Submitted:
18 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
Material and Methods
1. Introduction
2. Historical Perspective on Transplantation Therapies
3. Overview of Current Transplantation Therapies
4. Emerging Paradigms in Transplantation Therapeutics
5. Novel Cellular Therapies
6. Biotechnological Innovations in Transplantation
6.1. 3D Printing in Organ Transplantation
6.2. Nanotechnology in Immunosuppression Delivery
6.3. Bioengineering Solutions for Tissue Engineering
6.4. Wearable and Implantable Devices in Post-Transplant Monitoring
7. Clinical Trials and Case Studies
7.1. Overview of Ongoing Clinical Trials
7.1.1. CRISPR-Based Gene Editing in Allogeneic Stem Cell Transplantation
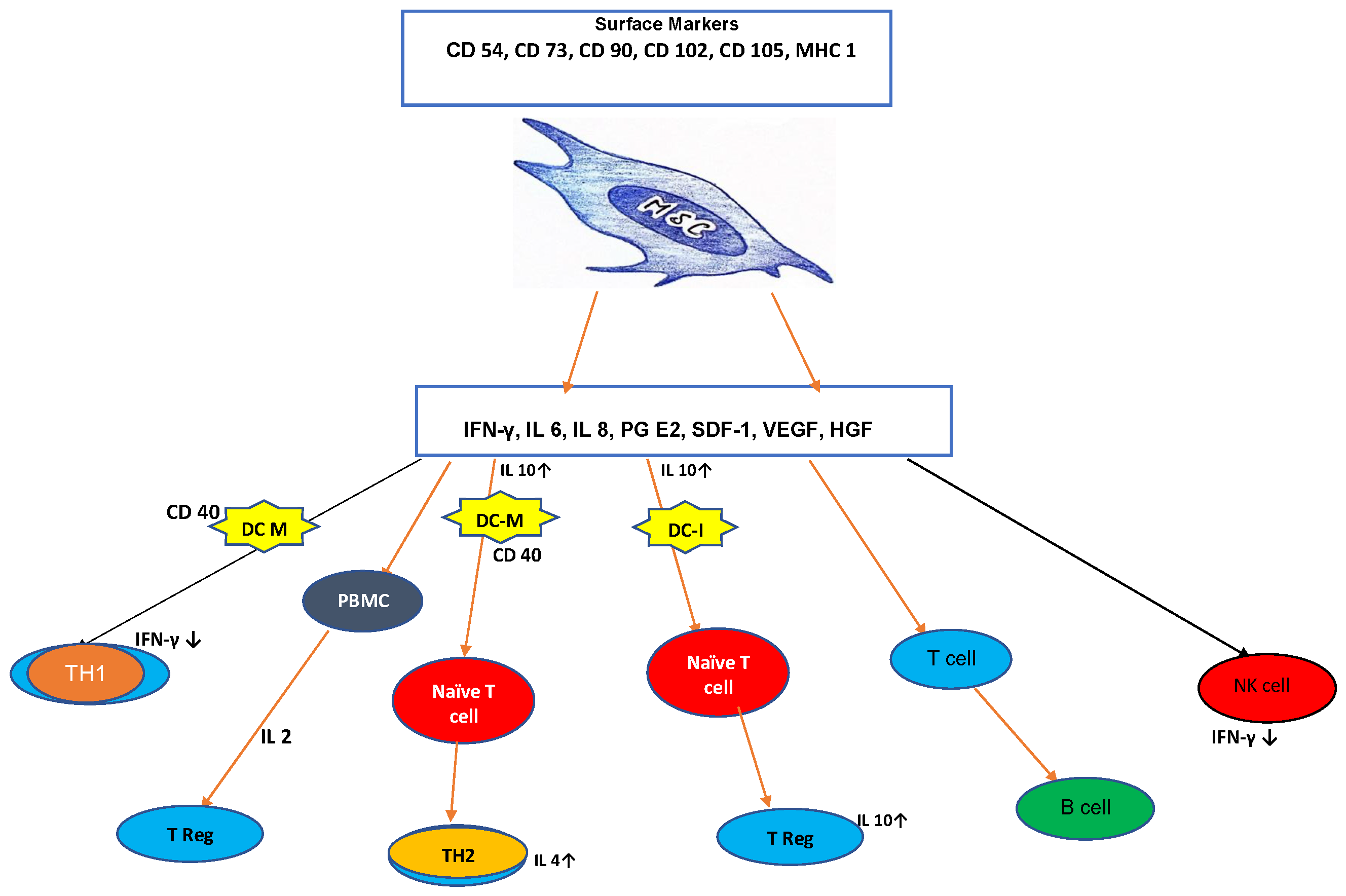
7.1.2. Mesenchymal Stem Cell (MSC) Therapies in Solid Organ Transplantation
7.2. Case Studies Highlighting Successful Implementation of Novel Therapies
7.2.1. Successful Transcoronary Infusion of Cardiac Progenitor Cells in Patients with Single Ventricle Physiology” (TICAP)
7.2.2. Successful Induction of Tolerance in Renal Transplantation with Regulatory T Cells (Tregs)
7.3. Challenges and Lessons Learned from Clinical Implementations
7.3.1. Immune-Related Adverse Events in Cellular Therapies
7.3.2. Hurdles in the Translation of Gene Editing Technologies to Clinical Settings
8. Ethical and Regulatory Considerations
8.1. Ethical Implications of Novel Therapeutic Approaches
8.1.1. Patient Autonomy and Informed Consent
8.1.2. Allocation of Limited Resources
8.2. Regulatory Frameworks and Guidelines
8.2.1. Regulatory Oversight and Approval
8.2.2. Adaptation of Regulations to Emerging Technologies
8.3. Balancing Innovation with Patient Safety
8.3.1. Risk-Benefit Assessment
8.3.2. Post-Marketing Surveillance and Long-Term Monitoring
9. Future Directions and Challenges
10. Conclusion
References
- Murray JE, Merrill JP, Harrison JH 1955. Renal homotransplantation in identical twins. Surg Forum 6: 432–436.
- Merrill JP, Murray JE, Harrison JH, Guild WR 1956. Successful homotransplantation of the human kidney between identical twins. JAMA 160: 277–282.
- Hillebrand, G. F., & Land, W. (1996). Renal transplantation: progress and prospects. Artificial Organs, 20(6), 403–407. [CrossRef]
- Toledo-Pereyra, L. H., & Palma-Vargas, J. (1999). Searching for history in transplantation: early modern attempts at surgical kidney grafting. Transplantation Proceedings, 31(7), 2945–2948. [CrossRef]
- Medawar, P. B. (1948). Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. PubMed, 29(1), 58–69.
- Shoskes, D. A., & Halloran, P. F. (1996b). Delayed graft function in renal transplantation: etiology, management and long-term significance. The Journal of Urology, 155(6), 1831–1840. [CrossRef]
- Jp, S. (2011). The History of Kidney Transplantation: Past, Present and Future (with Special References to the Belgian History). In InTech eBooks. [CrossRef]
- Vnucak, M., Granak, K., Beliancinova, M., Gala,et al. (2022). The impact of different induction immunosuppression protocols on patient survival, graft survival and acute graft rejection after kidney transplantation. Bratislava Medical Journal. [CrossRef]
- Swanevelder, J., Gordon, P., Brink, J., Gutsche, J. T., Dyer, R. A., & Augoustides, J. (2018). Fifty years: Reflections since the first successful heart transplant. Journal of Cardiothoracic and Vascular Anesthesia, 32(1), 14–18. [CrossRef]
- Miller CL, O JM, Allan JS, Madsen JC. Novel approaches for long-term lung transplant survival. Front Immunol. 2022 Jul 27;13:931251. doi: 10.3389/fimmu.2022.931251. PMID: 35967365; PMCID: PMC9363671.
- Current status of xenotransplantation research and the strategies for preventing xenograft rejection. Qiao Zhou, Ting-ting Li, Kaiwen Wang, Zhuowen Geng, et al.. 28 Jul 2022-Frontiers in Immunology.
- Contemporary review of heart transplant immunology and immunosuppressive therapy. Kamalesh Anbalakan, Kenneth Michael Yun-Chi Chew et al.- 01 Jun 2022-Proceedings of Singapore healthcare.
- Cellular therapies in organ transplantation. Martin J. Hoogduijn, Fadi Issa, Federica Casiraghi, Marlies E J Reinders - 15 Jan 2021-Transplant International.
- A comprehensive and contemporary review on immunosuppression therapy for heart transplantation. Livia Adams Goldraich, Santiago Alonso Tobar Leitão, Fernando Luís Scolari, et al., 01 Jan 2020-Current Pharmaceutical Design.
- Current state of organ transplant tolerance. Charles G. Rickert, James F. Markmann. 01 Aug 2019-Current Opinion in Organ Transplantation.
- The Fundamental Challenges in Organ Transplantation. Pål Dag Line. 24 Dec 2017-Vol. 1, Iss: 4, pp 1-1.
- Advances in organ preservation for transplantation. Ahmer Hameed, Ahmer Hameed, Wayne J. Hawthorne, Wayne J. Hawthorne. 01 Dec 2017-Anz Journal of Surgery.
- A Comprehensive Data-Driven Characterization of Organ Transplantation. Pinheiro Ferreira Silva, Diego Marconi,01 Jul 2018.
- Recent immunomodulatory strategies in transplantation. Ammar Ebrahimi, Fakher Rahim, 08 Oct 2014-Immunological Investigations.
- Bioengineering for Organ Transplantation: Progress and Challenges. Ted Welman, Sebastian Michel, Nicholas Segaren, Kumaran Shanmugarajah. 26 Aug 2015-Bioengineered bugs.
- Johnstone BH, Messner F, Brandacher G, Woods EJ. A Large-Scale Bank of Organ Donor Bone Marrow and Matched Mesenchymal Stem Cells for Promoting Immunomodulation and Transplant Tolerance. Front Immunol. 2021 Feb 26;12:622604. doi: 10.3389/fimmu.2021.622604. PMID: 33732244; PMCID: PMC7959805.
- Liao J, Shao K, Wang X, Wang X, Liu G. Immunomodulation and Mechanism of Transplantation Immune Tolerance Induced by Glucocorticoids.
- Hameed AM, Hawthorne WJ, Pleass HC. Advances in organ preservation for transplantation. ANZ J Surg. 2017 Dec;87(12):976-980. doi: 10.1111/ans.13713. Epub 2016 Aug 4. PMID: 27490874.
- Berkane Y, Hayau J, Filz von Reiterdank I, Kharga A, Charlès L, Mink van der Molen AB, Coert JH, Bertheuil N, Randolph MA, Cetrulo Jr CL, Longchamp A. Supercooling: a promising technique for prolonged preservation in solid organ transplantation, and early perspectives in vascularized composite allografts. Frontiers in Transplantation. 2023 Oct 23;2:1269706.
- Fung RK, Kerridge IH. Gene editing advance re-ignites debate on the merits and risks of animal to human transplantation. Intern Med J. 2016 Sep;46(9):1017-22. doi: 10.1111/imj.13183. PMID: 27633468.
- Fischer K, Schnieke A. How genome editing changed the world of large animal research. Front Genome Ed. 2023 Oct 11;5:1272687. doi: 10.3389/fgeed.2023.1272687. PMID: 37886655; PMCID: PMC10598601.
- Oberbauer R, Meyer TW. Precision medicine in transplantation and hemodialysis. Nephrol Dial Transplant. 2021 Jun 22;36(Suppl 2):31-36. doi: 10.1093/ndt/gfaa367. Erratum in: Nephrol Dial Transplant. 2021 Dec 31;37(1):199. PMID: 34153984; PMCID: PMC8216726.
- Sirota M, Sarwal MM. Transplantomics: Toward Precision Medicine in Transplantation Research. Transplantation. 2017 Aug;101(8):1777-1782. doi: 10.1097/TP.0000000000001664. PMID: 28121910.
- Girolami I, Pantanowitz L, Marletta S, Hermsen M, van der Laak J, Munari E, Furian L, Vistoli F, Zaza G, Cardillo M, Gesualdo L, Gambaro G, Eccher A. Artificial intelligence applications for pre-implantation kidney biopsy pathology practice: a systematic review. J Nephrol. 2022 Sep;35(7):1801-1808. doi: 10.1007/s40620-022-01327-8. Epub 2022 Apr 19. PMID: 35441256; PMCID: PMC9458558.
- Naruka V, Arjomandi Rad A, Subbiah Ponniah H, Francis J, Vardanyan R, Tasoudis P, Magouliotis DE, Lazopoulos GL, Salmasi MY, Athanasiou T. Machine learning and artificial intelligence in cardiac transplantation: A systematic review. Artif Organs. 2022 Sep;46(9):1741-1753. doi: 10.1111/aor.14334. Epub 2022 Jun 20. PMID: 35719121; PMCID: PMC9545856.
- Alagesan S, Griffin MD. Autologous and allogeneic mesenchymal stem cells in organ transplantation: what do we know about their safety and efficacy? Curr Opin Organ Transplant. 2014 Feb;19(1):65-72. doi: 10.1097/MOT.0000000000000043. PMID: 24370985.
- Deo D, Marchioni M, Rao P. Mesenchymal Stem/Stromal Cells in Organ Transplantation. Pharmaceutics. 2022 Apr 4;14(4):791. doi: 10.3390/pharmaceutics14040791. PMID: 35456625; PMCID: PMC9029865.
- Proics E, David M, Mojibian M, Speck M, Lounnas-Mourey N, Govehovitch A, Baghdadi W, Desnouveaux J, Bastian H, Freschi L, Privat G, Pouzet C, Grossi M, Heimendinger P, Abel T, Fenard D, Levings MK, Meyer F, Dumont C. Preclinical assessment of antigen-specific chimeric antigen receptor regulatory T cells for use in solid organ transplantation. Gene Ther. 2023 Apr;30(3-4):309-322. doi: 10.1038/s41434-022-00358-x. Epub 2022 Aug 5. PMID: 35931871; PMCID: PMC10113151.
- Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, June CH, Scheinberg P, Douek DC, Miller JS, Wagner JE, Blazar BR. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011 May 18;3(83):83ra41. doi: 10.1126/scitranslmed.3001809. PMID: 21593401; PMCID: PMC3551476.
- Bachanova V, Maakaron JE, Cichocki F, McKenna DH, Cao Q, DeFor TE, Janakiram M, Wangen R, Cayci Z, Grzywacz B, Simantov R. Gda-201, a novel metabolically enhanced allogeneic natural killer (NK) cell product yields high remission rates in patients with relapsed/refractory non-hodgkin lymphoma (NHL): 2-year survival and correlation with cytokine IL7. Blood. 2021 Nov 23;138:3854.
- Ma M, Badeti S, Kim JK, Liu D. Natural Killer (NK) and CAR-NK Cell Expansion Method using Membrane Bound-IL-21-Modified B Cell Line. J Vis Exp. 2022 Feb 8;(180):10.3791/62336. doi: 10.3791/62336. PMID: 35225261; PMCID: PMC10858653.
- Hsia GSP, Esposito J, da Rocha LA, Ramos SLG, Okamoto OK. Clinical Application of Human Induced Pluripotent Stem Cell-Derived Organoids as an Alternative to Organ Transplantation. Stem Cells Int. 2021 Feb 24;2021:6632160. doi: 10.1155/2021/6632160. PMID: 33679987; PMCID: PMC7929656.
- Goulart E. Stem Cell Technology in Organ Transplantation: A Novel Method for 3D Bioprinting Functional and Stable Liver Grafts Using Human iPS Cells Derived Cells. Methods Mol Biol. 2023;2575:269-274. doi: 10.1007/978-1-0716-2716-7_13. PMID: 36301480.
- Applications, advancements, and challenges of 3D bioprinting in organ transplantation.
- Guobin Huang, Yuanyuan Zhao, Dong Chen, Lai Wei, Zhiping Hu, Junbo Li, Xi Zhou, Bo Yang, Zhishui Chen Biomaterials Science 2024.
- Lim G, Choi D, Richardson EB. 3-D printing in organ transplantation. Hanyang Medical Reviews. 2014 Nov 1;34(4):158-64.
- Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013 Mar;60(3):691-9. doi: 10.1109/TBME.2013.2243912. Epub 2013 Jan 30. PMID: 23372076.
- Tripathi AS, Malakar K, Singh AK, Chaudhary I. 3D organ printing: A future prospect of medical sciences in organ transplantation. Innovare J. Life Sci. 2013 Oct 1;1:10-7.
- Tasciotti E, Cabrera FJ, Evangelopoulos M, Martinez JO, Thekkedath UR, Kloc M, Ghobrial RM, Li XC, Grattoni A, Ferrari M. The Emerging Role of Nanotechnology in Cell and Organ Transplantation. Transplantation. 2016 Aug;100(8):1629-38. doi: 10.1097/TP.0000000000001100. PMID: 27257995; PMCID: PMC4961523.
- Sanvicens N, Marco MP. Multifunctional nanoparticles--properties and prospects for their use in human medicine. Trends in biotechnology. 2008; 26(8):425–433. [PubMed: 18514941].
- Martinez JO, et al. Multistage Nanovectors Enhance the Delivery of Free and Encapsulated Drugs. Curr Drug Targets. 2014.
- Thierry B, et al. Immunotargeting of Functional Nanoparticles for MRI detection of Apoptotic Tumor Cells. Advanced materials. 2009; 21(5):541–545. [PubMed: 21161977].
- Muhammad F, et al. pH-Triggered controlled drug release from mesoporous silica nanoparticles via intracelluar dissolution of ZnO nanolids. Journal of the American Chemical Society. 2011; 133(23):8778–8781. [PubMed: 21574653].
- Letfullin RR, Iversen CB, George TF. Modeling nanophotothermal therapy: kinetics of thermal ablation of healthy and cancerous cell organelles and gold nanoparticles. Nanomedicine : nanotechnology, biology, and medicine. 2011; 7(2):137–145.
- Hedlund A, et al. Gd(2)O(3) nanoparticles in hematopoietic cells for MRI contrast enhancement. International journal of nanomedicine. 2011; 6:3233–3240. [PubMed: 22228991].
- Yigit MV, Moore A, Medarova Z. Magnetic nanoparticles for cancer diagnosis and therapy. Pharmaceutical research. 2012; 29(5):1180–1188. [PubMed: 22274558].
- Han J, Fu J, Schoch RB. Molecular sieving using nanofilters: past, present and future. Lab on a Chip. 2008; 8(1):23–33. [PubMed: 18094759].
- Hu Y, et al. Nanodevices in diagnostics. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2011; 3(1):11–32. [PubMed: 20229595].
- Fine D, et al. A robust nanofluidic membrane with tunable zero-order release for implantable dose specific drug delivery. Lab on a Chip. 2010; 10(22):3074–3083. [PubMed: 20697650].
- Orlando G, Soker S, Stratta RJ. Organ bioengineering and regeneration as the new Holy Grail for organ transplantation. Ann Surg. 2013 Aug;258(2):221-32. doi: 10.1097/SLA.0b013e31829c79cf. PMID: 23782908.
- Katari, Ravi S, Kyle P. McNamara, Carmine Gentile, Lauren Edgar, Tyler E. Callese, Daniel A. Igel, Joao P Zambon, Riccardo Tamburrini and Giuseppe Orlando. “Tissue Engineering and Regenerative Medicine Solutions for the Abdominal Organs.” (2017).
- Skowno JJ, Karpelowsky JS. Near-infrared spectroscopy for monitoring renal transplant perfusion. Pediatr Nephrol. 2014 Nov;29(11):2241-2. doi: 10.1007/s00467-014-2912-6. Epub 2014 Aug 15. PMID: 25119681.
- Ghidini F, Parolin M, De Corti F, Amigoni A, Fascetti Leon F, Benetti E, Gamba P. Can real-time near-infrared spectroscopy monitoring detect graft venous thrombosis after pediatric kidney transplantation? Pediatr Transplant. 2022 May;26(3):e14211. doi: 10.1111/petr.14211. Epub 2021 Dec 16. PMID: 34918432.
- Malakasioti G, Marks SD, Watson T, Williams F, Taylor-Allkins M, Mamode N, Morgan J, Hayes WN. Continuous monitoring of kidney transplant perfusion with near-infrared spectroscopy. Nephrol Dial Transplant. 2018 Oct 1;33(10):1863-1869. doi: 10.1093/ndt/gfy116. PMID: 29757424.
- Zeng S, Lei S, Qu C, Wang Y, Teng S, Huang P. CRISPR/Cas-based gene editing in therapeutic strategies for beta-thalassemia. Hum Genet. 2023 Dec;142(12):1677-1703. doi: 10.1007/s00439-023-02610-9. Epub 2023 Oct 25. PMID: 37878144.
- Lanza R, Russell DW, Nagy A. Engineering universal cells that evade immune detection. Nature Reviews Immunology. 2019 Dec;19(12):723-33.
- Deo D, Marchioni M, Rao P. Mesenchymal Stem/Stromal Cells in Organ Transplantation. Pharmaceutics. 2022 Apr 4;14(4):791. doi: 10.3390/pharmaceutics14040791. PMID: 35456625; PMCID: PMC9029865.
- Mohsin, Aisha & Javaid, Saima & Mehwish, Maryam & Imran, Rangraze. (2023). A review of the potential use of mesenchymal stem cell therapy for the management of COVID-19 infection. Bioscience Research. 19. 2250-2255.
- Abraham R, Vricella L, Hibino N. Cardiac tissue engineering for the treatment of hypoplastic left heart syndrome (HLHS). Transl Pediatr. 2023 Aug 30;12(8):1592-1600. doi: 10.21037/tp-23-127. Epub 2023 Aug 2. PMID: 37692536; PMCID: PMC10485645.
- Todo, S. , Yamashita K., Goto R., Zaitsu M., Nagatsu A., Oura T., Watanabe M., Aoyagi T., Suzuki T., Shimamura T., et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64:632–643. doi: 10.1002/hep.28459. [CrossRef].
- Trzonkowski, P. , Bieniaszewska M., Juścińska J., Dobyszuk A., Krzystyniak A., Marek N., Myśliwska J., Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin. Immunol. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [CrossRef].
- Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, et al.CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017 Dec 28;377(26):2531-2544. doi: 10.1056/NEJMoa1707447. Epub 2017 Dec 10. PMID: 29226797; PMCID: PMC5882485.
- Jennifer, A. Doudna, Emmanuelle Charpentier,The new frontier of genome engineering with CRISPR-Cas9.Science346,1258096(2014).DOI:10.1126/science.1258096.
- Gordon EJ, Veatch RM, Abt P, Reese PP. Organ donor intervention research informed consent - Timing and risk. Am J Transplant. 2020 Mar;20(3):906. doi: 10.1111/ajt.15758. Epub 2020 Jan 18. PMID: 31873971.
- Kute VB, Vanikar AV, Shah PR, Gumber MR, Patel HV, Engineer DP, Modi PR, Shah VR, Trivedi HL. Increasing access to kidney transplantation in countries with limited resources: the Indian experience with kidney paired donation. Nephrology (Carlton). 2014 Oct;19(10):599-604. doi: 10.1111/nep.12307. PMID: 24995599.
- Nassar A, Srivastava A, Hashmi SK, Aljurf M. Establishing an HSCT Program with Limited Resources. Establishing a Hematopoietic Stem Cell Transplantation Unit: A Practical Guide. 2018:257-70.
- Batra RK, Mulligan DC. Current status: meeting the regulatory goals of your liver transplant program. Curr Opin Organ Transplant. 2021 Apr 1;26(2):146-151. doi: 10.1097/MOT.0000000000000869. PMID: 33650996.
- Winkler M, Christians U. A risk-benefit assessment of tacrolimus in transplantation. Drug Saf. 1995 May;12(5):348-57. doi: 10.2165/00002018-199512050-00006. PMID: 7545405.
- Omar, M. O. , Abad Ali, Qabillie, Haji, A. I et al. (2024). Beyond Vision: Potential Role of AI-enabled Ocular Scans in the Prediction of Aging and Systemic Disorders: Role of AI-enabled Ocular Scans in the Prediction of Aging and Systemic Disorders. Siriraj Medical Journal, 76(2), 106–115. https://doi.org/10.33192/smj.v76i2.266303.
| Authors | Title | Summary | Limitations/Future directives |
|---|---|---|---|
| Qiao Zhou, Ting-ting Li, et.al | Current status of xenotransplantation research and the strategies for preventing xenograft rejection [11] 28 Jul 2022 |
The paper discusses xenotransplantation of pig organs as a potential alternative for organ transplantation, focusing on the mechanisms of immunological rejection and strategies for preventing xenograft rejection, such as gene editing and immunosuppressive regimens. | -Delayed xenograft rejection (DXR) and chronic rejection remain urgent issues in xenotransplantation. - Current status of xenotransplantation research and the strategies for preventing xenograft rejection. -Immune rejection is the major challenge. - Concerns about Porcine endogenous retroviruses (PERVs) transmission and ethical issues around xenotransplantation. |
| Kamalesh Anbalakan, Kenneth Michael et.al | Contemporary review of heart transplant immunology and immunosuppressive therapy [12] 01 Jun 2022 |
The paper provides an update on contemporary cardiac transplant medicine, focusing on immunosuppressive therapy and treatment of cardiac rejection. It highlights local practice differences from international counterparts and emphasizes the importance of individualized drug choices and strategies for preventing rejection. | - Heterogeneity in care and treatment protocols. -More studies are needed to improve outcomes and treatment protocols. -Challenges in the modern era of heart transplantation. \ -Side effects from over immunosuppression. |
| Martin J. Hoogduijn, Fadi Issa, et.al | Cellular therapies in organ transplantation [13] 15 Jan 2021 | The paper discusses the current state of cellular therapies in organ transplantation, emphasizing preclinical models and early clinical trials showing the safety and feasibility of cellular therapies. It addresses the challenges and future directions for improving outcomes in clinical transplantation. | -Timing and frequency of MSC injections need to be determined for future clinical trials. |
| Livia Adams Goldraich, Santiago Alonso Tobar Leitão, et.al | A comprehensive and contemporary review on immunosuppression therapy for heart transplantation [14] 01 Jan 2020 |
The paper provides a comprehensive overview of contemporary immunosuppression in heart transplantation, emphasizing individualized drug choices and practical approaches. It discusses clinical evidence for immunosuppressive drugs and highlights challenges in the modern era of heart transplantation. | -Lack of evidence and empirical observations. -Challenges in the modern era of heart transplantation. -Side effects from over immunosuppression. |
| Charles G. Rickert, James F. Markmann | Current state of organ transplant tolerance. [15] 01 Aug 2019 | The paper provides an overview of strategies for coping with the shortage of organ grafts for transplantation, focusing on extended criteria grafts, donation after circulatory death, and ex-vivo perfusion. It discusses their successes and limitations in improving organ quality and reducing graft loss. | -Shortage of organ grafts available for transplantation. -Increased risk of graft loss due to poor function. |
| Pål Dag Line | The Fundamental Challenges in Organ Transplantation [16] 24 Dec 2017 |
The paper proposes a comprehensive data-driven characterization of organ transplantation to uncover patterns of efficiency, equity, and awareness. It discusses the integration of available data sets and the trade-off between efficiency, equity, and awareness in organ transplantation. | -The state-of-the-art in organ transplantation lacks the characterization of awareness. -The trade-off between efficiency, equity, and awareness is not fully understood. |
| Ammar Ebrahimi, Fakher Rahim | Advances in organ preservation for transplantation [19] 08 Oct 2014 |
The paper evaluates recent clinical advances in immunosuppressive therapies for organ transplantation, emphasizing novel stem cell-based therapies and alternative therapeutic choices. It addresses the challenges and treatment-related adverse events associated with immunosuppressive therapies. | -Poor long-term survival and significant mortality in organ transplantation. -Treatment-related adverse events and high risk of chronic graft rejection. |
| Ted Welman, Sebastian Michel, Nicholas Segaren, Kumaran Shanmugarajah | Bioengineering for Organ Transplantation: Progress and Challenges [20] 26 Aug 2015 |
The paper provides a review of recent progress in organ bioengineering for transplantation, highlighting both successes and challenges. It discusses advances in decellularization and recellularization techniques and the future work needed for clinical translation of organ bioengineering. | -Difficulties in assessing cardiac function after circulatory cessation. -Decellularized scaffolds need to be immune system compatible. |
| Paradigm | Description | Key Advancements | Potential Impact |
|---|---|---|---|
| Immunomodulation and Tolerance Induction [21,22] |
Techniques to promote acceptance of transplanted tissue while reducing the use of immunosuppressive drugs. Involves Treg treatment and co-stimulation blocking. | Researching ways to achieve immunological tolerance for prolonged transplant survival without impacting general immune function. | Transforming immunosuppressive methods and enhancing long-term results. |
| Advances in Organ Preservation Techniques [23,24] |
Enhancements to prolong the durability of donated organs. Methods such as hypothermic perfusion, normothermic perfusion, and cryopreservation. | Possibility to expand the number of suitable donor organs, decrease ischemia-reperfusion harm, and improve transplant results. | Enhancing organ availability and increasing transplant success rates. |
| Gene Editing and Engineering in Transplantation [25,26] |
Utilizing gene editing technologies (e.g., CRISPR/Cas9) for precision manipulation of donor organs and recipient cells. | Targeting genetic factors in graft rejection and enhancing tolerance-inducing capabilities. | Personalizing transplantation medicine and improving compatibility between donors and recipients. |
| Biomaterials and Scaffold-Based Approaches [27] |
Application of biomaterials and scaffolds in tissue engineering and organ regeneration. | Offering structural support, fostering cell integration, and creating functioning tissues for transplantation. | Addressing issues with organ scarcity and improving the likelihood of successful transplantation. |
| Precision Medicine in Transplantation [28,29] |
Customizing treatment strategies according to specific individual traits. Includes analyzing genetic information, identifying specific biological markers, and utilizing sophisticated diagnostic instruments. | Enhancing immunosuppressive treatments, forecasting individual patient reactions, and enhancing transplant results. |
Customized treatment programs leading to improved patient results. |
| Artificial Intelligence and Machine Learning Applications [30,31] |
Integration of AI and ML for data analysis, risk prediction, and decision support. |
Improving the analysis of intricate datasets, enhancing organ matching algorithms, and forecasting post-transplant results. | Refining clinical decision-making and improving overall transplantation processes. |
| Cellular Therapy | Description | Mechanism of Action | Therapeutic Potential |
|---|---|---|---|
| Mesenchymal Stem Cells (MSCs) [32,33] |
Adaptable cell instruments with immune system-regulating characteristics. Display anti-inflammatory properties, inhibit immunological reactions against foreign tissue, and enhance tissue healing. |
Improving the success of transplanted tissue, reducing immune-related issues in both self and donor transplant scenarios. |
Current research focuses on enhancing the effectiveness of MSC therapy and its use in different transplantation situations. |
| Regulatory T Cells (Tregs) [34,35] |
Contribute significantly to immunological tolerance by inhibiting exaggerated immune reactions. Has the capacity to promote immunological tolerance, lower the chance of graft rejection, and decrease the necessity for immunosuppressive medications. | Utilizing Tregs for cellular treatment in transplantation. Methods to increase and include Tregs for enhanced therapeutic effectiveness. |
Actively researching Treg-based medicines in transplantation and improving procedures for wider use. |
| Natural Killer (NK) Cell Therapies [36,37] |
Main components of the innate immune system. Strive to utilize cytotoxic powers to target and eliminate alloreactive immune cells, hence minimizing the chance of graft rejection. | Studying methods using ex vivo expanded or genetically engineered NK cells for medicinal effectiveness. | Research is centered on comprehending NK cell activity in transplantation and enhancing strategies for clinical use. |
| Induced Pluripotent Stem Cells (iPSCs) [38,39] |
Revolutionary in the field of regenerative medicine. Convert somatic cells into pluripotent stem cells to create tissues and organs tailored for transplantation in individual patients. |
Possibilities for customized and immunologically compatible organ transplants despite safety and tumor formation issues. |
Current research focuses on improving iPSC-based methods, addressing safety issues, and broadening their use in transplantation. |
| Future directions and challenges | Modalities | Description |
|---|---|---|
| Potential Future Breakthroughs |
|
The development of CRISPR-based gene editing technologies has the potential to significantly impact transplantation therapies. Researchers want to create individualized immunomodulation by modifying genes linked to graft rejection to overcome immunological obstacles. Continued research on induced pluripotent stem cells (iPSCs) and 3D bioprinting to address the scarcity of organs. Advancements in organ regeneration and bioengineering may lead to the development of personalized organs, offering a long-term solution to the shortage of available organs. AI and ML integration in transplantation medicine for a shift towards precision medicine. AI algorithms can examine large datasets, anticipate individual patient reactions, and improve organ matching, thereby improving therapeutic approaches and outcomes. [74] |
| Anticipated Challenges and Hurdles |
|
The use of genetic engineering, particularly CRISPR-based technology, raises significant ethical issues. Discussions on the ethical considerations of genome manipulation for transplantation, such as unforeseen outcomes and social effects, provide significant problems that demand thorough ethical examination. The adoption of advanced treatments like gene editing and customized medicine poses issues regarding affordability and availability. To achieve fair access, we must tackle financial obstacles, develop efficient reimbursement methods, and reduce healthcare inequalities. Although bioengineered organs show potential, there are still worries about their extended safety and longevity. Challenges in stem cell-derived tissues involve vascularization, immunological compatibility, and the risk of tumorigenicity. Thorough, extended research is essential for evaluating long-lasting effectiveness and safety. |
| Collaboration and Interdisciplinary Approaches for Advancement |
|
Tackling complex issues and achieving advancements in transplantation treatments by working together across many disciplines. The convergence of expertise from several domains such as immunology, genetics, bioengineering, ethics, and data science is crucial for creating holistic solutions. Creating collaborative study groups and worldwide projects to combine resources, knowledge, and data. Collaborative clinical trials and setting worldwide standards can create a united effort to address issues and make advancements in transplantation medicine. It is crucial to include the viewpoints and experiences of patients and the general public for the success of future developments in transplantation treatments. Involving patients in research, incorporating their perspectives into study planning, and maintaining clear communication help create patient-focused solutions and build trust in new technology. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





