1. Introduction/Aim
During pregnancy, the thyroid undergoes dynamic changes to meet the increased demand for thyroid hormones, crucial for maternal and fetal health [
1]. Adequate iodine intake is essential for thyroid hormone synthesis, particularly during this period of heightened metabolic demand [
1]. The first trimester is the period of organogenesis whereas the third trimester is characterized by rapid fetal growth and development [
1]. These periods represent critical phases, where maternal thyroid function must be finely regulated to support fetal neurodevelopment and metabolic requirements, respectively. Central sensitivity to thyroid hormones refers to the sensitivity of the hypothalamic-pituitary-thyroid (HPT) axis to changes in circulating free thyroxine (fT4)[
2]. The relationship between fT4 levels and iodine intake is complex. Recent research works conducted in China have honed on the Thyroid Feedback Quantile-based Index (TFQI; a calculated parameter which is used to assess central sensitivity to thyroid hormones) in pregnancy, aiming to understand gestational complications. In the context of pre-pregnancy obesity and gestational diabetes mellitus (GDM), TFQI showed contrasting associations between obese and non-obese women[
3]. TFQI has stood out as an independent risk factor for fetal macrosomia in euthyroid pregnant women[
4]. Elevated TFQI levels were associated with increased neonatal thyroid-stimulating hormone (TSH), suggesting a potential risk factor for congenital hypothyroidism[
5]. Additionally, elevated TFQI during the first half of pregnancy has been linked to a lowered risk of GDM, possibly indicating a protective effect against this complication[
2]. Nevertheless, the course of TFQI during the second and third trimester of pregnancy, to the best of our knowledge, has not been evaluated. The aim of the present study was to assess central sensitivity to thyroid hormones in all trimesters of pregnancy and to evaluate it against iodine intake, the latter being a crucial component of thyroid hormone production.
2. Materials & Methods
This was a retrospective study where 1102 blood and urine samples were collected from pregnant women (with a mean age
+SD of 30.4
+4.6 years) during singleton pregnancies, in the three trimesters of pregnancy and at two-months postpartum. Women with known/diagnosed thyroid disease were excluded. Specifically, TSH & fT4 (Elecsys electrochemiluminescence assays, Roche, Basel, Switzerland) and 24-hour urinary iodine excretion (UI; colorimetric assay implementing the Sandell-Kolthoff reaction [
6]) were measured in each trimester and at postpartum. The TFQI was calculated to assess central sensitivity to thyroid hormones as follows: TFQI =
cdfFT4 - (1 -
cdfTSH), where
cdf is the cumulative distribution function of the parameter [
2]. Positive TFQI values indicate lower sensitivity to thyroid hormones while negative values indicate higher sensitivity. For the statistical analysis of TFQI by each time period, analysis of variance (ANOVA; with Tukey’s/Kramer’s post-hoc testing) was used, while Pearson's correlation was used to assess TFQI versus UI.
3. Results
Thyrotropin varied by time, being lower in the first trimester (p < 0.002) (
Table), whereas FT4, TFQI and UI showed no significant change (
Table).
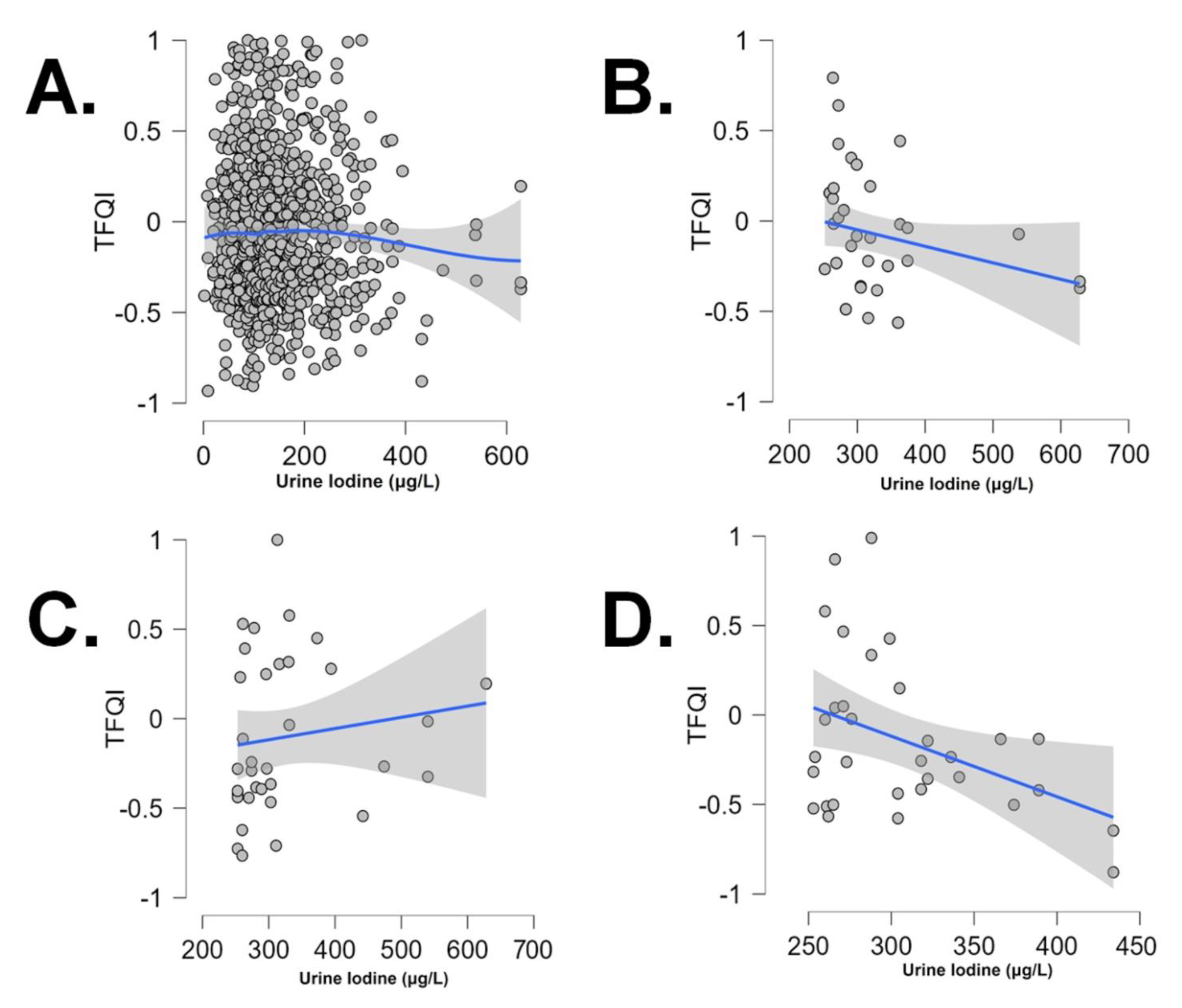
The TFQI vs UI correlation was negative and significant (Pearson r: +0.325, p: 0.04 and r: -0.399, p: 0.023), only for UI values over the threshold of 250 μg/L, in the first and third trimester, respectively (
Figure).
4. Discussion
TFQI is a new index reflecting central sensitivity to thyroid hormones. The latter has been associated with various health conditions, such as obesity, metabolic syndrome, impaired renal function, diabetes, and diabetes-related mortality [
7,
8,
9,
10]. Higher TFQI levels predict all-cause mortality in euthyroid individuals, independent of known risk factors [
11]. Elevated Premenopausal TFQI is linked to increased prevalence of type 2 diabetes, atrial fibrillation, ischemic heart disease, and hypertension [
12,
13]. Serum Adipocyte Fatty Acid-Binding Protein (A-FABP) levels correlate with TFQI, suggesting adipose tissue-HPT axis interaction [
14]. TFQI elevation is observed in Takotsubo syndrome [
15], and TFQI reductions are noted in schizophrenia subjects post-therapy initiation [
16]. Central sensitivity to thyroid hormones has a mitigated relationship with metabolic dysfunction-associated fatty liver disease (MAFLD) and dyslipidemia [
17]. Lower TFQI indicates higher sensitivity to thyroid hormones and, in our sample, in the first trimester (the critical period of organogenesis) and the third trimester (a period of intense fetal growth), it was inversely related to iodine intake, albeit over the normal threshold of UI values. These observed changes in TFQI may reflect different ways of central action of thyroid hormones, according to the phase of pregnancy.
In early pregnancy, before the fetal thyroid gland becomes fully functional, the fetus depends entirely on maternal transfer of thyroid hormones across the placenta to meet its thyroid hormone requirements. Maternal thyroid hormones, particularly thyroxine (T4), are transported across the placenta via passive diffusion, providing the fetus with a critical supply of thyroid hormones during early gestation when its own thyroid gland is still developing[
1]. However, as gestation progresses, typically by the end of the first trimester or early second trimester, the fetal thyroid gland becomes capable of synthesizing thyroid hormones independently. Fetal thyroid follicular cells begin to produce T4 and triiodothyronine (T3), which contribute to fetal circulating thyroid hormone levels[
1]. While fetal thyroid hormone production becomes increasingly important as pregnancy advances, maternal thyroid hormones continue to contribute to fetal thyroid hormone levels throughout gestation, albeit to a lesser extent. Maternal thyroid hormones still cross the placenta and supplement fetal thyroid hormone production, ensuring a continuous supply of thyroid hormones to support fetal growth and development.
TFQI was negatively correlated to iodine intake (as shown by UI) in the first and the third trimester, and over a normal threshold of UI values. Pregnancy induces physiological changes in thyroid hormone metabolism to meet the increased demand. The negative correlation between TFQI and UI levels during the first and third trimesters of pregnancy suggests potential underlying mechanisms and clinical implications. One possible explanation is that the increased demand for thyroid hormones during pregnancy, may enhance thyroid hormone sensitivity and iodine utilization as a compensatory mechanism. The negative correlation could be a reflection of this adaptive mechanism, where increased iodine leads to compensatory changes in thyroid hormone sensitivity (lower TFQI). The negative correlation between TFQI and UI could have significant implications for maternal and fetal health. Iodine deficiency and altered thyroid function during pregnancy have been linked to adverse outcomes, including impaired neurodevelopment, preterm birth, and miscarriage. The findings underscore the importance of adequate iodine supplementation during pregnancy. The World Health Organization recommends a daily iodine intake of 250 μg for pregnant women. It's plausible that the observed relationship between maternal TFQI and UI levels specifically during the third trimester could be influenced by the functional status of the fetal thyroid gland at this stage of pregnancy. In this context, the observed negative association between maternal TFQI (indicative of central sensitivity to thyroid hormone) and urine iodine levels during the third trimester may reflect the combined influence of maternal and fetal thyroid function, particularly over the normal threshold of 250 μg/L. At lower (normal and abnormally low) UI levels and across other time periods, the association of TFQI vs UI appears to be attenuated or absent.
Optimal maternal thyroid function contributes to a favorable intrauterine environment for fetal thyroid development and function. Adequate iodine intake, reflected by urine iodine levels, is crucial for supporting maternal and fetal thyroid hormone synthesis during pregnancy. The observed negative association between central sensitivity to thyroid hormone, as evaluated by the TFQI, and iodine nutritional status, as determined by UI, specifically during the first trimester and over UI levels of 250 μg/L, highlights the significance of optimal iodine intake in pregnancy-related thyroid function. Further research is warranted to elucidate the underlying mechanisms driving this association and to explore potential interventions aimed at optimizing iodine status and thyroid function in pregnant individuals, ultimately contributing to improved maternal and fetal health outcomes.
Disclosure of conflicts of interest:
The authors have no conflicting interests to declare.
Authors contributions:
Conceptualization, I.I. and E.K..; Methodology, I.I. , C.M. and E.K.; Software, I.I.; Validation, I.I., C.M., K.M. and E.K.; Formal Analysis, I.I. and E.K.; Investigation, M.A., E.M., C.K., E.V., I.M., K.M. and E.K.; Resources, K.M. and E.K; Data Curation, I.I. and M.A.; Writing – Original Draft Preparation, I.I. and E.K.; Writing – Review & Editing, I.I. and E.K.; Visualization, I.I.; Supervision, K.M. and E.K.; Project Administration, K.M. and E.K.
Funding
This research received no external funding.
References
- Ilias, I.; Milionis, C.; Koukkou, E. , Further understanding of thyroid function in pregnant women. Expert Rev Endocrinol Metab 2022, 17, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Laclaustra, M.; Moreno-Franco, B.; Lou-Bonafonte, J. M.; Mateo-Gallego, R.; Casasnovas, J. A.; Guallar-Castillon, P.; Cenarro, A.; Civeira, F. , Impaired Sensitivity to Thyroid Hormones Is Associated With Diabetes and Metabolic Syndrome. Diabetes Care 2019, 42, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhou, Y.; Liu, J.; Wang, Y.; Wang, G. , Maternal pre-pregnancy obesity modifies the association between first-trimester thyroid hormone sensitivity and gestational Diabetes Mellitus: a retrospective study from Northern China. Diabetol Metab Syndr 2023, 15, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, J.; Yuan, N.; Zhang, X. , Relationship between the Central and Peripheral Thyroid Sensitivity Indices and Fetal Macrosomia: A Cohort Study of Euthyroid Pregnant Women in China. Diagnostics 2023, 13, 2013. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, Y.; Zhang, S.-j.; Li, G.; Xiang, Y. T.; Zhang, W.-z.; Pan, W.-j.; Chen, W.-q.; Hao, Y.-t.; Ling, W.-h.; Liu, Z.-m. , Higher maternal thyroid resistance indices were associated with increased neonatal thyroid-stimulating hormone— analyses based on the Huizhou mother-infant cohort. Front. Endocrinol. 2022, 13, 937430. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J. T.; Crutchfield, H. E.; Gutekunst, R.; Dunn, A. D. , Two simple methods for measuring iodine in urine. Thyroid 1993, 3, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Corica, D.; Marzuillo, P.; Di Sessa, A.; Licenziati, M. R.; Faienza, M. F.; Calcaterra, V.; Franco, F.; Maltoni, G.; Valerio, G.; Wasniewska, M. , Sensitivity to thyroid hormones and reduced glomerular filtration in children and adolescents with overweight or obesity. Horm Res Paediatr 2023. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Chai, Y.; Zhang, Y.; Zhang, L.; Zhang, H. , Thyroid function and thyroid homeostasis parameters are associated with increased urinary albumin excretion in euthyroid individuals over 60 years old from NHANES. Front. Endocrinol. 2024, 14, 1285249. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, X.; Du, Z.; Tian, L. , Impaired Sensitivity to Thyroid Hormones is Associated with the Risk of Diabetic Nephropathy in Euthyroid Patients with Type 1 Diabetes Mellitus. Diabetes Metab Syndr Obes 2024, 17, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Jin, C.; Wang, H.; Lai, Y.; Li, J.; Shan, Z. , Subclinical hypothyroidism increases insulin resistance in normoglycemic people. Front. Endocrinol. 2023, 14, 1106968. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S. P.; Valdés, S.; Maldonado-Araque, C.; Lago, A.; Ocon, P.; Calle, A.; Castaño, L.; Delgado, E.; Menéndez, E.; Franch-Nadal, J.; Gaztambide, S.; Girbés, J.; Chaves, F.; Garcia-Serrano, S.; Garcia-Escobar, E.; Fernandez-García, J. C.; Olveira, G.; Colomo, N.; Rojo-Martínez, G. , Thyroid hormone resistance index and mortality in euthyroid subjects: Di@bet.es study. European Journal of Endocrinology 2022, 186, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, J.; An, Y.; Liu, J.; Wang, Y.; Wang, G.; Leng, S. , Impaired sensitivity to thyroid hormones is associated with hyperuricemia in a Chinese euthyroid population. Front. Endocrinol. 2023, 14, 1132543. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhao, X.; Xu, X.; Zhang, Z.; Zhang, X. , Association Between Thyroid Parameters and Subclinical Atherosclerosis in Hospitalised Euthyroid Patients with Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes 2023, 16, 3163–3171. [Google Scholar] [CrossRef] [PubMed]
- Polak, A. M.; Łebkowska, A.; Krentowska, A.; Buczyńska, A.; Adamski, M.; Krętowski, A. J.; Kowalska, I.; Adamska, A. , Elevated Serum Concentration of Adipocyte Fatty Acid-Binding Protein Correlates with the Markers of Abdominal Obesity Independently of Thyroid Hormones in Non-Obese Women with Polycystic Ovary Syndrome. JCM 2023, 12, 4610. [Google Scholar] [CrossRef] [PubMed]
- Aweimer, A.; El-Battrawy, I.; Akin, I.; Borggrefe, M.; Mügge, A.; Patsalis, P. C.; Urban, A.; Kummer, M.; Vasileva, S.; Stachon, A.; Hering, S.; Dietrich, J. W. , Abnormal thyroid function is common in takotsubo syndrome and depends on two distinct mechanisms: results of a multicentre observational study. Journal of Internal Medicine 2021, 289, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guan, Q.; Shi, J.; Sun, J.; Wang, Q.; Yang, J.; Retnakaran, R.; Han, J.; Zhang, X.; Hao, W.; Huang, X.; Zhang, R.; Zhai, D.; Wen, S. W. , Impaired central set point of thyroid homeostasis during quetiapine treatment in the acute phase of schizophrenia. Schizophrenia Research 2022, 241, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xing, Y.; Nie, Q.; Li, Z.; Meng, C.; Ma, H. , Association Between Sensitivity to Thyroid Hormones and Metabolic Dysfunction-Associated Fatty Liver Disease in Euthyroid Subjects: A Cross-Sectional Study. Diabetes Metab Syndr Obes 2023, 16, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).