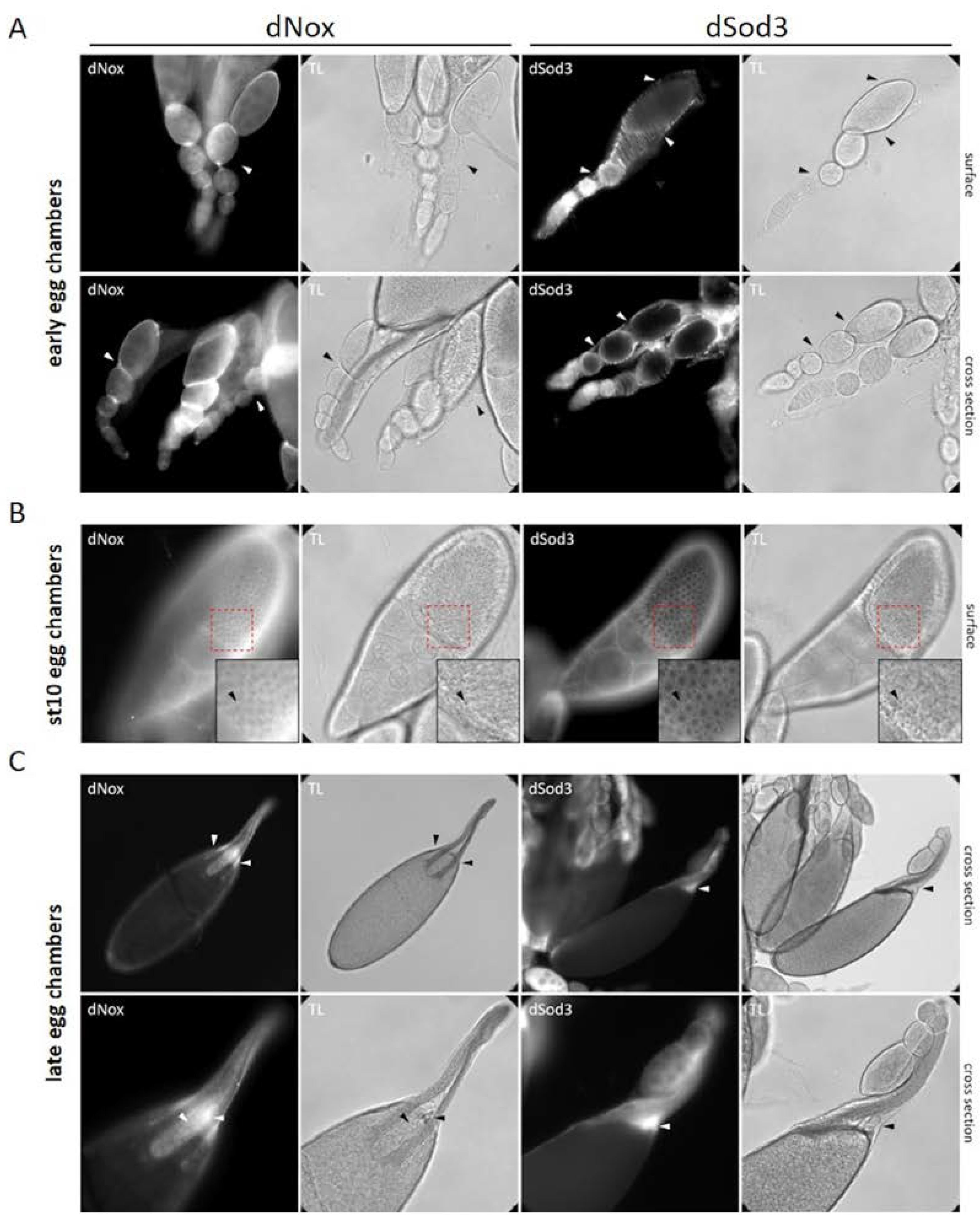
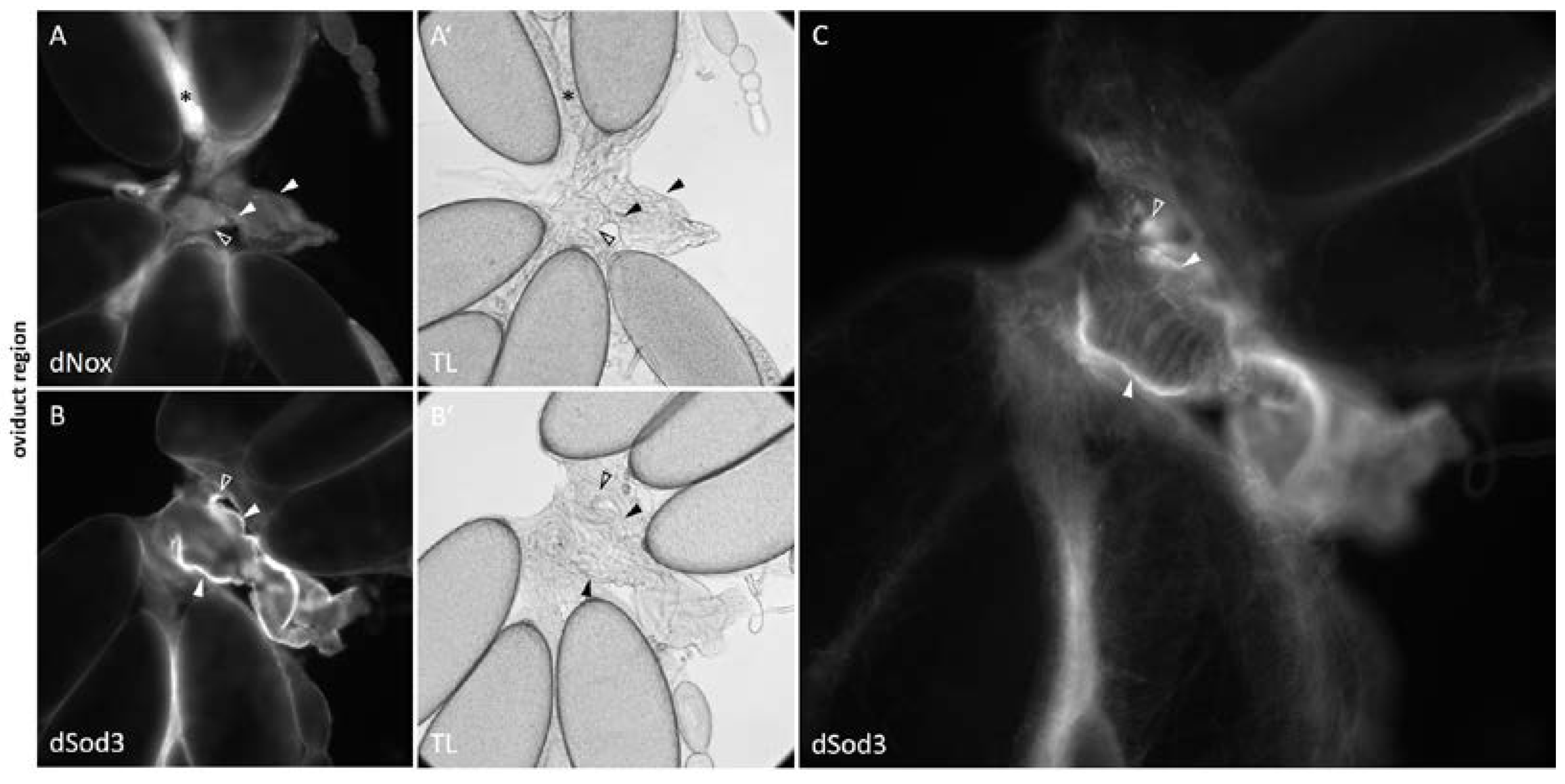
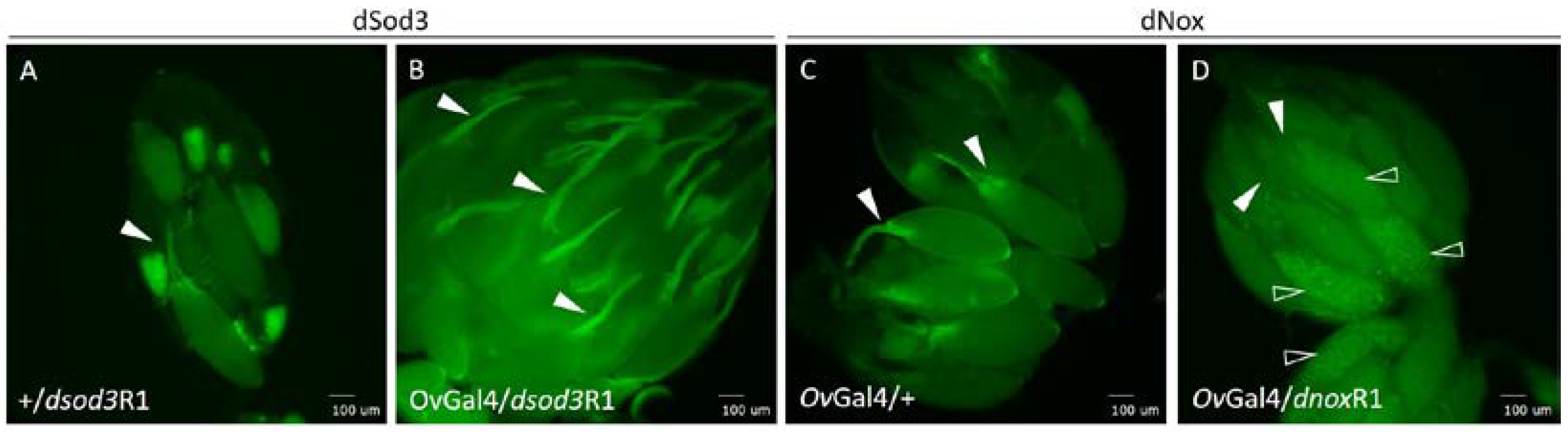
Localization of dNox and dSod3 in Drosophila Ovaries
Redox Differences in Ovaries
3. Discussion
4. Conclusions
5. Materials and Methods
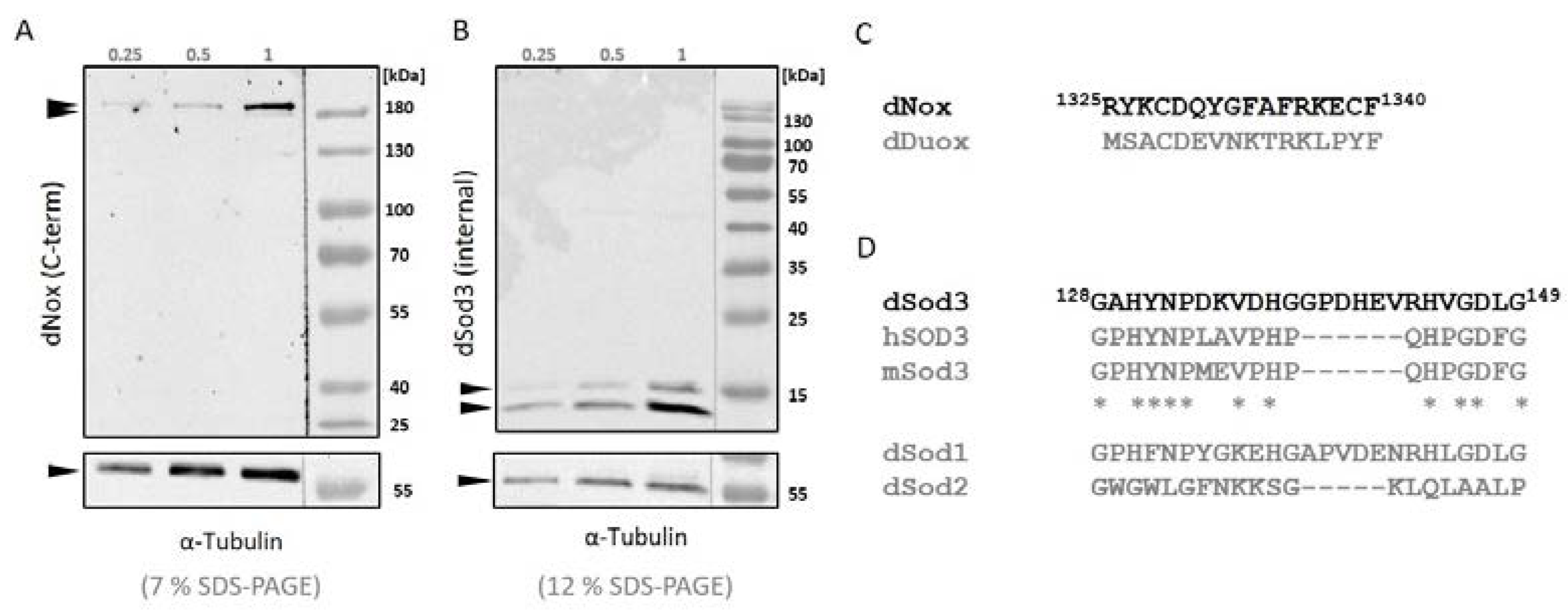
Antibodies
Immunoblotting and Immunostaining
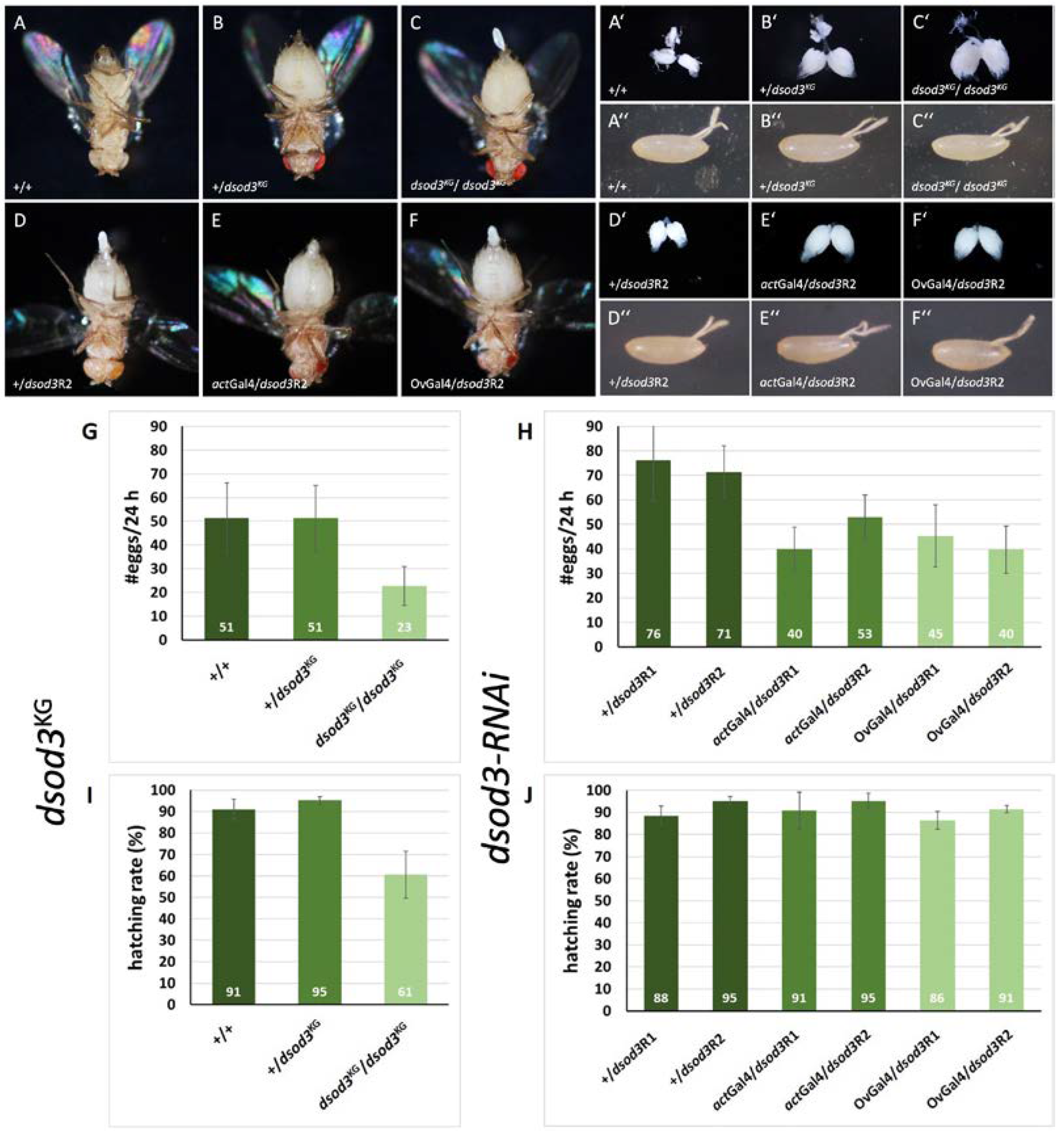
Fly Strains
Egg Laying/Egg Hatching Analysis
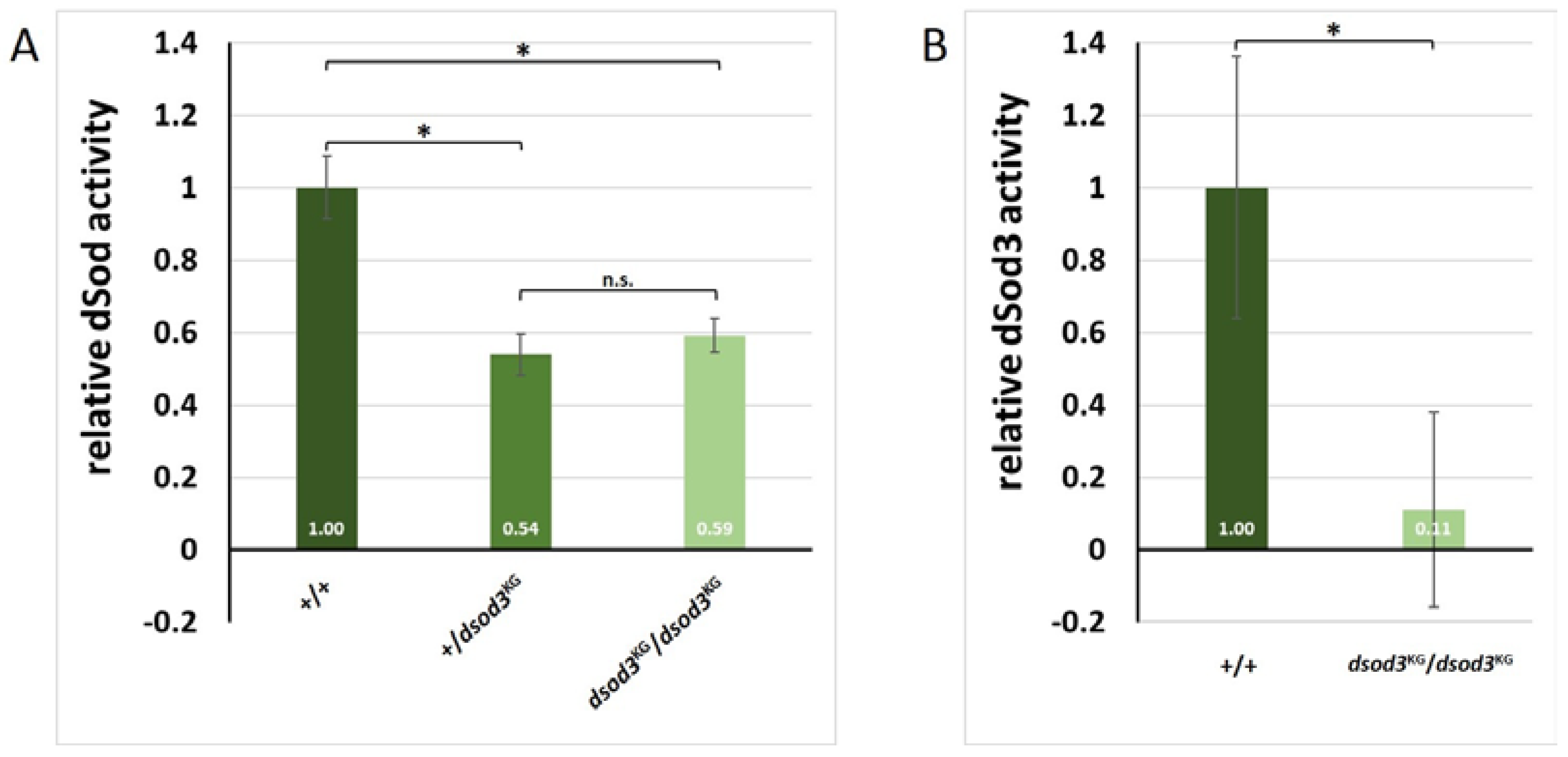
dSod Activity Assay/dSod3 Activity Assay
H2O2 Sensor Measurements
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Autréaux, B.; Toledano, M.B. ROS as Signalling Molecules: Mechanisms That Generate Specificity in ROS Homeostasis. Nat Rev Mol Cell Biol 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Finkel, T. Signal Transduction by Reactive Oxygen Species. Journal of Cell Biology 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V. V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining Roles of Specific Reactive Oxygen Species (ROS) in Cell Biology and Physiology. Nat Rev Mol Cell Biol 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat Rev Mol Cell Biol 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Sugino, N. Reactive Oxygen Species in Ovarian Physiology; 2005; Vol. 4;
- Shkolnik, K.; Tadmor, A.; Ben-Dor, S.; Nevo, N.; Galiani, D.; Dekel, N. Reactive Oxygen Species Are Indispensable in Ovulation. Proc Natl Acad Sci U S A 2011, 108, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, G.; Chen, M.; Zuo, T.; Xu, W.; Liu, X. The Role of Antioxidant Enzymes in the Ovaries. Oxid Med Cell Longev 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Gao, Y.; Feng, Z.; Zhang, B.; Na, Z.; Li, D. Reactive Oxygen Species and Ovarian Diseases: Antioxidant Strategies. Redox Biol 2023, 62. [Google Scholar] [CrossRef]
- Rizzo, A.; Roscino, M.T.; Binetti, F.; Sciorsci, R.L. Roles of Reactive Oxygen Species in Female Reproduction. Reproduction in Domestic Animals 2012, 47, 344–352. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for Measuring Reactive Oxygen Species and Oxidative Damage in Cells and in Vivo. Nat Metab 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. SUPEROXIDE DISMUTASE MULTIGENE FAMILY: A COMPARISON OF THE CuZn-SOD (SOD1), Mn-SOD (SOD2), AND EC-SOD (SOD3) GENE STRUCTURES, EVOLUTION, AND EXPRESSION; 2002.
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid Redox Signal 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Marklund, S.L. Human Copper-Containing Superoxide Dismutase of High Molecular Weight (Lung/Superoxide Radical/Extracellular Fluids); 1982; Vol. 79;
- Marklund, S.L.; Holme, E.; Hellner, L. Superoxide Dismutase in Extracellular Fluids. Clinica Chimica Acta 1982, 126, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.; Stevenson, D.; Gomes, F.; Silva-carvalho, J.L.; Almeida, H. Superoxide Dismutase Expression in Human Cumulus Oophorus Cells. Mol Hum Reprod 2009, 15, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Talat, A.; Satyanarayana, P.; Anand, P. Association of Superoxide Dismutase Level in Women with Polycystic Ovary Syndrome. Journal of Obstetrics and Gynecology of India 2022, 72, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. Journal of Cell Biology 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.R. REPRODUCTION REVIEW Drosophila Melanogaster as a Model for Nutrient Regulation of Ovarian Function. 2020. [CrossRef]
- Landis, G.N.; Tower, J. Superoxide Dismutase Evolution and Life Span Regulation. Mech Ageing Dev 2005, 126, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, O.H.; Degnan, S.M. Distribution and Diversity of ROS-Generating Enzymes across the Animal Kingdom, with a Focus on Sponges (Porifera). BMC Biol 2022, 20. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.P.; Campbellt, S.D.; Michaud, D.; Charbonneaut, M.; Hilliker, A.J. Null Mutation of Copper/Zinc Superoxide Dismutase in Drosophila Confers Hypersensitivity to Paraquat and Reduced Longevity; 1989; Vol. 86;
- Duttaroy, A.; Parkes, T.; Emtage, P.; Kirby, K.; Boulianne, G.L.; Wang, X.; Hilliker, A.J.; Phillips, I.P. The Manganese Superoxide Dismutase Gene of Drosophila: Structure, Expression, and Evidence for Regulation by MAP Kinase; Mary Ann Liebert, Inc. Pp, 1997; Vol. 16;
- Kirby, K.; Hu, J.; Hilliker, A.J.; Phillips, J.P. RNA Interference-Mediated Silencing of Sod2 in Drosophila Leads to Early Adult-Onset Mortality and Elevated Endogenous Oxidative Stress.
- Jung, I.; Kim, T.Y.; Kim-Ha, J. Identification of Drosophila SOD3 and Its Protective Role against Phototoxic Damage to Cells. FEBS Lett 2011, 585, 1973–1978. [Google Scholar] [CrossRef]
- Blackney, M.J.; Cox, R.; Shepherd, D.; Parker, J.D. Cloning and Expression Analysis of Drosophila Extracellular Cu Zn Superoxide Dismutase. Biosci Rep 2014, 34, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Chintapalli, V.R.; Wang, J.; Dow, J.A.T. Using FlyAtlas to Identify Better Drosophila Melanogaster Models of Human Disease. Nat Genet 2007, 39, 715–720. [Google Scholar] [CrossRef]
- Eichhorn, S.W.; Subtelny, A.O.; Kronja, I.; Kwasnieski, J.C.; Orr-Weaver, T.L.; Bartel, D.P. MRNA Poly(A)-Tail Changes Specified by Deadenylation Broadly Reshape Translation in Drosophila Oocytes and Early Embryos. 2016. [CrossRef]
- Li, W.; Young, J.F.; Sun, J. NADPH Oxidase-Generated Reactive Oxygen Species in Mature Follicles Are Essential for Drosophila Ovulation. Proc Natl Acad Sci U S A 2018, 115, 776–7770. [Google Scholar] [CrossRef]
- Lambeth, J.D. NOX Enzymes and the Biology of Reactive Oxygen. Nat Rev Immunol 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. 2007. [CrossRef]
- Brown, D.I.; Griendling, K.K. Nox Proteins in Signal Transduction. Free Radic Biol Med 2009, 47, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Jaquet, V.; Krause, K.H. NOX5: From Basic Biology to Signaling and Disease. Free Radic Biol Med 2012, 52, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Anagnostopoulou, A.; Rios, F.; Montezano, A.C.; Camargo, L.L. NOX5: Molecular Biology and Pathophysiology. Exp Physiol 2019, 104, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Ritsick D; Edens W; Finnerty V; Lambeth D Nox Regulation of Smooth Muscle Contraction. Free Radic Biol Med 2007, 43, 31–38. [CrossRef] [PubMed]
- Diebold, B.A.; Wilder, S.G.; De Deken, X.; Meitzler, J.L.; Doroshow, J.H.; McCoy, J.W.; Zhu, Y.; Lambeth, J.D. Guidelines for the Detection of NADPH Oxidases by Immunoblot and RT-QPCR. In Methods in Molecular Biology; Humana Press Inc., 2019; Vol. 1982, pp. 191–229.
- Gutscher, M.; Sobotta, M.C.; Wabnitz, G.H.; Ballikaya, S.; Meyer, A.J.; Samstag, Y.; Dick, T.P. Proximity-Based Protein Thiol Oxidation by H2O2-Scavenging Peroxidases. Journal of Biological Chemistry 2009, 284, 31532–31540. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.C.; Barata, A.G.; Großhans, J.; Teleman, A.A.; Dick, T.P. In Vivo Mapping of Hydrogen Peroxide and Oxidized Glutathione Reveals Chemical and Regional Specificity of Redox Homeostasis. Cell Metab 2011, 14, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of Oxidative Stress in Female Reproduction. Reproductive Biology and Endocrinology 2005, 3. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sekhon, L.; Shah, R. Redox Considerations in Female Reproductive Function and Assisted Reproduction: From Molecular Mechanisms to Health Implications. Antioxid Redox Signal 2008, 10, 1375–1403. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Chang, X.; He, F.; Ma, J. Modeling Obesity-Associated Ovarian Dysfunction in Drosophila. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Brookheart, R.T.; Duncan, J.G. Drosophila Melanogaster: An Emerging Model of Transgenerational Effects of Maternal Obesity. Mol Cell Endocrinol 2016, 435, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Bestetti, I.; Barbieri, C.; Sironi, A.; Specchia, V.; Yatsenko, S.A.; de Donno, M.D.; Caslini, C.; Gentilini, D.; Crippa, M.; Larizza, L.; et al. Targeted Whole Exome Sequencing and Drosophila Modelling to Unveil the Molecular Basis of Primary Ovarian Insufficiency. Human Reproduction 2021, 36, 2975–2991. [Google Scholar] [CrossRef] [PubMed]
- Elis, S.; Desmarchais, A.; Cardona, E.; Fouchecourt, S.; Dalbies-Tran, R.; Nguyen, T.; Thermes, V.; Maillard, V.; Papillier, P.; Uzbekova, S.; et al. Genes Involved in Drosophilamelanogaster Ovarian Function Are Highly Conserved throughout Evolution. Genome Biol Evol 2018, 10, 2629–2642. [Google Scholar] [CrossRef] [PubMed]
- Buck, T.; Hack, C.T.; Berg, D.; Berg, U.; Kunz, L.; Mayerhofer, A. The NADPH Oxidase 4 Is a Major Source of Hydrogen Peroxide in Human Granulosa-Lutein and Granulosa Tumor Cells. Sci Rep 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Buck, T. Role of ROS and ROS Generating Enzymes in the Human Ovary, 2018.
- Szeles, Z.; Petheő, G.L.; Szikora, B.; Kacskovics, I.; Geiszt, M. A Novel Monoclonal Antibody Reveals the Enrichment of NADPH Oxidase 5 in Human Splenic Endothelial Cells. Sci Rep 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Kampfer, C.; Saller, S.; Windschüttl, S.; Berg, D.; Berg, U.; Mayerhofer, A. Pigment-Epithelium Derived Factor (PEDF) and the Human Ovary: A Role in the Generation of ROS in Granulosa Cells. Life Sci 2014, 97, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jevitt, A.; Chatterjee, D.; Xie, G.; Wang, X.F.; Otwell, T.; Huang, Y.C.; Deng, W.M. A Single-Cell Atlas of Adult Drosophila Ovary Identifies Transcriptional Programs and Somatic Cell Lineage Regulating Oogenesis. PLoS Biol 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Piacenza, L.; Zeida, A.; Trujillo, M.; Radi, R. THE SUPEROXIDE RADICAL SWITCH IN THE BIOLOGY OF NITRIC OXIDE AND PEROXYNITRITE. Physiol Rev 2022, 102, 1881–1906. [Google Scholar] [CrossRef]
- Mindrinos, M.N.; Petri, W.H.; Galanopoulos, V.K.; Lombard, M.F.; Margaritis, L.H. Crosslinking of the Drosophila Chorion Involves a Peroxidase; 1980; Vol. 189;
- Konstandi, O.A.; Papassideri, I.S.; Stravopodis, D.J.; Kenoutis, C.A.; Hasan, Z.; Katsorchis, T.; Wever, R.; Margaritis, L.H. The Enzymatic Component of Drosophila Melanogaster Chorion Is the Pxd Peroxidase. Insect Biochem Mol Biol 2005, 35, 1043–1057. [Google Scholar] [CrossRef]
- Keramaris, K.E.; Konstantopoulos, K.; Margaritis, L.H.; Velentzas, A.D.; Papassideri, I.S.; Stravopodis, D.J. Exploitation of Drosophila Choriogenesis Process as a Model Cellular System for Assessment of Compound Toxicity: The Phloroglucinol Paradigm. Sci Rep 2020, 10. [Google Scholar] [CrossRef]
- Margaritis, L.H.; Kafatos, F.C.; Petri, W.H. The Eggshell of Drosophila Melanogaster I. Fine Structure of the Layers and Regions of the Wild Type Eggshell. J. Cell Sci 1980, 43, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Margaritis L The Eggshell of Drosophila Melanogaster III. Covalent Crosslinking of the Chorion Proteins Involves Endogenous Hydrogen Peroxide. Tissue Cell 1985, 17, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Keramaris, K.E.; Margaritis, L.H.; Zografou, E.N.; Tsiropoulos, G.J. Egg Laying Suppression in Drosophila Melanogaster (Diptera: Drosophilidae) and Dacus (Bactrocera) Oleae (Diptera: Tephritidae) by Phloroglucinol, a Peroxidase Inhibitor; 1996; Vol. 86;
- Suzuki, T.; Sugino, N.; Fukaya, T.; Sugiyama, S.; Uda, T.; Takaya, R.; Yajima, A.; Sasano, H. Superoxide Dismutase in Normal Cycling Human Ovaries: Immunohistochemical Localization and Characterization, 1999.
- Combelles, C.M.H.; Holick, E.A.; Paolella, L.J.; Walker, D.C.; Wu, Q. Profiling of Superoxide Dismutase Isoenzymes in Compartments of the Developing Bovine Antral Follicles. Reproduction 2010, 139, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Agrahari, G.; Kim, T.Y. Insights into Superoxide Dismutase 3 in Regulating Biological and Functional Properties of Mesenchymal Stem Cells. Cell Biosci 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Stralin, P.; Karlsson, K.; Johansson, B.O.; Marklund, S.L. The Interstitium of the Human Arterial Wall Contains Very Large Amounts of Extracellular Superoxide Dismutase, 1995.
- Hudson, A.M.; Petrella, L.N.; Tanaka, A.J.; Cooley, L.
- Deshpande, S.A.; Rohrbach, E.W.; Asuncion, J.D.; Harrigan, J.; Eamani, A.; Schlingmann, E.H.; Suto, D.J.; Lee, P.T.; Schweizer, F.E.; Bellen, H.J.; et al. Regulation of Drosophila Oviduct Muscle Contractility by Octopamine. iScience 2022, 25. [Google Scholar] [CrossRef] [PubMed]
- Deady, L.D.; Sun, J. A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Drosophila Ovulation. PLoS Genet 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Deady, L.D.; Shen, W.; Mosure, S.A.; Spradling, A.C.; Sun, J. Matrix Metalloproteinase 2 Is Required for Ovulation and Corpus Luteum Formation in Drosophila. PLoS Genet 2015, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Camargo, L.D.L.; Persson, P.; Rios, F.J.; Harvey, A.P.; Anagnostopoulou, A.; Palacios, R.; Gandara, A.C.P.; Alves-Lopes, R.; Neves, K.B.; et al. NADPH Oxidase 5 Is a Pro-Contractile Nox Isoform and a Point of Cross-Talk for Calcium and Redox Signaling-Implications in Vascular Function. J Am Heart Assoc 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Budani, M.C.; Tiboni, G.M. Novel Insights on the Role of Nitric Oxide in the Ovary: A Review of the Literature. Int J Environ Res Public Health 2021, 18, 1–19. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S. juan; Guo, L. ya; Zhang, Z.; Zhang, J. bin; Wang, X. meng; Meng, X. bo; Zhang, M. ying; Zhang, K. ke; Chen, L. lin; et al. Nitric Oxide Synthase and Its Function in Animal Reproduction: An Update. Front Physiol 2023, 14. [Google Scholar] [CrossRef]
- Ekerhovd, E.; Brännström, M.; Alexandersson, M. Evidence for Nitric Oxide Mediation of Contractile Activity in Isolated Strips of the Human Fallopian Tube A.Norström NO Is Highly Unstable, with a Half-Life of Seconds in Buffer; 1997; Vol. 12;
- Berisha, B.; Schams, D.; Sinowatz, F.; Rodler, D.; Pfaffl, M.W. Hypoxia-Inducible Factor-1alpha and Nitric Oxide Synthases in Bovine Follicles Close to Ovulation and Early Luteal Angiogenesis. Reproduction in Domestic Animals 2020, 55, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Fattman, C.L.; Schaefer, L.M.; Oury, T.D. Extracellular Superoxide Dismutase in Biology and Medicine. Free Radic Biol Med 2003, 35, 236–256. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels1; 1971; Vol. 44;
- Lee, P.-T.; Zirin, J.; Kanca, O.; Lin, W.-W.; Schulze, K.L.; Li-Kroeger, D.; Tao, R.; Devereaux, C.; Hu, Y.; Chung, V.; et al. A Gene-Specific T2A-GAL4 Library for Drosophila. 2018. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




