1. Introduction
Cardiac autonomic neuropathy (CAN) is a severe complication of diabetes mellitus (DM) strongly linked to a nearly five-fold higher risk of cardiovascular mortality [
1]. Factors leading to diabetic complications, such as genetic predisposition, environmental signals, insulin resistance, immune dysfunction and inflammation, intracellular fuel excess, and comorbidities, have a similar impact on both microvascular and macrovascular complications [
2]. Elucidation of the common mechanism of cell damage in DM put to rest any misconceptions that microvascular disease and macrovascular disease constitute distinct disease entities [
3,
4].
CAN represents a manageable complication of T2DM, posing an elevated risk of cardiovascular mortality [
5]. Patients with CAN have a high degree of underdiagnosis. This may occur because of a lack of physician awareness of the disease, the often late onset mild or absent symptoms of the disease, and the lack of specific CAN diagnostic tests [
6]
. Various cross-sectional studies conducted in Europe and the United States have revealed a wide range of prevalence rates. For instance, studies have shown rates ranging from 16.7% in insulin-dependent diabetic patients participating in the Diabetes Control and Complications Trial (DCCT) cohort to as high as 60% in a community-based sample of type 2 diabetic patients over the age of 65 in Rochester, Minnesota [
7,
8].
Among the many diagnostic tools available, Ewing and Sudoscan stand out as promising methods for evaluating autonomic neuropathy and its implications for cardiovascular health. Patients with Type 2 Diabetes Mellitus (T2DM) are a significant cohort in which these assessments have particular relevance to the increased cardiovascular risk inherent in the condition. Conditions like ischemic heart disease and stroke contribute significantly to the global burden of public health as they rank as the primary causes of mortality worldwide. Annually, cardiovascular diseases (CVDs) account for approximately 17.9 million deaths, making up 31% of all global fatalities [
9]. Therefore, it is imperative that public health policies prioritize cardiovascular prevention, as well as the early identification and management of established cardiovascular risk factors (CVRF), such as dyslipidemia, T2DM, and hypertension [
10]. Cardiovascular diseases represent the primary contributors to both morbidity and mortality among individuals diagnosed with T2DM [
11].
In patients aged ≥40 years with T2DM without coronary artery atherosclerotic disease (ASCVD) or severe target organ damage (TOD), it is recommended to estimate the 10-year cardiovascular risk using the SCORE2-Diabetes algorithm. In the 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice, the ADVANCE or DIAL models have been suggested for estimating cardiovascular disease risk in patients with diabetes [
12,
13,
14]. These models have been developed based on a limited set of studies and have not been systematically recalibrated to contemporary rates of cardiovascular disease, meaning they are not ideal for use in contemporary European populations. To address these limitations, the current guidelines recommend using the SCORE2-Diabetes model, which extends the regionally recalibrated European SCORE2 10-year risk model to enable use in individuals aged 40 to 69 years with T2DM without ASCVD or severe TOD, and to estimate the individual 10-year risk of fatal and non-fatal cardiovascular events [
15].
On the other hand, a useful tool in detecting chronic microvascular complications of type 2 diabetes mellitus, which represent additional CVRF, is the measure of the electrochemical activity of the sweat glands in the hands and feets, using Sudoscan. Its results can provide valuable information for diagnosing and monitoring neuropathy and assessing cardiovascular risk in diabetic patients [
8].
CAN, a prevalent and thoroughly studied type of diabetic autonomic neuropathy, is of particular concern due to its life-threatening implications, such as arrhythmias, silent myocardial ischemia, and sudden death [
16]. The prevalence of CAN is expected to rise due to the diabetes epidemic and its early and widespread onset. CAN plays a definite prognostic role in cardiovascular mortality and morbidity.
Managing T2DM demands a comprehensive strategy encompassing lifestyle adjustments and glycemic regulation, alongside mitigating cardiovascular risk factors through targeted interventions informed by cardiovascular risk assessments [
17]. Additionally, employing glucose-lowering medications known for their cardiovascular benefits, such as SGLT2 inhibitors [
18] and GLP-1 receptor agonists [
19], is essential.
This study aimed to explore the subtle correlation between the Ewing test, Sudoscan- cardiovascular autonomic neuropathy score, and cardiovascular risk calculated using SCORE 2 Diabetes in individuals with T2DM. By elucidating this correlation, we aim to shed light on new avenues for comprehensive cardiovascular risk assessment.
2. Materials and Methods
This study was cross-sectional, effectuated between June 2019 and June 2020. Ethical approval for the study was obtained from the local Ethics Committee of “Nicolae Malaxa” Clinical Hospital. Informed consent was obtained from all participating patients.
Study Population
The inclusion criteria encompass patients diagnosed with T2DM, and overweight/obese individuals aged 40 to 69 years. Conversely, exclusion criteria involve patients who have not signed informed consent, those with other diabetes types (type 1 diabetes, latent autoimmune diabetes of adults, maturity-onset diabetes of the young), individuals aged below 40 years or above 69 years, pregnant women, patients diagnosed with neoplasms within the past five years, those with stroke sequelae, history of myocardial infarction, pelvic limb amputations, pre-existing chronic kidney disease predating diabetes diagnosis, and patients with neuropathy caused by alternative factors (alcoholism, vitamin B12 deficiency).
Examination of patients:Data on anthropometric indices, including height, weight, body mass index (BMI), waist circumference, waist-to-hip ratio, blood pressure values in supine and orthostatic positions, heart rate in supine and orthostatic positions, and smoking status, were recorded.
Measurement of biochemical parameters:The following samples were collected from venous plasma after 8 hours of fasting: serum glucose, glycated hemoglobin HbA1c, total cholesterol, high-density lipoproteins cholesterol (HDLc), LDL cholesterol, triglycerides, bilirubin, C-reactive protein, serum creatinine, potassium, magnesium, chloride, sodium, calcium, urea, glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), gamma-glutamyl transferase (GGT) and urinary albumin-to-creatinine ratio (ACR).
Diagnosis of CAN
The evaluation of cardiac autonomic neuropathy involved conducting an electrocardiogram and assessing the QTc interval in the morning prior to medication intake. CART (cardiovascular reflex tests) was also performed, encompassing assessments of heart rate variability during deep breathing, the Valsalva maneuver, and orthostatic changes. Furthermore, measurements of orthostatic systolic and diastolic blood pressure will be conducted during an isometric effort. The Heart rate variability index (HRVi) was also measured. The results of CART were categorized as usual if no abnormal findings were detected. They were considered to indicate mild dysfunction if one out of the five tests was abnormal, moderate dysfunction if two or three of the tests were abnormal, and severe dysfunction if more than three tests were abnormal. All these measurements were performed using the ESP-01-PA Ewing Tester neuropathic measuring and analyzing system with an ECG module. At the same time, the diagnosis of CAN was evaluated by performing a sweat test using Sudoscan assessment. During the test, the patient places their hands and feet on the electrodes. The test takes 3 minutes to perform, is painless, and requires no subject preparation. Additionally, Sudoscan incorporates built-in algorithms that integrate electrochemical skin conductance with age to generate a score estimating the current risks of CAN (Sudoscan-CAN score) and chronic kidney disease (Sudoscan-Nephro score).
We have also calculated the SCORE2-Diabetes for the patients included in the study. SCORE2-Diabetes is a new algorithm developed, calibrated, and validated to predict 10-year risk of CVD in individuals with type 2 diabetes that enhances identification of individuals at higher risk of developing CVD across Europe [
20]. Sex-specific competing risk-adjusted models were used, incorporating traditional risk factors, such as age, smoking, systolic blood pressure, total and HDL-cholesterol alongside diabetes-related variables including age at diabetes onset, glycated hemoglobin (HbA1c), and estimated glomerular filtration rate (eGFR) based on creatinine.
The statistical analysis of the population was conducted using IBM SPSS v.20. Continuous variables usually distributed were presented as mean ± SD (standard deviation), and non-normal variables were expressed as median (interquartile range [IQR]). In contrast, categorical variables were reported as absolute counts and percentages. Statistical significance was determined at a 95% confidence interval. Analysis of variance (ANOVA) was employed for comparisons among groups for quantitative variables, while the χ2 test was utilized for categorical variables—multiple linear regression was used to estimate the independent correlation of the SCORE2-Diabetes risk with results of Sudoscan parameters.
3. Results
The cohort comprised 211 patients diagnosed with T2DM, without established ASCVD, 51.6% being male (n=109), with a mean age of 58.35±7.18 years and a mean weight of 90.36±17 kg. The prevalence of CAN in our group was 67.2% (n=142).
In the study group, according SCORE2-Diabetes, four patients (1.9%) was classified with moderate cardiovascular risk, thirty-five (16.6%) with high risk, and one hundred seventy-two (81.5%) with very high cardiovascular risk. Patient’s baseline characteristics are presented in
Table 1.
Patiets with very high cardiovascular risk were older and with longer diabetes duration. However, there were no notable differences in height, weight, or waist circumference across risk categories. Elevated levels of FPG and HbA1c were observed in higher-risk groups. Lipid levels, including TC, HDL-c, TGL, and LDL-c, did not significantly vary among risk groups. Lower eGFR was decreased parallel with increased CVR categories. While GGT showed a marginal difference across risk groups, overall, factors such as age, diabetes duration, FPG, HbA1c, and eGFR were identified as key indicators of cardiovascular risk in diabetic patients (
Table 1).
There are significant variations in SBP while lying down among the three risk categories, with higher levels detected in higher-risk groups (p=0.041). There's no notable difference in DBP in the lying position (p= 0.065). There are no substantial differences in SBP and DBP while standing across the risk categories (p>0.05). During handgrip exercises, SBP doesn't significantly differ across risk groups (p=0.494), yet there's a marginal difference in DBP (p=0.073) and a significant difference in heart rate (p=0.046) (
Table 2).
The statistical analysis of Sudoscan's parameters in relation to cardiovascular risk reveals significant differences. Specifically, the Sudoscan CAN-score exhibits significant variance among risk groups (p=0.002), indicating a potential correlation between sudomotor dysfunction and cardiac autonomic neuropathy in individuals at higher risk of cardiovascular complications. Similarly, the Sudoscan Nephro-score displays a notable difference across risk categories, with lower scores observed in higher-risk groups (p=0.001), suggesting a potential link between sudomotor dysfunction and renal function in individuals with increased cardiovascular risk. However, scores for Sudoscan parameters related to the feet and hands do not show significant differences across risk categories. These findings suggest that Sudoscan-derived measures may serve as valuable indicators of cardiovascular risk, particularly in assessing cardiac autonomic function and nephropathy in diabetic populations (
Table 3).
The statistical analysis of the frequency of chronic diabetes complications based on cardiovascular risk indicates significant differences among the various risk categories. For cardiovascular autonomic neuropathy (CAN), the prevalence increases with the degree of cardiovascular risk, with the highest frequency observed in those with a risk greater than 20% (69.80%). The same trend is observed for diabetic polyneuropathy (DPN), chronic kidney disease (CKD), and diabetic retinopathy (DR), where the frequency of complications significantly increases with higher cardiovascular risk. These findings underscore the importance of proper monitoring and management of cardiovascular risk in addressing chronic diabetes complications (
Table 4).
Correlation of Sudoscan with SCORE 2 - Diabetes
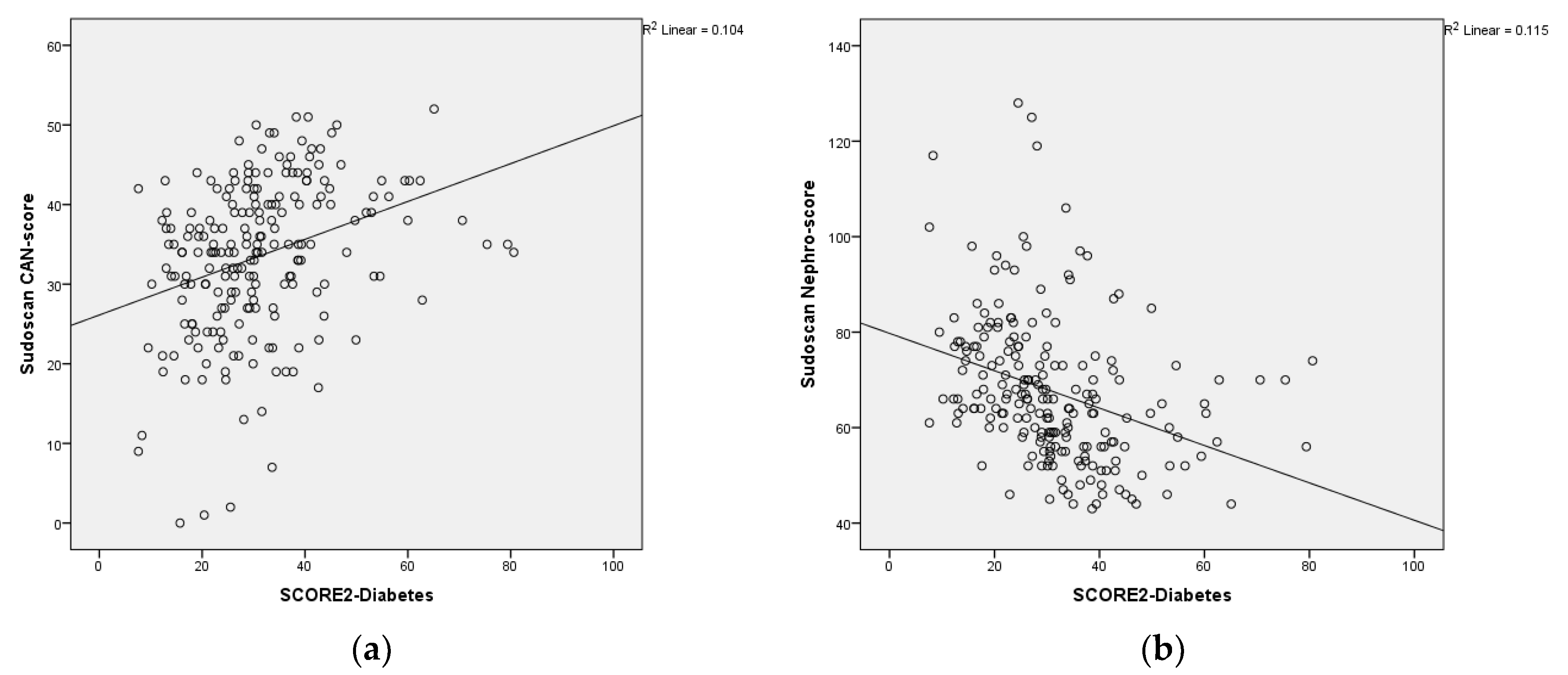
The scatterplot shows the relationship between the Sudoscan CAN-score, Sudoscan Nephro-score and the SCORE2-Diabetes. Sudoscan CAN-score showed a signiflicantly positive correlation with SCORE 2-Diabetes and, Sudoscan Nephro-score score showed a significantly negative correlation with SCORE 2-Diabetes (
Figure 1a,b).
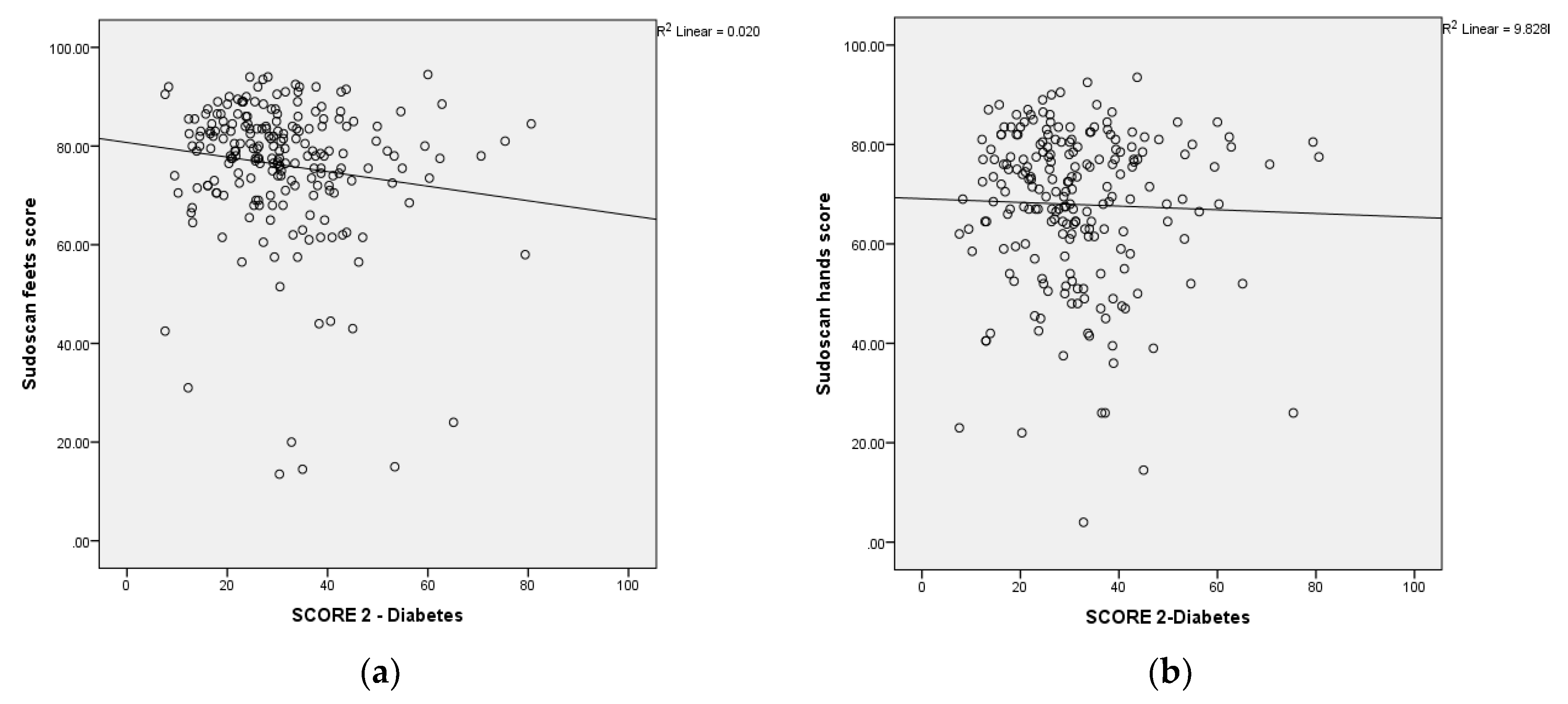
The scatterplot shows the relationship between the Sudoscan feets-score, Sudoscan hands-score and the SCORE2-Diabetes. Sudoscan feets-score and Sudoscan hands-score showed a significantly negative correlation with SCORE 2-Diabetes (
Figure 2a,b).
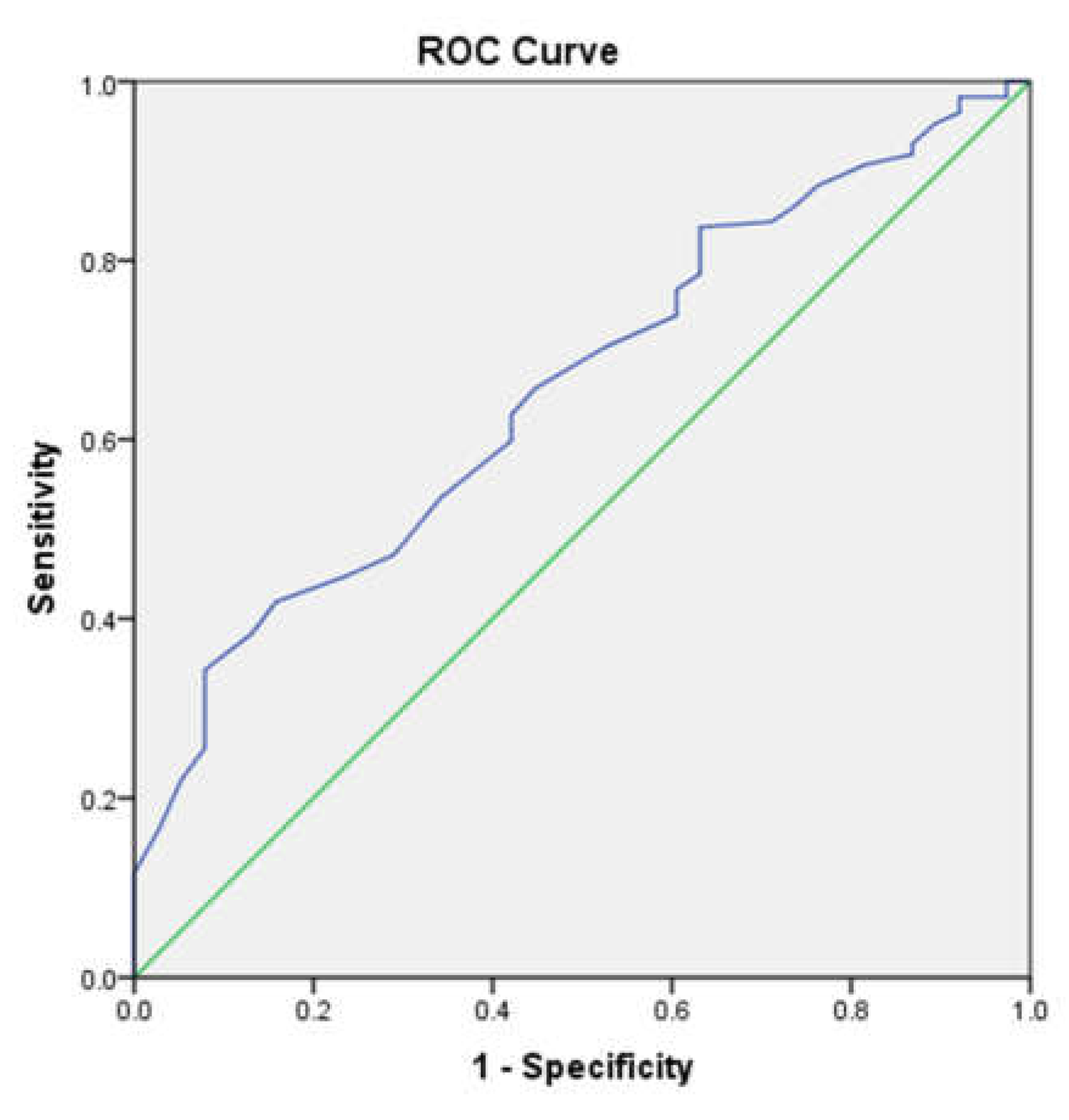
The area under the receiver operating characteristic (ROC) curve of the Sudoscan-CAN score to predict very high cardiovascular risk was 0.657 (95%CI: 0.569-0.745) of the total square (
Figure 3). The Sudoscan-CAN score cut-off was 39.5, and the test had 34.3% sensitivity and 79% specificity to detect very high cardiovascular risk.
The multiple linear regression analysis examining clinical factors associated with SCORE2-Diabetes in patients with T2DM reveals several significant associations. Age and diabetes duration exhibit positive correlations with SCORE2-Diabetes, with standardized β-coefficients of 0.413 and 0.179, respectively, both statistically significant (p < 0.001). Similarly, HbA1c, LDL-c Sudoscan feet score, Sudoscan CAN-score, Ewing test score, and SBP in the supine position also demonstrate positive associations with SCORE2-Diabetes, with significant p-values (<0.05). Conversely, eGFR and Sudoscan Nephro-score display negative associations with SCORE2-Diabetes, suggesting that higher eGFR and Sudoscan Nephro-scores are associated with lower SCORE2-Diabetes values. These findings highlight the importance of age, diabetes duration, HbA1c, LDL-c, Sudoscan parameters, eGFR, and SBP in predicting SCORE2-Diabetes in patients with T2DM (
Table 5).
1. Discussion
The findings of the study highlight the significant cardiovascular risk faced by patients diagnosed with T2DM. The prevalence of CAN was significant within our studied patient cohort, being 67.2%. What is important to mention is that 69.8% of patients with very high cardiovascular risk also associate CAN. With a substantial portion of the cohort (81.5%) classified as having very high cardiovascular risk according to SCORE2-Diabetes, it underscores the critical need for comprehensive risk assessment and management strategies in this population.
In a meta-analysis conducted in 2003, Maser et al. synthesized the evidence base to evaluate the relationship between CAN and the risk of mortality in diabetes. CAN was associated with future risk of mortality both in cases of definite CAN and possible CAN, with a stronger association observed in definite CAN cases [
21].
Also, Ewing et al. illustrated a 2.5-year mortality rate of 27.5%, which escalated by 25.5% after 5 years in patients with diabetes and definite CAN [
22]. This stands in stark contrast to patients with diabetes and a normal autonomic function test (AFT), who exhibited a mortality rate of only 15% over the same 5-year period. Additionally, CAN also serves as a prognostic indicator for cardiovascular events (CVE) and mortality in the context of intensive glycemic control in type 2 diabetes. This was evidenced by the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), Veterans Affairs Diabetes Trial (VADT), and ACCORD Studies [
23,
24,
25].
Our study introduces the utilization of the Ewing Test and Sudoscan Cardiovascular Autonomic Neuropathy Score as adjunctive tools for assessing cardiovascular risk in T2DM patients. This integration of novel assessment methods provides a more comprehensive evaluation beyond traditional risk scoring algorithms, potentially offering additional insights into cardiovascular health in this population.
Vinik et al. explored the correlation between CAN and subsequent cardiovascular events. They analyzed fatal or non-fatal incidents such as myocardial infarction, heart failure, ventricular tachycardia or fibrillation necessitating resuscitation, angina pectoris, or the need for coronary revascularization. They discovered a link between CAN and major cardiovascular events and found that the relative risks associated with CAN was 3.4 (p<0.05) [
26].
The observed correlations between Sudoscan CAN-score and Sudoscan Nephro-score with SCORE2-Diabetes shed light on the interplay between autonomic neuropathy, renal function, and cardiovascular risk in T2DM patients. The positive correlation of Sudoscan CAN-score with cardiovascular risk suggests a potential association between autonomic dysfunction and increased cardiovascular risk. The identification of significant associations between various clinical parameters and SCORE2-Diabetes underscores the multifactorial nature of cardiovascular risk in T2DM patients. This highlights the importance of comprehensive risk stratification strategies that consider not only traditional risk factors but also novel markers such as autonomic function and renal health in guiding therapeutic interventions and optimizing outcomes.
The findings regarding the correlation between Sudoscan feets-score and Sudoscan hands-score with SCORE2-Diabetes suggest a potential role for Sudoscan as a non-invasive tool for assessing peripheral neuropathy in T2DM patients and predicting cardiovascular risk.
Further research is warranted to elucidate the clinical utility of Sudoscan scores in risk stratification and guiding therapeutic interventions in this population.
While the study provides valuable insights into the relationship between Ewing Test, Sudoscan scores, and cardiovascular risk in T2DM patients, certain limitations need to be acknowledged. Firstly, these include the relatively small sample size and cross-sectional design. Secondly, all participants included in our research are from Romania, a very-high-risk European region in terms of CV mortality [
27]. Also, the study enrolled patients from a single center.
Future prospective studies with larger sample sizes and longitudinal follow-up are needed to validate these findings and explore the utility of novel assessment tools in improving cardiovascular outcomes in T2DM patients.
6. Conclusions
The impact of microvascular damage in diabetic patients may contribute to the development of cardiovascular diseases. Sudomotor dysfunction is a marker of this damage and may help the identification of high-risk patients. As a measure of sudomotor function, Sudoscan represents a potentially valuable and non-invasive method of assessing microvascular function. Studies have demonstrated that Sudoscan can detect early peripheral neuropathy in diabetic and pre-diabetic patients, with correlation to nerve fiber density in skin biopsy samples. The predictive accuracy and diagnostic performance of Sudoscan in identifying cardiovascular risk in diabetic and other patients at risk of microvascular damage is yet to be clearly established. This is the first study to evaluate the relationship between Sudoscan and cardiovascular risk. We have established that Sudoscan is a reliable measure of measuring sudomotor function in patients with Type 2 diabetes. Considering that nearly 70% of patients with very high cardiovascular risk associate with CAN, it is important to assess this diagnosis through a rapid diagnostic method. This supports our hypothesis that increased cardiovascular risk is associated with sudomotor damage and that Sudoscan is an effective and non-invasive measure of identifying such risk.
Author Contributions
Conceptualization, A.E.N and E.R; methodology A.E.N, C.D, G.R.; software A.E.N, E.R; validation, A.E.N, C.D. and G.R.; formal analysis, A.E.N, E.R; investigation, F.R.; resources, A.E.N., E.R, C.D. and O.A.P; data curation, C.S, O.A.P; writing—original draft preparation, A.E.N and E.R; writing—review and editing, A.E.N, E.R.; visualization, F.R.; supervision, G.R.; project administration, A.E.N.; funding acquisition, A.E.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of “Nicolae Malaxa” Clinical Hospital, Bucharest, Romania (approval number 2145 on 7 March 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the hospital’s privacy policy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Serhiyenko VA, Serhiyenko AA. Cardiac autonomic neuropathy: Risk factors, diagnosis and treatment. In World J Diabetes [Internet]. 2018 Jan 1 [cited 2024 Apr 11];9(1):1. Available from: /pmc/articles/PMC5763036/. [CrossRef]
- Schwartz SS, Epstein S, Corkey BE, Grant SFA, Gavin III JR, Aguilar RB, et al. A Unified Pathophysiological Construct of Diabetes and its Complications. In Trends in Endocrinology & Metabolism, 2nd ed.; Editor 1, A., Editor 2, B., Eds.; Publisher: Publisher Location, Country, 2017; Volume 28, pp. 645–55.
- Shah MS, Brownlee M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. In Circ Res [Internet]. 2016 May 27 [cited 2024 Apr 16];118(11):1808–29. Available from: https://pubmed.ncbi.nlm.nih.gov/27230643/.
- Brownlee, M. The Pathobiology of Diabetic Complications A Unifying Mechanism. In Banting Lecture [Internet]. 2004 [cited 2024 Apr 16]; Available from: http://diabetesjournals.org/diabetes/article-pdf/54/6/1615/381945/zdb00605001615.pdf.
- Nica AE, Rusu E, Dobjanschi CG, Rusu F, Parliteanu OA, Sivu C, et al. The Importance of Evaluating Sudo-motor Function in the Diagnosis of Cardiac Autonomic Neuropathy. In Cureus [Internet]. 2024 Mar 29 [cited 2024 Apr 17];16(3). Available from: https://www.cureus.com/articles/239801-the-importance-of-evaluating-sudomotor-function-in-the-diagnosis-of-cardiac-autonomic-neuropathy.
- Bönhof GJ, Herder C, Ziegler D. Diagnostic Tools, Biomarkers, and Treatments in Diabetic polyneuropathy and Cardiovascular Autonomic Neuropathy. In Curr Diabetes Rev. 2022 Jun;18(5). [CrossRef]
- Hicks CW, Wang D, Matsushita K, Windham BG, Selvin E. Peripheral Neuropathy and All-Cause and Car-diovascular Mortality in US Adults.
- Eleftheriadou A, Williams S, Nevitt S, Brown E, Roylance R, Wilding JPH, et al. The prevalence of cardiac autonomic neuropathy in prediabetes: a systematic review. [cited 2024 Apr 11]. [CrossRef]
- Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. In The Lancet. 2018 Nov;392(10159):1736–88. [CrossRef]
- Caussy C, Aubin A, Loomba R. The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. In Curr Diab Rep. 2021 May 19;21(5):15. [CrossRef]
- Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. In Nat Rev Cardiol. 2023 Oct 16;20(10):685–95. [CrossRef]
- Berkelmans GFN, Gudbjörnsdottir S, Visseren FLJ, Wild SH, Franzen S, Chalmers J, et al. Prediction of indi-vidual life-years gained without cardiovascular events from lipid, blood pressure, glucose, and aspirin treatment based on data of more than 500 000 patients with Type 2 diabetes mellitus. In Eur Heart J. 2019 Sep 7;40(34):2899–906. [CrossRef]
- Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardio-vascular disease prevention in clinical practice. In Eur Heart J. 2021 Sep 7;42(34):3227–337.
- Kengne AP, Patel A, Marre M, Travert F, Lievre M, Zoungas S, et al. Contemporary model for cardiovascular risk prediction in people with type 2 diabetes. In European Journal of Cardiovascular Prevention & Rehabilita-tion. 2011 Jun 28;18(3):393–8. [CrossRef]
- Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. In Eur Heart J. 2023 Oct 14;44(39):4043–140. [CrossRef]
- Gavan DE, Gavan A, Bondor CI, Florea B, Bowling FL, Inceu GV, et al. SUDOSCAN, an Innovative, Simple and Non-Invasive Medical Device for Assessing Sudomotor Function. In Sensors. 2022 Oct 6;22(19):7571. [CrossRef]
- Wing RR, Bahnson JL, Bray GA, Clark JM, Coday M, Egan C, et al. Long-term Effects of a Lifestyle Interven-tion on Weight and Cardiovascular Risk Factors in Individuals With Type 2 Diabetes Mellitus: Four-Year Results of the Look AHEAD Trial. In Arch Intern Med [Internet]. 2010 Sep 27 [cited 2024 Mar 17];170(17):1566–75. Available from: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/226013.
- Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic re-view and network meta-analysis of randomised controlled trials. In BMJ [Internet]. 2021 Jan 13 [cited 2024 Mar 17];372. Available from: https://www.bmj.com/content/372/bmj.m4573. [CrossRef]
- Marsico F, Paolillo S, Gargiulo P, Bruzzese D, Dell’Aversana S, Esposito I, et al. Effects of glucagon-like pep-tide-1 receptor agonists on major cardiovascular events in patients with Type 2 diabetes mellitus with or without established cardiovascular disease: a meta-analysis of randomized controlled trials. In Eur Heart J [In-ternet]. 2020 Sep 14 [cited 2024 Mar 17];41(35):3346–58. [CrossRef]
- Pennells L, Kaptoge S, Østergaard HB, Read SH, Carinci F, Franch-Nadal J, et al. SCORE2-Diabetes: 10-year cardiovascular risk estimation in type 2 diabetes in Europe. In Eur Heart J. 2023 Jul 21;44(28):2544–56. [CrossRef]
- Maser RE, Mitchell BD, Vinik AI, Freeman R. The Association Between Cardiovascular Autonomic Neurop-athy and Mortality in Individuals With Diabetes A meta-analysis. In Diabetes Care [Internet]. 2003 [cited 2024 Apr 16];26:1895–901. Available from: http://diabetesjournals.org/care/article-pdf/26/6/1895/591627/dc0603001895.pdf. [CrossRef]
- Ewing DJ, Campbell IW, Clarke BF. MORTALITY IN DIABETIC AUTONOMIC NEUROPATHY. In The Lancet. 1976 Mar 20;307(7960):601–3. [CrossRef]
- Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, et al. Effects of Cardiac Autonomic Dysfunction on Mortality Risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial AND THE ACCORD STUDY GROUP*. 2010 [cited 2024 Apr 16]; Available from: http://creativecommons.
- Pop-Busui, R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. In Diabetes Care [Internet]. 2010 Feb [cited 2024 Apr 16];33(2):434–41. Available from: https://pubmed.ncbi.nlm.nih.gov/20103559/. [CrossRef]
- Zoungas S, Arima H, Gerstein HC, Holman RR, Woodward M, Reaven P, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual partici-pant data from randomised controlled trials. In Lancet Diabetes Endocrinol. 2017 Jun;5(6):431–7. [CrossRef]
- Vinik AI, Ziegler D. Diabetic Cardiovascular Autonomic Neuropathy. In 2007 [cited 2024 Apr 16]; Available from: http://www.circulationaha.org.
- Luca SA, Bungau RM, Lazar S, Potre O, Timar B. To What Extent Does Cardiovascular Risk Classification of Patients with Type 2 Diabetes Differ between European Guidelines from 2023, 2021, and 2019? A Cross-Sectional Study. In Medicina 2024, Vol 60, Page 334 [Internet]. 2024 Feb 16 [cited 2024 Apr 17];60(2):334. Available from: https://www.mdpi.com/1648-9144/60/2/334/htm. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).