Submitted:
22 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Thermostable α-Amylases
Structural Characteristics and Catalytic Mechanism of α-Amylases
Factors Contributing to Stability in Thermostable α-Amylases
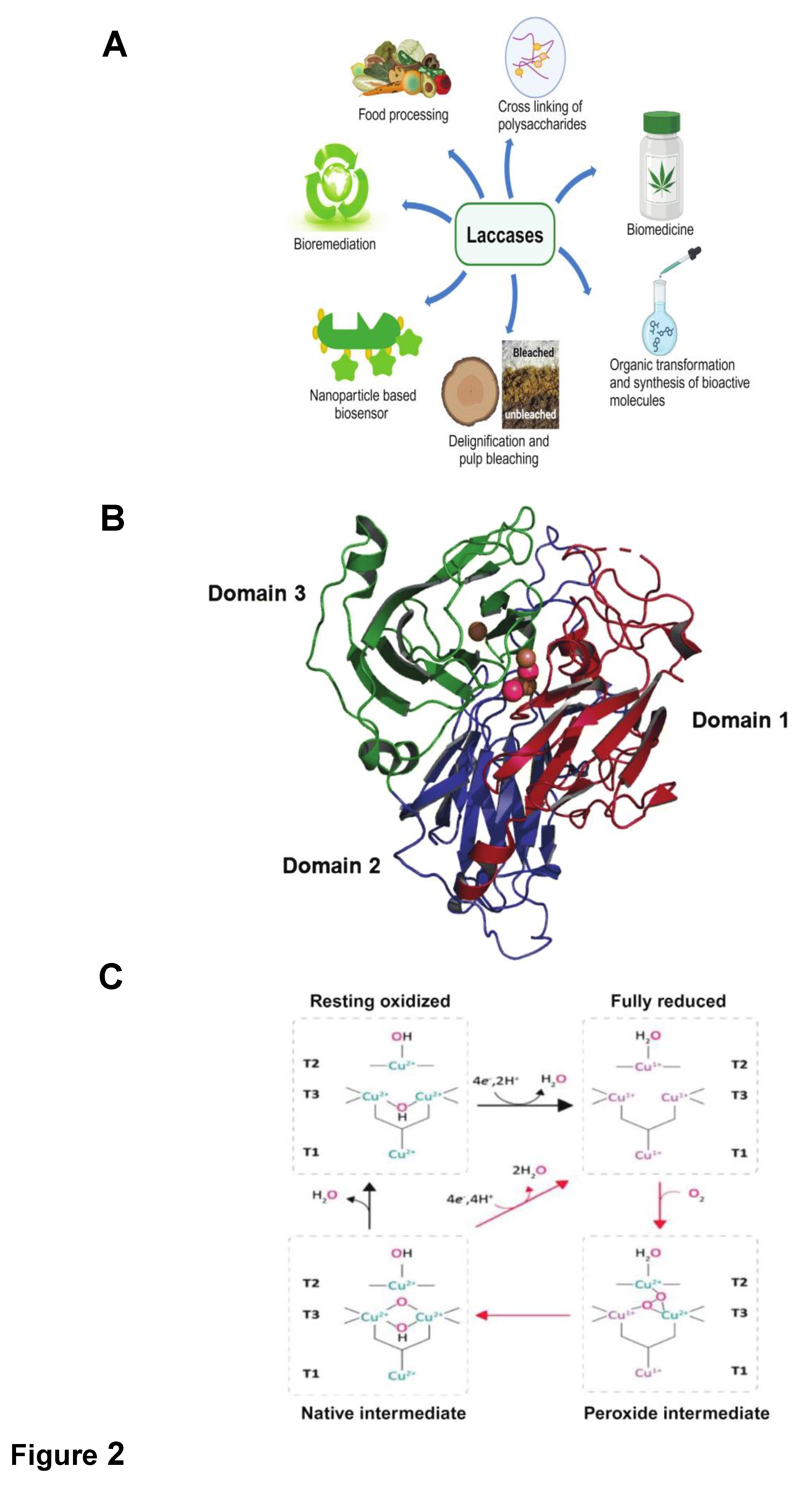
Thermostable Laccases
Structural Characteristics and Catalytic Mechanism of Laccases
- Type-1 Copper Reduction by Reducing Substrate: Laccase initiates the reaction by accepting electrons from the substrate, reducing the Type-1 copper center.
- Internal Electron Transfer: Electron transfer occurs from Type-1 to Type-2 and Type-3 copper centers, forming a trinuclear cluster.
- Reduction of Oxygen to Water: The trinuclear copper cluster reduces molecular oxygen to water, concluding the catalytic cycle.
Structure-Function Relationship among Laccases
Major Strategies to Enhance Thermostability
Current Challenges, Research Aims and Recent Advances in the Field of Thermostable α-Amylases and Laccases
Future Directions in the Field of Thermostable Enzymes
Concluding Remarks
Acknowledgements
Compliance with ethical standards
Disclosure of potential conflicts of interest:
Research involving human participants and/or animals
Informed consent
References
- Vieille, C.; Zeikus Gregory, J. Hyperthermophilic Enzymes: Sources, Uses, and Molecular Mechanisms for Thermostability. Microbiology and Molecular Biology Reviews 2001, 65, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Che Hussian, C.H.A.; Leong, W.Y. Thermostable enzyme research advances: a bibliometric analysis. Journal of Genetic Engineering and Biotechnology 2023, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Mateljak, I.; Alcalde, M. Engineering a Highly Thermostable High-Redox Potential Laccase. ACS Sustainable Chemistry & Engineering 2021, 9, 9632–9637. [Google Scholar] [CrossRef]
- Ferreira, A.V.F.; Silva, F.F.; Silva, A.A.M.; Azevedo, L.S.; da Fonseca, S.T.D.; Camilo, N.H.; Dos Santos, K.P.E.; de Carvalho, L.C.; Tarabal, V.S.; da Silva, J.O.; et al. Recent Patents on the Industrial Application of Alpha-amylases. Recent Pat Biotechnol 2020, 14, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Da Lage, J.L.; Danchin, E.G.; Casane, D. Where do animal alpha-amylases come from? An interkingdom trip. FEBS Lett 2007, 581, 3927–3935. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Zheng, C.; Liu, B.; Yin, Q.; Cao, Y.; Yao, J. Leucine Affects α-Amylase Synthesis through PI3K/Akt-mTOR Signaling Pathways in Pancreatic Acinar Cells of Dairy Calves. J Agric Food Chem 2018, 66, 5149–5156. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.; Kimmel, A.R. mTORC1/AMPK responses define a core gene set for developmental cell fate switching. BMC Biol 2019, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.; Majithia, A.R.; Rosel, D.; Liao, X.H.; Khurana, T.; Kimmel, A.R. Integrated actions of mTOR complexes 1 and 2 for growth and development of Dictyostelium. Int J Dev Biol 2019, 63, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial α-amylases: a biotechnological perspective. Process Biochemistry 2003, 38, 1599–1616. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Madhavan, A.; Beevi, U.S.; Mathew, A.K.; Abraham, A.; Pandey, A.; Kumar, V. Molecular improvements in microbial α-amylases for enhanced stability and catalytic efficiency. Bioresour Technol 2017, 245, 1740–1748. [Google Scholar] [CrossRef]
- Prakash, O.; Jaiswal, N. alpha-Amylase: an ideal representative of thermostable enzymes. Appl Biochem Biotechnol 2010, 160, 2401–2414. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Jaiswal, N. A highly efficient and thermostable α-amylase from soya bean seeds. Biotechnol Appl Biochem 2010, 57, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishna, G.; Nirmala, M. Cereal α-amylases—an overview. Carbohydrate Polymers 2005, 60, 163–173. [Google Scholar] [CrossRef]
- Singh, K.; Ahmad, F.; Singh, V.K.; Kayastha, K.; Kayastha, A.M. Purification, biochemical characterization and Insilico modeling of α-amylase from Vicia faba. Journal of Molecular Liquids 2017, 234, 133–141. [Google Scholar] [CrossRef]
- Drula, E.; Garron, M.L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res 2022, 50, D571–d577. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Research 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Mareček, F.; Janeček, Š. A Novel Subfamily GH13_46 of the α-Amylase Family GH13 Represented by the Cyclomaltodextrinase from Flavobacterium sp. No. 92. Molecules 2022, 27, 8735. [Google Scholar] [CrossRef]
- Janeček, Š. Advances in Amylases—What’s Going on? Molecules 2023, 28, 7268. [Google Scholar] [CrossRef]
- Janeček, Š.; Svensson, B. How many α-amylase GH families are there in the CAZy database? 6, -10. [CrossRef]
- Ben Mabrouk, S.; Aghajari, N.; Ben Ali, M.; Ben Messaoud, E.; Juy, M.; Haser, R.; Bejar, S. Enhancement of the thermostability of the maltogenic amylase MAUS149 by Gly312Ala and Lys436Arg substitutions. Bioresour Technol 2011, 102, 1740–1746. [Google Scholar] [CrossRef]
- Li, Z.; Duan, X.; Wu, J. Improving the thermostability and enhancing the Ca(2+) binding of the maltohexaose-forming α-amylase from Bacillus stearothermophilus. J Biotechnol 2016, 222, 65–72. [Google Scholar] [CrossRef]
- Desse Haki, G.; Anceno, A.; Rakshit, S. Atypical Ca2+-independent, raw-starch hydrolysing α-amylase from Bacillus sp. GRE1: Characterization and gene isolation. World Journal of Microbiology and Biotechnology 2008, 24, 2517–2524. [Google Scholar] [CrossRef]
- Haki, G.D.; Rakshit, S.K. Developments in industrially important thermostable enzymes: a review. Bioresour Technol 2003, 89, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ito, N.; Yuuki, T.; Yamagata, H.; Udaka, S. Amino Acid Residues Stabilizing a Bacillus α-Amylase against Irreversible Thermoinactivation*. Journal of Biological Chemistry 1989, 264, 18933–18938. [Google Scholar] [CrossRef] [PubMed]
- Khemakhem, B.; Ali, M.B.; Aghajari, N.; Juy, M.; Haser, R.; Bejar, S. The importance of an extra loop in the B-domain of an α-amylase from B. stearothermophilus US100. Biochemical and Biophysical Research Communications 2009, 385, 78–83. [Google Scholar] [CrossRef]
- Ben Ali, M.; Khemakhem, B.; Robert, X.; Haser, R.; Bejar, S. Thermostability enhancement and change in starch hydrolysis profile of the maltohexaose-forming amylase of Bacillus stearothermophilus US100 strain. Biochem J 2006, 394, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Declerck, N.; Machius, M.; Wiegand, G.; Huber, R.; Gaillardin, C. Probing structural determinants specifying high thermostability in Bacillus licheniformis alpha-amylase. J Mol Biol 2000, 301, 1041–1057. [Google Scholar] [CrossRef]
- Choi, J.M.; Han, S.S.; Kim, H.S. Industrial applications of enzyme biocatalysis: Current status and future aspects. Biotechnol Adv 2015, 33, 1443–1454. [Google Scholar] [CrossRef]
- Sivaramakrishnan, S.; Gangadharan, D.; Nampoothiri, K.M.; Soccol, C.R.; Pandey, A. α-Amylases from Microbial Sources -- An Overview on Recent Developments. Food Technology & Biotechnology 2006, 44, 173–184. [Google Scholar]
- Greenwood, C.T.; MacGregor, A.W. THE ISOLATION OF α-AMYLASE FROM BARLEY AND MALTED BARLEY, AND A STUDY OF THE PROPERTIES AND ACTION-PATTERNS OF THE ENZYMES. Journal of the Institute of Brewing 1965, 71, 405–417. [Google Scholar] [CrossRef]
- Tripathi, P.; Lo Leggio, L.; Mansfeld, J.; Ulbrich-Hofmann, R.; Kayastha, A.M. Alpha-amylase from mung beans (Vigna radiata)--correlation of biochemical properties and tertiary structure by homology modelling. Phytochemistry 2007, 68, 1623–1631. [Google Scholar] [CrossRef]
- Nakamura, Y.; Koizumi, R.; Uchino, M.; Sato, H.; Takano, K. Purification and Characterization of α-Amylase from Potato Tuber. Food Preservation Science 2010, 36, 271–276. [Google Scholar] [CrossRef]
- Sarker, G.K.; Hasan, S.; Nikkon, F.; Mosaddik, A.; Sana, N.K.; Rahman, H.; Park, S.; Lee, D.-S.; Cho, S.K. Purification, characterization, and biochemical properties of α-amylase from potato. Journal of the Korean Society for Applied Biological Chemistry 2010, 53, 8–14. [Google Scholar] [CrossRef]
- Khare, S.; Prakash, O. Purification and Biochemical Characterization of α-Amylase from Radish (Raphanus sativus L.) Seeds Using Response surface methodology. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences 2019, 89, 79–88. [Google Scholar] [CrossRef]
- Amid, M.; Manap, M.Y.A. Purification and characterisation of a novel amylase enzyme from red pitaya (Hylocereus polyrhizus) peel. Food Chemistry 2014, 165, 412–418. [Google Scholar] [CrossRef]
- Singh, K.; Kayastha, A.M. A-amylase from wheat (Triticum aestivum) seeds: its purification, biochemical attributes and active site studies. Food Chem 2014, 162, 1–9. [Google Scholar] [CrossRef]
- Posoongnoen, S.; Thummavongsa, T. Purification and characterization of thermostable α-amylase from germinating Sword bean (Canavalia gladiata (Jacq.) DC.) seeds. Plant Biotechnol (Tokyo) 2020, 37, 31–38. [Google Scholar] [CrossRef]
- Banner, D.W.; Bloomer, A.C.; Petsko, G.A.; Phillips, D.C.; Pogson, C.I.; Wilson, I.A.; Corran, P.H.; Furth, A.J.; Milman, J.D.; Offord, R.E.; et al. Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5Å resolution: using amino acid sequence data. Nature 1975, 255, 609–614. [Google Scholar] [CrossRef]
- Svensson, B.; Søgaard, M. Protein engineering of amylases. Biochem Soc Trans 1992, 20, 34–42. [Google Scholar] [CrossRef]
- MacGregor, E.A. α-Amylase structure and activity. Journal of Protein Chemistry 1988, 7, 399–415. [Google Scholar] [CrossRef]
- Nielsen, J.E.; Borchert, T.V. Protein engineering of bacterial α-amylases. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 2000, 1543, 253–274. [Google Scholar] [CrossRef]
- van der Maarel, M.J.; van der Veen, B.; Uitdehaag, J.C.; Leemhuis, H.; Dijkhuizen, L. Properties and applications of starch-converting enzymes of the alpha-amylase family. J Biotechnol 2002, 94, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, Z.-L.; Hanzlik, S.; Cook, E.; Shen, Q.J. Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid-inducible WRKY gene. Plant Molecular Biology 2007, 64, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Kuriki, T.; Imanaka, T. The concept of the alpha-amylase family: structural similarity and common catalytic mechanism. J Biosci Bioeng 1999, 87, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Vihinen, M.; Mäntsälä, P. Microbial amylolytic enzymes. Crit Rev Biochem Mol Biol 1989, 24, 329–418. [Google Scholar] [CrossRef] [PubMed]
- Violet, M.; Meunier, J.C. Kinetic study of the irreversible thermal denaturation of Bacillus licheniformis alpha-amylase. Biochem J 1989, 263, 665–670. [Google Scholar] [CrossRef]
- Hwang, K.Y.; Song, H.K.; Chang, C.; Lee, J.; Lee, S.Y.; Kim, K.K.; Choe, S.; Sweet, R.M.; Suh, S.W. Crystal structure of thermostable alpha-amylase from Bacillus licheniformis refined at 1.7 A resolution. Mol Cells 1997, 7, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Machius, M.; Wiegand, G.; Huber, R. Crystal structure of calcium-depleted Bacillus licheniformis alpha-amylase at 2.2 A resolution. J Mol Biol 1995, 246, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Stein, E.A.; Sumerwell, W.N.; Fischer, E.H. Metal content of alpha-amylases of various origins. J Biol Chem 1959, 234, 2901–2905. [Google Scholar] [CrossRef]
- Boel, E.; Brady, L.; Brzozowski, A.M.; Derewenda, Z.; Dodson, G.G.; Jensen, V.J.; Petersen, S.B.; Swift, H.; Thim, L.; Woldike, H.F. Calcium binding in alpha-amylases: an X-ray diffraction study at 2.1-A resolution of two enzymes from Aspergillus. Biochemistry 1990, 29, 6244–6249. [Google Scholar] [CrossRef]
- Hsiu, J.; Fischer, E.H.; Stein, E.A. Alpha-Amylases as Calcium-Metalloenzymes. II. Calcium and the Catalytic Activity. Biochemistry 1964, 3, 61–66. [Google Scholar] [CrossRef]
- Larson, S.B.; Greenwood, A.; Cascio, D.; Day, J.; McPherson, A. Refined molecular structure of pig pancreatic alpha-amylase at 2.1 A resolution. J Mol Biol 1994, 235, 1560–1584. [Google Scholar] [CrossRef] [PubMed]
- Buisson, G.; Duée, E.; Haser, R.; Payan, F. Three dimensional structure of porcine pancreatic alpha-amylase at 2.9 A resolution. Role of calcium in structure and activity. The EMBO Journal 1987, 6, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.X.; Wang, Y.B.; Pan, Y.J.; Li, W.F. Differences in amino acids composition and coupling patterns between mesophilic and thermophilic proteins. Amino Acids 2008, 34, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.A.; Bort, B.R.; Martínez, J.; Randez-Gil, F.; Buesa, C.; Sanz, P. Purification and characterization of a new alpha-amylase of intermediate thermal stability from the yeast Lipomyces kononenkoae. Biochem Cell Biol 1995, 73, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Declerck, N.; Machius, M.; Joyet, P.; Wiegand, G.; Huber, R.; Gaillardin, C. Hyperthermostabilization of Bacillus licheniformis alpha-amylase and modulation of its stability over a 50 degrees C temperature range. Protein Eng 2003, 16, 287–293. [Google Scholar] [CrossRef]
- Torrance, J.W.; MacArthur, M.W.; Thornton, J.M. Evolution of binding sites for zinc and calcium ions playing structural roles. Proteins: Structure, Function, and Bioinformatics 2008, 71, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Gupta, J.K.; Soni, S.K. A novel raw starch digesting thermostable α-amylase from Bacillus sp. I-3 and its use in the direct hydrolysis of raw potato starch. Enzyme and Microbial Technology 2005, 37, 723–734. [Google Scholar] [CrossRef]
- Khajeh, K.; Ranjbar, B.; Naderi-Manesh, H.; Ebrahim Habibi, A.; Nemat-Gorgani, M. Chemical modification of bacterial alpha-amylases: changes in tertiary structures and the effect of additional calcium. Biochim Biophys Acta 2001, 1548, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.S.; Sticher, L.; van Huystee, R.; Wagner, D.; Jones, R.L. The calcium requirement for stability and enzymatic activity of two isoforms of barley aleurone alpha-amylase. J Biol Chem 1989, 264, 19392–19398. [Google Scholar] [CrossRef] [PubMed]
- Hmidet, N.; Bayoudh, A.; Berrin, J.G.; Kanoun, S.; Juge, N.; Nasri, M. Purification and biochemical characterization of a novel α-amylase from Bacillus licheniformis NH1: Cloning, nucleotide sequence and expression of amyN gene in Escherichia coli. Process Biochemistry 2008, 43, 499–510. [Google Scholar] [CrossRef]
- Asoodeh, A.; Chamani, J.; Lagzian, M. A novel thermostable, acidophilic α-amylase from a new thermophilic “Bacillus sp. Ferdowsicous” isolated from Ferdows hot mineral spring in Iran: Purification and biochemical characterization. International Journal of Biological Macromolecules 2010, 46, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Satyanarayana, T. High maltose-forming, Ca2+-independent and acid stable α-amylase from a novel acidophilic bacterium, Bacillus acidicola. Biotechnol Lett 2010, 32, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Noorwez, S.M.; Satyanarayana, T. Production and partial characterization of thermostable and calcium-independent alpha-amylase of an extreme thermophile Bacillus thermooleovorans NP54. Lett Appl Microbiol 2000, 31, 378–384. [Google Scholar] [CrossRef]
- Tanaka, A.; Hoshino, E. Secondary calcium-binding parameter of Bacillus amyloliquefaciens alpha-amylase obtained from inhibition kinetics. J Biosci Bioeng 2003, 96, 262–267. [Google Scholar] [CrossRef]
- Mehta, D.; Satyanarayana, T. Biochemical and molecular characterization of recombinant acidic and thermostable raw-starch hydrolysing α-amylase from an extreme thermophile Geobacillus thermoleovorans. Journal of Molecular Catalysis B: Enzymatic 2013, 85-86, 229–238. [Google Scholar] [CrossRef]
- Liao, S.M.; Liang, G.; Zhu, J.; Lu, B.; Peng, L.X.; Wang, Q.Y.; Wei, Y.T.; Zhou, G.P.; Huang, R.B. Influence of Calcium Ions on the Thermal Characteristics of α-amylase from Thermophilic Anoxybacillus sp. GXS-BL. Protein Pept Lett 2019, 26, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Wyss, M. Engineering proteins for thermostability: the use of sequence alignments versus rational design and directed evolution. Current Opinion in Biotechnology 2001, 12, 371–375. [Google Scholar] [CrossRef]
- Arnold, F.H. Engineering proteins for nonnatural environments. The FASEB Journal 1993, 7, 744–749. [Google Scholar] [CrossRef]
- Bibi, M.; Yasmin, A.; Blanford, C.; Safdar, N. Production and characterization of a highly stable laccase from extreme thermophile Geobacillus stearothermophilus MB600 isolated from hot spring of Gilgit Balitistan (Pakistan). Journal of Taibah University for Science 2023, 17, 2268903. [Google Scholar] [CrossRef]
- Hoopes, J.T.; Dean, J.F.D. Ferroxidase activity in a laccase-like multicopper oxidase from Liriodendron tulipifera. Plant Physiology and Biochemistry 2004, 42, 27–33. [Google Scholar] [CrossRef]
- McCaig, B.C.; Meagher, R.B.; Dean, J.F. Gene structure and molecular analysis of the laccase-like multicopper oxidase (LMCO) gene family in Arabidopsis thaliana. Planta 2005, 221, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Leatham, G.F.; Stahmann, M.A. Studies on the Laccase of Lentinus edodes: Specificity, Localization and Association with the Development of Fruiting Bodies. Microbiology 1981, 125, 147–157. [Google Scholar] [CrossRef]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Nagai, M.; Kawata, M.; Watanabe, H.; Ogawa, M.; Saito, K.; Takesawa, T.; Kanda, K.; Sato, T. Important role of fungal intracellular laccase for melanin synthesis: purification and characterization of an intracellular laccase from Lentinula edodes fruit bodies. Microbiology 2003, 149, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, K.; Streibel, M.; Jahn, B.; Haase, G.; Brakhage, A.A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genetics and Biology 2003, 38, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Givaudan, A.; Effosse, A.; Faure, D.; Potier, P.; Bouillant, M.-L.; Bally, R. Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: Evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiology Letters 1993, 108, 205–210. [Google Scholar] [CrossRef]
- Malliga, P.; Uma, L.; Subramanian, G. Lignolytic activity of the cyanobacterium Anabaena azollae ML2 and the value of coir waste as a carrier for BGA biofertilizer. Microbios 86.
- Martins, L.O.; Soares, C.M.; Pereira, M.M.; Teixeira, M.; Costa, T.; Jones, G.H.; Henriques, A.O. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem 2002, 277, 18849–18859. [Google Scholar] [CrossRef]
- Arias, M.E.; Arenas, M.; Rodríguez, J.; Soliveri, J.; Ball Andrew, S.; Hernández, M. Kraft Pulp Biobleaching and Mediated Oxidation of a Nonphenolic Substrate by Laccase from Streptomyces cyaneus CECT 3335. Applied and Environmental Microbiology 2003, 69, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Endo, K.; Ito, M.; Tsujibo, H.; Miyamoto, K.; Inamori, Y. A thermostable laccase from Streptomyces lavendulae REN-7: purification, characterization, nucleotide sequence, and expression. Biosci Biotechnol Biochem 2003, 67, 2167–2175. [Google Scholar] [CrossRef]
- Solano, F.; Lucas-Elío, P.; Fernández, E.; Sanchez-Amat, A. Marinomonas mediterranea MMB-1 Transposon Mutagenesis: Isolation of a Multipotent Polyphenol Oxidase Mutant. Journal of Bacteriology 2000, 182, 3754–3760. [Google Scholar] [CrossRef]
- Dwivedi, U.N.; Singh, P.; Pandey, V.P.; Kumar, A. Structure–function relationship among bacterial, fungal and plant laccases. Journal of Molecular Catalysis B: Enzymatic 2011, 68, 117–128. [Google Scholar] [CrossRef]
- Held, C.; Kandelbauer, A.; Schroeder, M.; Cavaco-Paulo, A.; Guebitz, G.M. Biotransformation of phenolics with laccase containing bacterial spores. Environmental Chemistry Letters 2005, 3, 74–77. [Google Scholar] [CrossRef]
- Rosconi, F.; Fraguas, L.F.; Martínez-Drets, G.; Castro-Sowinski, S. Purification and characterization of a periplasmic laccase produced by Sinorhizobium meliloti. Enzyme and Microbial Technology 2005, 36, 800–807. [Google Scholar] [CrossRef]
- McMahon, A.M.; Doyle, E.M.; Brooks, S.; O’Connor, K.E. Biochemical characterisation of the coexisting tyrosinase and laccase in the soil bacterium Pseudomonas putida F6. Enzyme and Microbial Technology 2007, 40, 1435–1441. [Google Scholar] [CrossRef]
- Singh Arora, D.; Kumar Sharma, R. Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol 2010, 160, 1760–1788. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.E.; Andanson, J.M.; Verney, V. Improving laccase thermostability with aqueous natural deep eutectic solvents. Int J Biol Macromol 2020, 163, 919–926. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal Chim Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Messerschmidt, A.; Huber, R. The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin Modelling and structural relationships. European Journal of Biochemistry 1990, 187, 341–352. [Google Scholar] [CrossRef]
- Solomon, E.I.; Baldwin, M.J.; Lowery, M.D. Electronic structures of active sites in copper proteins: contributions to reactivity. Chemical Reviews 1992, 92, 521–542. [Google Scholar] [CrossRef]
- Palmieri, G.; Cennamo, G.; Faraco, V.; Amoresano, A.; Sannia, G.; Giardina, P. Atypical laccase isoenzymes from copper supplemented Pleurotus ostreatus cultures. Enzyme and Microbial Technology 2003, 33, 220–230. [Google Scholar] [CrossRef]
- Leontievsky, A.A.; Vares, T.; Lankinen, P.; Shergill, J.K.; Pozdnyakova, N.N.; Myasoedova, N.M.; Kalkkinen, N.; Golovleva, L.A.; Cammack, R.; Thurston, C.F.; et al. Blue and yellow laccases of ligninolytic fungi. FEMS Microbiol Lett 1997, 156, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Aza, P.; Camarero, S. Fungal Laccases: Fundamentals, Engineering and Classification Update. Biomolecules 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Piontek, K.; Antorini, M.; Choinowski, T. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-A resolution containing a full complement of coppers. J Biol Chem 2002, 277, 37663–37669. [Google Scholar] [CrossRef] [PubMed]
- Gianfreda, L.; Xu, F.; Bollag, J.-M. Laccases: A Useful Group of Oxidoreductive Enzymes. Bioremediation Journal 1999, 3, 1–26. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Purification of a thermostable laccase from Leucaena leucocephala using a copper alginate entrapment approach and the application of the laccase in dye decolorization. Process Biochemistry 2014, 49, 1196–1204. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Purification of a thermostable alkaline laccase from papaya (Carica papaya) using affinity chromatography. Int J Biol Macromol 2015, 72, 326–332. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Immobilization of papaya laccase in chitosan led to improved multipronged stability and dye discoloration. Int J Biol Macromol 2016, 86, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; O'Malley, D.M.; Whetten, R.; Sederoff, R.R. A Laccase Associated with Lignification in Loblolly Pine Xylem. Science 1993, 260, 672–674. [Google Scholar] [CrossRef]
- Liu, L.; Dean, J.F.D.; Friedman, W.E.; Eriksson, K.-E.L. A laccase-like phenoloxidase is correlated with lignin biosynthesis in Zinnia elegans stem tissues. The Plant Journal 1994, 6, 213–224. [Google Scholar] [CrossRef]
- Sterjiades, R.; Dean, J.F.; Eriksson, K.E. Laccase from Sycamore Maple (Acer pseudoplatanus) Polymerizes Monolignols. Plant Physiol 1992, 99, 1162–1168. [Google Scholar] [CrossRef]
- Ranocha, P.; McDougall, G.; Hawkins, S.; Sterjiades, R.; Borderies, G.; Stewart, D.; Cabanes-Macheteau, M.; Boudet, A.-M.; Goffner, D. Biochemical characterization, molecular cloning and expression of laccases – a divergent gene family – in poplar. European Journal of Biochemistry 1999, 259, 485–495. [Google Scholar] [CrossRef]
- Sato, Y.; Wuli, B.; Sederoff, R.; Whetten, R. Molecular Cloning and Expression of Eight Laccase cDNAs in Loblolly Pine (Pinus taeda)*. Journal of Plant Research 2001, 114, 147–155. [Google Scholar] [CrossRef]
- Ranocha, P.; Chabannes, M.; Chamayou, S.; Danoun, S.; Jauneau, A.; Boudet, A.M.; Goffner, D. Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar. Plant Physiol 2002, 129, 145–155. [Google Scholar] [CrossRef]
- Camarero, S.; Galletti, G.C.; Martínez, A.T. Preferential degradation of phenolic lignin units by two white rot fungi. Appl Environ Microbiol 1994, 60, 4509–4516. [Google Scholar] [CrossRef] [PubMed]
- Colaneri, M.J.; Vitali, J. Copper dynamics in doped metal-bis(histidine) complexes. J Phys Chem A 2014, 118, 4688–4694. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, R.; Behera, R.; Padhi, S.K.; Guru, R.K. Computational Phylogenetic Study and Data Mining Approach to Laccase Enzyme Sequences. 2013.
- Li, K.; Xu, F.; Eriksson, K.E. Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl Environ Microbiol 1999, 65, 2654–2660. [Google Scholar] [CrossRef] [PubMed]
- Gorbacheva, M.A.; Shumakovich, G.P.; Morozova, O.V.; Strel’tsov, A.V.; Zaitseva, E.A.; Shleev, S.V. Comparative study of biocatalytic reactions of high and low redox potential fungal and plant laccases in homogeneous and heterogeneous reactions. Moscow University Chemistry Bulletin 2008, 63, 94–98. [Google Scholar] [CrossRef]
- Madhavi; Lele, S. Laccase: Properties and Applications. BioResources 2009, 4. [Google Scholar] [CrossRef]
- Hakulinen, N.; Kiiskinen, L.-L.; Kruus, K.; Saloheimo, M.; Paananen, A.; Koivula, A.; Rouvinen, J. Crystal structure of a laccase from Melanocarpus albomyces with an intact trinuclear copper site. Nature Structural Biology 2002, 9, 601–605. [Google Scholar] [CrossRef]
- Awasthi, M.; Jaiswal, N.; Singh, S.; Pandey, V.P.; Dwivedi, U.N. Molecular docking and dynamics simulation analyses unraveling the differential enzymatic catalysis by plant and fungal laccases with respect to lignin biosynthesis and degradation. J Biomol Struct Dyn 2015, 33, 1835–1849. [Google Scholar] [CrossRef]
- Yun, J.; Kang, S.; Park, S.; Yoon, H.; Kim, M.-J.; Heu, S.; Ryu, S. Characterization of a Novel Amylolytic Enzyme Encoded by a Gene from a Soil-Derived Metagenomic Library. Applied and Environmental Microbiology 2004, 70, 7229–7235. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Satyanarayana, T. Bacterial and Archaeal α-Amylases: Diversity and Amelioration of the Desirable Characteristics for Industrial Applications. Frontiers in Microbiology 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Khozeymeh Nezhad, M.; Aghaei, H. Tosylated cloisite as a new heterofunctional carrier for covalent immobilization of lipase and its utilization for production of biodiesel from waste frying oil. Renewable Energy 2021, 164, 876–888. [Google Scholar] [CrossRef]
- Varriale, S.; Delorme, A.E.; Andanson, J.-M.; Devemy, J.; Malfreyt, P.; Verney, V.; Pezzella, C. Enhancing the Thermostability of Engineered Laccases in Aqueous Betaine-Based Natural Deep Eutectic Solvents. ACS Sustainable Chemistry & Engineering 2022, 10, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.E.; Andanson, J.-M.; Verney, V. Improving laccase thermostability with aqueous natural deep eutectic solvents. International Journal of Biological Macromolecules 2020, 163, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Pouyan, S.; Lagzian, M.; Sangtarash, M.H. Enhancing thermostabilization of a newly discovered α-amylase from Bacillus cereus GL96 by combining computer-aided directed evolution and site-directed mutagenesis. Int J Biol Macromol 2022, 197, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, L.; Xiao, Y.; Li, J.; Long, L.; Wang, F.; Zhang, S. Identification and thermoadaptation engineering of thermostability conferring residue of deep sea bacterial α-amylase AMY121. Journal of Molecular Catalysis B: Enzymatic 2016, 126, 56–63. [Google Scholar] [CrossRef]
- Yuan, S.; Yan, R.; Lin, B.; Li, R.; Ye, X. Improving thermostability of Bacillus amyloliquefaciens alpha-amylase by multipoint mutations. Biochem Biophys Res Commun 2023, 653, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, R.; He, B.; Du, Q. Improving the thermostability of alpha-amylase by combinatorial coevolving-site saturation mutagenesis. BMC Bioinformatics 2012, 13, 263. [Google Scholar] [CrossRef]
- Yang, Y.; Ghatge, S.; Hur, H.-G. Improvement of thermoalkaliphilic laccase (CtLac) by a directed evolution and application to lignin degradation. Applied Microbiology and Biotechnology 2023, 107, 273–286. [Google Scholar] [CrossRef]
- Li, Z.; Duan, X.; Chen, S.; Wu, J. Improving the reversibility of thermal denaturation and catalytic efficiency of Bacillus licheniformis α-amylase through stabilizing a long loop in domain B. PLOS ONE 2017, 12, e0173187. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Escribano, D.; Pliego-Magán, R.; de Salas, F.; Aza, P.; Gentili, P.; Ihalainen, P.; Levée, T.; Meyer, V.; Petit-Conil, M.; Tapin-Lingua, S.; et al. Tailor-made alkaliphilic and thermostable fungal laccases for industrial wood processing. Biotechnology for Biofuels and Bioproducts 2022, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, N.G.; Rahman, R.; Normi, Y.M.; Oslan, S.N.; Shariff, F.M.; Leow, T.C. Recent advances in simultaneous thermostability-activity improvement of industrial enzymes through structure modification. Int J Biol Macromol 2023, 232, 123440. [Google Scholar] [CrossRef] [PubMed]
- Declerck, N.; Machius, M.; Joyet, P.; Wiegand, G.; Huber, R.; Gaillardin, C. Hyperthermostabilization of Bacillus licheniformis α-amylase and modulation of its stability over a 50°C temperature range. Protein Engineering, Design and Selection 2003, 16, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Grzybowska, B.; Szweda, P.; Synowiecki, J. Cloning of the thermostable alpha-amylase gene from Pyrococcus woesei in Escherichia coli: isolation and some properties of the enzyme. Mol Biotechnol 2004, 26, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Kaveh, S.; Mohammad Bagher Hashemi, S. Structure-based modification of a-amylase by conventional and emerging technologies: Comparative study on the secondary structure, activity, thermal stability and amylolysis efficiency. Food Chem 2024, 437, 137903. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.S.O.; Pandey, A.; Rao, S.S.; Sukumaran, R.K. Cellulase production through solid-state tray fermentation, and its use for bioethanol from sorghum stover. Bioresource Technology 2017, 242, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Dubessay, P.; Record, E.; Audonnet, F.; Michaud, P. Recent advances in laccase activity assays: A crucial challenge for applications on complex substrates. Enzyme Microb Technol 2024, 173, 110373. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Pawar, R.G.; Rathod, V.K. Recent advances in enzyme extraction strategies: A comprehensive review. Int J Biol Macromol 2017, 101, 931–957. [Google Scholar] [CrossRef]
- Panwar, V.; Dey, B.; Sheikh, J.N.; Dutta, T. Thermostable bacterial laccase for sustainable dyeing using plant phenols. RSC Advances 2022, 12, 18168–18180. [Google Scholar] [CrossRef]
- Chai, K.P.; Othman, N.F.B.; Teh, A.-H.; Ho, K.L.; Chan, K.-G.; Shamsir, M.S.; Goh, K.M.; Ng, C.L. Crystal structure of Anoxybacillus α-amylase provides insights into maltose binding of a new glycosyl hydrolase subclass. Scientific Reports 2016, 6, 23126. [Google Scholar] [CrossRef]
- Abdella, M.A.A.; El-Sherbiny, G.M.; El-Shamy, A.R.; Atalla, S.M.M.; Ahmed, S.A. Statistical optimization of chemical modification of chitosan-magnetic nano-particles beads to promote Bacillus subtilis MK1 α-amylase immobilization and its application. Bulletin of the National Research Centre 2020, 44, 40. [Google Scholar] [CrossRef]
- Enguita, F.J.; Martins, L.O.; Henriques, A.O.; Carrondo, M.A. Crystal structure of a bacterial endospore coat component. A laccase with enhanced thermostability properties. J Biol Chem 2003, 278, 19416–19425. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cellular and Molecular Life Sciences 2015, 72, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Kherouf, M.; Habbeche, A.; Benamia, F.; Saoudi, B.; Kerouaz, B.; Ladjama, A. Statistical optimization of a novel extracellular alkaline and thermostable amylase production from thermophilic Actinomadura keratinilytica sp. Cpt29 and its potential application in detergent industry. Biocatalysis and Agricultural Biotechnology 2021, 35, 102068. [Google Scholar] [CrossRef]
- Timilsina, P.M.; Pandey, G.R.; Shrestha, A.; Ojha, M.; Karki, T.B. Purification and characterization of a noble thermostable algal starch liquefying alpha-amylase from Aeribacillus pallidus BTPS-2 isolated from geothermal spring of Nepal. Biotechnology Reports 2020, 28, e00551. [Google Scholar] [CrossRef] [PubMed]
- Slavić, M.Š.; Kojić, M.; Margetić, A.; Stanisavljević, N.; Gardijan, L.; Božić, N.; Vujčić, Z. Highly stable and versatile α-amylase from Anoxybacillus vranjensis ST4 suitable for various applications. International Journal of Biological Macromolecules 2023, 249, 126055. [Google Scholar] [CrossRef]
- Du, R.; Song, Q.; Zhang, Q.; Zhao, F.; Kim, R.C.; Zhou, Z.; Han, Y. Purification and characterization of novel thermostable and Ca-independent α-amylase produced by Bacillus amyloliquefaciens BH072. Int J Biol Macromol 2018, 115, 1151–1156. [Google Scholar] [CrossRef]
- Priyadarshini, S.; Pradhan, S.K.; Ray, P. Production, characterization and application of thermostable, alkaline α-amylase (AA11) from Bacillus cereus strain SP-CH11 isolated from Chilika Lake. Int J Biol Macromol 2020, 145, 804–812. [Google Scholar] [CrossRef]
- Afrisham, S.; Badoei-Dalfard, A.; Namaki-Shoushtari, A.; Karami, Z. Characterization of a thermostable, CaCl2-activated and raw-starch hydrolyzing alpha-amylase from Bacillus licheniformis AT70: Production under solid state fermentation by utilizing agricultural wastes. Journal of Molecular Catalysis B: Enzymatic 2016, 132, 98–106. [Google Scholar] [CrossRef]
- Hmidet, N.; El-Hadj Ali, N.; Haddar, A.; Kanoun, S.; Alya, S.-K.; Nasri, M. Alkaline proteases and thermostable α-amylase co-produced by Bacillus licheniformis NH1: Characterization and potential application as detergent additive. Biochemical Engineering Journal 2009, 47, 71–79. [Google Scholar] [CrossRef]
- Fincan, S.A.; Özdemir, S.; Karakaya, A.; Enez, B.; Mustafov, S.D.; Ulutaş, M.S.; Şen, F. Purification and characterization of thermostable α-amylase produced from Bacillus licheniformis So-B3 and its potential in hydrolyzing raw starch. Life Sciences 2021, 264, 118639. [Google Scholar] [CrossRef]
- Arikan, B. Highly thermostable, thermophilic, alkaline, SDS and chelator resistant amylase from a thermophilic Bacillus sp. isolate A3-15. Bioresour Technol 2008, 99, 3071–3076. [Google Scholar] [CrossRef]
- Gupta, N.; Paul, J.S.; Jadhav, S.K. Biovalorizing agro-waste 'de-oiled rice bran' for thermostable, alkalophilic and detergent stable α-amylase production with its application as laundry detergent additive and textile desizer. Int J Biol Macromol 2024, 256, 128470. [Google Scholar] [CrossRef]
- Prakash, B.; Vidyasagar, M.; Madhukumar, M.S.; Muralikrishna, G.; Sreeramulu, K. Production, purification, and characterization of two extremely halotolerant, thermostable, and alkali-stable α-amylases from Chromohalobacter sp. TVSP 101. Process Biochemistry 2009, 44, 210–215. [Google Scholar] [CrossRef]
- Shukla, R.J.; Singh, S.P. Characteristics and thermodynamics of α-amylase from thermophilic actinobacterium, Laceyella sacchari TSI-2. Process Biochemistry 2015, 50, 2128–2136. [Google Scholar] [CrossRef]
- Santorelli, M.; Maurelli, L.; Pocsfalvi, G.; Fiume, I.; Squillaci, G.; La Cara, F.; Del Monaco, G.; Morana, A. Isolation and characterisation of a novel alpha-amylase from the extreme haloarchaeon Haloterrigena turkmenica. Int J Biol Macromol 2016, 92, 174–184. [Google Scholar] [CrossRef]
- Michelin, M.; Silva, T.M.; Benassi, V.M.; Peixoto-Nogueira, S.C.; Moraes, L.A.B.; Leão, J.M.; Jorge, J.A.; Terenzi, H.F.; Polizeli, M.d.L.T.M. Purification and characterization of a thermostable α-amylase produced by the fungus Paecilomyces variotii. Carbohydrate Research 2010, 345, 2348–2353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-c.; Hu, H.-f.; Ma, J.-w.; Yan, Q.-j.; Liu, H.-j.; Jiang, Z.-q. A novel high maltose-forming α-amylase from Rhizomucor miehei and its application in the food industry. Food Chemistry 2020, 305, 125447. [Google Scholar] [CrossRef]
- Edoamodu, C.E.; Nwodo, U.U. Enterobacter sp. AI1 produced a thermo-acidic-tolerant laccase with a high potential for textile dyes degradation. Biocatalysis and Agricultural Biotechnology 2021, 38, 102206. [Google Scholar] [CrossRef]
- Allala, F.; Bouacem, K.; Boucherba, N.; Azzouz, Z.; Mechri, S.; Sahnoun, M.; Benallaoua, S.; Hacene, H.; Jaouadi, B.; Bouanane-Darenfed, A. Purification, biochemical, and molecular characterization of a novel extracellular thermostable and alkaline α-amylase from Tepidimonas fonticaldi strain HB23. Int J Biol Macromol 2019, 132, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Zhao, N.; Ma, J.W.; Liu, J.; Yan, Q.J.; Jiang, Z.Q. High-level expression of a novel α-amylase from Thermomyces dupontii in Pichia pastoris and its application in maltose syrup production. Int J Biol Macromol 2019, 127, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Dror, A.; Fishman, A. ENGINEERING NON-HEME MONO- AND DIOXYGENASES FOR BIOCATALYSIS. Computational and Structural Biotechnology Journal 2012, 2, e201209011. [Google Scholar] [CrossRef] [PubMed]
- Maphupha, M.; Juma, W.P.; de Koning, C.B.; Brady, D. A modern and practical laccase-catalysed route suitable for the synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles. RSC Adv 2018, 8, 39496–39510. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Couto, S.; Lardizábal, P.M.d. Laccases for Denim Bleaching: An Eco-Friendly Alternative. The Open Textile Journal 2012, 5, 1–7. [Google Scholar] [CrossRef]
- de Salas, F.; Pardo, I.; Salavagione, H.J.; Aza, P.; Amougi, E.; Vind, J.; Martínez, A.T.; Camarero, S. Advanced Synthesis of Conductive Polyaniline Using Laccase as Biocatalyst. PLoS One 2016, 11, e0164958. [Google Scholar] [CrossRef]
- Call, H.P.; Mücke, I. History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems (Lignozym®-process). Journal of Biotechnology 1997, 53, 163–202. [Google Scholar] [CrossRef]
- Cindy; Mendoza, D. M.; Konadu, K.T.; Ichinose, H.; Sasaki, K. Multiple laccase-mediator system treatments for carbonaceous matter degradation in double refractory gold ore. Hydrometallurgy 2023, 221, 106129. [Google Scholar] [CrossRef]
- Wellington, K.W.; Govindjee, V.P.; Steenkamp, P. A laccase-catalysed synthesis of triaminated cyclohexa-2,4-dienones from catechol. Journal of Catalysis 2018, 368, 306–314. [Google Scholar] [CrossRef]
- Hämäläinen, V.; Grönroos, T.; Suonpää, A.; Heikkilä, M.W.; Romein, B.; Ihalainen, P.; Malandra, S.; Birikh, K.R. Enzymatic Processes to Unlock the Lignin Value. Front Bioeng Biotechnol 2018, 6, 20. [Google Scholar] [CrossRef]
- Othman, A.M.; Elsayed, M.A.; Elshafei, A.M.; Hassan, M.M. Purification and biochemical characterization of two isolated laccase isoforms from Agaricus bisporus CU13 and their potency in dye decolorization. Int J Biol Macromol 2018, 113, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Mehandia, S.; Sharma, S.C.; Arya, S.K. Isolation and characterization of an alkali and thermostable laccase from a novel Alcaligenes faecalis and its application in decolorization of synthetic dyes. Biotechnology Reports 2020, 25, e00413. [Google Scholar] [CrossRef] [PubMed]
- Diamantidis, G.; Effosse, A.; Potier, P.; Bally, R. Purification and characterization of the first bacterial laccase in the rhizospheric bacterium Azospirillum lipoferum. Soil Biology and Biochemistry 2000, 32, 919–927. [Google Scholar] [CrossRef]
- Khan, S.I.; Sahinkaya, M.; Colak, D.N.; Zada, N.S.; Uzuner, U.; Belduz, A.O.; Çanakçi, S.; Khan, A.Z.; Khan, S.; Badshah, M.; et al. Production and characterization of novel thermostable CotA-laccase from Bacillus altitudinis SL7 and its application for lignin degradation. Enzyme and Microbial Technology 2024, 172, 110329. [Google Scholar] [CrossRef]
- Sondhi, S.; Kaur, R.; Madan, J. Purification and characterization of a novel white highly thermo stable laccase from a novel Bacillus sp. MSK-01 having potential to be used as anticancer agent. Int J Biol Macromol 2021, 170, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Leung, I.K.H. Novel Thermophilic Bacterial Laccase for the Degradation of Aromatic Organic Pollutants. Frontiers in Chemistry 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Ayothiraman, S.; Dhakshinamoorthy, V. Production of highly thermo-tolerant laccase from novel thermophilic bacterium Bacillus sp. PC-3 and its application in functionalization of chitosan film. J Biosci Bioeng 2019, 127, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Patel, A.K.; Singhania, R.R.; Tsai, C.H.; Chen, S.Y.; Chen, C.W.; Dong, C.D. Heterologous expression of bacterial CotA-laccase, characterization and its application for biodegradation of malachite green. Bioresour Technol 2021, 340, 125708. [Google Scholar] [CrossRef] [PubMed]
- Basheer, S.; Rashid, N.; Akram, M.S.; Akhtar, M. A highly stable laccase from Bacillus subtilis strain R5: gene cloning and characterization. Biosci Biotechnol Biochem 2019, 83, 436–445. [Google Scholar] [CrossRef]
- Xu, G.; Wang, J.; Yin, Q.; Fang, W.; Xiao, Y.; Fang, Z. Expression of a thermo- and alkali-philic fungal laccase in Pichia pastoris and its application. Protein Expr Purif 2019, 154, 16–24. [Google Scholar] [CrossRef]
- Ibrahim, V.; Mendoza, L.; Mamo, G.; Hatti-Kaul, R. Blue laccase from Galerina sp.: Properties and potential for Kraft lignin demethylation. Process Biochemistry 2011, 46, 379–384. [Google Scholar] [CrossRef]
- Murugesan, K.; Nam, I.-H.; Kim, Y.-M.; Chang, Y.-S. Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture. Enzyme and Microbial Technology 2007, 40, 1662–1672. [Google Scholar] [CrossRef]
- Alshiekheid, M.A.; Umar, A.; Ameen, F.; Alyahya, S.A.; Dufossé, L. Biodegradation of chromium by laccase action of Ganoderma multipileum. Journal of King Saud University - Science 2023, 35, 102948. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Guo, W.; Jia, L.; Fu, Y.; Gui, S.; Lu, F. Cloning, expression, and characterization of a thermostable and pH-stable laccase from Klebsiella pneumoniae and its application to dye decolorization. Process Biochemistry 2017, 53, 125–134. [Google Scholar] [CrossRef]
- Callejón, S.; Sendra, R.; Ferrer, S.; Pardo, I. Cloning and characterization of a new laccase from Lactobacillus plantarum J16 CECT 8944 catalyzing biogenic amines degradation. Applied Microbiology and Biotechnology 2016, 100, 3113–3124. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, A.B.; de Aquino Saraiva, R.; de Siqueira, V.M.; Yogui, G.T.; de Souza Bezerra, R.; de Assis, C.R.D.; Sousa, M.S.B.; de Souza Buarque, D. Shrimp laccase degrades polycyclic aromatic hydrocarbons from an oil spill disaster in Brazil: A tool for marine environmental bioremediation. Marine Pollution Bulletin 2023, 194, 115445. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Xu, W.; Zhang, W.; Guang, C.; Mu, W. Efficient removal of sulfonamides and tetracyclines residues by the laccase-mediator system employing a novel laccase from Lysinibacillus fusiformis. Journal of Environmental Chemical Engineering 2022, 10, 108809. [Google Scholar] [CrossRef]
- Liu, N.; Shen, S.; Jia, H.; Yang, B.; Guo, X.; Si, H.; Cao, Z.; Dong, J. Heterologous expression of Stlac2, a laccase isozyme of Setosphearia turcica, and the ability of decolorization of malachite green. Int J Biol Macromol 2019, 138, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, D.; Wu, Z.; Li, D.; Cai, Y.; Lu, Y.; Zhao, X.; Xue, H. Multiple Tolerance and Dye Decolorization Ability of a Novel Laccase Identified from Staphylococcus Haemolyticus. J Microbiol Biotechnol 2020, 30, 615–621. [Google Scholar] [CrossRef]
- Blánquez, A.; Ball, A.S.; González-Pérez, J.A.; Jiménez-Morillo, N.T.; González-Vila, F.; Arias, M.E.; Hernández, M. Laccase SilA from Streptomyces ipomoeae CECT 3341, a key enzyme for the degradation of lignin from agricultural residues? PLoS One 2017, 12, e0187649. [Google Scholar] [CrossRef]
- Brander, S.; Mikkelsen, J.D.; Kepp, K.P. TtMCO: A highly thermostable laccase-like multicopper oxidase from the thermophilic Thermobaculum terrenum. Journal of Molecular Catalysis B: Enzymatic 2015, 112, 59–65. [Google Scholar] [CrossRef]
- Navas, L.E.; Martínez, F.D.; Taverna, M.E.; Fetherolf, M.M.; Eltis, L.D.; Nicolau, V.; Estenoz, D.; Campos, E.; Benintende, G.B.; Berretta, M.F. A thermostable laccase from Thermus sp. 2.9 and its potential for delignification of Eucalyptus biomass. AMB Express 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.K.; Khatri, M.; Dhawaria, M.; Kurmi, A.; Pandey, D.; Ghosh, S.; Jain, S.l. Potential of Trametes maxima IIPLC-32 derived laccase for the detoxification of phenolic inhibitors in lignocellulosic biomass prehydrolysate. International Biodeterioration & Biodegradation 2018, 133, 1–8. [Google Scholar] [CrossRef]
- Zheng, F.; An, Q.; Meng, G.; Wu, X.J.; Dai, Y.C.; Si, J.; Cui, B.K. A novel laccase from white rot fungus Trametes orientalis: Purification, characterization, and application. Int J Biol Macromol 2017, 102, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Ai, M.Q.; Wang, F.F.; Huang, F. Purification and Characterization of a Thermostable Laccase from Trametes trogii and Its Ability in Modification of Kraft Lignin. J Microbiol Biotechnol 2015, 25, 1361–1370. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Immobilization of papaya laccase in chitosan led to improved multipronged stability and dye discoloration. International Journal of Biological Macromolecules 2016, 86, 288–295. [Google Scholar] [CrossRef]
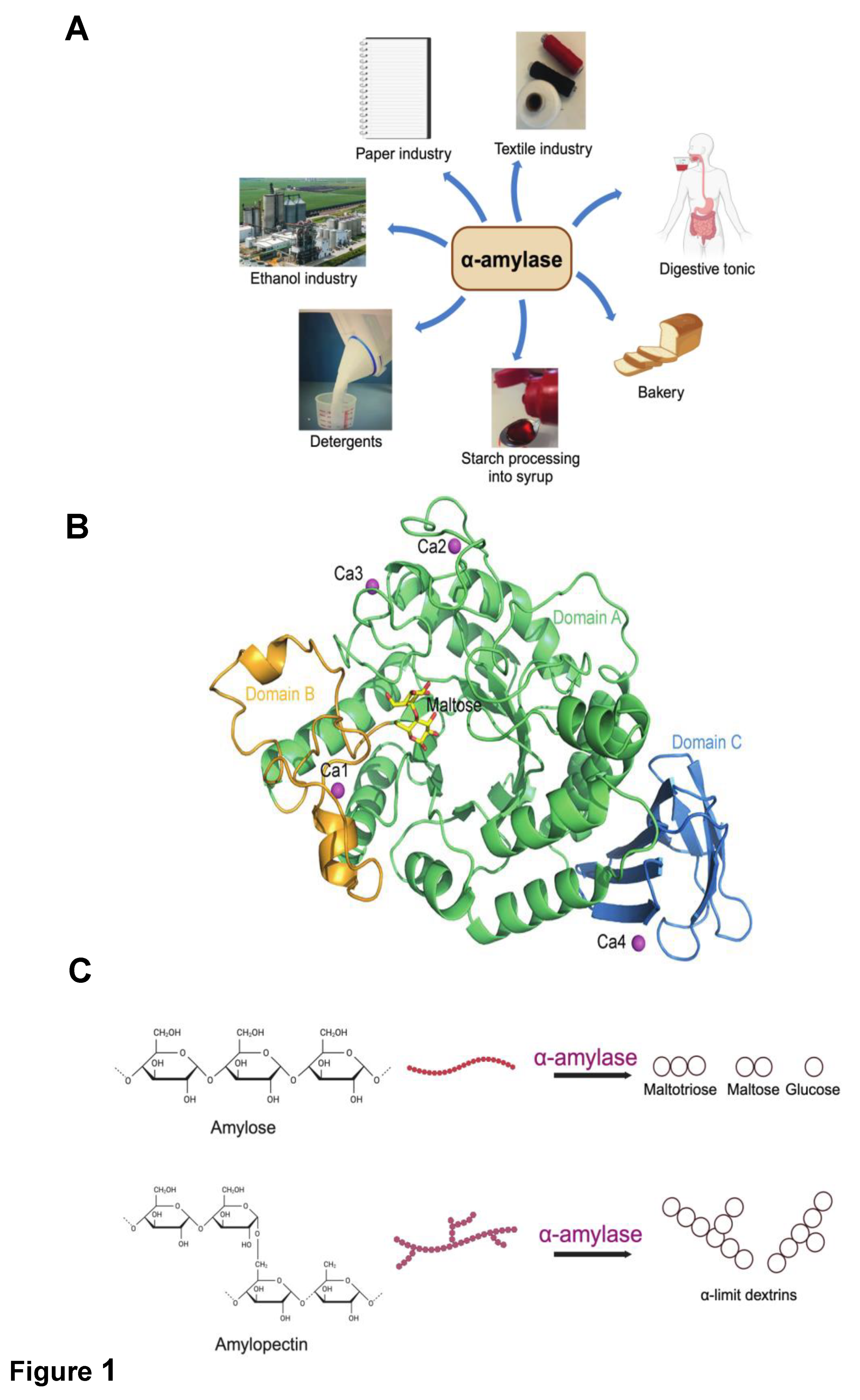
| Microbial source of α amylases |
Commercial Name of α- amylase |
Manufacturer | Industrial applications |
|---|---|---|---|
| Aspergillus oryzae | Fructamyl® FHT | Erbslöh Geisenheim AG |
Beverage industry |
| Bacillus licheniformis | Liquozyme® SC DC | Novozymes | Liquefaction for ethanol production |
| Bacillus amyloliquefaciens | BAN® | Novozymes | Oat starch liquefaction |
| Bacillus licheniformis | Termamyl® | Novozymes | Adjunct liquefaction |
| Aspergillus oryzae | Fungamyl | Novozymes | Baking |
| Bacillus subtilis | Validase BAA | IMCD Germany | Food and Feed |
| Bacillus subtilis | ZylozymeTM AA | Kemin Industries | Biofuel |
| Bacillus licheniformis | Bioconvert ALKA | Noor Enzymes | Biofuel |
| Genetically modified microorganism | Stainzyme® Plus Evity® 48 T | Novozymes | Detergent |
| Genetically modified microorganism | Aquazym® | Novozymes | Textile |
| Source of α-amylases | Optimum Temperature | Industrial applications | References |
|---|---|---|---|
| Actinomadura keratinilytica sp. Cpt29 | 70 °C | Laundry detergent additive | [138] |
| Aeribacillus pallidus BTPS- 2 | 70 °C | Starch liquefcation | [139] |
| Anoxybacillus vranjensis ST4 | 60–80 °C | Starch hydrolysis | [140] |
| Bacillus amyloliquefaciens BH072 | 60 °C | Food processing | [141] |
| Bacillus cereus SP- CH11 | 65 °C | Food processing | [142] |
| Bacillus licheniformis AT70 | 60 °C | Starch degradation | [143] |
| Bacillus licheniformis NH1 strain | 70 °C | Laundry detergent additive | [144] |
| Bacillus licheniformis So-B3 | 70 °C | Hydrolyzing raw starch | [145] |
| Bacillus sp. isolate A3-15 | 100 °C | Textile industry | [146] |
| Bacillus tequilensis TB5 | 60 °C | Textile de-sizer | [147] |
| Chromohalobacter sp. TVSP 101 | 65 °C | Starch hydrolysis | [148] |
| Geobacillus thermoleovorans | 80 °C | Improvement of washing efficiency of detergents | [149] |
| Germinated wheat seeds (Triticum aestivum) |
68 °C | Starch processing | [36] |
| Haloterrigena turkmenica | 55 °C | Agricultural residues treatment | [150] |
| Paecilomyces variotii | 60 °C | Starch degradation | [151] |
| Rhizomucor miehei | 75 °C | Food processing | [152] |
| Rhizopus oligosporus | 60 °C | Laundry detergent additive | [153] |
| Soybean (Glycine max) seeds | 75 °C | Starch liquefaction | [12] |
| Tepidimonas fonticaldi strain HB23 | 80 °C | Laundry detergent additive | [154] |
| Thermomyces dupontii | 60 °C | Maltose syrup production | [155] |
| Source of Laccases | Commercial Name of Laccase | Manufacturer | Industrial applications |
|---|---|---|---|
| Myceliophthora thermophila laccase expressed in Aspergillus oryzae | Denilite™ I Denilite™ II |
Novozymes [156] Novozymes |
|
| Zylite | Zytex Biotech Private Limited [156] | Textile | |
| Ecostone LC10 | AB Enzymes GmbH | ||
| IndiStar | Genencor International Inc. | ||
| Novoprime Base 268 | Novozymes [157] | ||
| Primagreen Ecofade LT100 | Genencor International Inc. [158] | ||
| Novozym® 51,003 | Novozymes [159] | ||
| White-rot fungi (Phanerochaete chrysosporium, Trametes versicolor) | Lignozym® Process Laccase Y120 Novozym® 51,003 |
IBB Netzwerk GmbH [160] Amano Enzyme [161] Novozymes [159] |
Paper Food processing |
| Filamentous fungi and yeasts | Suberase® | Novozymes [162] | Brewing |
| Genetically engineered bacterial laccase | MetZyme® LIGNO™ | MetZen [163] | Bio-refinery |
| Source of Laccases | Optimum Temperature | Industrial applications | References |
|---|---|---|---|
| Agaricus bisporus CU13 | 55 °C | Decolorization of synthetic dyes | [164] |
| Alcaligenes faecalis XF1 | 80 °C | Decolorization of synthetic dyes | [165] |
| Azospirillum lipoferum | 70 °C | Ecological role in the process of root colonization | [166] |
| Bacillus altitudinis SL7 | 55 °C | Bioremediation of lignin contaminated wastewater from pulp and paper industries | [167] |
| Bacillus sp. MSK-01 | 75 °C | Proposed as an anti-proliferative agent to cancer cells | [168] |
| Bacillus sp. PC- 3 | 60 °C | Functionalization of chitosan film for antimicrobial activity | [169,170] |
| Bacillus subtilis | 60 °C | Biodegradation of the fungicide | [171] |
| Bacillus subtilis strain R5 | 55 °C | Degradation of synthetic dyes | [172] |
|
Caldalkalibacillus thermarum TA2.A1 |
70 °C | Lignin degradation | [123] |
| Coprinopsis cinerea | 70 °C | Wastewater treatment | [173] |
| Enterobactersp. AI1 | 60 °C | Degradation and detoxification of synthetic dyes | [153] |
| Galerina sp. HC1 | 60 °C | Demethylation of lignin | [174] |
| Ganoderma lucidum KMK2 | 60 °C | Decolorization of reactive dyes | [175] |
| Ganoderma multipileum | 70 °C | Biodegradation of chromium | [176] |
| Geobacillus stearothermophilus MB600 | 90 ℃ | Biodegradation of pollutants | [70] |
| Geobacillus yumthangensis | 60 °C | Degradation of organic pollutants | [169] |
| Klebsiella pneumoniae | 70 ℃ | Decolorization of synthetic dyes | [177] |
| Lactobacillus plantarumJ16 CECT 8944 | 60 °C | Eliminating toxic compounds present in fermented food and beverages | [178] |
| Litopenaeus vannamei | >90 °C | Marine bioremediation | [179] |
| Lysinibacillus fusiformis | 80 °C | Removal of sulfonamides and tetracyclines residues | [180] |
| Setosphearia turcica | 60 °C | Decolorization of malachite green | [181] |
| Staphylococcus haemolyticus | 60 °C | Textile finishing | [182] |
| Streptomyces ipomoeae CECT 3341 | 60 ± 6 °C | Decolorization and detoxification of textile dyes | [183] |
| Thermobaculum terrenum | 80 °C | Protein engineering studies | [184] |
| Thermus sp. 2.9 | 70 °C | Delignification of Eucalyptus biomass | [185] |
| Trametes maxima IIPLC- 32 | 50–70 °C | Detoxification of phenolic inhibitors in lignocellulosic biomass | [186] |
| Trametes orientalis | 80 °C | Decolorization and bioremediation of synthetic dyes | [187] |
| Trametes trogii | 70 °C | Modification of kraft lignin | [188] |
| Leucaena leucocephala | 80 °C | Decolorization of synthetic dyes | [98,189] |
| Carica papaya | 70 °C | Dye decolorization | [98,189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




