Submitted:
18 April 2024
Posted:
22 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
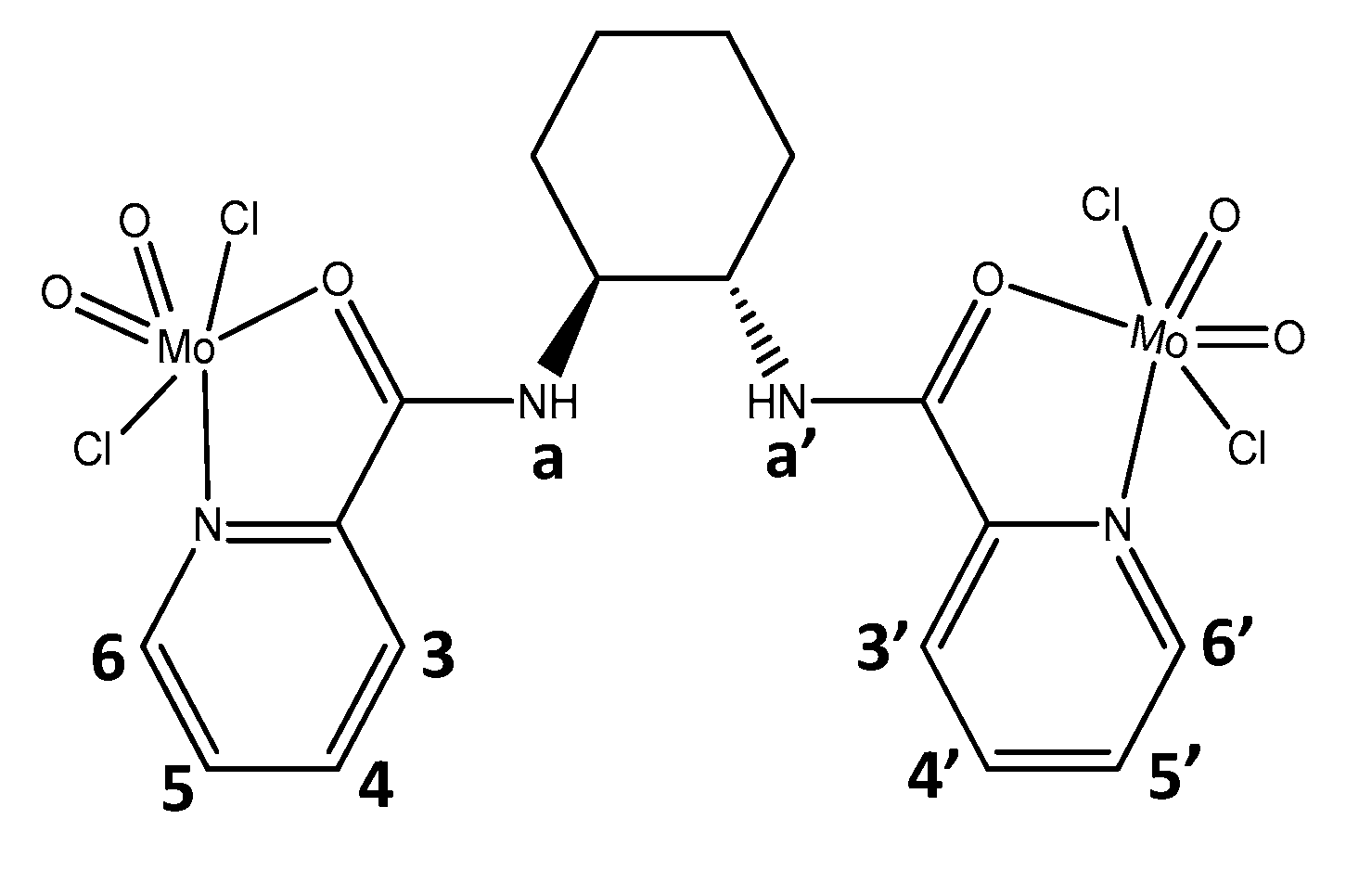
2.3. Catalyst Synthesis
2.4. Oxidative Desulfurization Studies (ODS)
3. Results and Discussion
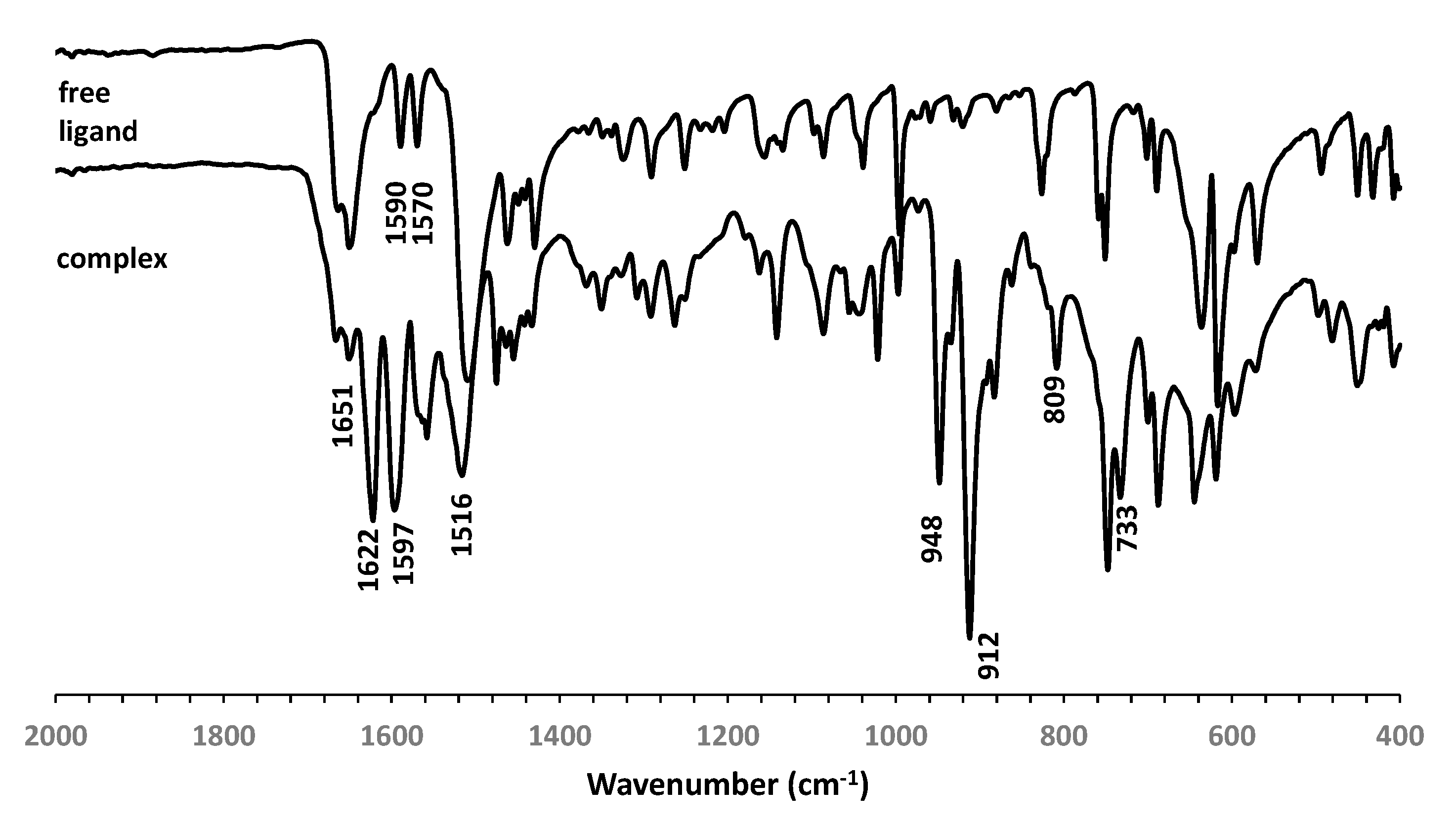
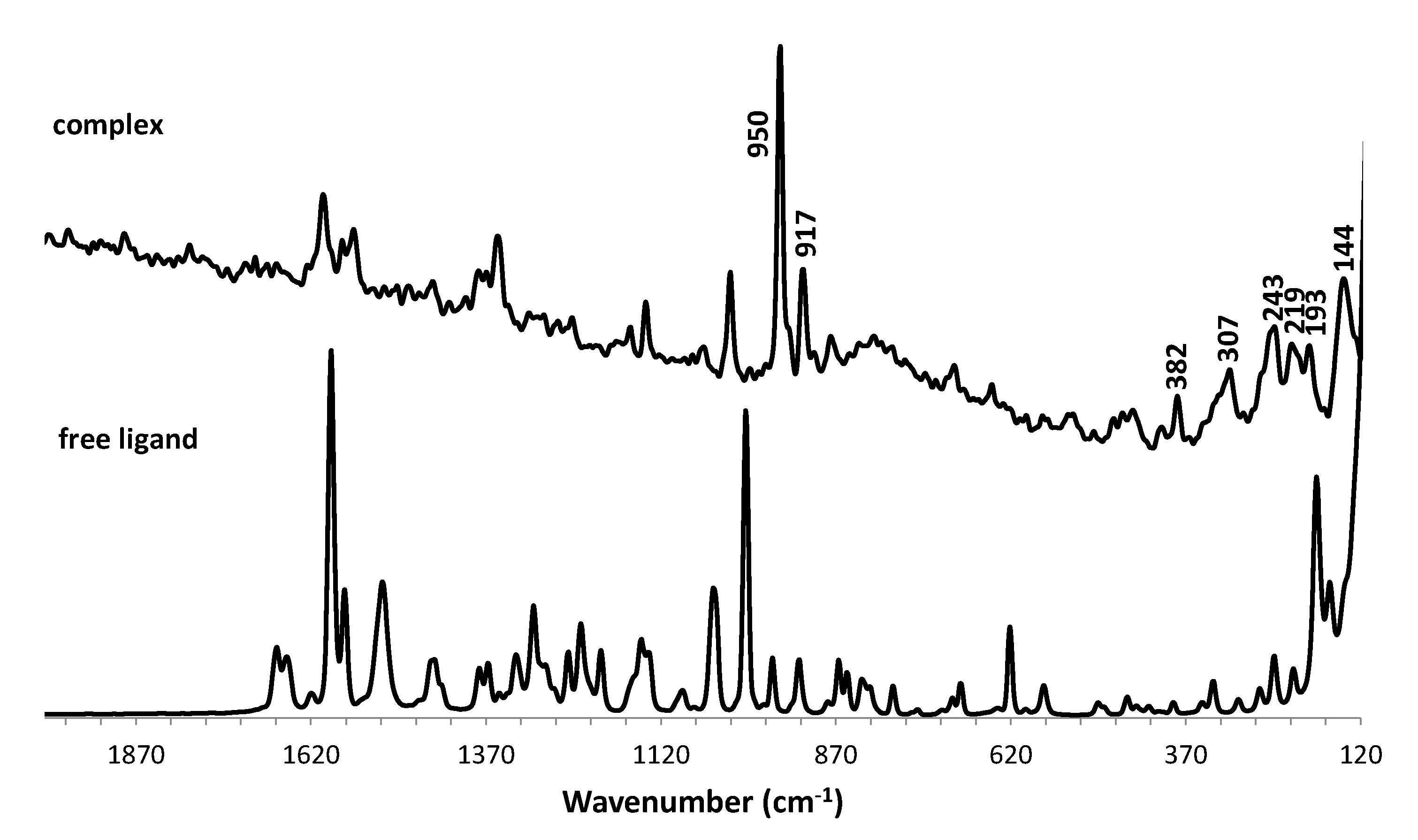
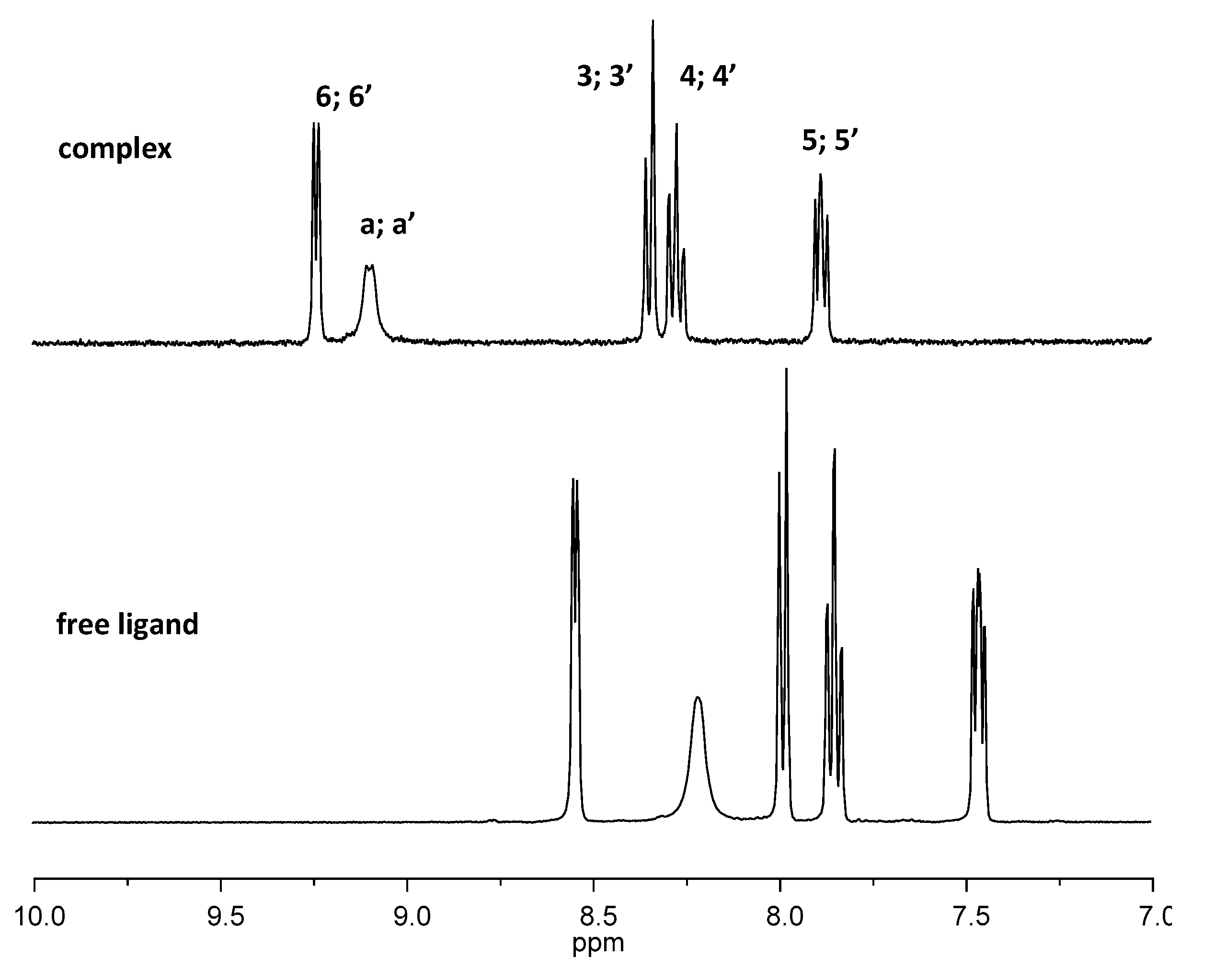
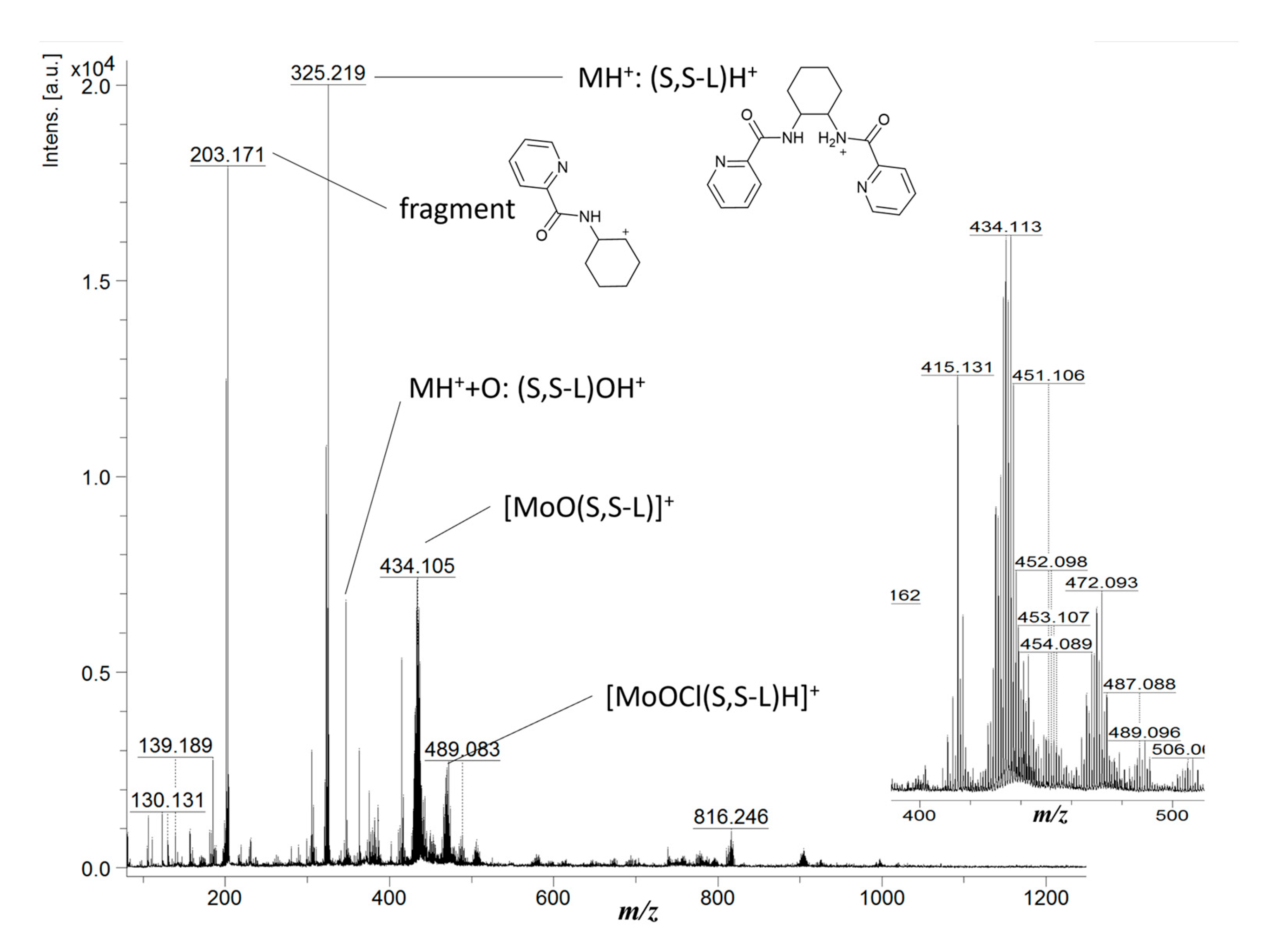
3.1. Catalyst Characterization
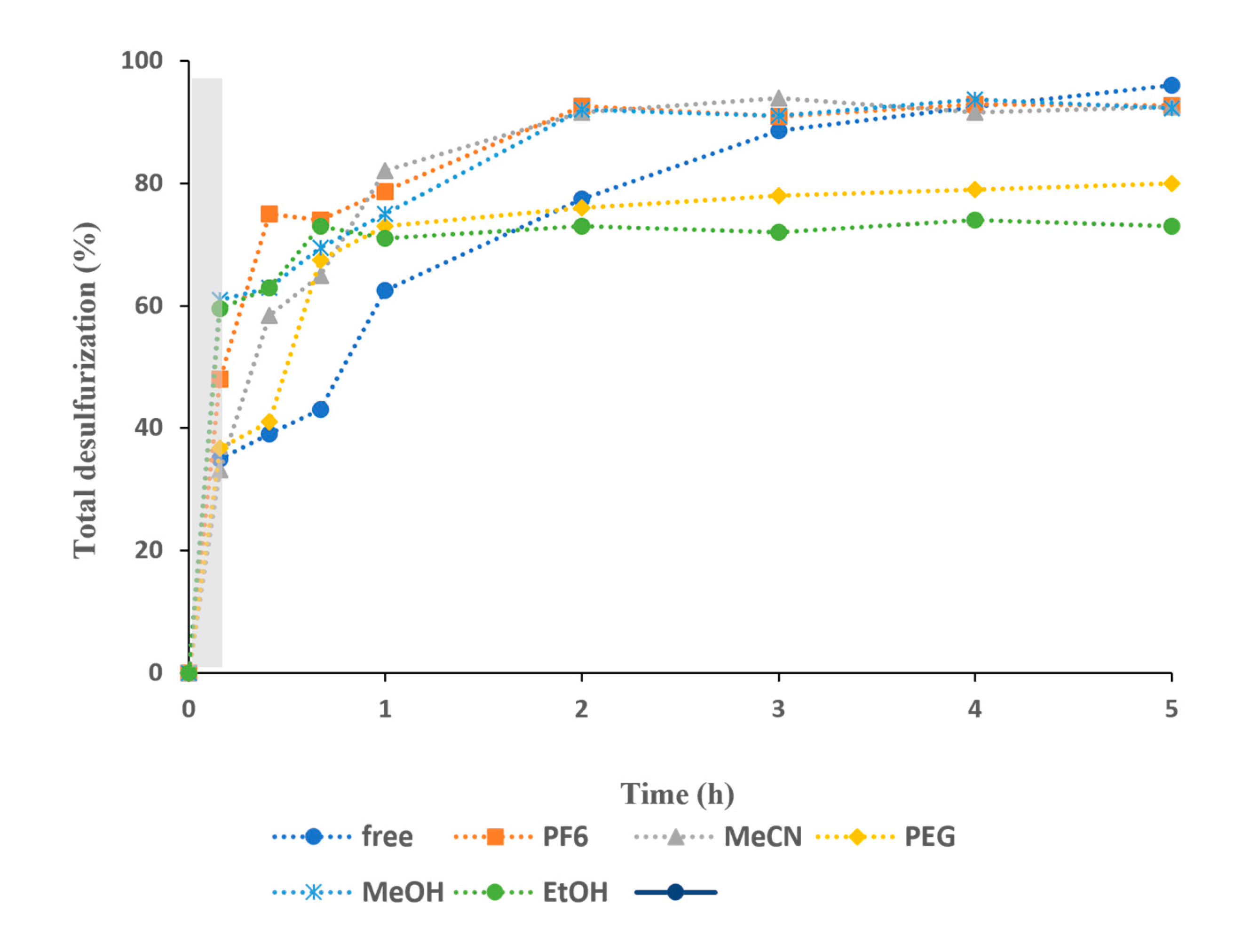
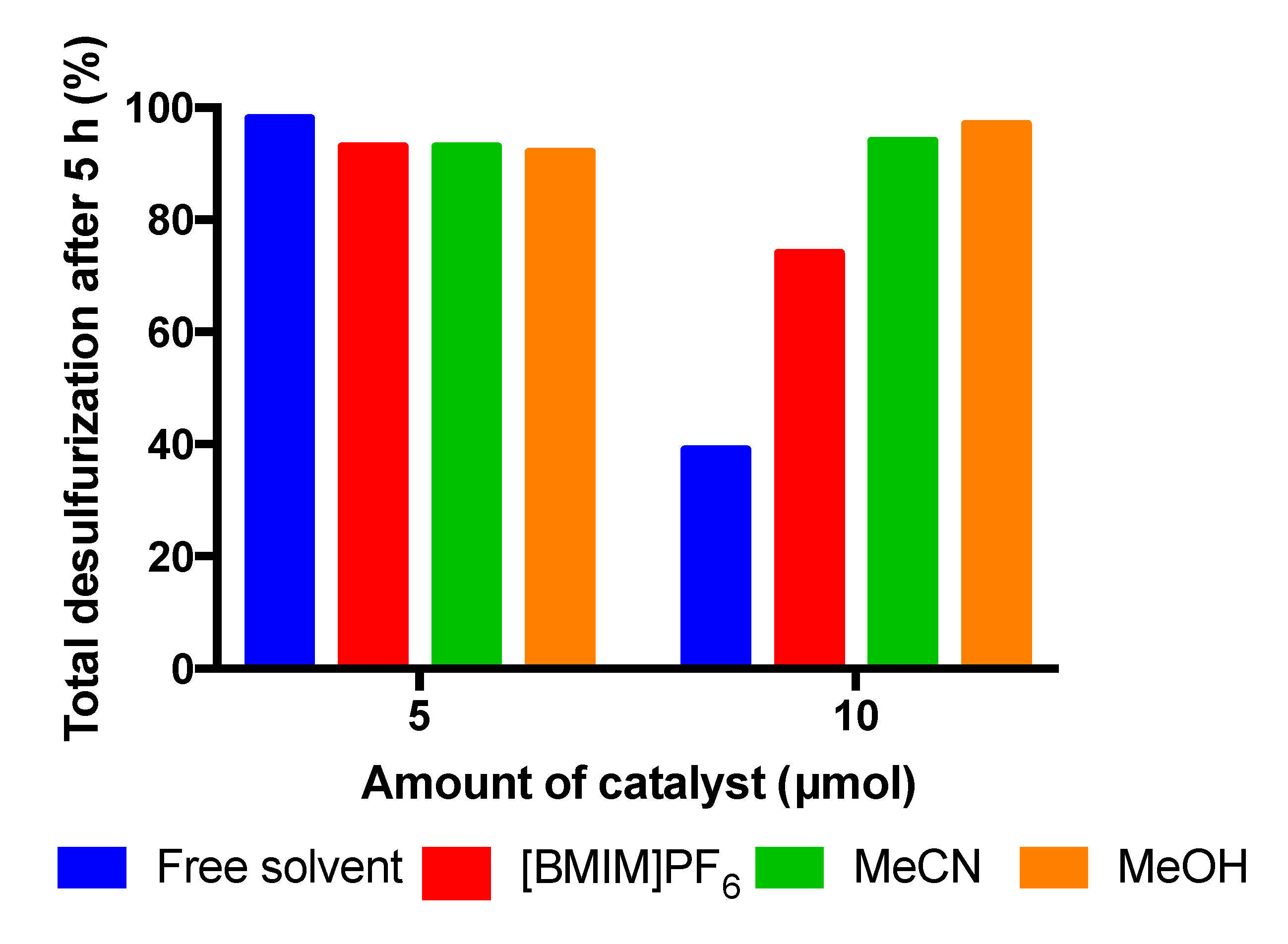
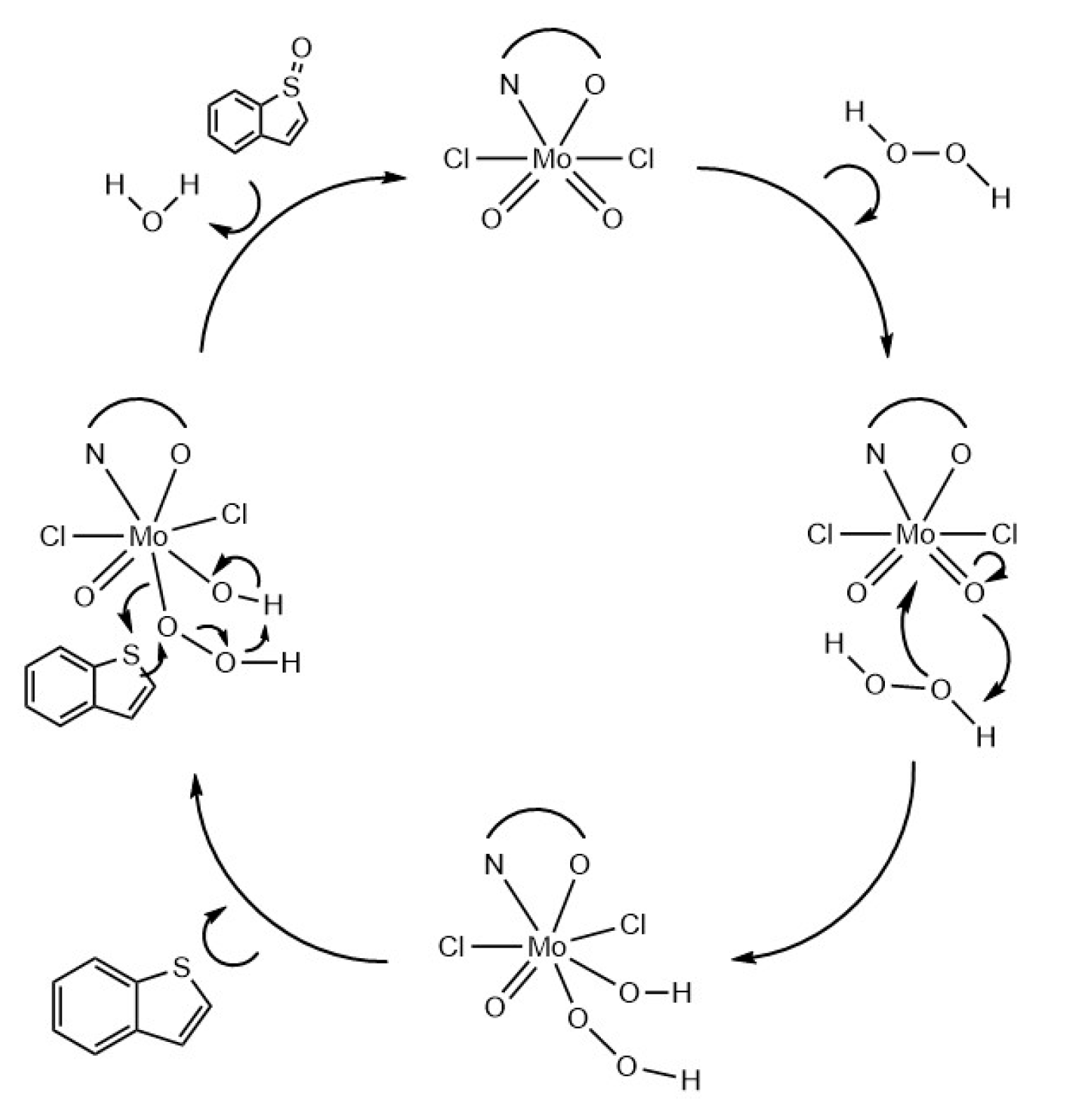
3.2. Desulfurization Studies
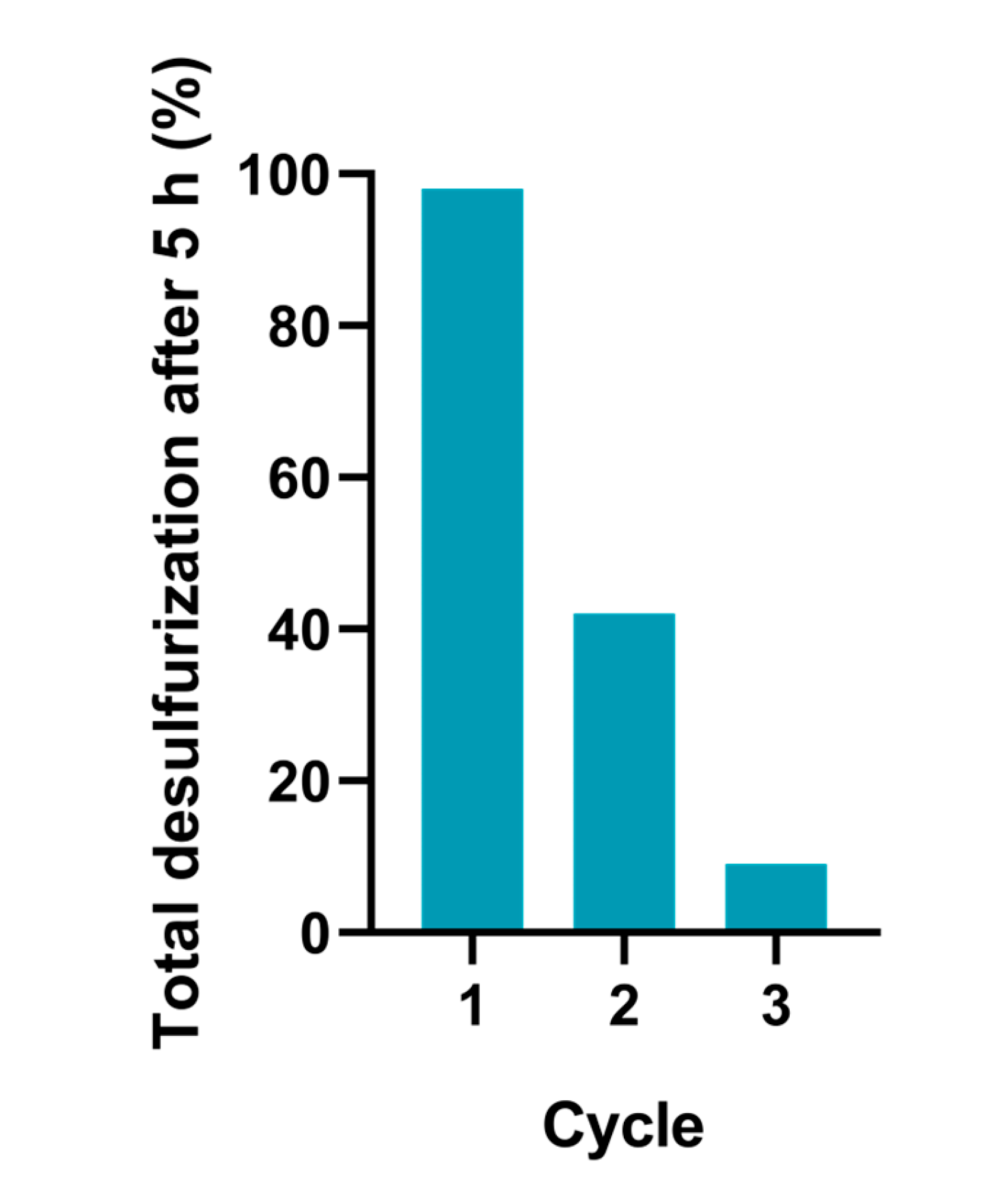
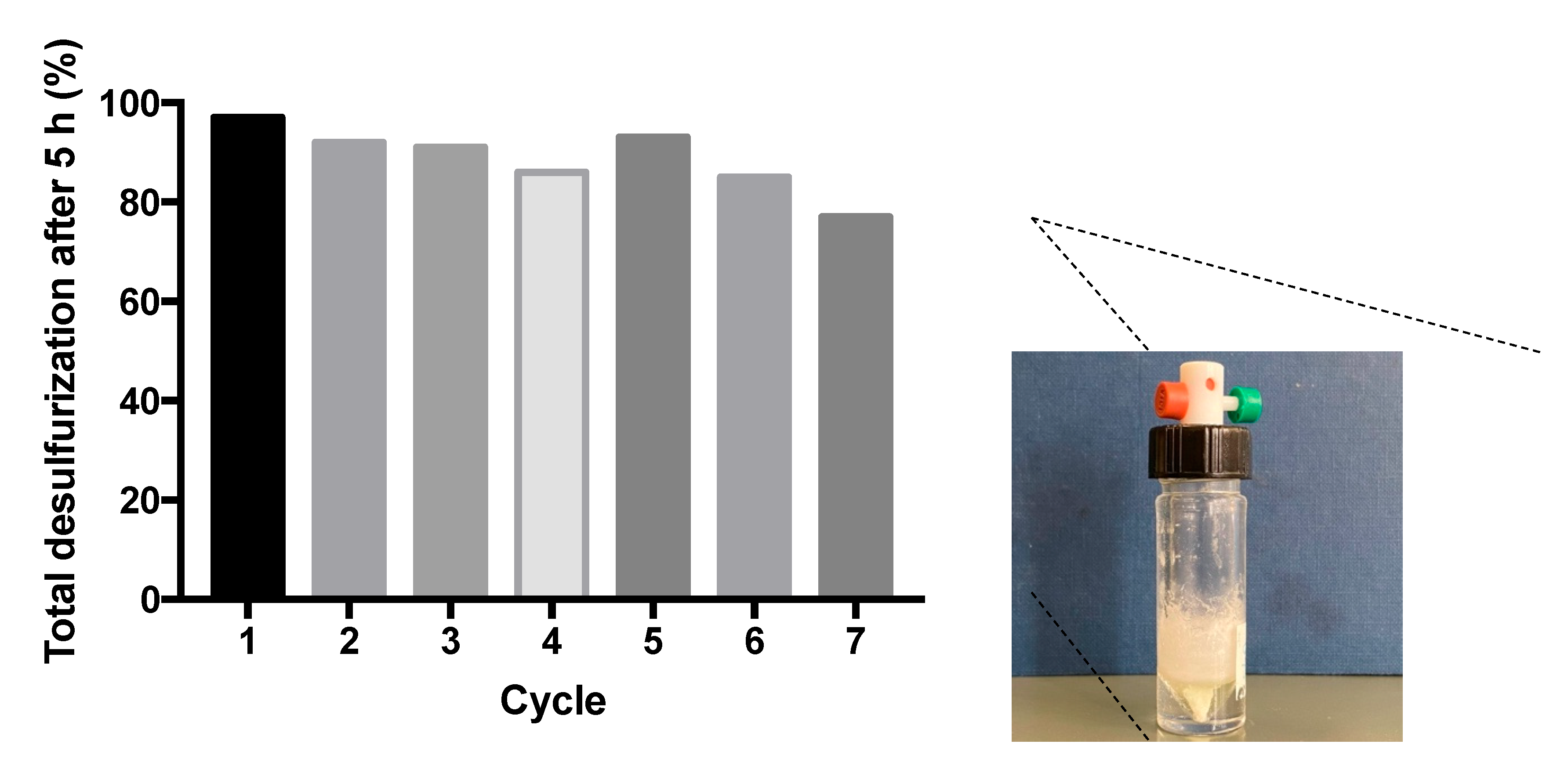
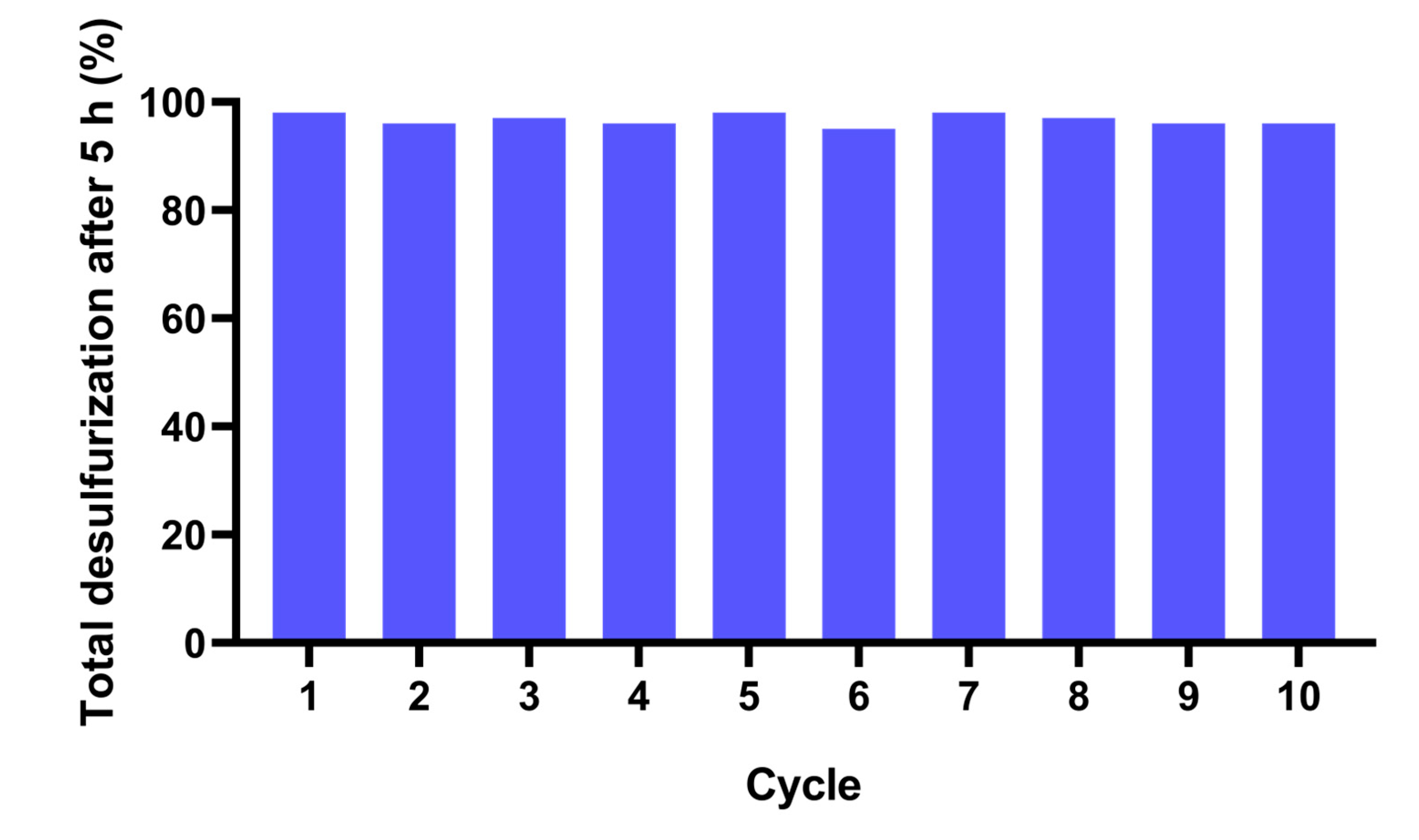
3.3. Reusing Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
References
- aY. Zhang, R. Wang, Applied Catalysis B: Environmental 2018, 234, 247-259; bY. Gu, W. Xu, Y. Sun, Catalysis Today 2020.
- Tugrul Albayrak, A. Tavman, Ultrasonics Sonochemistry 2022, 83, 105845.
- . aY. Tu, T. Li, G. Yu, L. Wei, L. Ta, Z. Zhou, Z. Ren, Energy & Fuels 2019, 33, 8503-8510; bN. S. El-Gendy, J. G. Speight, Handbook of refinery desulfurization, Vol. 140, CRC Press, 2015.
- aD. Juliao, A. C. Gomes, L. Cunha-Silva, M. Pillinger, I. S. Gonçalves, S. S. Balula, Journal of Organometallic Chemistry 2022, 967; bD. Juliao, A. C. Gomes, L. Cunha-Silva, M. Pillinger, A. D. Lopes, R. Valença, J. C. Ribeiro, I. S. Gonçalves, S. S. Balula, Catalysis Communications 2019, 128; cD. Juliao, A. C. Gomes, M. Pillinger, I. S. Gonçalves, S. S. Balula, Chemical Engineering & Technology 2020, 43, 1774-1783; dD. Juliao, A. C. Gomes, M. Pillinger, A. D. Lopes, R. Valença, J. C. Ribeiro, I. S. Gonçalves, S. S. Balula, Journal of Molecular Liquids 2020, 309; eD. Juliao, A. C. Gomes, M. Pillinger, R. Valenca, J. C. Ribeiro, I. S. Goncalves, S. S. Balula, Applied Organometallic Chemistry 2020, 34; fD. Juliao, A. C. Gomes, M. Pillinger, R. Valença, J. C. Ribeiro, I. S. Gonçalves, S. S. Balula, Dalton Transactions 2016, 45, 15242-15248; gR. G. Faria, D. Silva, F. Mirante, S. Gago, L. Cunha-Silva, S. S. Balula, Catalysts 2024, 14; hY. Gao, C. M. Granadeiro, L. Cunha-Silva, J. S. Zhao, S. S. Balula, Catalysis Science & Technology 2023, 13, 4785-4801; iD. F. Silva, R. G. Faria, I. Santos-Vieira, L. Cunha-Silva, C. M. Granadeiro, S. S. Balula, Catalysis Today 2023, 423.
- D. Julião, A. C. Gomes, L. Cunha-Silva, M. Pillinger, A. D. Lopes, R. Valença, J. C. Ribeiro, I. S. Gonçalves, S. S. Balula, Catalysis Communications 2019, 128, 105704.
- D. Julião, A. C. Gomes, M. Pillinger, A. D. Lopes, R. Valença, J. C. Ribeiro, I. S. Gonçalves, S. S. Balula, Journal of Molecular Liquids 2020, 309, 113093.
- F. Ferella, L. Biancalana, F. Marchetti, M. Crucianelli, Catalysis Today 2020, 357, 646-654.
- D. Juliao, A. C. Gomes, M. Pillinger, R. Valença, J. C. Ribeiro, I. S. Gonçalves, S. S. Balula, Applied Catalysis B-Environmental 2018, 230, 177-183.
- Belda, C. Moberg, Coordination Chemistry Reviews 2005, 249, 727-740.
- M. Palucki, J. Um, N. Yasuda, D. Conlon, F.-R. Tsay, F. Hartner, Y. Hsiao, B. Marcune, S. Karady, D. Hughes, P. Dormer, P. Reider, The Journal of organic chemistry 2002, 67, 5508-5516.
- S. W. Krska, D. L. Hughes, R. A. Reamer, D. J. Mathre, Y. Sun, B. M. Trost, Journal of the American Chemical Society 2002, 124, 12656-12657.
- aR. L. Chapman, R. S. Vagg, Inorganica Chimica Acta 1979, 33, 227-234; bT. F. Zafiropoulos, S. P. Perlepes, P. V. Ioannou, J. M. Tsangaris, A. G. Galinos, Zeitschrift für Naturforschung B 1981, 36, 87-93; cT. F. Zafiropoulos, S. P. Perlepes, P. V. Ioannou, J. M. Tsangaris, A. G. Galinos, 1981, 36, 87-93.
- aS. Gago, J. E. Rodríguez-Borges, C. Teixeira, A. M. Santos, J. Zhao, M. Pillinger, C. D. Nunes, Ž. Petrovski, T. M. Santos, F. E. Kühn, C. C. Romão, I. S. Gonçalves, Journal of Molecular Catalysis A: Chemical 2005, 236, 1-6; bF. E. Kühn*, A. M. Santos, A. D. Lopes, I. S. Gonçalves, J. E. Rodrı́guez-Borges, M. Pillinger, C. C. Romão*, Journal of Organometallic Chemistry 2001, 621, 207-217.
- C. Coelho, M. Nolasco, S. S. Balula, M. M. Antunes, C. C. L. Pereira, F. A. Almeida Paz, A. A. Valente, M. Pillinger, P. Ribeiro-Claro, J. Klinowski, I. S. Gonçalves, Inorganic Chemistry 2011, 50, 525-538.
- X. Bullock, C. S. Jamieson, P. Moënne-Loccoz, B. Taylor, J. A. M. Gonzalez, E. A. Draves, L. Y. Kuo, Inorganic Chemistry 2021, 60, 7762-7772.
- aR. Wang, G. Zhang, H. Zhao, Catalysis Today 2010, 149, 117-121; bY. Chen, Q. Tian, Y. Tian, J. Cui, G. Wang, Applied Sciences 2021, 11; cI. Kozhevnikov, Catalysts for fine chemical synthesis, catalysis by polyoxometalates, Vol. 2, Wiley, 2002.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




