Submitted:
22 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Conventional 2-Dimensional (2-D) Sonographic Examinations
2.3. Volume Acquisition
2.4. Determination of Power Doppler Indices
2.5. Procedure of Amniocentesis
2.6. Samples
2.7. Enzyme-linked Immunosorbent Assay (ELISA)
2.8. Data and Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huppertz, B. The anatomy of the normal placenta. J Clin Pathol. 2008, 61. [Google Scholar] [CrossRef]
- Oravecz O, Romero R, Tóth E, Kapitány J, Posta M, Gallo DM, et al. Placental galectins regulate innate and adaptive immune responses in pregnancy. Front Immunol. 2022, 13. [CrossRef]
- Balogh A, Toth E, Romero R, Parej K, Csala D, Szenasi NL, et al. Placental galectins are key players in regulating the maternal adaptive immune response. Front Immunol. 2019, 10. [CrossRef]
- Sammar M, Drobnjak T, Mandala M, Gizurarson S, Huppertz B, Meiri H. Galectin 13 (PP13) facilitates remodeling and structural stabilization of maternal vessels during pregnancy. Int J Mol Sci. 2019, 20. [CrossRef]
- Than NG, Pick E, Bellyei S, Szigeti A, Burger O, Berente Z, et al. Functional analyses of placental protein 13/galectin-13. Eur J Biochem. 2004, 271. [CrossRef]
- Bhati T, Ray A, Arora R, Siraj F, Parvez S, Rastogi S. Galectins are critical regulators of cytokine signalling at feto-maternal interface in infection-associated spontaneous preterm birth. Placenta. 2023, 138. [CrossRef]
- Vokalova L, Balogh A, Toth E, Van Breda S V., Schäfer G, Hoesli I, et al. Placental Protein 13 (Galectin-13) Polarizes Neutrophils Toward an Immune Regulatory Phenotype. Front Immunol. 2020 Feb;11. [CrossRef]
- Kliman HJ, Sammar M, Grimpel YI, Lynch SK, Milano KM, Pick E, et al. Placental protein 13 and decidual zones of necrosis: An immunologic diversion that may be linked to preeclampsia. Reproductive Sciences. 2012, 19. [CrossRef]
- Huppertz B, Sammar M, Chefetz I, Neumaier-Wagner P, Bartz C, Meiri H. Longitudinal determination of serum placental protein 13 during development of preeclampsia. Fetal Diagn Ther. 2008, 24. [CrossRef]
- Kazatsker MM, Sharabi-Nov A, Meiri H, Sammour R, Sammar M. Augmented Placental Protein 13 in Placental-Associated Extracellular Vesicles in Term and Preterm Preeclampsia Is Further Elevated by Corticosteroids. Int J Mol Sci. 2023, 24. [CrossRef]
- Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983, 4. [CrossRef]
- Suzuki T, Iizuka T, Kagami K, Matsumoto T, Yamazaki R, Daikoku T, et al. Laeverin/aminopeptidase Q induces indoleamine 2,3-dioxygenase-1 in human monocytes. iScience. 2023, 26. [CrossRef]
- Fujiwara H, Ono M, Sato Y, Imakawa K, Iizuka T, Kagami K, et al. Promoting roles of embryonic signals in embryo implantation and placentation in cooperation with endocrine and immune systems. Int J Mol Sci. 2020, 21. [CrossRef]
- Maruyama M, Hattori A, Goto Y, Ueda M, Maeda M, Fujiwara H, et al. Laeverin/aminopeptidase Q, a novel bestatin-sensitive leucine aminopeptidase belonging to the M1 family of aminopeptidases. Journal of Biological Chemistry. 2007, 282. [CrossRef]
- Fujiwara, H. Membrane-Bound Peptidases Regulate Human Extravillous Trophoblast Invasion. Placenta. 2007, 28(SUPPL.). [CrossRef]
- Nystad M, Sitras V, Larsen M, Acharya G. Placental expression of aminopeptidase-Q (laeverin) and its role in the pathophysiology of preeclampsia. Am J Obstet Gynecol. 2014, 211. [CrossRef]
- Sitras V, Paulssen RH, Grønaas H, Leirvik J, Hanssen TA, Vårtun Å, et al. Differential Placental Gene Expression in Severe Preeclampsia. Placenta. 2009, 30. [CrossRef]
- Nystad M, Sitras V, Flo K, Widnes C, Vårtun Å, Wilsgaard T, et al. Longitudinal reference ranges for maternal plasma laeverin, and its role as a potential biomarker of preeclampsia. BMC Pregnancy Childbirth. 2016, 16. [CrossRef]
- Nystad M, Sitras V, Nordbakken CV, Pedersen MI, Acharya G. Laeverin protein expression in normal and preeclamptic placentas using tissue microarray analysis. Acta Obstet Gynecol Scand. 2018, 97. [CrossRef]
- Fujiwara H, Higuchi T, Yamada S, Hirano T, Sato Y, Nishioka Y, et al. Human extravillous trophoblasts express laeverin, a novel protein that belongs to membrane-bound gluzincin metallopeptidases. Biochem Biophys Res Commun. 2004, 313, 962–968.
- Pihl K, Sørensen S, Nystad M, Acharya G, Jørgensen FS. Maternal serum laeverin (aminopeptidase Q) measured in the first trimester of pregnancy does not predict preeclampsia. Journal of Maternal-Fetal and Neonatal Medicine. 2019, 32. [CrossRef]
- Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol. 1985, 151:333–7.
- K. Joubert. Magyar születéskori testtömeg- és testhossz-standardok az 1990-96. évi országos élveszületési adatok alapján. Magy Noorv Lapja. 2000, 63:155–63.
- Suranyi A, Kozinszky Z, Molnar A, Nyari T, Bito T, Pal A. Placental three-dimensional power Doppler indices in mid-pregnancy and late pregnancy complicated by gestational diabetes mellitus. Prenat Diagn. 2013, 33, 952–958.
- Molnar A, Suranyi A, Nyari T, Nemeth G, Pal A. Examination of placental three-dimensional power Doppler indices and perinatal outcome in pregnancies complicated by intrauterine growth restriction. Int J Gynaecol Obstet. 2015, 129, 5–8.
- Lai PK, Wang YA, Welsh AW. Reproducibility of regional placental vascularity/perfusion measurement using 3D power Doppler. Ultrasound Obstet Gynecol. 2010, 36, 202–209.
- Vincze M, Sikovanyecz J, Molnár A, Földesi I, Surányi A, Várbíró S, et al. Predictive Capabilities of Human Leukocyte Antigen-G and Galectin-13 Levels in the Amniotic Fluid and Maternal Blood for the Pregnancy Outcome. Medicina (Lithuania). 2024, 60. [CrossRef]
- Nystad M, Sitras V, Acharya G. PP030. Transmission electron microscopy reveals leakage of laeverin into the villous capillaries and ectopic expression in the cytoplasm instead of cell membrane of the trophoblasts in preeclamptic placentas. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health. 2013, 3. [CrossRef]
- Sammar M, Nisemblat S, Fleischfarb Z, Golan A, Sadan O, Meiri H, et al. Placenta-bound and body fluid PP13 and its mRNA in normal pregnancy compared to preeclampsia, HELLP and preterm delivery. Placenta. 2011, 32(SUPPL. 1). [CrossRef]
- Huppertz, B. The Placenta: From Development to Disease. In: Kay H, Nelson M, Wang Y, editors., Wiley-Blac. Singapore: Wiley-Blackwell; 2011, pp 36–42.
- Horie A, Fujiwara H, Sato Y, Suginami K, Matsumoto H, Maruyama M, et al. Laeverin/aminopeptidase Q induces trophoblast invasion during human early placentation. Human Reproduction. 2012, 27. [CrossRef]
- Demir R, Seval Y, Huppertz B. Vasculogenesis and angiogenesis in the early human placenta. Acta Histochem. 2007, 109, 257–265.
- Nicolaides KH, Bindra R, Turan OM, Chefetz I, Sammar M, Meiri H, et al. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound in Obstetrics and Gynecology. 2006, 27. [CrossRef]
- Khalil A, Cowans NJ, Spencer K, Goichman S, Meiri H, Harrington K. First trimester maternal serum placental protein 13 for the prediction of pre-eclampsia in women with a priori high risk. Prenat Diagn. 2009, 29. [CrossRef]
- Vasilache I-A, Carauleanu A, Socolov D, Matasariu R, Pavaleanu I, Nemescu D. Predictive performance of first trimester serum galectin-13/PP-13 in preeclampsia screening: A systematic review and meta-analysis. Exp Ther Med. 2022, 23. [CrossRef]
- Suranyi A, Altorjay A, Kaiser L, Nyari T, Nemeth G. Evaluation of placental vascularization by three-dimensional ultrasound examination in second and third trimester of pregnancies complicated by chronic hypertension, gestational hypertension or pre-eclampsia. Pregnancy Hypertens. 2017 Apr;8:51–9.
- Dahlstrom B, Romundstad P, Oian P, Vatten LJ, Eskild A. Placenta weight in pre-eclampsia. Acta Obstet Gynecol Scand. 2008, 87, 608–611.
- Unverdorben L, Hüttenbrenner R, Knabl J, Jeschke U, Hutter S. Galectin-13/PP-13 expression in term placentas of gestational diabetes mellitus pregnancies. Placenta. 2015, 36. [CrossRef]
- Központi Statisztikai Hivatal (Central Statistical Office H. KSH database 22.1.1.7. Live births by main characteristics of mother and newborn 1980-2022] [Internet]. 2023.
| Maternal age (years)* | 34.53 ± 6.03 |
| Number of nulliparous women in the study** | 21 (33.9) |
| BMI at the time of genetical consultation (kg/m2)* | 27.14 ± 6.03 |
| Nuchal translucency at first trimester genetic sonography (mm) | 1.97 ± 0.61 |
| Gestational age at nuchal translucency (weeks) | 12.75 ± 0.58 |
| Gestational age at the time of amniocentesis (weeks)* | 18.22 ± 1.35 |
| Birthweight (grams)* | 3406.29 ± 559.44 |
| Gestational age at delivery (weeks)* | 39.04 ± 1.41 |
| Fetal biometry | |
| Gestational age at the time of amniocentesis (weeks) | 18.22 ± 1.35 |
| Head circumference (mm) | 151.02 ± 15.47 |
| Head circumference (percentile) | 51.23 ± 29.62 |
| Abdominal circumference (mm) | 127.86 ± 15.65 |
| Abdominal circumference (percentile) | 49.44 ± 28.30 |
| Femur length (mm) | 26.90 ± 4.70 |
| Femur length (percentile) | 54.66 ± 31.37 |
| Estimated Birthweight (grams) | 238.69 ± 69.82 |
| Estimated Birthweight (percentile) | 50.93 ± 26.62 |
| Placental sonography | |
| Placental volume (mm3) | 230.38 ± 93.96 |
| VI | 13.90 ± 5.09 |
| FI | 45.44 ± 24.02 |
| VFI | 7.77 ± 3.43 |
| Gal-13 concentration in serum (pg/mL) | 204.23 ± 171.34 |
| Gal-13 concentration in amniotic fluid (pg/mL) | 8.68 ± 9.85 |
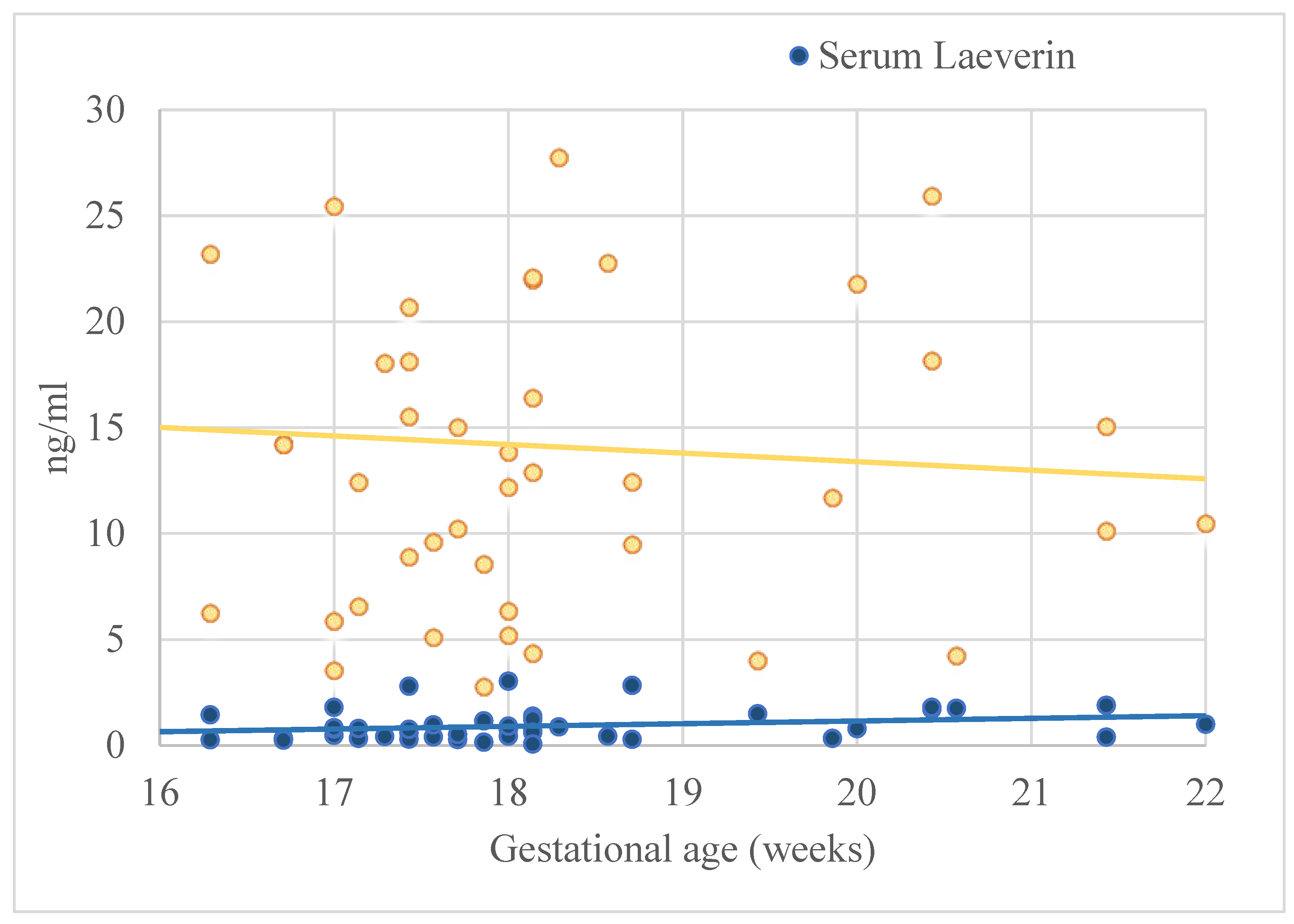
| Laeverin concentration in serum (ng/mL) | 0.93 ± 0.74 |
| Laeverin in amniotic fluid (ng/mL) | 14.11 ± 9.18 |
| Laeverin in serum | Laeverin in amniotic fluid | ||||||||
| Univariate linear regression | Multivariate linear regression | Univariate linear regression | Multivariate linear regression | ||||||
| β | CI | β | CI | β | CI | β | CI | ||
| Gal-13 in serum | -0.38* | -0.01- -0.01* | -0.31 | -0.01-0.01 | -0.15 | -0.06-0.02 | -0.11 | -0.06-0.03 | |
| Gal-13 in amniotic fluid | -0.32* | -0.04-0.01* | -0.36 | -0.06-0.01 | -0.13 | -0.44-0.19 | -0.16 | -0.53-0.23 | |
| Laeverin in serum | - | - | - | - | 0.03 | -3.59-4.26 | -0.01 | -4.29-4.20 | |
| Gal-13 in serum | Gal-13 in amniotic fluid | ||||||||
| Univariate linear regression | Multivariate linear regression | Univariate linear regression | Multivariate linear regression | ||||||
| β | CI | β | CI | β | CI | β | CI | ||
| Gal-13 in serum | - | - | - | - | 0.04 | -0.01-0.02 | -0.11 | -0.06-0.03 | |
| Laeverin serum level | Laeverin in amniotic fluid | |||||||
| Univariate linear regression | Multivariate linear regression | Univariate linear regression | Multivariate linear regression | |||||
| β | CI | β | CI | β | CI | β | CI | |
| Clinical and obstetrical characteristics | ||||||||
| Maternal age | -0.34* | -0.06-0.01* | -0.15 | -0.06-0.03 | -0.11 | -0.63-0.31 | -0.14 | -0.47-0.19 |
| Previous parity | -0.04 | -0.29-0.22 | 0.03 | -0.26-0.32 | 0.01 | -3.13-3.31 | 0.01 | -3.62-3.72 |
| BMI at the time of genetical consultation (kg/m2) | -0.19 | -0.06-0.01 | -0.14 | -0.05-0.02 | -0.18 | -0.71-0.19 | -0.18 | -0.75-0.23 |
| Birthweight (grams) | -0.01 | 0.01-0.01 | 0.02 | 0.01-0.01 | 0.01 | -0.01-0.01 | 0.01 | -0.01-0.01 |
| Birthweight (percentile) | -0.19 | -0.01-0.01 | -0.15 | -0.01-0.01 | -0.03 | -0.11-0.09 | -0.03 | -0.11-0.09 |
| GA at delivery (weeks) | 0.39* | 0.01-0.07* | 0.41* | 0.01-0.07* | 0.04 | -0.36-0.47 | -0.15 | -0.47-0.19 |
| GA at the time of amniocentesis (weeks) | 0.24 | -0.01-0.04 | 0.17 | -0.01-0.04 | -0.06 | -0.35-0.24 | -0.15 | -0.47-0.19 |
| Fetal sonography at the time of amniocentesis | ||||||||
| Head circumference (mm) | 0.18 | -0.01-0.02 | -0.21 | -0.04-0.02 | 0.03 | -0.17-0.21 | -0.03 | -0.23-0.19 |
| Head circumference (percentile) | -0.32* | -0.01-0.01* | -0.15 | -0.01-0.01 | 0.19 | -0.04-0.16 | 0.21 | -0.04-0.17 |
| Abdominal circumference (mm) | 0.15 | -0.02-0.03 | -0.26 | -0.08-0.05 | 0.55* | 0.02-0.56** | 0.11 | -0.18-0.27 |
| Abdominal circumference (percentile) | -0.08 | -0.02-0.03 | -0.05 | -0.02-0.02 | -0.19 | -0.18-0.08 | -0.18 | -0.16-0.07 |
| Femur length (mm) | 0.24 | -0.02-0.09 | -0.12 | -0.16-0.13 | 0.20 | -0.23-0.76 | -0.11 | -0.66-0.38 |
| Femur length (percentile) | -0.07 | -0.01-0.01 | -0.07 | -0.01-0.01 | 0.29 | -0.02-0.15 | 0.16 | -0.04-0.11 |
| Estimated fetal weight (grams) | 0.15 | -0.01-0.01 | -0.51 | -0.03-0.02 | 0.48* | 0.01-0.68* | 0.22 | -0.03-0.07 |
| Estimated fetal weight (percentile) | -0.14 | -0.02-0.01 | -0.08 | -0.02-0.02 | 0.09 | -0.11-0.16 | 0.04 | -0.12-0.14 |
| Placental sonography at the time of amniocentesis | ||||||||
| Placental volume (mm3) | 0.06 | -0.01-0.01 | 0.17 | -0.01-0.01 | 0.32* | 0.01-0.46* | 0.42 | 0.01-0.07 |
| VI | 0.07 | -0.04-0.05 | 0.12 | -0.03-0.06 | 0.05 | -0.47-0.63 | 0.05 | -0.51-0.69 |
| FI | -0.14 | -0.01-0.01 | -0.14 | -0.01-0.01 | 0.01 | -0.10-0.10 | -0.01 | -0.11-0.11 |
| VFI | 0.05 | -0.06-0.08 | 0.12 | -0.05-0.10 | -0.06 | -1.06-0.74 | -0.03 | -1.05-0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





