1. Introduction
Large-cell neuroendocrine carcinoma (LCNEC), with high malignancy and poor prognosis, is a rare malignant tumor of the lungs. LCNEC has an incidence of approximately 3% and usually occurs in smokers and middle-aged and older male patients [
1]. The treatment of advanced LCNEC has no unified standard treatment plan at home and abroad at present, although the chemotherapy regimen of small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) is typically used in clinical treatment. Research has suggested that the biological behavior of LCNEC, such as the evident heterogeneity in morphology and biology, is similar to that of SCLC. Most scholars choose SCLC chemotherapy, and the American Society of Clinical Oncology (ASCO) guidelines recommend the EP chemotherapy regimen (a commonly used chemotherapy regimen for SCLC) [
2]. Existing treatment regimens are ineffective because there are no standardized treatments. Thus, there is an imperative need for further prospective multicenter clinical trials to explore effective treatment strategies for pulmonary LCNEC.
Neuroendocrine neoplasms are divided into neuroendocrine tumors (NET) and neuroendocrine carcinomas (NEC), including LCNEC. Treatment of NET involves somatostatin analogs, chemotherapy, systemic targeted agents, and peptide receptor- targeted therapies [
3]. NET can benefit from molecular targeted therapy [
4], for example, antiangiogenic tyrosine kinase inhibitors (solfantinib) or mTOR inhibitors (everolimus). Currently, chemotherapy is the most effective treatment for NEC. There was little benefit from targeted therapy alone for NEC [
5,
6]. Therefore, we explored a combination of treatments with different mechanisms of action for pulmonary LCNEC. Everolimus is an inhibitor of the mTOR pathway, which has a dual effect on both antitumor activity and enhanced tumor immunity [
7]. Studies have shown that everolimus has a definite effect on the treatment of NET of the gastrointestinal tract or pancreas (G1 and G2). However, the objective response rate (ORR) was approximately 2%–4.8%, and the progression-free survival (PFS) and overall survival (OS) were not significantly different from those of the placebo [
8,
9]. The mTOR pathway regulates cell proliferation through related signaling pathways [
10]. Studies have shown that mutations in the mTOR pathway are present in LCNEC. Currently, there are no specific recommendations for combination therapy with chemotherapy or targeted therapy for pulmonary LCNEC. This study aimed to adopt a combination treatment regimen with everolimus and EP chemotherapy. Our objectives include observing therapeutic efficacy, assessing the pros and cons of adverse reactions, and evaluating differences in survival and prognosis compared to EP chemotherapy alone. The study also aimed to explore an effective new treatment strategy for advanced pulmonary LCNEC.
2. Materials and Methods
2.1. Research Design
This was a multicenter, randomized, controlled, phase II clinical study of everolimus combined with first-line chemotherapy for the treatment of advanced pulmonary LCNEC. The main observation target was disease control rate (DCR), and the secondary observation targets were ORR, PFS, OS, adverse events (AEs), quality of life (QOL), and exploratory indicators. From June 1, 2020, to February 29, 2024, patients were randomly divided into experimental and control groups, with 20 patients in each group. During the three-year follow-up period, the experimental group received the mTOR inhibitor everolimus 5 mg daily plus intravenous infusion of the EP regimen chemotherapy every 3 weeks for 4–6 cycles, whereas the control group only received the EP regimen chemotherapy, and the infusion method was the same as that of the experimental group.
2.2. Patient Selection
All eligible patients were untreated and histologically and immunohistochemically confirmed to have advanced pulmonary LCNEC (Syn+, CgA+, and CD56+). All patients were enrolled at the Chongqing University Cancer Hospital or the top three hospitals in Chongqing.
Inclusion criteria: 1) Patients who volunteered to participate in the study and signed an informed consent form. 2) The Eastern Cooperative Oncology Group Performance Status (ECOG PS) was ≤2. 3) The expected survival time was greater than 3 months. 4) Patient over 18 years old with pathologically verified advanced or metastatic LCNEC. 5) The patient had at least one evaluable lesion (RECIST 1.1). 6) The patient had received no prior chemotherapy or other targeted therapies. 7) The major organ function within 7 days prior to treatment met the following criteria for routine blood tests (without blood transfusion within 14 days): hemoglobin ≥ 90 g/L, neutrophils ≥ 1.5×109/L, platelets ≥ 80×109/L; alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤ 2 upper limit of normal (ULN), or ≤ 5 ULN if accompanied by liver metastasis; total bilirubin (TBIL) ≤ 1.5 ULN; serum creatinine (Cr) ≤ 1.5 mg/dL/1.5 ULN or creatinine clearance rate (Ccr) ≥ 60 mL/min. Doppler ultrasound assessment indicated left ventricular ejection fraction (LVEF) ≥ the threshold of normal (50%). 8) Women of reproductive age who agreed to use contraception measures (such as intrauterine devices, contraceptives, or condoms) during the study period and within 6 months after the study ended, negative for serum or urine pregnancy test, and were not breastfeeding. Male patients also agreed to use contraceptive measures during the study period and within 6 months after the study ended.
Exclusion criteria: Patients with any of the following conditions were not enrolled in this study: 1) combined with other malignancies; 2) multiple factors that affect oral medication (e.g. inability to swallow, post-gastrointestinal resection, chronic diarrhea, etc.); 3) receiving other targeted drugs during treatment; 4) any severe and/or uncontrolled diseases, including active or uncontrolled severe infection (≥ Common Toxicity Criteria (CTC) AE grade 2 infection), liver cirrhosis, decompensated liver disease, active hepatitis or chronic hepatitis requiring antiviral treatment, a history of immunodeficiency, including HIV-positivity or other acquired and congenital immunodeficiency disorders, diabetes with poor glycemic control (fasting blood glucose (FBG) >10 mmol/L), poorly controlled hypertension (systolic pressure ≥ 150 mmHg, diastolic blood pressure ≥ 100 mmHg), myocardial infarction, severe arrhythmia, ≥ Grade 2 congestive heart failure (New York Heart Association rating), renal failure requiring hemodialysis or peritoneal dialysis, arteriovenous thrombosis events within 6 months, such as cerebrovascular accident, deep venous thrombosis and pulmonary embolism, pregnant or breastfeeding, unable to understand the content of the experiment and unable to cooperate and refused to sign the informed consent.
2.3. Observation of Research Results
2.3.1. Safety
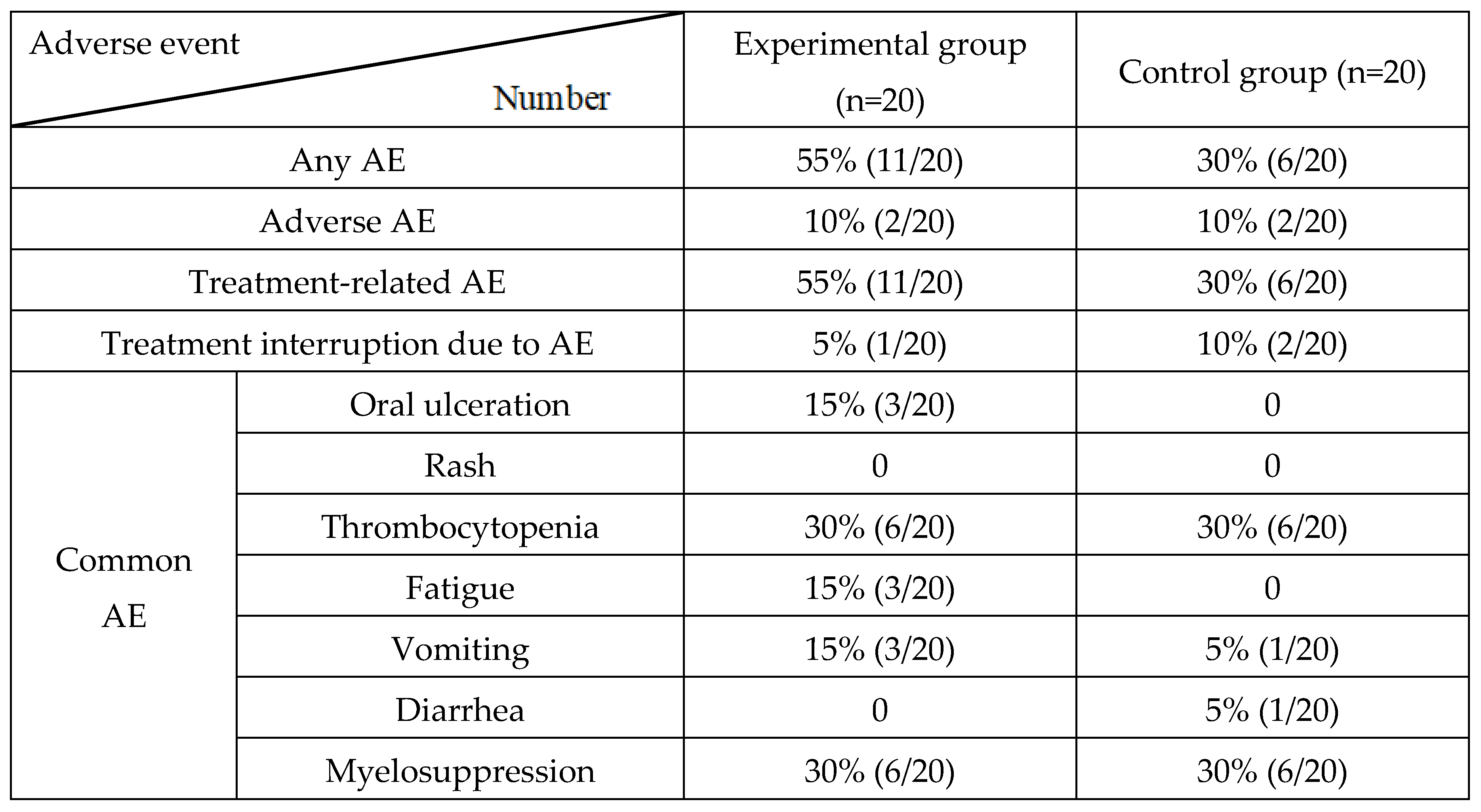
After screening and each treatment cycle, the patients underwent physical examination, and the following parameters were assessed: vital signs, ECOG PS, hematological analysis, biochemical analysis, and urinalysis. All AEs were reported throughout the study. The AEs were graded according to the National Cancer Institute General Toxicity Standard v5.0.
2.3.2. Validity
Tumor evaluation was performed at the end of each of the two cycles, including evaluation of the curative effect and adverse reactions. The optimal treatment response for each patient was assessed according to RECIST. Computed tomography (CT) or magnetic resonance imaging (MRI) was primarily used to evaluate the stage and effect of the study. However, other imaging methods have not been accepted yet.
2.3.3. Biomarker Analysis
During screening, tumor tissues were obtained for mutation analysis and formalin-fixed paraffin-embedded tissue sections were dissected and microdissected. DNA lysates were prepared from microcut tissues, gene mutations of ten patients were assessed by next-generation sequencing (NGS), and tumor microenvironment of one patient with superior effect was detected by multiple immunohistochemistry (mIHC).
2.3.4. Statistical Analysis
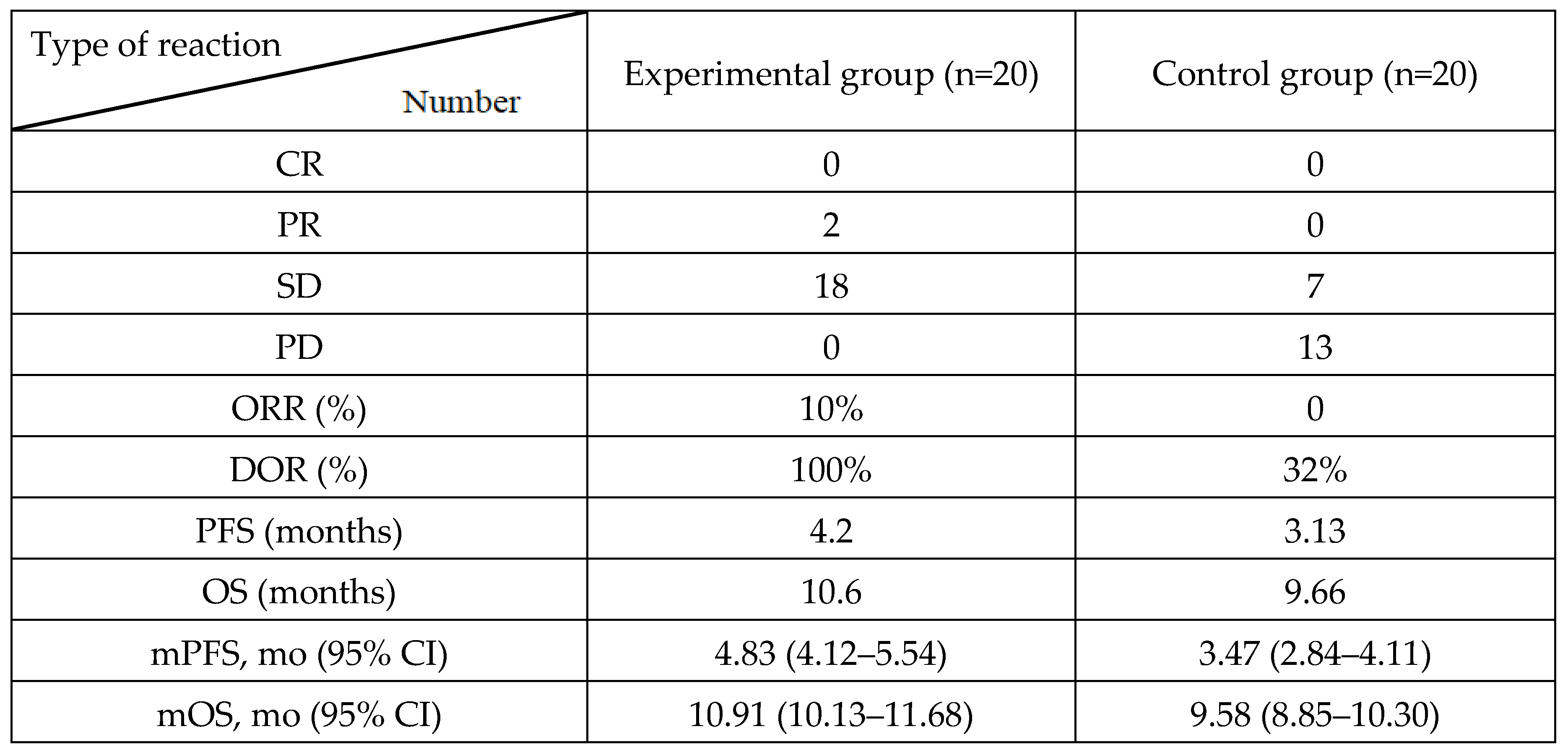
A multidisciplinary team evaluated each patient’s clinical status. According to the RECIST criteria 1.1, tumor response was assessed as Complete Response (CR), Partial Response (PR), Stable Disease (SD), or Progressive Disease (PD), and the ORR was the sum of CR and PR. DCR was defined as SD and ORR.
This study was designed for optimal efficacy; the primary endpoint was DCR, and the secondary endpoints were PFS, OS, and ORR. According to the literature, the DCR of EP in the treatment of LCNEC was 60%–75%. Taking the DCR of 68% as the historical data for calculation, assuming that DCR of LCNEC in the treatment of everolimus combined with EP was 100%, and assuming that the unilateral α=0.025. With an 80% confidence (β=0.2) and a 1:1 randomization ratio, it is expected that a total of 40 participants will be required to test for statistically significant differences. Forty participants were included in this study, reaching the minimum sample size standard. The sample size was calculated using R version 4.3.2.
A logistic regression model was used calculate odds ratios and
P value calculation to compare the ORR and DCR between the two treatment groups. PFS and OS were estimated using the Kaplan–Meier method, and changes in tumors from baseline were analyzed using GraphPad Prism 8. The results are presented as a waterfall diagram. A two-sided log-rank test was used to compare the treatment arms. The HR was estimated using a stratified Cox proportional hazards model. Statistical significance was set at P < 0.05. This trial was registered at
https://www.medicalresearch.org.cn/index (MR-50-23-022133).
4. Discussion
LCNEC is a rare, malignant lung tumor with no unified standard first-line treatment plan, and only evidence-based recommendations for small samples are available [
11,
12,
13,
14]. Therefore, more prospective, multicenter, large randomized controlled clinical trials are needed to explore the treatment strategies. This study provides new ideas and options for the clinical treatment of pulmonary LCNEC.
The EP chemo-regimen is the present standard regimen of first-line therapy recommended by ASCO for pulmonary SCLC [
2], but there is no standard therapeutic regimen for advanced pulmonary LCNEC. Based on the synergistic mechanism of chemotherapy and targeted therapy, this study explored whether chemotherapy combined with targeted therapy is effective for pulmonary LCNEC.
We enrolled 40 patients with pulmonary LCNEC who were divided into experimental and control groups receiving EP chemotherapy with or without everolimus. The results showed that the therapeutic effect of the combined group was better than that of the chemotherapy group, with ORR of 15% vs. 0, DCR of 100% vs. 32%, PFS of 4.20 vs. 3.13 months, and OS of 10.60 vs. 9.66 months. Tumor shrinkage was greater in the experimental group than in the control group. Although adverse reactions in the combined group were slightly higher than those in the chemotherapy group, the P values were not significantly different. These results indicate that the combination of chemotherapy and targeted therapy can benefit patients with LCNEC, and that adverse reactions can be controlled. Currently, there are several limited clinical trials on LCNEC with different therapeutic effects. A single-arm study conducted in 2017 showed that the everolimus +TC regimen (paclitaxel + carboplatin) was effective in 49 patients with LCNEC, with an ORR of 45%, DCR of 74%, PFS of 4.4 months, and OS of 9.9 months [
15]. The ORR in our study was not as high as that in the above study, but the DCR reached 100%, which was obvious superior to that in this trial. The PFS was similar, and the survival time was slightly longer than that in the study. Our study had a 100% DCR, which indicated that the EP regimen could EP regimen could stabilize more LCNEC tumors than the TC regimen, whereas the TC regimen may be superior to the EP regimen in terms of tumor regression. The PFS and OS rate were similar between the two trials. The reasons for the differences among different studies may be related to different chemotherapy regimens, TNM stages, ethnicities, tumor heterogeneity, and the length and effectiveness of the follow-up. Other trials including some patients with LCNEC, administered simple chemotherapy and showed different results. A phase II clinical trial enrolling in stage IV/IIIB LCNEC had a PFS of 5.2 months(95%CI, 3.1–6.6)and OS of 7.7 months(95%CI, 6.0–9.6)after receiving EP chemotherapy [
16]. In addition, LCNEC patients who were administered with " NSCLC-t" chemotherapy had PFS of 6.7 months (95% CI 1.08–2.56; p=0.020) and OS of 8.5 months (95% CI 7.0–9.9) [
17]. Another trial compared with the effects of cisplatin and carboplatin combined with Etoposide, with similar ORR (44% vs. 33%), DCR (75% vs. 70%), PFS (6.0 months vs 5.0 months), and OS (13.0 months vs 10 months) [
18]. These studies had a PFS of 5–6 months, which was longer than our control group result because the enrolled patients did not have simple LCNEC but had a different TNM stage or different pathological types. Interestingly, the OS in our study was superior to that in those trials, which indicated that EP combined with everolimus may provide useful benefits for patients with pulmonary LCNEC.
In this study, the treatment regimen adopted in the experimental and control groups was effective for pulmonary LCNEC; however, the efficacy in the experimental group was better than that in the control group. The PFS or OS in combined the treatment group was longer than those in the control group. There was a significant difference in PFS. Unfortunately, OS did not attain a significant value. It has been suggested that although chemotherapy combined with targeted therapy has better local control of patients' tumors, the survival benefit of patients is still limited owing to the obvious tumor heterogeneity of lung LCNEC. Therefore, clinical trials are required to explore more effective treatment strategies.
In our study, the combination of chemotherapy and targeted therapy, namely the combination of etoposide, cisplatin, and everolimus, improved the efficacy of pulmonary LCNEC treatments and prolonged the disease progression time of patients. These findings indicated that the combination of chemotherapy and targeted therapy had a synergistic effect. Cisplatin is an alkylating agent that damages the molecular structure of DNA in tumor cells and crosslinks its bases [
19],while etoposide is an inhibitor of topoisomerase II. This mainly changes the topological structure of DNA via catalysis and affects the S phase of the tumor cell cycle [
20]. In addition to directly killing tumor cells and breaking the DNA double strand, chemotherapeutic drugs can also release tumor antigens to antigen presenting cells (APCs) to further promote the activation of T cells.
MTOR, a serine/threonine (Ser/Thr) protein kinase, is upregulated in some human tumors. As an inhibitor of the mTOR pathway, everolimus binds to the intracellular protein FKBP12 to form the inhibitory complex mTORC1, which inhibits mTOR activity. This results in decreased activity of the transcription regulatory factor ribosomal S6 kinase 1 (S6K1) and the eukaryotic extension factor elF-4E binding protein (4E-BP). Therefore, translation and synthesis of proteins related to the cell cycle, angiogenesis, glycolysis, and other functions are disrupted, and the proliferation of tumor cells is inhibited [
21]. In addition to inhibiting cell proliferation, everolimus regulates and enhances CD8+ T cell-mediated tumor immunity [
22]. According to the above theories, chemotherapy in this regimen enables tumor cells to release tumor antigens and promotes the activation of the T-cell signaling pathway. Moreover, on the one hand, mTOR inhibitors inhibit the proliferation of tumor cells. However, they may also regulate the tumor microenvironment and activate CD8
+ T cells to mediate antitumor immunity.
Currently, targeted gene mutation therapy for lung LCNEC is still lacking, and it is necessary to identify effective targeted points. Mutations in various molecular markers of lung LCNEC have been detected by genome sequencing, including p53 mutations and loss of heterozygosity (LOH) mutations, with very high incidences of 72% and 82%, respectively [
23,
24]. Miyoshi et al. [
24] performed a sequencing study on lung LCNECs and found abnormal mutations in various genes and pathways, including
P53 (71%),
RB1(26%),
PI3K/AKT/mTOR (15%),
KRAS (6%),
FGFR1 (5%),
KIT (4%),
ERBB2 (4%),
72 HRAS (1%), and
EGFR (1%). However, therapeutically targeted drugs are not available for the treatment of LCNEC.
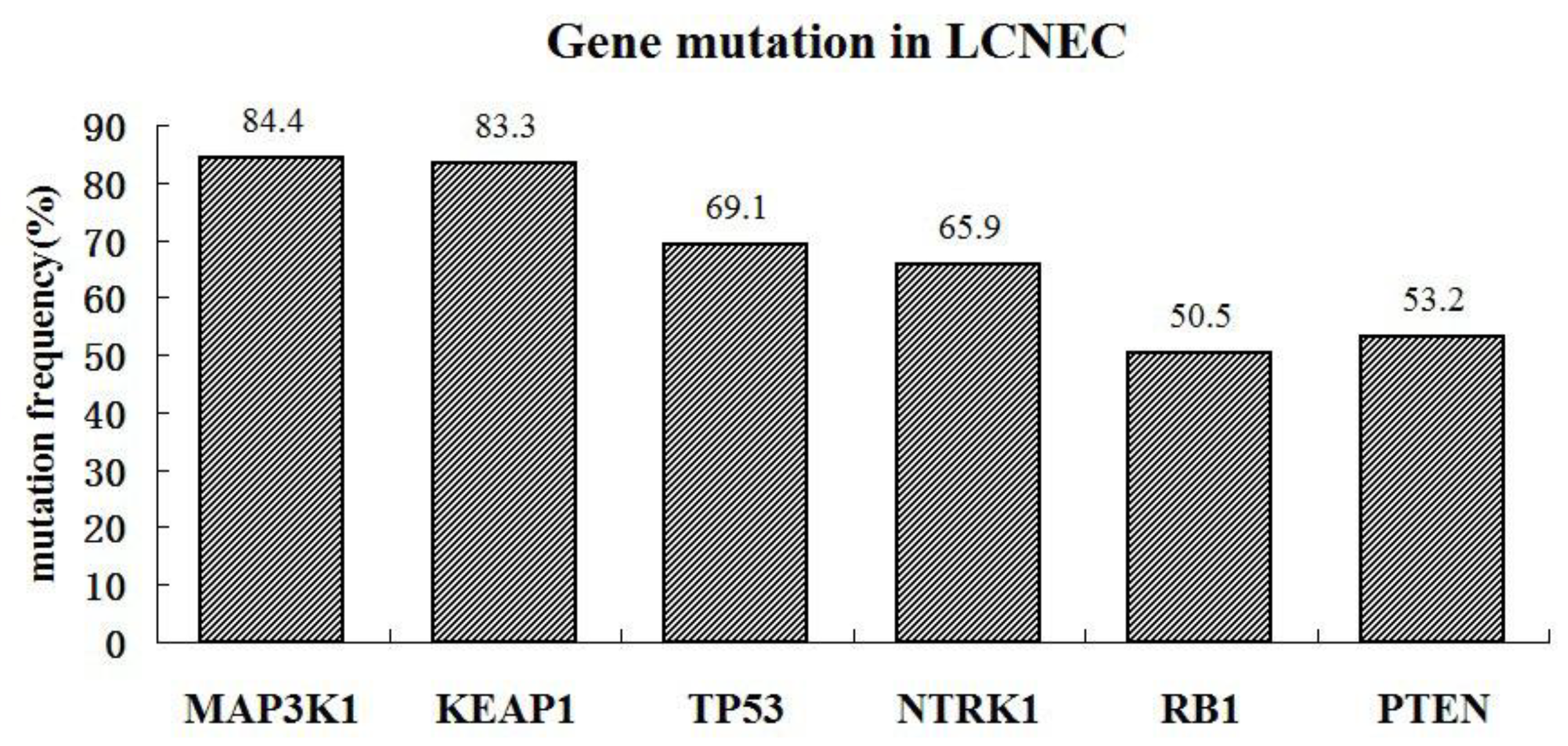
We detected gene mutations in tissue samples and found that TP53, RB1, MAP3K1, NTRK1, KEAP1, and PTEN genes had a high mutation abundance (> 50%). For these gene mutations, no corresponding targeted drugs are available in clinical practice, and only traditional chemotherapy can be used to control tumor growth. The TMB was also determined, and some patients had a high TMB (50%, 5/10). Moreover, TP53 positively correlated with immunity, whereas KEAP1, PTEN, NTRK1, and BRAF negatively correlated. These results suggest that patients with mutations in TMB or genes that are positively associated with immunity may benefit from immunotherapy.
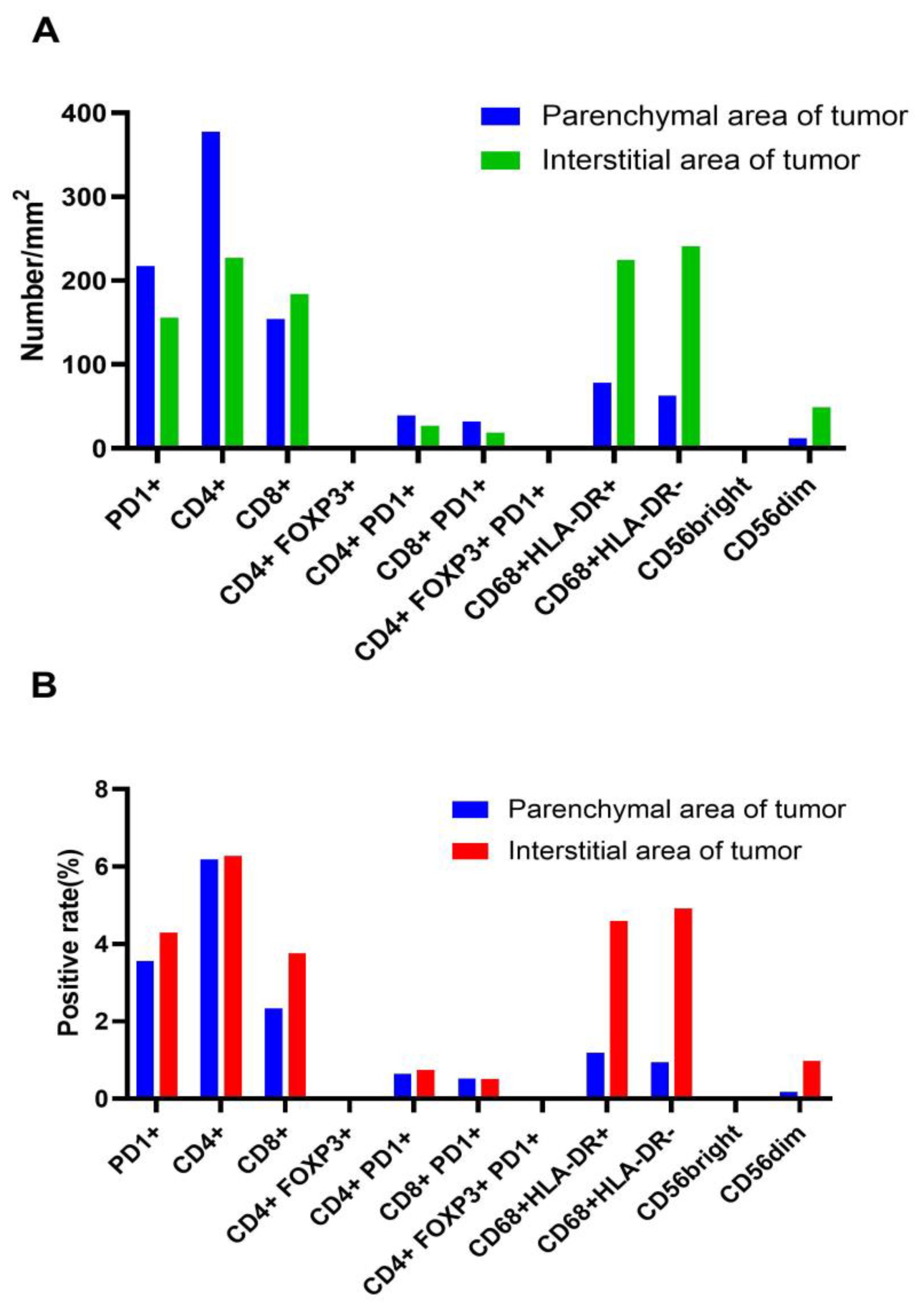
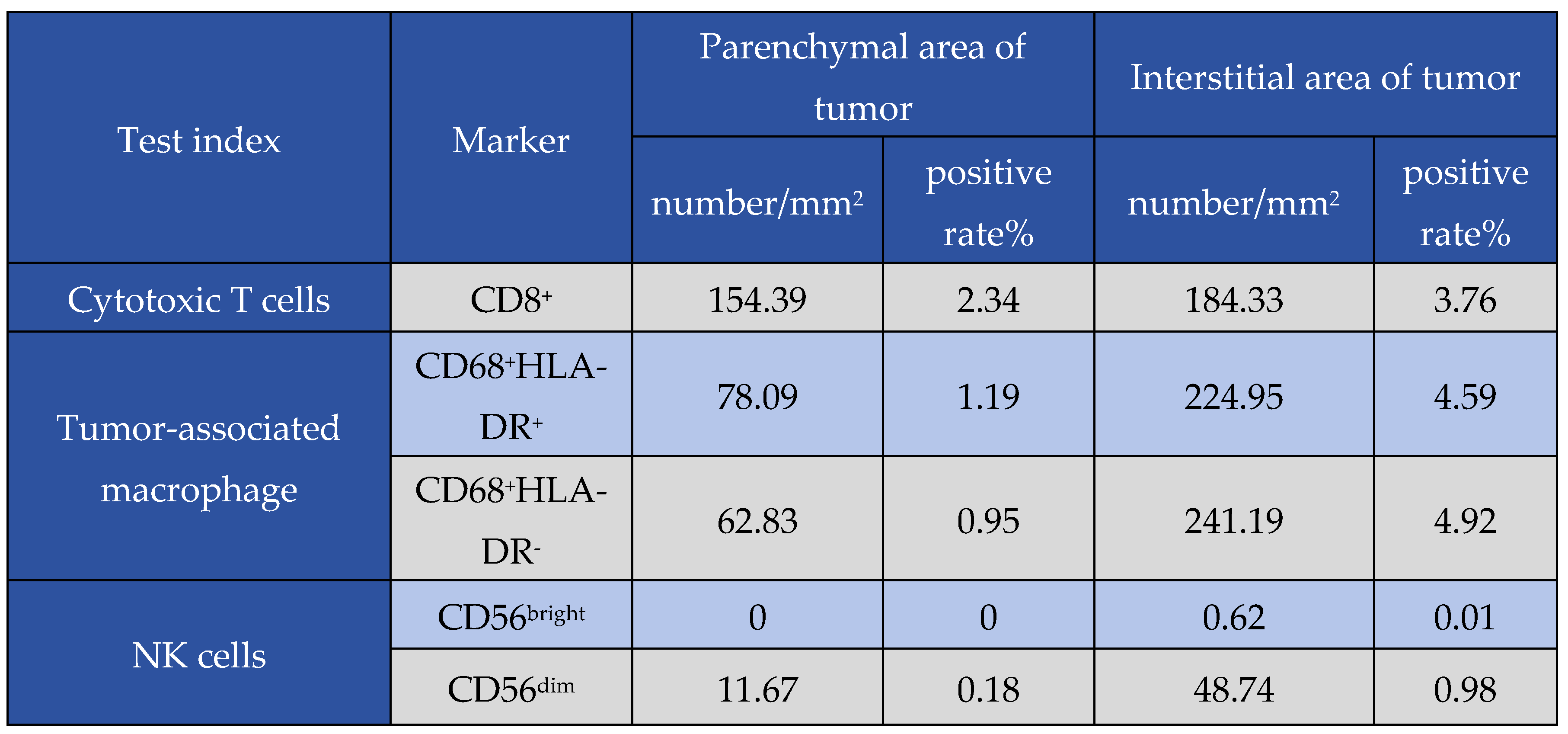
In addition, the tumor microenvironment was assessed in one patient in the combination group with an optimum therapy response. The patient showed a relatively high density of CD4+ and CD8+ T cells, a low number of depleted T cells, a slightly higher density of M1-type macrophages than M2-type macrophages, and a higher number of dim-type NK cells than bright-type NK cells. These results indicate a relatively low expression of immune exhaustion- and immunosuppression-related antigens in this patient, suggesting that the TME was an antitumor condition. Cells expressing FOXP3 and NK cells of the bright type suggest a relatively low state of immune depletion, and the patient's tumor microenvironment is an antitumor environment. The patient was treated with chemotherapy combined with everolimus, suggesting that the combined regimen improved the tumor microenvironment. This was beneficial for immune cells to play an antitumor role in order that the patient could achieve survival benefits. Owing to the limited number of specimens, the tumor microenvironment of this patient before treatment could not be determined. However, based on the treatment efficacy results, it is presumed that the tumor microenvironment is immunosuppressive. Because pulmonary LCNEC is a rare tumor, the efficacy in the study cases was also different. Thus, tumor microenvironment assessments were not performed in all cases. The results can be extended to more patients in the future to further analyze changes in the tumor microenvironment before and after treatment.
Abbreviations
AEs: adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ASCO, American Society of Clinical Oncology; Ccr, creatinine clearance rate; Cr, serum creatinine; DCR, disease control rate; DLTs, dose-limiting toxicities; 4E-BP, elF-4E binding protein; EP, etoposide and cisplatin; LCNEC, large cell neuroendocrine carcinoma; NEC, neuroendocrine carcinomas; NET, neuroendocrine tumors; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PD, disease progression; PFS, the progression-free survival; QOL, quality of life; S6K1, ribosomal S6 kinase 1; SAEs, serious adverse effects; SCLC, small cell lung cancer; TAMs, tumor-associated macrophages; TMB, tumor mutational burden; ULN, upper limit of normal.
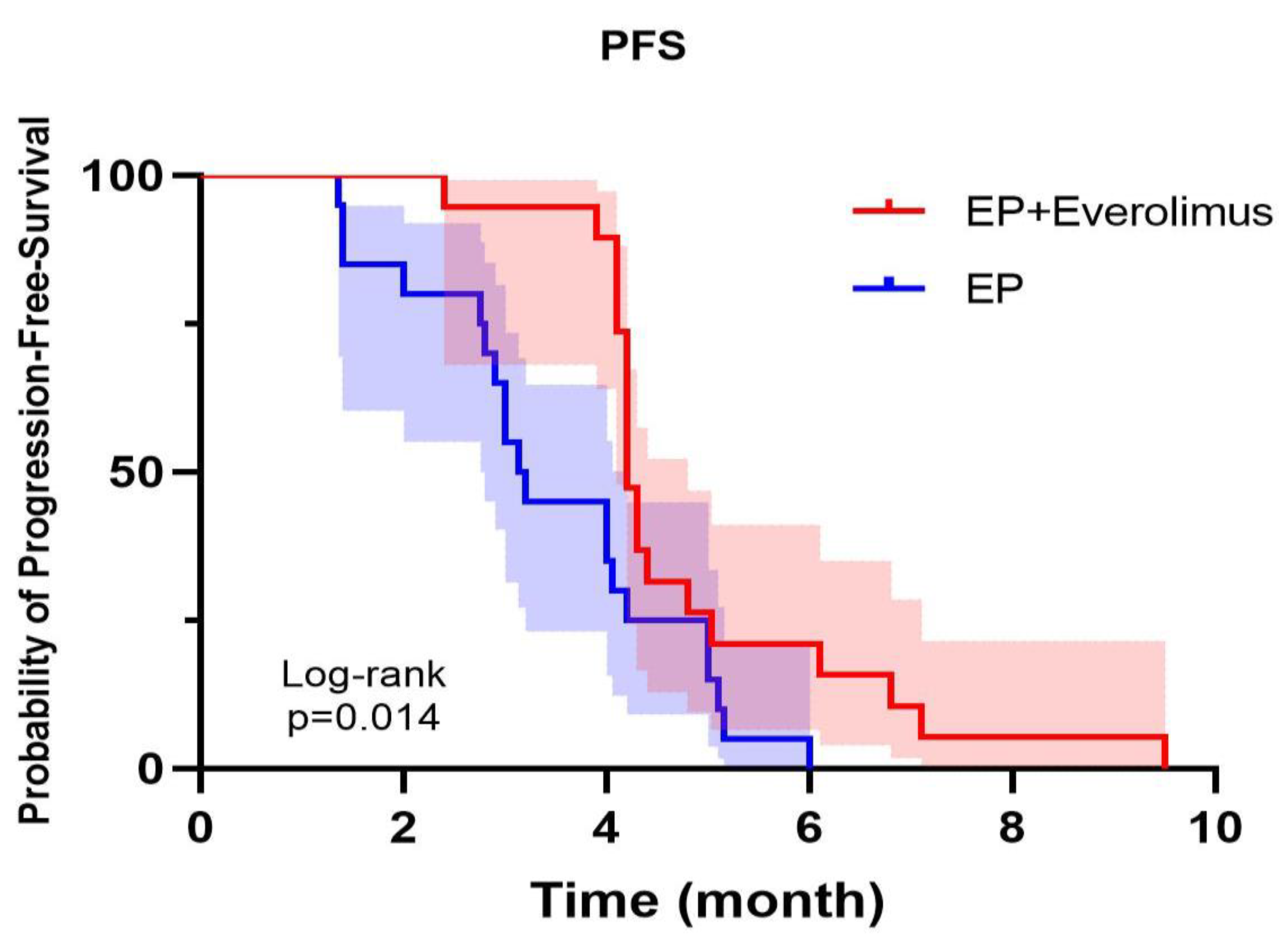
Figure 1.
In the Kaplan–Meier curve, the experiment group showes better progression-free survival than the control group (P=0.014).
Figure 1.
In the Kaplan–Meier curve, the experiment group showes better progression-free survival than the control group (P=0.014).
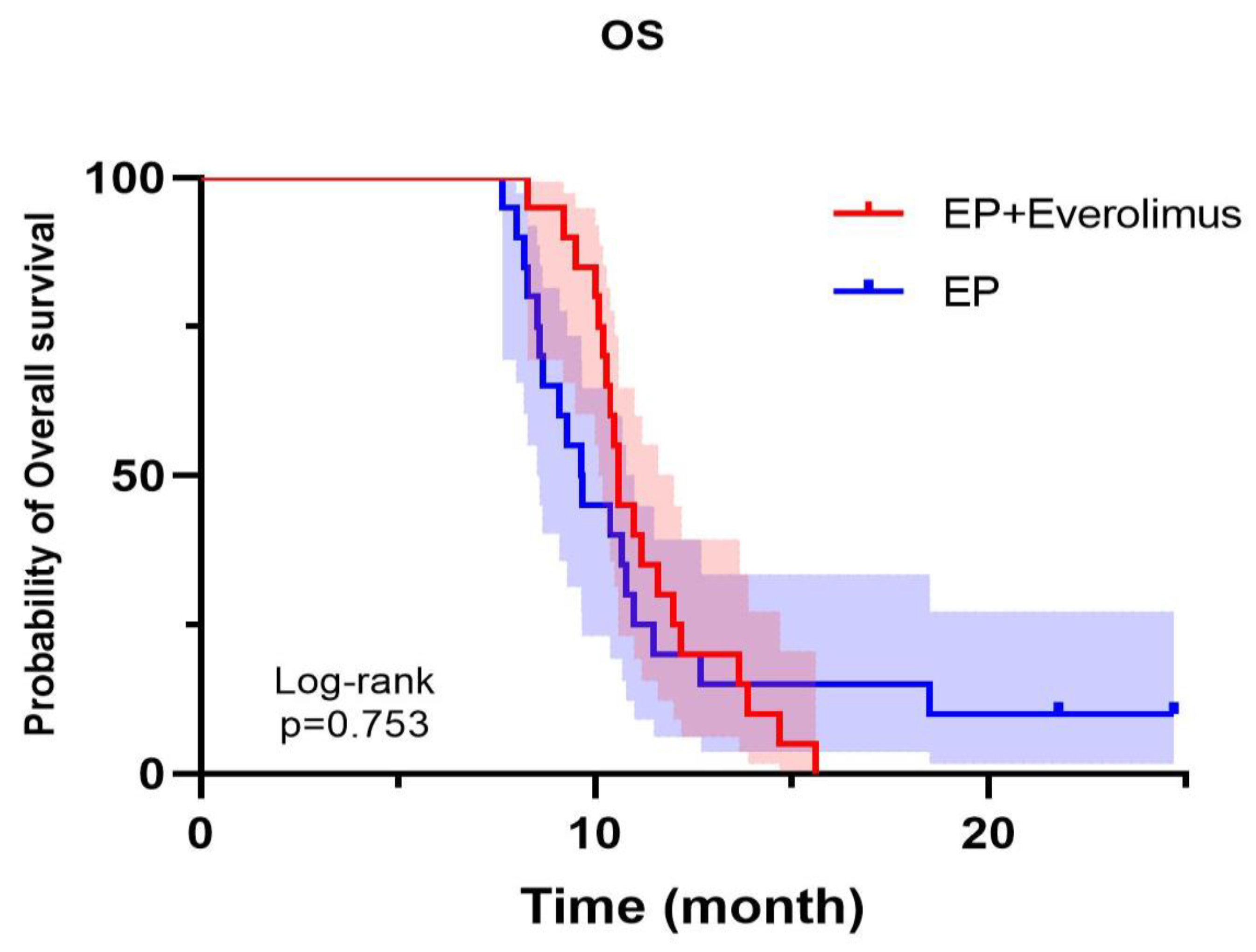
Figure 2.
Overall survival of patients in the experiment group (20 cases) is longer than that in the control group (17 cases) (P=0.0386).
Figure 2.
Overall survival of patients in the experiment group (20 cases) is longer than that in the control group (17 cases) (P=0.0386).
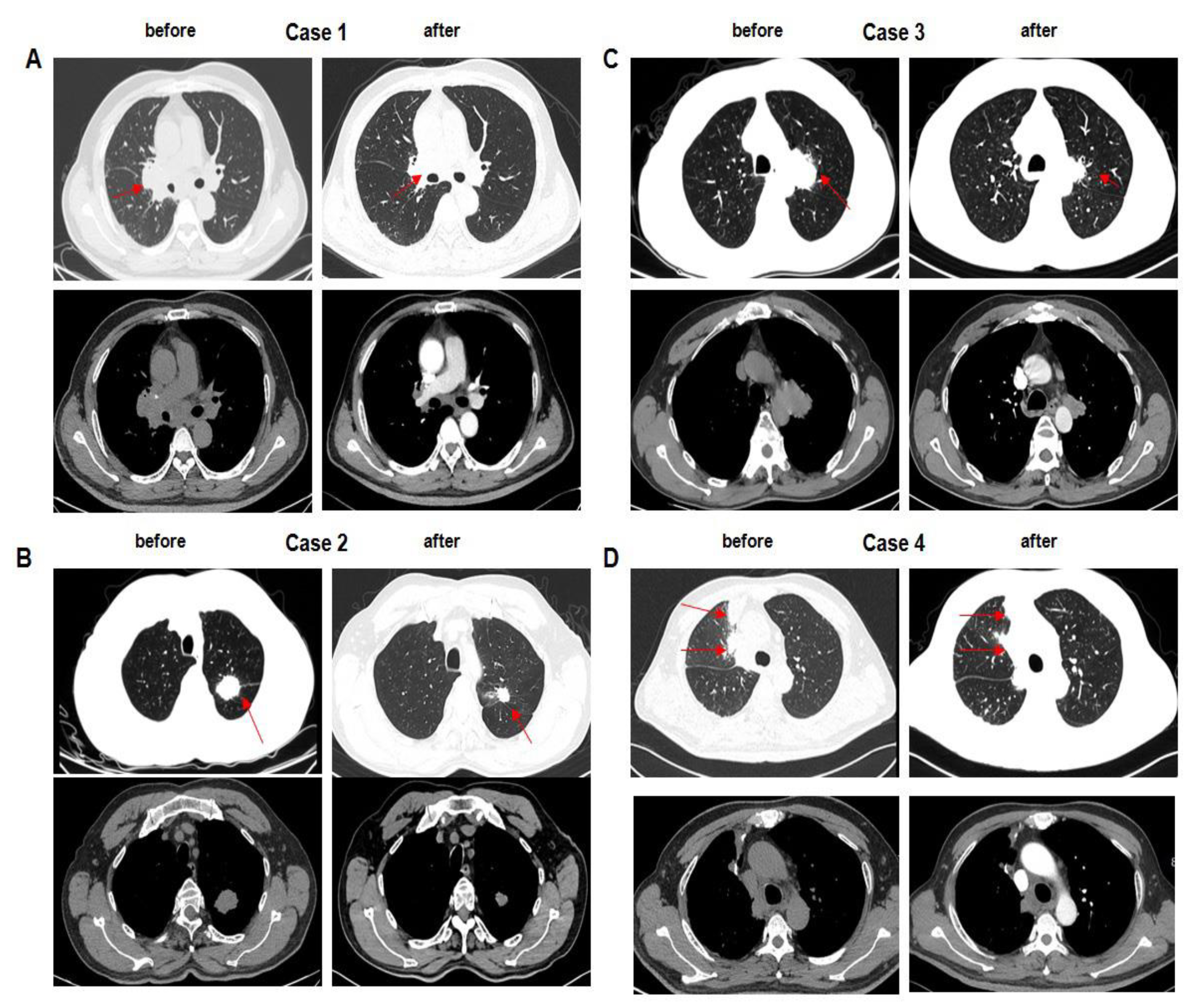
Figure 3.
A–C. Baseline of cases 1 to 3 before therapy in the experimental group is shown. After two cycles of EP chemotherapy combined with everolimus, tumor volume in the right lung obviously shrunk, especially up to 47% in case 1. D. Case 4 received only chemotherapy. After two cycles of EP chemotherapy, tumor volume in the right lung was becoming a little smaller than that before treatment. Compared to case 1 to case 3, the extent of tumor regression was limited.
Figure 3.
A–C. Baseline of cases 1 to 3 before therapy in the experimental group is shown. After two cycles of EP chemotherapy combined with everolimus, tumor volume in the right lung obviously shrunk, especially up to 47% in case 1. D. Case 4 received only chemotherapy. After two cycles of EP chemotherapy, tumor volume in the right lung was becoming a little smaller than that before treatment. Compared to case 1 to case 3, the extent of tumor regression was limited.
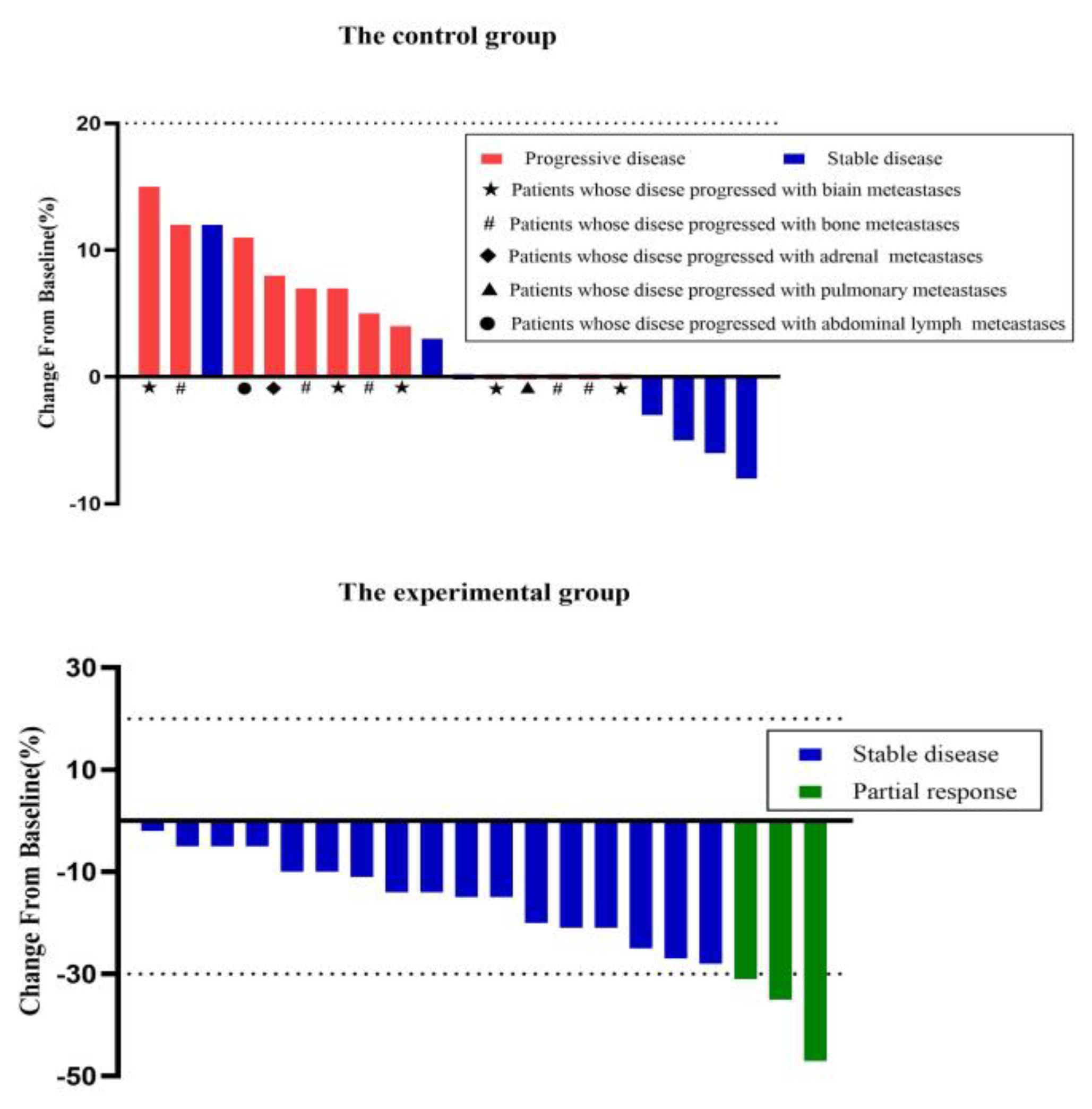
Figure 4.
The percentage of tumor change from baseline between the experimental group and control group. Tumor shrinkage of the experiment group was superior to the control group.
Figure 4.
The percentage of tumor change from baseline between the experimental group and control group. Tumor shrinkage of the experiment group was superior to the control group.
Figure 5.
Various gene mutation was found to occur in LCNEC. The genes of the most common mutation include MAP3K1, KEAP1, TP53, NTRK1, RB1, and PTEN.
Figure 5.
Various gene mutation was found to occur in LCNEC. The genes of the most common mutation include MAP3K1, KEAP1, TP53, NTRK1, RB1, and PTEN.
Figure 6.
The tumor microenvironment of one patient after combined regimen therapy, including PD1 cells, helper T cells (CD4+), cytotoxic T cells (CD8+), regulatory T cells (Tregs), depleted T cells, tumor-associated macrophages (TAMs), and NK cells, was detected via mIHC (multiple immunohistochemistry,) using in situ lung tumor tissue specimens.
Figure 6.
The tumor microenvironment of one patient after combined regimen therapy, including PD1 cells, helper T cells (CD4+), cytotoxic T cells (CD8+), regulatory T cells (Tregs), depleted T cells, tumor-associated macrophages (TAMs), and NK cells, was detected via mIHC (multiple immunohistochemistry,) using in situ lung tumor tissue specimens.
Table 1.
Baseline characteristics of patients.
Table 1.
Baseline characteristics of patients.
| Characteristics |
Experimental group |
Control group |
| Number of patients (n) |
20 |
20 |
| Mean age (years) |
55.8 ± 8.8 |
58.8 ± 6.3 |
| Gender |
Male |
16 (80%) |
19 (95%) |
| Female |
4 (20%) |
1 (5%) |
| Median time after diagnosis (months) |
2.34 |
2.63 |
| Pathological |
LCNEC |
LCNEC |
| Metastatic tissue sites |
brain, bone, adrenal, lymph, pulmonary, abdominal |
None |
EGOG
score |
0 |
0 |
0 |
| 1 |
20 |
20 |
| Smoking |
Yes |
17 (85%) |
19 (95%) |
| No |
0 |
0 |
Table 2.
Adverse event (AE).
Table 2.
Adverse event (AE).
Table 3.
Reaction after treatment.
Table 3.
Reaction after treatment.
Table 4.
Results of a patient's tumor microenvironment.
Table 4.
Results of a patient's tumor microenvironment.
Table 5.
Results of a patient's tumor microenvironment.
Table 5.
Results of a patient's tumor microenvironment.