Submitted:
22 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
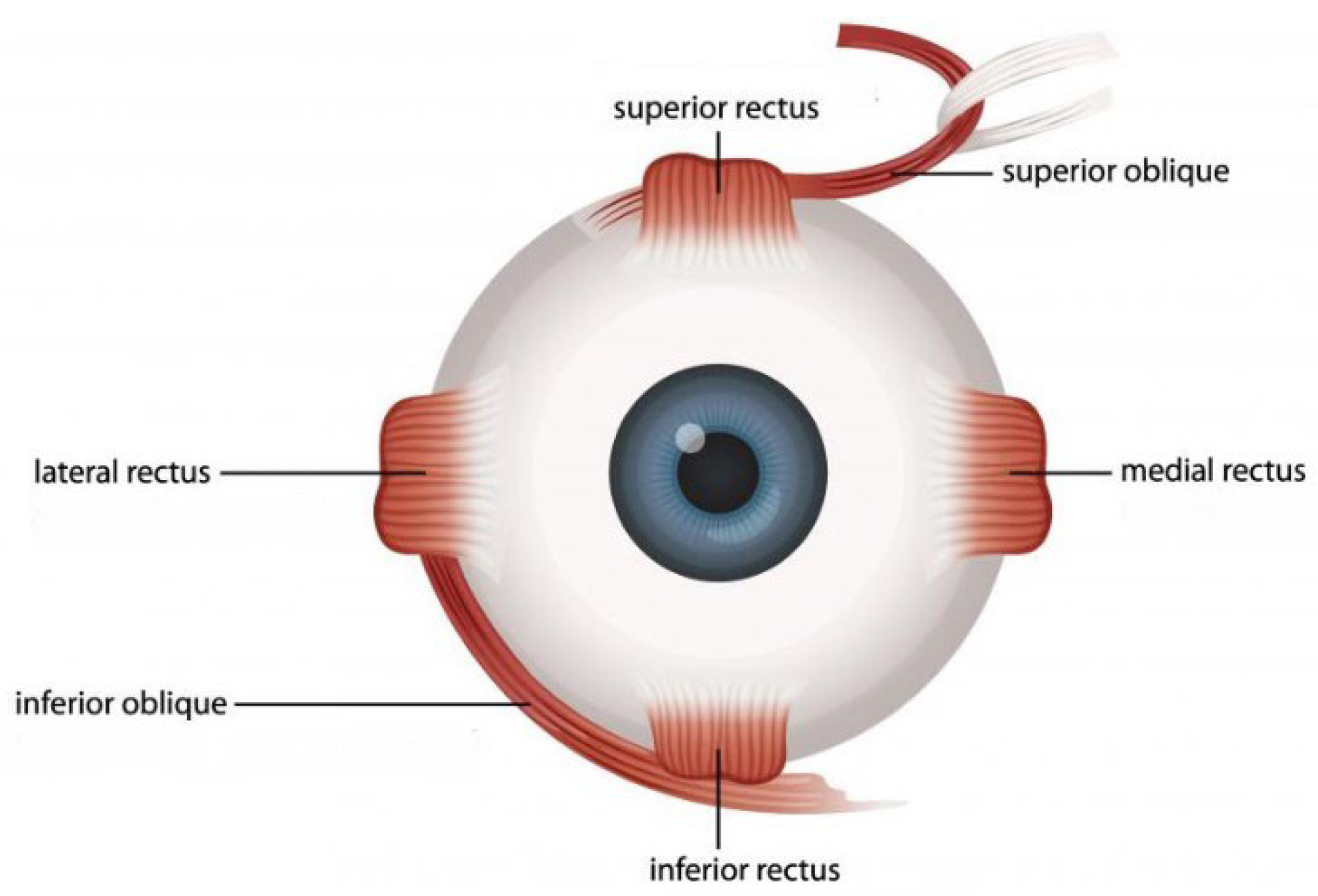
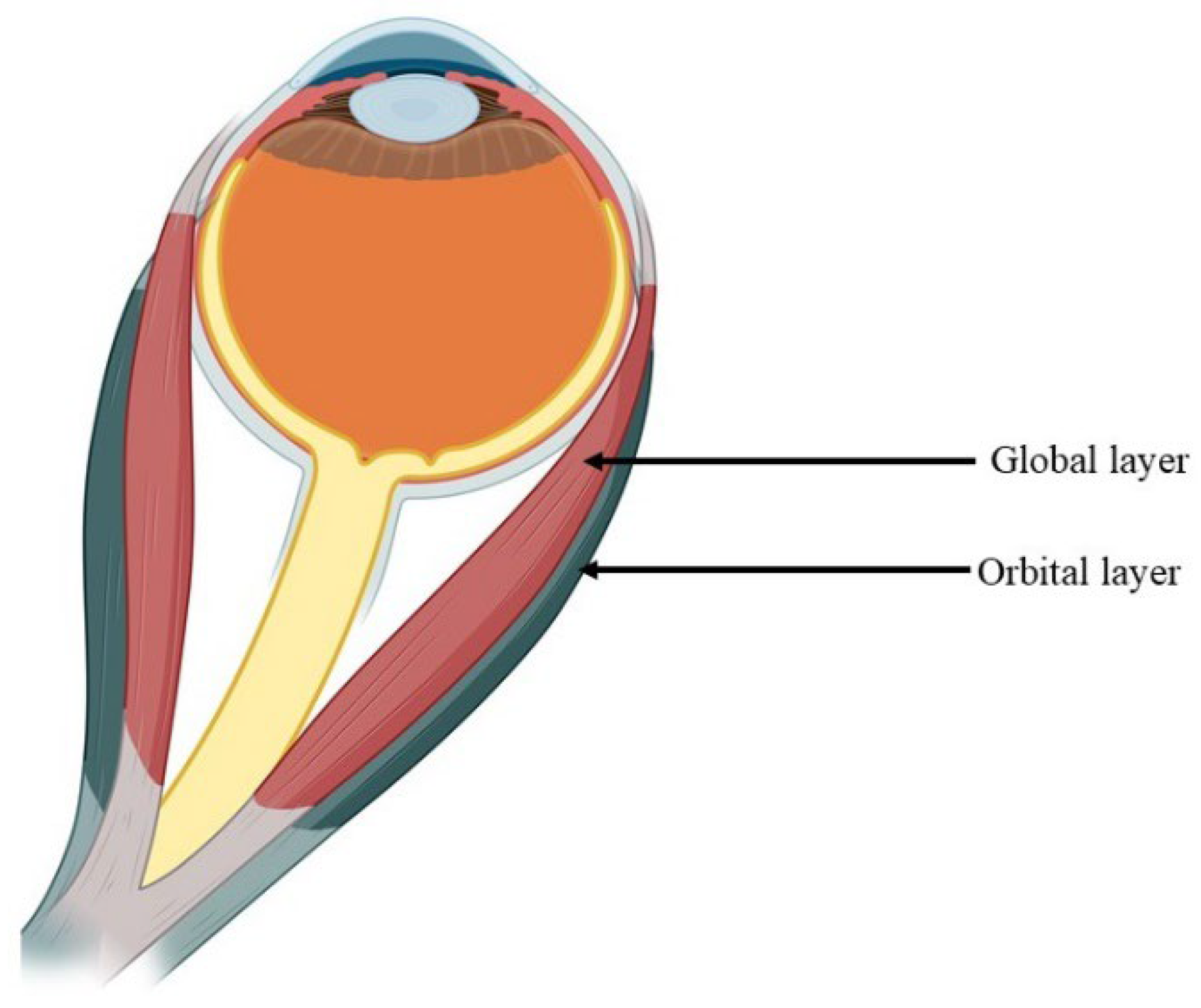
1.1. Structural Characteristics of the EOM
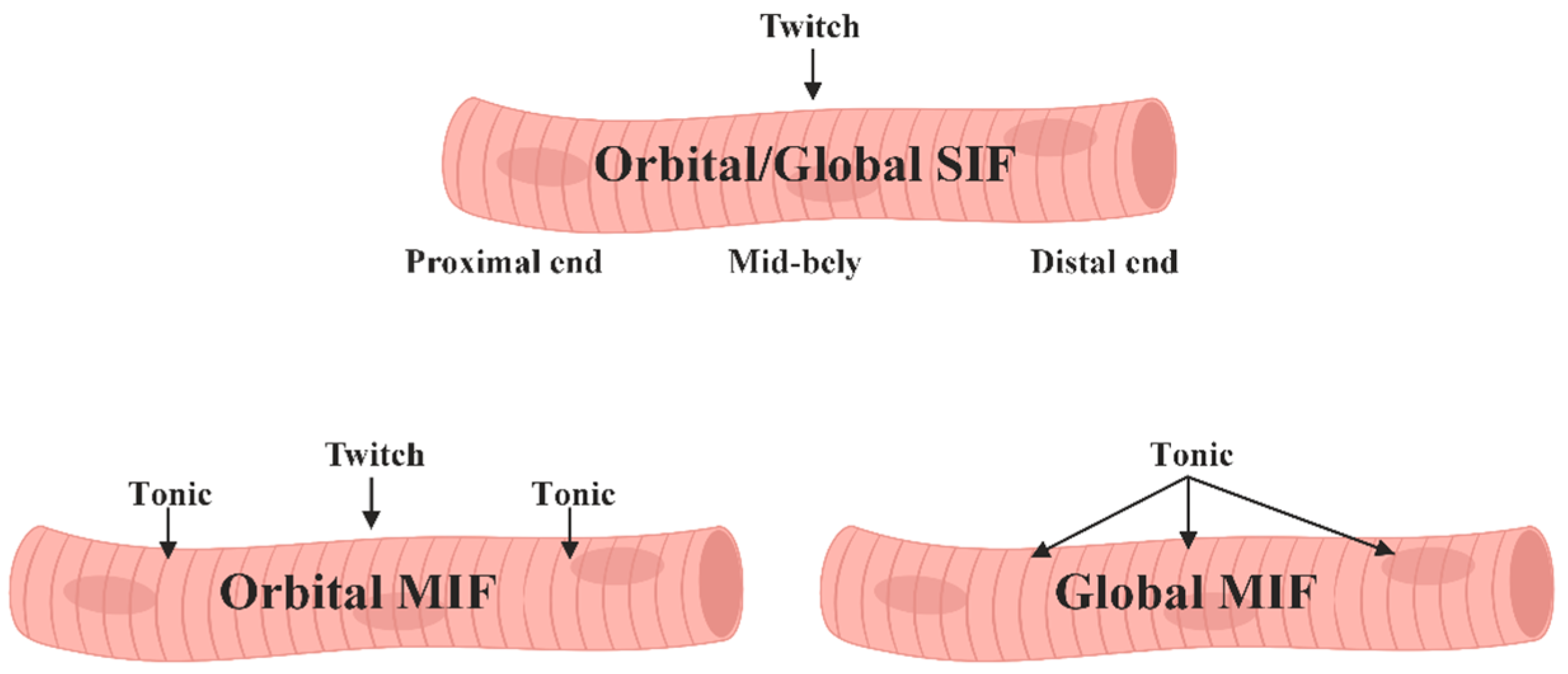
1.2. Muscle Fibers of Extraocular Muscles
- Singly innervated, fast-twitch, and fatigue-resistant in the "orbital" layer.
- Multiply innervated with both fast-twitch and slow-twitch fibers, exhibiting variable fatigue resistance in the "orbital" layer.
- "Red," singly innervated, fast-twitch, and fatigue-resistant in the "global" layer.
- "White," singly innervated, fast-twitch, and exhibiting low fatigue susceptibility in the "global" layer.
- "Intermediate," singly innervated, fast-twitch with moderate fatigue resistance in the "global" layer.
- Multiply innervated, slow-twitch, and fatigue-resistant in the "global" layer.
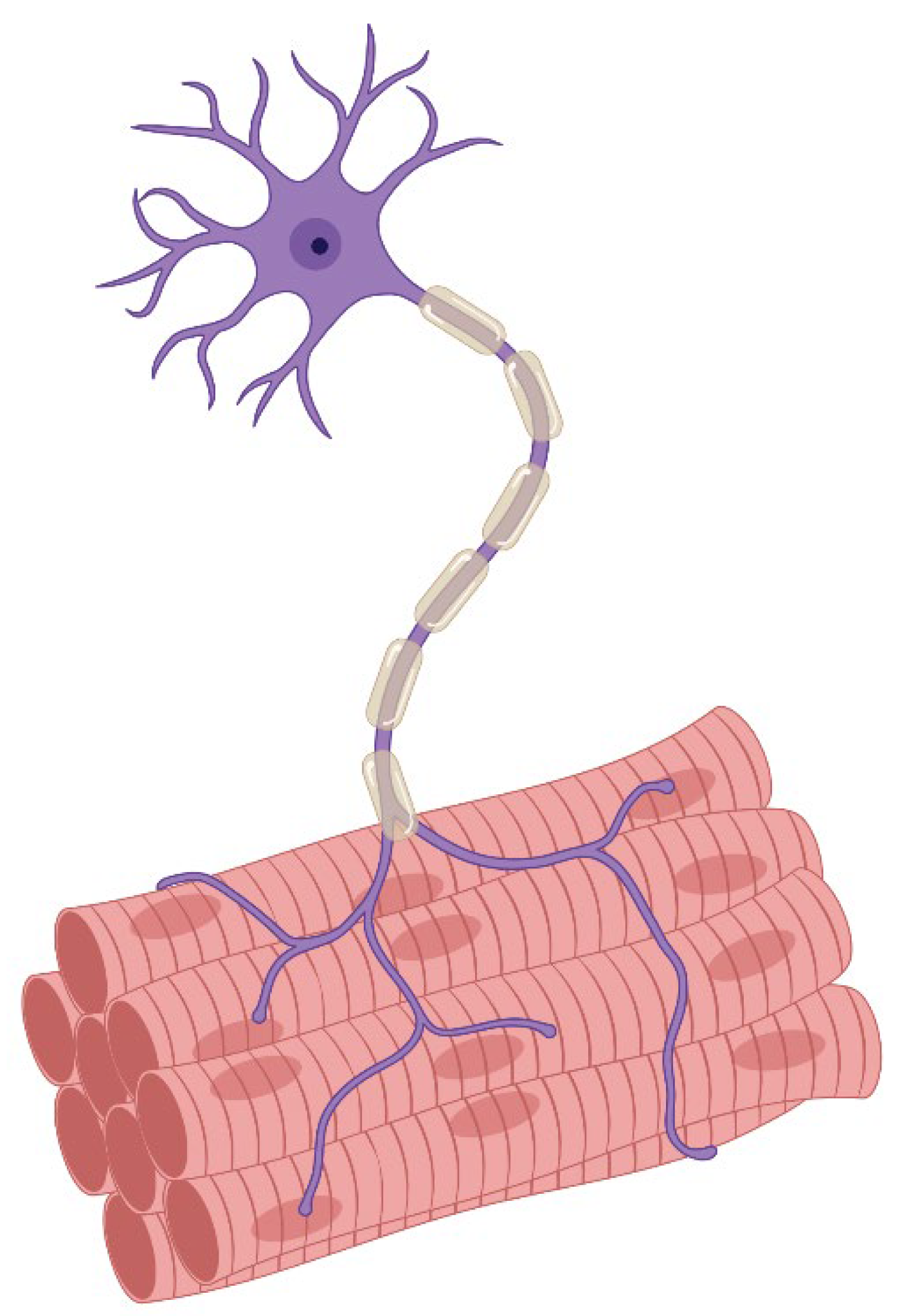
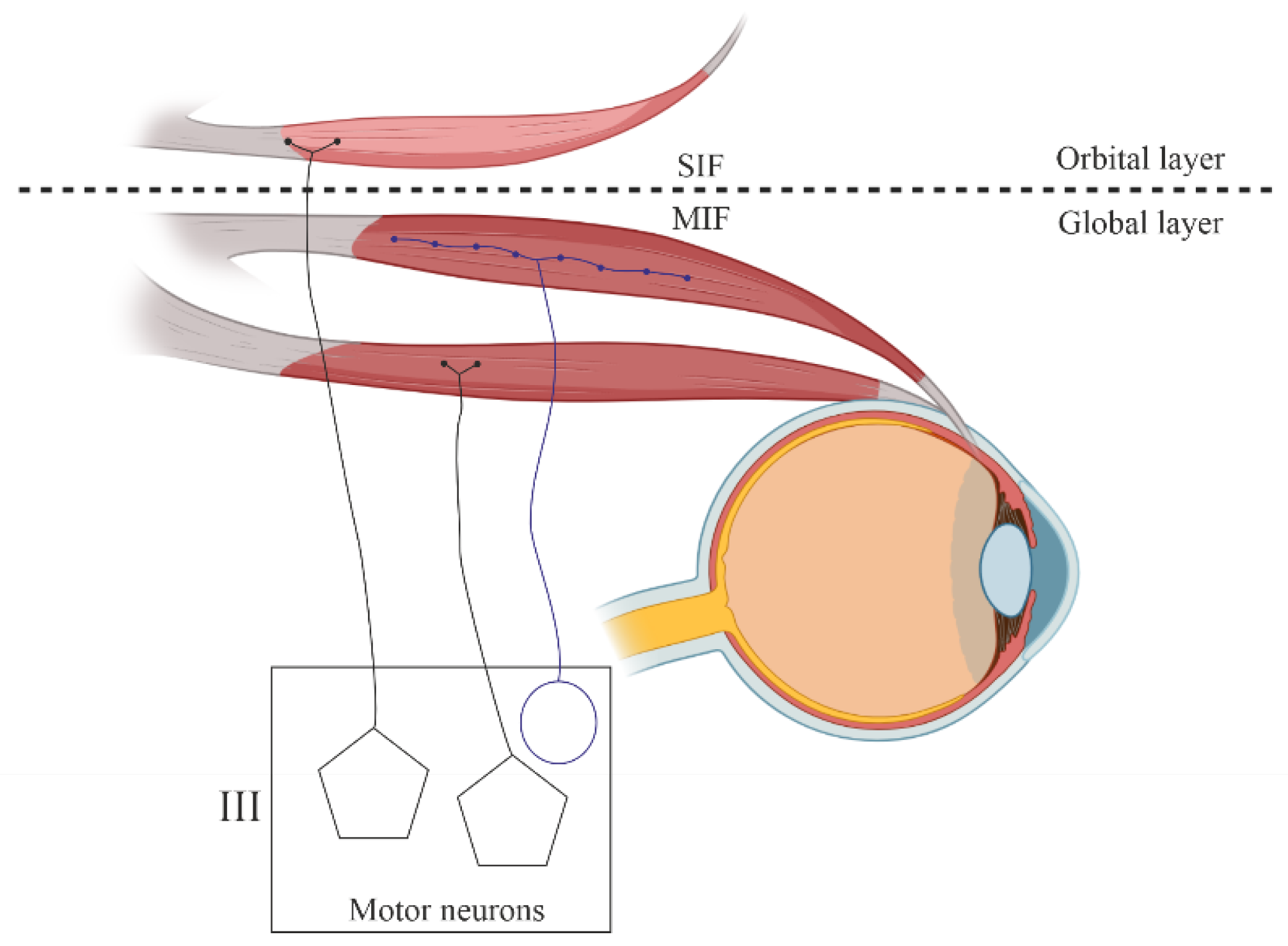
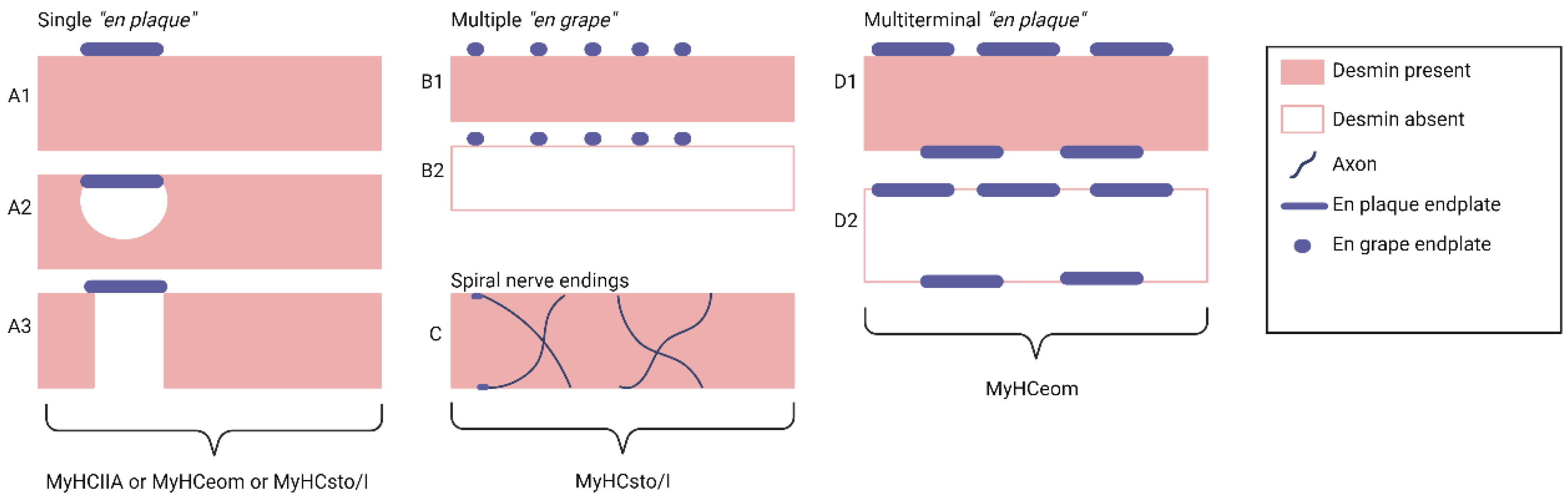
1.3. Motor Innervation
- 1.
- Singly innervated (twitch) muscle fibers, corresponding to fast skeletal MF. This type constitutes the dominant population in both the "global" (90%) and "orbital" (80%) layers; electrical stimulation of the innervating axon triggers a twitch response based on the "all-or-nothing" principle.
- 2.
- 3.
- Multiply innervated MF in the "orbital" layer (20%) with NMJ en grappe and en plaque, exhibiting corresponding dynamics of contraction in the central and distal parts upon electrical stimulation [80].
1.4. Cytoskeleton and Basal Membrane of EOM
1.5. Connective Tissue
1.6. Muscle Fiber Metabolism and Antioxidant Capacity
1.7. Protection, regeneration and Aging of EOM
1.8. Response to Acute Damage by Botulinum Toxin in EOM
2. EOM in Diseases
2.1. The Duchenne Muscular Dystrophy
2.2. Amyotrophic Lateral Sclerosis
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kjellgren, D.; Thornell, L.E.; Andersen, J.; Pedrosa-Domellöf, F. Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci 2003, 44, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Kjellgren, D.; Ryan, M.; Ohlendieck, K.; Thornell, L.E.; Pedrosa-Domellöf, F. Sarco(endo)plasmic reticulum Ca2+ ATPases (SERCA1 and -2) in human extraocular muscles. Invest Ophthalmol Vis Sci 2003, 44, 5057–5062. [Google Scholar] [CrossRef] [PubMed]
- L, Muralidhar. Amyotrophic Lateral Sclerosis - Recent Advances and Therapeutic Challenges. IntechOpen, 2020. Crossref.
- Stuelsatz, P.; Shearer, A.; Li, Y.; Muir, L.A.; Ieronimakis, N.; Shen, Q.W.; Kirillova, I.; Yablonka-Reuveni, Z. Extraocular muscle satellite cells are high performance myo-engines retaining efficient regenerative capacity in dystrophin deficiency. Dev Biol 2015, 397, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Fitzpatrick, K.; McLoon, L.K. Extraocular Muscle Repair and Regeneration. Curr Ophthalmol Rep 2017, 5, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.D.; Gorospe, J.R.; Felder, E.; Bogdanovich, S.; Pedrosa-Domellöf, F.; Ahima, R.S.; Rubinstein, N.A.; Hoffman, E.P.; Khurana, T.S. Expression profiling reveals metabolic and structural components of extraocular muscles. Physiol Genomics 2002, 9, 71–84. [Google Scholar] [CrossRef]
- Sadeh, M. Extraocular muscles. In: ENgel AG, Franzini-Armstrong C, eds. Myology.New York, McGraw-Hill, 1994, 119–127.
- Ziermann, J.M.; Diogo, R.; Noden, D.M. Neural crest and the patterning of vertebrate craniofacial muscles. Genesis 2018, 56, e23097. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, B.L.; Gallina, D.; Thompson, H.; Kasprick, D.S.; Lucarelli, M.J.; Dootz, G.; Nelson, C.; McGonnell, I.M.; Kahana, A. Development of extraocular muscles requires early signals from periocular neural crest and the developing eye. Arch Ophthalmol 2011, 129, 1030–1041. [Google Scholar] [CrossRef]
- Noden, D.M.; Trainor, P.A. Relations and interactions between cranial mesoderm and neural crest populations. J Anat 2005, 207, 575–601. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N., Motlagh, M., Singh G. Anatomy, Head and Neck, Eye Arteries. In: StatPearls. Treasure Island (FL): StatPearls Publishing; July 24, 2023.
- Azzam, D., Cypen, S., Tao J. Anatomy, Head and Neck: Eye Ophthalmic Vein. In: StatPearls. Treasure Island (FL): StatPearls Publishing; July 24, 2023.
- Olitsky, S.E.; Coats, D.K. Complications of Strabismus Surgery. Middle East Afr J Ophthalmol 2015, 22, 271–278. [Google Scholar] [CrossRef]
- Joyce, C., Le, P.H., Peterson, D.C. Neuroanatomy, Cranial Nerve 3 (Oculomotor). In: StatPearls. Treasure Island (FL): StatPearls Publishing; March 27, 2023.
- Zanganeh, T., Legault, G.L. Extraocular Muscle Management With Orbital and Globe Trauma. In: StatPearls. Treasure Island (FL): StatPearls Publishing; July 17, 2023.
- Lacey, H.; Oliphant, H.; Smith, C.; Koenig, M.; Rajak, S. Topographical anatomy of the annulus of Zinn. Sci Rep 2022, 12, 1064. [Google Scholar] [CrossRef]
- Noden, D.M. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat 1983, 168, 257–276. [Google Scholar] [CrossRef]
- Tzahor, E.; Kempf, H.; Mootoosamy, R.C.; Poon, A.C.; Abzhanov, A.; Tabin, C.J.; Dietrich, S.; Lassar, A.B. Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev 2003, 17, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- von Scheven, G.; Alvares, L.E.; Mootoosamy, R.C.; Dietrich, S. Neural tube derived signals and Fgf8 act antagonistically to specify eye versus mandibular arch muscles. Development 2006, 133, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Gage, P.J.; Suh, H.; Camper, S.A. Dosage requirement of Pitx2 for development of multiple organs. Development 1999, 126, 4643–4651. [Google Scholar] [CrossRef]
- Schilling, T.F.; Walker, C.; Kimmel, C.B. The chinless mutation and neural crest cell interactions in zebrafish jaw development. Development 1996, 122, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Rinon, A.; Lazar, S.; Marshall, H.; Büchmann-Møller, S.; Neufeld, A.; Elhanany-Tamir, H.; Taketo, M.M.; Sommer, L.; Krumlauf, R.; Tzahor, E. Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development 2007, 134, 3065–3075. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Liu, J.X.; Brännström, T.; Andersen, P.M.; Stål, P.; Pedrosa-Domellöf, F. Human extraocular muscles in ALS. Invest Ophthalmol Vis Sci 2010, 51, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Mühlendyck, H. [Age-dependent changes in transverse sections of muscle fibres from the exterior eye muscles in man]. Z Gerontol 1979, 12, 46–59. [Google Scholar] [PubMed]
- Henson, C.; Staunton, H.; Brett, F.M. Does ageing have an effect on midbrain premotor nuclei for vertical eye movements. Mov Disord 2003, 18, 688–694. [Google Scholar] [CrossRef]
- Stål, P.; Eriksson, P.O.; Schiaffino, S.; Butler-Browne, G.S.; Thornell, L.E. Differences in myosin composition between human oro-facial, masticatory and limb muscles: enzyme-, immunohisto- and biochemical studies. J Muscle Res Cell Motil 1994, 15, 517–534. [Google Scholar] [CrossRef]
- Hoogenraad, T.U.; Jennekens, F.G.; Tan, K.E. Histochemical fibre types in human extraocular muscles, an investigation of inferior oblique muscle. Acta Neuropathol 1979, 45, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Budak, M.T.; Bogdanovich, S.; Wiesen, M.H.; Lozynska, O.; Khurana, T.S.; Rubinstein, N.A. Layer-specific differences of gene expression in extraocular muscles identified by laser-capture microscopy. Physiol Genomics 2004, 20, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.F.; Porter, J.D. Structural organization of the extraocular muscles. Rev Oculomot Res 1988, 2, 33–79. [Google Scholar] [PubMed]
- Spencer, R.F.; Porter, J.D. Biological organization of the extraocular muscles. Prog Brain Res 2006, 151, 43–80. [Google Scholar]
- Porter, J.D.; Burns, L.A.; May, P.J. Morphological substrate for eyelid movements: innervation and structure of primate levator palpebrae superioris and orbicularis oculi muscles. J Comp Neurol 1989, 287, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Rossi, A.C.; Smerdu, V.; Leinwand, L.A.; Reggiani, C. Developmental myosins: expression patterns and functional significance. Skelet Muscle 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Martin, L.J. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum Mol Genet 2010, 19, 2284–2302. [Google Scholar] [CrossRef]
- Wieczorek, D.F.; Periasamy, M.; Butler-Browne, G.S.; Whalen, R.G.; Nadal-Ginard, B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol 1985, 101, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Yu Wai Man, C.Y.; Chinnery, P.F.; Griffiths, P.G. Extraocular muscles have fundamentally distinct properties that make them selectively vulnerable to certain disorders. Neuromuscul Disord 2005, 15, 17–23. [Google Scholar] [CrossRef]
- Briggs, M.M.; Schachat, F. Early specialization of the superfast myosin in extraocular and laryngeal muscles. J Exp Biol 2000, 203, 2485–2494. [Google Scholar] [CrossRef]
- Briggs, M.M.; Schachat, F. The superfast extraocular myosin (MYH13) is localized to the innervation zone in both the global and orbital layers of rabbit extraocular muscle. J Exp Biol 2002, 205, 3133–3142. [Google Scholar] [CrossRef]
- Rossi, A.C.; Mammucari, C.; Argentini, C.; Reggiani, C.; Schiaffino, S. Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles. J Physiol 2010, 588, 353–364. [Google Scholar] [CrossRef]
- Lee, L.A.; Karabina, A.; Broadwell, L.J.; Leinwand, L.A. The ancient sarcomeric myosins found in specialized muscles. Skelet Muscle 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Mascarello, F.; Toniolo, L.; Cancellara, P.; Reggiani, C.; Maccatrozzo, L. Expression and identification of 10 sarcomeric MyHC isoforms in human skeletal muscles of different embryological origin. Diversity and similarity in mammalian species. Ann Anat 2016, 207, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Kranjc, B.S.; Sketelj, J.; Albis, A.D.; Ambroz, M.; Erzen, I. Fibre types and myosin heavy chain expression in the ocular medial rectus muscle of the adult rat. J Muscle Res Cell Motil 2000, 21, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Stirn Kranjc, B.; Smerdu, V.; Erzen, I. Histochemical and immunohistochemical profile of human and rat ocular medial rectus muscles. Graefes Arch Clin Exp Ophthalmol 2009, 247, 1505–1515. [Google Scholar] [CrossRef]
- Rushbrook, J.I.; Weiss, C.; Ko, K.; Feuerman, M.H.; Carleton, S.; Ing, A.; Jacoby, J. Identification of alpha-cardiac myosin heavy chain mRNA and protein in extraocular muscle of the adult rabbit. J Muscle Res Cell Motil 1994, 15, 505–515. [Google Scholar] [CrossRef]
- Rubinstein, N.A.; Hoh, J.F. The distribution of myosin heavy chain isoforms among rat extraocular muscle fiber types. Invest Ophthalmol Vis Sci 2000, 41, 3391–3398. [Google Scholar]
- Bicer, S.; Reiser, P.J. Myosin isoform expression in dog rectus muscles: patterns in global and orbital layers and among single fibers. Invest Ophthalmol Vis Sci 2009, 50, 157–167. [Google Scholar] [CrossRef]
- McLoon, L.K., Andrade, F. Craniofacial Muscles: A New Framework for Understanding the Effector Side of Craniofacial Muscle Control. L.K. McLoon and F. Andrade, editors. Springer New York, New York, NY, 2012.
- Mayr, R. Structure and distribution of fibre types in the external eye muscles of the rat. Tissue Cell 1971, 3, 433–462. [Google Scholar] [CrossRef]
- Rashed, R.M.; El-Alfy, S.H.; Mohamed, I.K. Histochemical analysis of muscle fiber types of rat superior rectus extraocular muscle. Acta Histochem 2010, 112, 536–545. [Google Scholar] [CrossRef]
- Rashed, R.M.; El-Alfy, S.H. Ultrastructural organization of muscle fiber types and their distribution in the rat superior rectus extraocular muscle. Acta Histochem 2012, 114, 217–225. [Google Scholar] [CrossRef]
- Wasicky, R.; Ziya-Ghazvini, F.; Blumer, R.; Lukas, J.R.; Mayr, R. Muscle fiber types of human extraocular muscles: a histochemical and immunohistochemical study. Invest Ophthalmol Vis Sci 2000, 41, 980–990. [Google Scholar] [PubMed]
- Shear, T.D.; Martyn, J.A. Physiology and biology of neuromuscular transmission in health and disease. J Crit Care 2009, 24, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Lepore, E.; Casola, I.; Dobrowolny, G.; Musarò, A. Neuromuscular Junction as an Entity of Nerve-Muscle Communication. Cells 2019, 8, 906. [Google Scholar] [CrossRef]
- Hughes, B.W.; Kusner, L.L.; Kaminski, H.J. Molecular architecture of the neuromuscular junction. Muscle Nerve 2006, 33, 445–46. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Levine, D.N.; Tsairis, P.; Zajac, F.E. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 1973, 234, 723–748. [Google Scholar] [CrossRef]
- Ogata, T.; Yamasaki, Y. Scanning electron-microscopic study on the three-dimensional structure of motor endplates of the slow (tonic) muscle fibers in the frog, Rana n. nigromaculata. Cell Tissue Res 1988, 252, 211–213. [Google Scholar] [CrossRef]
- Boyd-Clark, L.C.; Briggs, C.A.; Galea, M.P. Comparative histochemical composition of muscle fibres in a pre- and a postvertebral muscle of the cervical spine. J Anat 2001, 199, 709–716. [Google Scholar] [CrossRef]
- Callister, R.J.; Callister, R.; Peterson, E.H. Design and control of the head retractor muscle in a turtle, Pseudemys (Trachemys) scripta: I. Architecture and histochemistry of single muscle fibers. J Comp Neurol 1992, 325, 405–421. [Google Scholar] [CrossRef]
- Э Sosnicki, A.A.; Lutz, G.J.; Rome, L.C.; Goble, D.O. Histochemical and molecular determination of fiber types in chemically skinned single equine skeletal muscle fibers. J Histochem Cytochem 1989, 37, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Sanchís-Moysi, J.; Idoate, F.; Olmedillas, H.; Guadalupe-Grau, A.; Alayón, S.; Carreras, A.; Dorado, C.; Calbet, J.A. The upper extremity of the professional tennis player: muscle volumes, fiber-type distribution and muscle strength. Scand J Med Sci Sports 2010. [CrossRef] [PubMed]
- Smith M. Neurological Rehabilitation. 5th ed. Darcy A. Umphred St. Louis, MO.; Mosby Elsevier. 2007; ISBN-13: 978-0-323-03306-0.
- Kuffler, S.W.; Vaughan Williams, E.M. Properties of the 'slow' skeletal muscles fibres of the frog. J Physiol 1953, 121, 318–340. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, S.W.; Vaughan Williams, E.M. Small-nerve junctional potentials; the distribution of small motor nerves to frog skeletal muscle, and the membrane characteristics of the fibres they innervate. J Physiol 1953, 121, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.; Pilar, G. Slow fibres in the extraocular muscles of the cat. J Physiol 1963, 169, 780–798. [Google Scholar] [CrossRef] [PubMed]
- Hess, A. Two Kinds of Extrafusal Muscle Fibers and Their Nerve Endings in the Garter Snake. Am J Anat 1963, 113, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Hess, A. Further morphological observations of 'en plaque' and 'en grappe' nerve endings on mammalian extrafusal muscle fibers with the cholinesterase technique. Rev Can Biol 1962, 21, 241–248. [Google Scholar] [PubMed]
- Dietert, S.E. The demonstration of different types of muscle fibers in human extraocular muscle fibers in human extraocular muscle by electron microscopy and cholinesterase staining. Invest Ophthalmol 1965, 4, 51–63. [Google Scholar] [PubMed]
- Lichtman, J.W.; Wilkinson, R.S.; Rich, M.M. Multiple innervation of tonic endplates revealed by activity-dependent uptake of fluorescent probes. Nature 1985, 314, 357–359. [Google Scholar] [CrossRef]
- Ridge, R.M. Different types of extrafusal muscle fibres in snake costocutaneous muscles. J Physiol 1971, 217, 393–418. [Google Scholar] [CrossRef]
- Oda, K. Motor innervation and acetylcholine receptor distribution of human extraocular muscle fibres. J Neurol Sci 1986, 74, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, C. Motor innervation of extraocular muscle. J Physiol 1960, 153, 522–526. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol Rev 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Liu, J.X.; Domellöf, F.P. A Novel Type of Multiterminal Motor Endplate in Human Extraocular Muscles. Invest Ophthalmol Vis Sci 2018, 59, 539–548. [Google Scholar] [CrossRef]
- Carry, M.R.; Ringel, S.P.; Starcevich, J.M. Mitochondrial morphometrics of histochemically identified human extraocular muscle fibers. Anat Rec 1986, 214, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Demer, J.L.; Oh, S.Y.; Poukens, V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci 2000, 41, 1280–1290. [Google Scholar]
- Porter, J.D.; Baker, R.S. Muscles of a different 'color': the unusual properties of the extraocular muscles may predispose or protect them in neurogenic and myogenic disease. Neurology 1996, 46, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Pachter, B.R. Fiber composition of the superior rectus extraocular muscle of the rhesus macaque. J Morphol 1982, 174, 237–250. [Google Scholar] [CrossRef]
- Chiarandini, D.J.; Davidowitz, J. Structure and function of extraocular muscle fibers. Curr Top Eye Res 1979, 1, 91–142. [Google Scholar]
- Khanna, S.; Porter, J.D. Evidence for rectus extraocular muscle pulleys in rodents. Invest Ophthalmol Vis Sci 2001, 42, 1986–1992. [Google Scholar]
- Chiarandini, D.J.; Stefani, E. Electrophysiological identification of two types of fibres in rat extraocular muscles. J Physiol 1979, 290, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, J.; Chiarandini, D.J.; Stefani, E. Electrical properties and innervation of fibers in the orbital layer of rat extraocular muscles. J Neurophysiol 1989, 61, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, H.J.; Kusner, L.L.; Block, C.H. Expression of acetylcholine receptor isoforms at extraocular muscle endplates. Invest Ophthalmol Vis Sci 1996, 37, 345–351. [Google Scholar]
- Gu, Y.; Hall, Z.W. Immunological evidence for a change in subunits of the acetylcholine receptor in developing and denervated rat muscle. Neuron 1988, 1, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Fraterman, S.; Khurana, T.S.; Rubinstein, N.A. Identification of acetylcholine receptor subunits differentially expressed in singly and multiply innervated fibers of extraocular muscles. Invest Ophthalmol Vis Sci 2006, 47, 3828–3834. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, F.; Vicini, S.; Schuetze, S.M. Embryonic acetylcholine receptors guarantee spontaneous contractions in rat developing muscle. Nature 1988, 335, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Witzemann, V.; Schwarz, H.; Koenen, M.; Berberich, C.; Villarroel, A.; Wernig, A.; Brenner, H.R.; Sakmann, B. Acetylcholine receptor epsilon-subunit deletion causes muscle weakness and atrophy in juvenile and adult mice. Proc Natl Acad Sci U S A 1996, 93, 13286–13291. [Google Scholar] [CrossRef]
- Liu, Y.; Padgett, D.; Takahashi, M.; Li, H.; Sayeed, A.; Teichert, R.W.; Olivera, B.M.; McArdle, J.J.; Green, W.N.; Lin, W. Essential roles of the acetylcholine receptor gamma-subunit in neuromuscular synaptic patterning. Development 2008, 135, 1957–1967. [Google Scholar] [CrossRef]
- Hoffmann, K.; Muller, J.S.; Stricker, S.; Megarbane, A.; Rajab, A.; Lindner, T.H.; Cohen, M.; Chouery, E.; Adaimy, L.; Ghanem, I.; Delague, V.; Boltshauser, E.; Talim, B.; Horvath, R.; Robinson, P.N.; Lochmüller, H.; Hübner, C.; Mundlos, S. Escobar syndrome is a prenatal myasthenia caused by disruption of the acetylcholine receptor fetal gamma subunit. Am J Hum Genet 2006, 79, 303–312. [Google Scholar] [CrossRef]
- Morgan, N.V.; Brueton, L.A.; Cox, P.; Greally, M.T.; Tolmie, J.; Pasha, S.; Aligianis, I.A.; van Bokhoven, H.; Marton, T.; Al-Gazali, L.; Morton, J.E.; Oley, C.; Johnson, C.A.; Trembath, R.C.; Brunner, H.G.; Maher, E.R. Mutations in the embryonal subunit of the acetylcholine receptor (CHRNG) cause lethal and Escobar variants of multiple pterygium syndrome. Am J Hum Genet 2006, 79, 390–395. [Google Scholar] [CrossRef]
- Vogt, J.; Morgan, N.V.; Rehal, P.; Faivre, L.; Brueton, L.A.; Becker, K.; Fryns, J.P.; Holder, S.; Islam, L.; Kivuva, E.; Lynch, S.A.; Touraine, R.; Wilson, L.C.; MacDonald, F.; Maher, E.R. CHRNG genotype-phenotype correlations in the multiple pterygium syndromes. J Med Genet 2012, 49, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Mishina, M.; Takai, T.; Imoto, K.; Noda, M.; Takahashi, T.; Numa, S.; Methfessel, C.; Sakmann, B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature 1986, 321, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Bär, H.; Strelkov, S.V.; Sjöberg, G.; Aebi, U.; Herrmann, H. The biology of desmin filaments: how do mutations affect their structure, assembly, and organisation. J Struct Biol 2004, 148, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Askanas, V.; Bornemann, A.; Engel, W.K. Immunocytochemical localization of desmin at human neuromuscular junctions. Neurology 1990, 40, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Clemen, C.S.; Herrmann, H.; Strelkov, S.V.; Schröder, R. Desminopathies: pathology and mechanisms. Acta Neuropathol 2013, 125, 47–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Pedrosa Domellöf, F. Complex Correlations Between Desmin Content, Myofiber Types, and Innervation Patterns in the Human Extraocular Muscles. Invest Ophthalmol Vis Sci 2020, 61, 15. [Google Scholar] [CrossRef] [PubMed]
- Janbaz, A.H.; Lindström, M.; Liu, J.X.; Pedrosa Domellöf, F. Intermediate filaments in the human extraocular muscles. Invest Ophthalmol Vis Sci 2014, 55, 5151–5159. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Brännström, T.; Andersen, P.M.; Pedrosa-Domellöf, F. Different impact of ALS on laminin isoforms in human extraocular muscles versus limb muscles. Invest Ophthalmol Vis Sci 2011, 52, 4842–4852. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Brännström, T.; Andersen, P.M.; Pedrosa-Domellöf, F. Distinct changes in synaptic protein composition at neuromuscular junctions of extraocular muscles versus limb muscles of ALS donors. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- McLoon, L.K.; Harandi, V.M.; Brännström, T.; Andersen, P.M.; Liu, J.X. Wnt and extraocular muscle sparing in amyotrophic lateral sclerosis. Invest Ophthalmol Vis Sci 2014, 55, 5482–5496. [Google Scholar] [CrossRef]
- Harandi, V.M.; Gaied, A.R.; Brännström, T.; Pedrosa Domellöf, F.; Liu, J.X. Unchanged Neurotrophic Factors and Their Receptors Correlate With Sparing in Extraocular Muscles in Amyotrophic Lateral Sclerosis. Invest Ophthalmol Vis Sci 2016, 57, 6831–6842. [Google Scholar] [CrossRef] [PubMed]
- Agbulut, O.; Li, Z.; Mouly, V.; Butler-Browne, G.S. Analysis of skeletal and cardiac muscle from desmin knock-out and normal mice by high resolution separation of myosin heavy-chain isoforms. Biol Cell 1996, 88, 131–135. [Google Scholar]
- Balogh, J.; Merisckay, M.; Li, Z.; Paulin, D.; Arner, A. Hearts from mice lacking desmin have a myopathy with impaired active force generation and unaltered wall compliance. Cardiovasc Res 2002, 53, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Kjellgren, D.; Thornell, L.E.; Virtanen, I.; Pedrosa-Domellöf, F. Laminin isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci 2004, 45, 4233–4239. [Google Scholar] [CrossRef] [PubMed]
- McLoon, L.K.; Vicente, A.; Fitzpatrick, K.R.; Lindström, M.; Pedrosa Domellöf, F. Composition, Architecture, and Functional Implications of the Connective Tissue Network of the Extraocular Muscles. Invest Ophthalmol Vis Sci 2018, 59, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Dennhag, N.; Domellöf, F.P. Understanding the extraocular muscles: connective tissue, motor endplates and the cytoskeleton. Biochem (Lond) 2020, 42, 52–57. [Google Scholar] [CrossRef]
- Smith, T.J. Insights into the role of fibroblasts in human autoimmune diseases. Clin Exp Immunol 2005, 141, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Bednarczuk, T.; Gopinath, B.; Ploski, R.; Wall, J.R. Susceptibility genes in Graves' ophthalmopathy: searching for a needle in a haystack. Clin Endocrinol (Oxf) 2007, 67, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J. Novel aspects of orbital fibroblast pathology. J Endocrinol Invest 2004, 27, 246–253. [Google Scholar] [CrossRef]
- Gage, P.J.; Rhoades, W.; Prucka, S.K.; Hjalt, T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci 2005, 46, 4200–4208. [Google Scholar] [CrossRef]
- Kusner, L.L.; Young, A.; Tjoe, S.; Leahy, P.; Kaminski, H.J. Perimysial fibroblasts of extraocular muscle, as unique as the muscle fibers. Invest Ophthalmol Vis Sci 2010, 51, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.P.; Chamberlain-Banoub, J.; Neal, J.W.; Song, W.; Mizuno, M.; Harris, C.L. The membrane attack pathway of complement drives pathology in passively induced experimental autoimmune myasthenia gravis in mice. Clin Exp Immunol 2006, 146, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, H.J.; Kusner, L.L.; Richmonds, C.; Medof, M.E.; Lin, F. Deficiency of decay accelerating factor and CD59 leads to crisis in experimental myasthenia. Exp Neurol 2006, 202, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.J.; Afifiyan, N.; Sand, D.; Naik, V.; Said, J.; Pollock, S.J.; Chen, B.; Phipps, R.P.; Goldberg, R.A.; Smith, T.J.; Douglas, R.S. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci 2009, 50, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Laban-Guceva, N.; Bogoev, M.; Antova, M. Serum concentrations of interleukin (IL-)1alpha, 1beta, 6 and tumor necrosis factor (TNF-) alpha in patients with thyroid eye disease (TED). Med Arh 2007, 61, 203–206. [Google Scholar] [PubMed]
- Kaminski, H.J.; Li, Z.; Richmonds, C.; Ruff, R.L.; Kusner, L. Susceptibility of ocular tissues to autoimmune diseases. Ann N Y Acad Sci 2003, 998, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.D.; Khanna, S.; Kaminski, H.J.; Rao, J.S.; Merriam, A.P.; Richmonds, C.R.; Leahy, P.; Li, J.; Andrade, F.H. Extraocular muscle is defined by a fundamentally distinct gene expression profile. Proc Natl Acad Sci U S A 2001, 98, 12062–12067. [Google Scholar] [CrossRef]
- Nguyen, B.; Gopinath, B.; Tani, J.; Wescombe, L.; Wall, J.R. Peripheral blood T lymphocyte sensitisation against calsequestrin and flavoprotein in patients with Graves' ophthalmopathy. Autoimmunity 2008, 41, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, R.J.; Chow, C.K.; St Clair, D.K.; Porter, J.D. Extraocular, limb and diaphragm muscle group-specific antioxidant enzyme activity patterns in control and mdx mice. J Neurol Sci 1996, 139, 180–186. [Google Scholar] [CrossRef]
- McLoon, L.K.; Wirtschafter, J. Activated satellite cells in extraocular muscles of normal adult monkeys and humans. Invest Ophthalmol Vis Sci 2003, 44, 1927–1932. [Google Scholar] [CrossRef]
- Formicola, L.; Marazzi, G.; Sassoon, D.A. The extraocular muscle stem cell niche is resistant to ageing and disease. Front Aging Neurosci 2014, 6, 328. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, R.M.; Quattrocelli, M.; Pietrangelo, T.; Di Filippo, E.S.; Maccatrozzo, L.; Cassano, M.; Mascarello, F.; Barthélémy, I.; Blot, S.; Sampaolesi, M.; Fulle, S. Myogenic potential of canine craniofacial satellite cells. Front Aging Neurosci 2014, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Carrero-Rojas, G.; Benítez-Temiño, B.; Pastor, A.M.; Davis López de Carrizosa, M.A. Muscle Progenitors Derived from Extraocular Muscles Express Higher Levels of Neurotrophins and their Receptors than other Cranial and Limb Muscles. Cells 2020, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Renault, V.; Piron-Hamelin, G.; Forestier, C.; DiDonna, S.; Decary, S.; Hentati, F.; Saillant, G.; Butler-Browne, G.S.; Mouly, V. Skeletal muscle regeneration and the mitotic clock. Exp Gerontol 2000, 35, 711–719. [Google Scholar] [CrossRef]
- Kallestad, K.M.; Hebert, S.L.; McDonald, A.A.; Daniel, M.L.; Cu, S.R.; McLoon, L.K. Sparing of extraocular muscle in aging and muscular dystrophies: a myogenic precursor cell hypothesis. Exp Cell Res 2011, 317, 873–885. [Google Scholar] [CrossRef]
- Hebert, S.L.; Daniel, M.L.; McLoon, L.K. The role of Pitx2 in maintaining the phenotype of myogenic precursor cells in the extraocular muscles. PLoS One 2013, 8, e58405. [Google Scholar] [CrossRef] [PubMed]
- McGeachie, J.K.; Grounds, M.D. The timing between skeletal muscle myoblast replication and fusion into myotubes, and the stability of regenerated dystrophic myofibres: an autoradiographic study in mdx mice. J Anat 1999, 194 (Pt 2), 287–295. [Google Scholar] [CrossRef] [PubMed]
- Heslop, L.; Morgan, J.E.; Partridge, T.A. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci 2000, 113 (Pt 12), 2299–2308. [Google Scholar] [CrossRef]
- Girolamo, D.D.; Benavente-Diaz, M.; Murolo, M.; Grimaldi, A.; Lopes, P.T.; Evano, B.; Kuriki, M.; Gioftsidi, S.; Laville, V.; Tinevez, J.Y.; Letort, G.; Mella, S.; Tajbakhsh, S.; Comai, G. Extraocular muscle stem cells exhibit distinct cellular properties associated with non-muscle molecular signatures. Development 2024, 151, dev202144. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, G.; Dieter, L.; Hjalt, T.A.; Andrade, F.H.; Stahl, J.S.; Kaminski, H.J. An altered phenotype in a conditional knockout of Pitx2 in extraocular muscle. Invest Ophthalmol Vis Sci 2009, 50, 4531–4541. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, D.; Kaminski, H.J. Pitx2 regulates myosin heavy chain isoform expression and multi-innervation in extraocular muscle. J Physiol 2011, 589, 4601–4614. [Google Scholar] [CrossRef]
- Benítez-Temiño, B.; Davis-López de Carrizosa, M.A.; Morcuende, S.; Matarredona, E.R.; de la Cruz, R.R.; Pastor, A.M. Functional Diversity of Neurotrophin Actions on the Oculomotor System. Int J Mol Sci 2016, 17, 2016. [Google Scholar] [CrossRef] [PubMed]
- McLoon, L.K.; Christiansen, S.P. Increasing extraocular muscle strength with insulin-like growth factor II. Invest Ophthalmol Vis Sci 2003, 44, 3866–3872. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.Y.; von Bartheld, C.S. Expression of insulin-like growth factor 1 isoforms in the rabbit oculomotor system. Growth Horm IGF Res 2011, 21, 228–232. [Google Scholar] [CrossRef]
- Davis-López de Carrizosa, M.A.; Morado-Díaz, C.J.; Tena, J.J.; Benítez-Temiño, B.; Pecero, M.L.; Morcuende, S.R.; de la Cruz, R.R.; Pastor, A.M. Complementary actions of BDNF and neurotrophin-3 on the firing patterns and synaptic composition of motoneurons. J Neurosci 2009, 29, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, C.L.; Fleuriet, J.; Walton, M.M.; Mustari, M.J.; McLoon, L.K. Adaptation of slow myofibers: the effect of sustained BDNF treatment of extraocular muscles in infant nonhuman primates. Invest Ophthalmol Vis Sci 2015, 56, 3467–3483. [Google Scholar] [CrossRef]
- Agarwal, A.B.; Feng, C.Y.; Altick, A.L.; Quilici, D.R.; Wen, D.; Johnson, L.A.; von Bartheld, C.S. Altered Protein Composition and Gene Expression in Strabismic Human Extraocular Muscles and Tendons. Invest Ophthalmol Vis Sci 2016, 57, 5576–5585. [Google Scholar] [CrossRef] [PubMed]
- Harandi, V.M.; Lindquist, S.; Kolan, S.S.; Brännström, T.; Liu, J.X. Analysis of neurotrophic factors in limb and extraocular muscles of mouse model of amyotrophic lateral sclerosis. PLoS One 2014, 9, e109833. [Google Scholar] [CrossRef]
- Steljes, T.P.; Kinoshita, Y.; Wheeler, E.F.; Oppenheim, R.W.; von Bartheld, C.S. Neurotrophic factor regulation of developing avian oculomotor neurons: differential effects of BDNF and GDNF. J Neurobiol 1999, 41, 295–315. [Google Scholar] [CrossRef]
- Chen, J.; von Bartheld, C.S. Role of exogenous and endogenous trophic factors in the regulation of extraocular muscle strength during development. Invest Ophthalmol Vis Sci 2004, 45, 3538–3545. [Google Scholar] [CrossRef]
- Hassan, S.M.; Jennekens, F.G.; Veldman, H. Botulinum toxin-induced myopathy in the rat. Brain 1995, 118 (Pt 2), 533–545. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.J.; Vanden Noven, S.; Muccio, D.; Wallace, N. Axotomy-like changes in cat motoneuron electrical properties elicited by botulinum toxin depend on the complete elimination of neuromuscular transmission. J Neurosci 1991, 11, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Kranjc, B.S.; Sketelj, J.; D'Albis, A.; Erzen, I. Long-term changes in myosin heavy chain composition after botulinum toxin a injection into rat medial rectus muscle. Invest Ophthalmol Vis Sci 2001, 42, 3158–3164. [Google Scholar] [PubMed]
- Spencer, R.F.; McNeer, K.W. Botulinum toxin paralysis of adult monkey extraocular muscle. Structural alterations in orbital, singly innervated muscle fibers. Arch Ophthalmol 1987, 105, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Ugalde, I.; Christiansen, S.P.; McLoon, L.K. Botulinum toxin treatment of extraocular muscles in rabbits results in increased myofiber remodeling. Invest Ophthalmol Vis Sci 2005, 46, 4114–4120. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowski, B.; Pulliam, C.; Betta, N.D.; Kardon, G.; Olwin, B.B. Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet Muscle 2015, 5, 42. [Google Scholar] [CrossRef]
- Karpati, G.; Carpenter, S. Small-caliber skeletal muscle fibers do not suffer deleterious consequences of dystrophic gene expression. Am J Med Genet 1986, 25, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.H.; Porter, J.D.; Kaminski, H.J. Eye muscle sparing by the muscular dystrophies: lessons to be learned. Microsc Res Tech 2000, 48, 192–203. [Google Scholar] [CrossRef]
- Kuang, S.; Chargé, S.B.; Seale, P.; Huh, M.; Rudnicki, M.A. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol 2006, 172, 103–113. [Google Scholar] [CrossRef]
- Khurana, T.S.; Prendergast, R.A.; Alameddine, H.S.; Tomé, F.M.; Fardeau, M.; Arahata, K.; Sugita, H.; Kunkel, L.M. Absence of extraocular muscle pathology in Duchenne's muscular dystrophy: role for calcium homeostasis in extraocular muscle sparing. J Exp Med 1995, 182, 467–475. [Google Scholar] [CrossRef]
- Matsumura, K.; Ervasti, J.M.; Ohlendieck, K.; Kahl, S.D.; Campbell, K.P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature 1992, 360, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Caligari, M.; Godi, M.; Guglielmetti, S.; Franchignoni, F.; Nardone, A. Eye tracking communication devices in amyotrophic lateral sclerosis: impact on disability and quality of life. Amyotroph Lateral Scler Frontotemporal Degener 2013, 14, 546–552. [Google Scholar] [CrossRef]
- Kubota, M.; Sakakihara, Y.; Uchiyama, Y.; Nara, A.; Nagata, T.; Nitta, H.; Ishimoto, K.; Oka, A.; Horio, K.; Yanagisawa, M. New ocular movement detector system as a communication tool in ventilator-assisted Werdnig-Hoffmann disease. Dev Med Child Neurol 2000, 42, 61–64. [Google Scholar]
- Pun, S.; Santos, A.F.; Saxena, S.; Xu, L.; Caroni, P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci 2006, 9, 408–419. [Google Scholar] [CrossRef]
- Hegedus, J.; Putman, C.T.; Gordon, T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 2007, 28, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Donaghy, C.; Pinnock, R.; Abrahams, S.; Cardwell, C.; Hardiman, O.; Patterson, V.; McGivern, R.C.; Gibson, J.M. Slow saccades in bulbar-onset motor neurone disease. J Neurol 2010, 257, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Kim, J.I.; Lim, Y.M.; Kim, K.K. Abnormal Oculomotor Functions in Amyotrophic Lateral Sclerosis. J Clin Neurol 2018, 14, 464–471. [Google Scholar] [CrossRef]
- Tjust, A.E.; Danielsson, A.; Andersen, P.M.; Brännström, T.; Pedrosa Domellöf, F. Impact of Amyotrophic Lateral Sclerosis on Slow Tonic Myofiber Composition in Human Extraocular Muscles. Invest Ophthalmol Vis Sci 2017, 58, 3708–3715. [Google Scholar] [CrossRef]
| Type of isoform | Name |
|---|---|
| Fast isoforms of MyHC | MyHCIIa (Myh2); MyHCIIb (Myh4); MyHCIIx (Myh1); MyHCeom (Myh13). |
| Slow isoforms of MyHC | МyHCI-MyHC-B/slow (Myh7); MyHCα-cardiac (Myh6); MyHCsto (MyH7B) |
| MyHC isoforms related to development | MyHCemb (Myh3); MyHCneonatal (Myh8) |
| The ancient sarcomeric MyHC | MYH14/7b (MyH7B), MyH15 (Myh15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





