1. Introduction
Mouse spermatozoa are refractory to sperm head DNA damage and can be thus easily stored with different approaches. In the mouse, for example, the offspring were born even when spermatozoa were recovered from cadavers kept in the freezer (– 80oC) for up to twelve months [
1], from frozen testicular sections [
2], from spermatozoa frozen without cryoprotectants [
3], or when lyophilized spermatozoa were used for ICSI [
4]. Eventually the spermatozoa stored in the fridge in different simple solutions can give rise to offspring [
5]. Therefore, although apparently “dead”, their DNA is not seriously damaged and when injected into metaphase II (MII) oocytes the sperm head decondenses and forms the paternal pronucleus (male PN; mPN) that contributes to normal embryo development.
The spermatozoa of other mammalian species are not so extensively characterized when compared to the mouse. Moreover, it seems, that the resistance to DNA damage is much lower in male germ cells of some other mammalian species even when their spermatozoa are frozen with classical, conventional schemes [
6]. An excellent example is the horse and related species like the rhinoceros where in some males the spermatozoa show very low resistance even to conventional freezing (“poor freezers”) while some others can be conserved relatively well (“good freezers”) [
7,
8].
There are several approaches how to assess the sperm DNA damage [
9]. However, these approaches typically need a high number of spermatozoa. The interspecific ICSI is another possible approach that can be used when the number of spermatozoa is quite low or they are difficult to be isolated (i.e. from frozen sections or from permafrost cadavers) and also because the ovulated oocytes of given species are not readily available and if so, they must be typically matured in vitro and this decreases their quality [
10,
11,
12]. Beside this, the ICSI approach does not work well in domestic animal (bovine, ovine, porcine, etc.) – the horse is the exception although even here the results are still suboptimal [
13].
It has been shown that after xenogenic ICSI the injected sperm heads decondense and form mPNs in which the level of DNA damage can be assessed by labeling the zygotes with anti-γH2AX antibody [
10]. Moreover, the interspecific zygotes can be karyotyped during the first mitotic division where abnormal karyotypes also indicate DNA damage [
12]. Also, the presence of micronuclei (MNs) as a relevant criterium can be used in the two-cell stage interspecific embryos as it has been shown that MNs are very frequent in embryos originating from oocytes fertilized with sperm with damaged DNA [
14].
In our experiments we have used the xenogenic ICSI (frozen horse sperm x mouse oocytes) for the assessment of DNA damage after sperm freezing/thawing. We do believe that this approach can accelerate the progress of sperm freezing optimization in the horse, to better define good and bad freezers as well as to find some alternative approaches that can lead to satisfactory long-term sperm storage in those species where these approaches were not yet tested.
2. Materials and Methods
Mouse metaphase II (MII) oocytes were obtained from PMSG (5 I.U., Bioveta, Ivanovice, CZ) stimulated BDF1 females that were induced to ovulate by applying hCG (5 I.U., Sigma, Prague, CZ) approximately 44 - 46 h after PMSG. The oocytes were isolated from oviduct ampullae after about 15 h post hCG. Their cumuli were dissolved in Hyaluronidase/M2 (Merck, Prague, CZ) solution and only healthy-looking oocytes were incubated in KSOM (Merck, Prague, CZ) at 5% CO2 in air/37oC before used for ICSI.
Horse frozen spermatozoa were obtained from four stallions. After thawing, when evaluated with Casa, the stallion I. has been classified as a good freezer (motility about 50%) whilst stallions II., III. and IV. as bad freezers (motility less than 10%). From every stallion we had several frozen semen doses from independent collections.
As controls we used conventionally (LN2) frozen epididymal mouse spermatozoa (motile and immotile after thawing) and immotile spermatozoa kept in PBS/BSA [
5].
Sperm heads isolated either from immotile or motile mouse/horse spermatozoa were injected into mouse MII oocytes essentially as described previously [
10,
15]. The injected oocytes (zygotes) used for γH2AX labelling, i.e. to detect DNA damage, were then incubated in KSOM medium (Merck, Prague, CZ) for about 5 – 6h, i.e. before the onset of DNA replication (S-phase). Then their zonae pellucidae were dissolved in Acid Tyrode´s solution (Merck, Prague, CZ) and the zygotes were fixed in 4% paraformaldehyde in phosphate buffered saline (PFA/PBS) for 15 minutes and then kept in PBS until further use. For DNA damage evaluation we labelled the zygotes with anti-γH2AX antibody. Zygotes were first permeabilized in 0.5% Triton X-100 (TX100) in PBS for 15 minutes. Unspecific antibody binding was then prevented by incubating the zygotes in PBS supplemented with 1% bovine serum albumin (PBS/BSA) for 2-3 hrs. Thereafter the specimens were incubated with the first antibody (anti-γH2AX 1:100 in 0.2%TX100/PBS/1%BSA; Upstate, Merck, Prague, CZ) overnight at +4oC. Then the zygotes were washed several times in 0.2%TX100/PBS/1%BSA and transferred into the second antibody (FITC conjugated donkey anti-mouse IgG 1:200; Jackson Immuno Research, Cambridge, U.K. or Alexa Fluor 488 donkey anti mouse IgG 1:1000; AbCam, Cambridge, U.K.) and incubated in it for 2hrs. Then the zygotes were washed several times in PBS/BSA and mounted in ProLong Gold antifade reagent with DAPI (Invitrogen/ThermoFisher Scientific, Prague, CZ) and inspected and evaluated under the appropriate fluorescence on IX71 Olympus microscope.
For micronuclei (MNs) assessment the intra- and inter-specific zygotes with both pronuclei (mPN and fPN) were cultured in KSOM for approximately 24 hrs and then fixed in 4% PFA/0.1%TX100/PBS for 15 min. Before labeling, the samples were kept in PBS at +4°C. The presence of MNs was detected by labeling the nuclei/MNs with anti-lamin B antibody (1:200, Santa Cruz, Dallas, TX, U.S.A.), as the second antibody we used (Texas Red conjugated donkey anti-rabbit IgG, 1: 200, Jackson Immuno Research, Cambridge, U.K.) The procedure of labeling was essentially similar as for γH2AX.
The experiments were repeated at least three times.
Unless stated otherwise all chemicals were purchased from Merck/Sigma Aldrich, Prague, Czech Republic.
3. Results
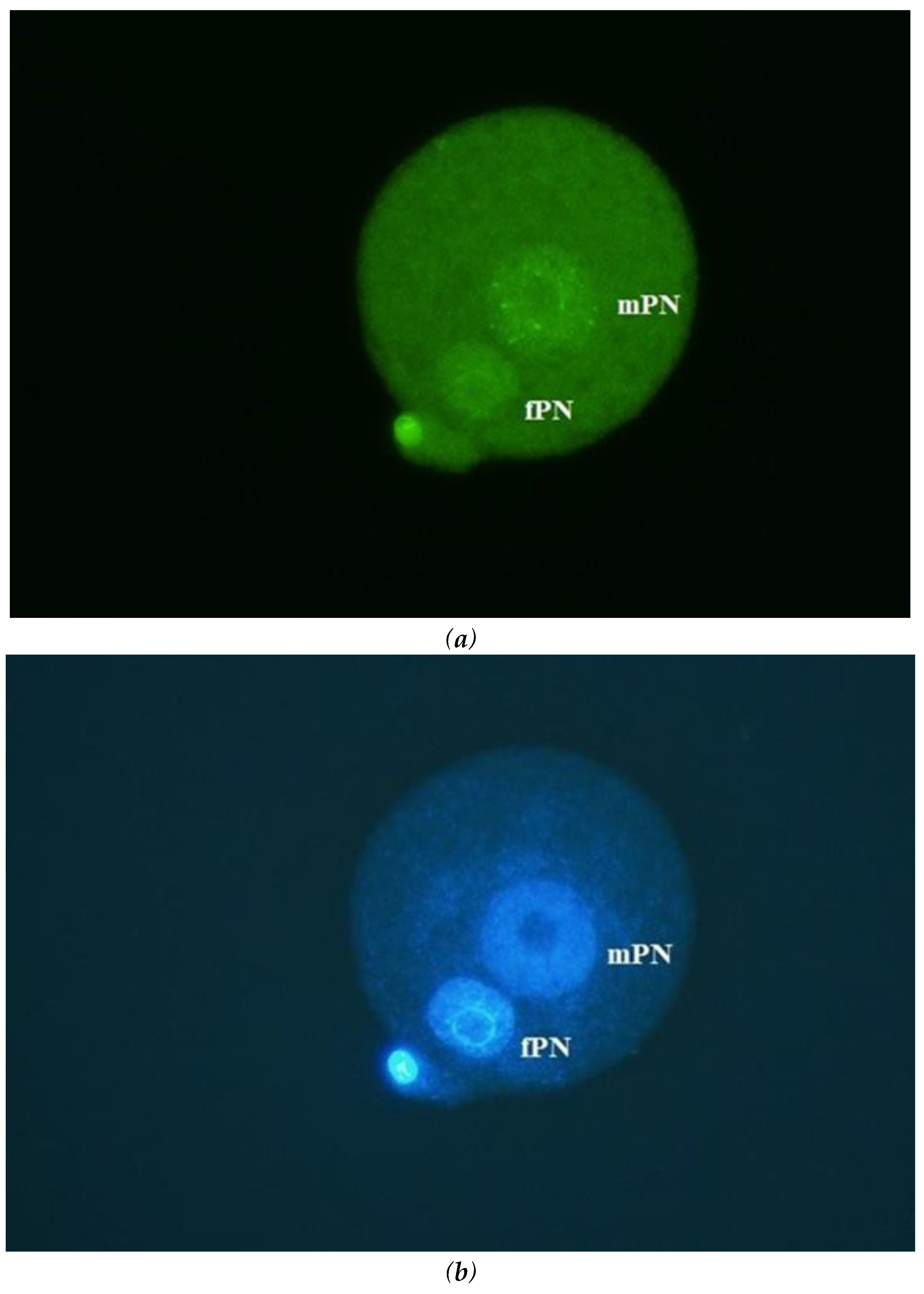
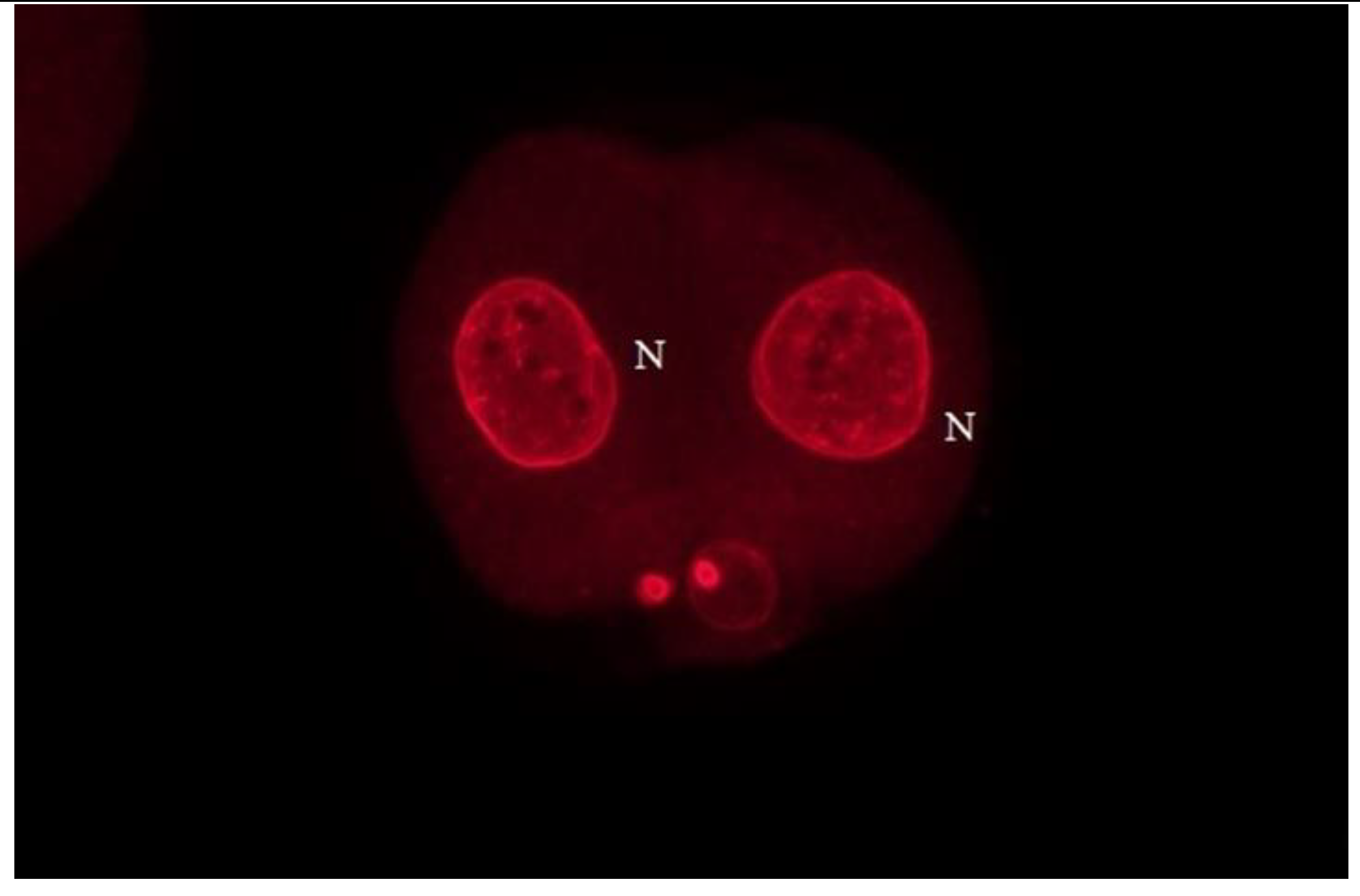
Briefly, in control mouse ICSI zygotes (in total, we have injected 235 mouse ovulated oocytes), the damage of DNA in mPNs was essentially undetectable (
Figure 1a, b) and if so, it was very weak, irrespective of the origin and motility of spermatozoa (stored either in liquid nitrogen or PBS/BSA at +4°C; motile/immotile). In a separate set of ICSI experiments, almost all zygotes cleaved (59/65) to the two-cell stage with regular blastomere nuclei. MNs were only exceptionally detected (5/59) in blastomeres. There were no differences depending on the origin of spermatozoa. These results confirm previously published results that mouse sperm head DNA is very resistant to damage.
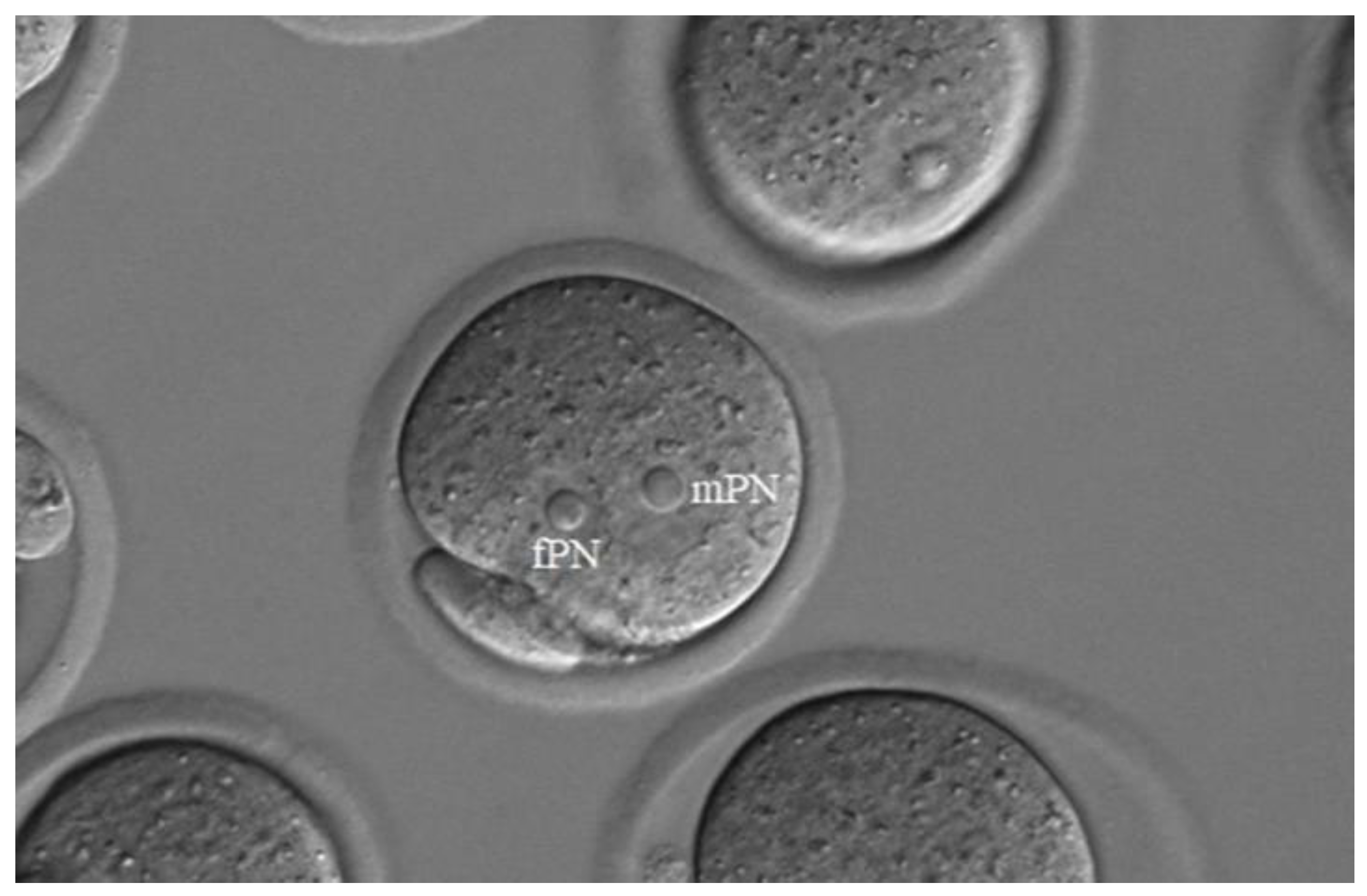
In interspecific ICSI (horse sperm x mouse oocytes) we have injected 2420 oocytes for direct evaluation of DNA damage in mPNs (2239 oocytes survived and 2156 contained two PNs). The horse sperm heads readily decondensed in mouse MII oocytes and formed well visible mPNs that were slightly larger than the female ones, fPNs (
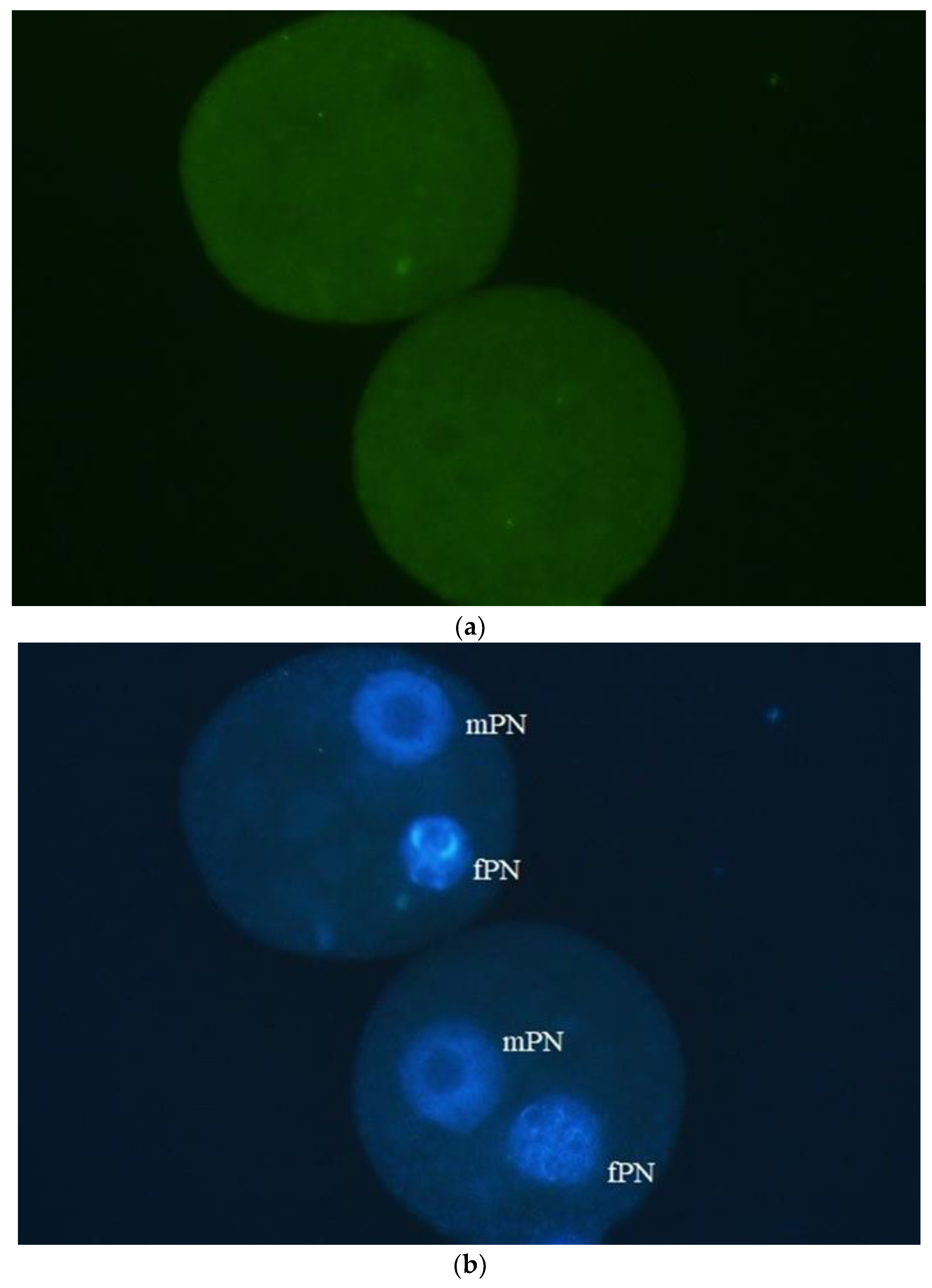
Figure 2). The same situation as in the mouse, i.e. no DNA damage in mPNs, we detected in interspecific zygotes when motile horse spermatozoa, irrespectively of which stallion was used for ICSI. In almost all paternal (horse) PNs no fluorescence signal, which would be indicating DNA damage, was detected and if so, it was very weak (
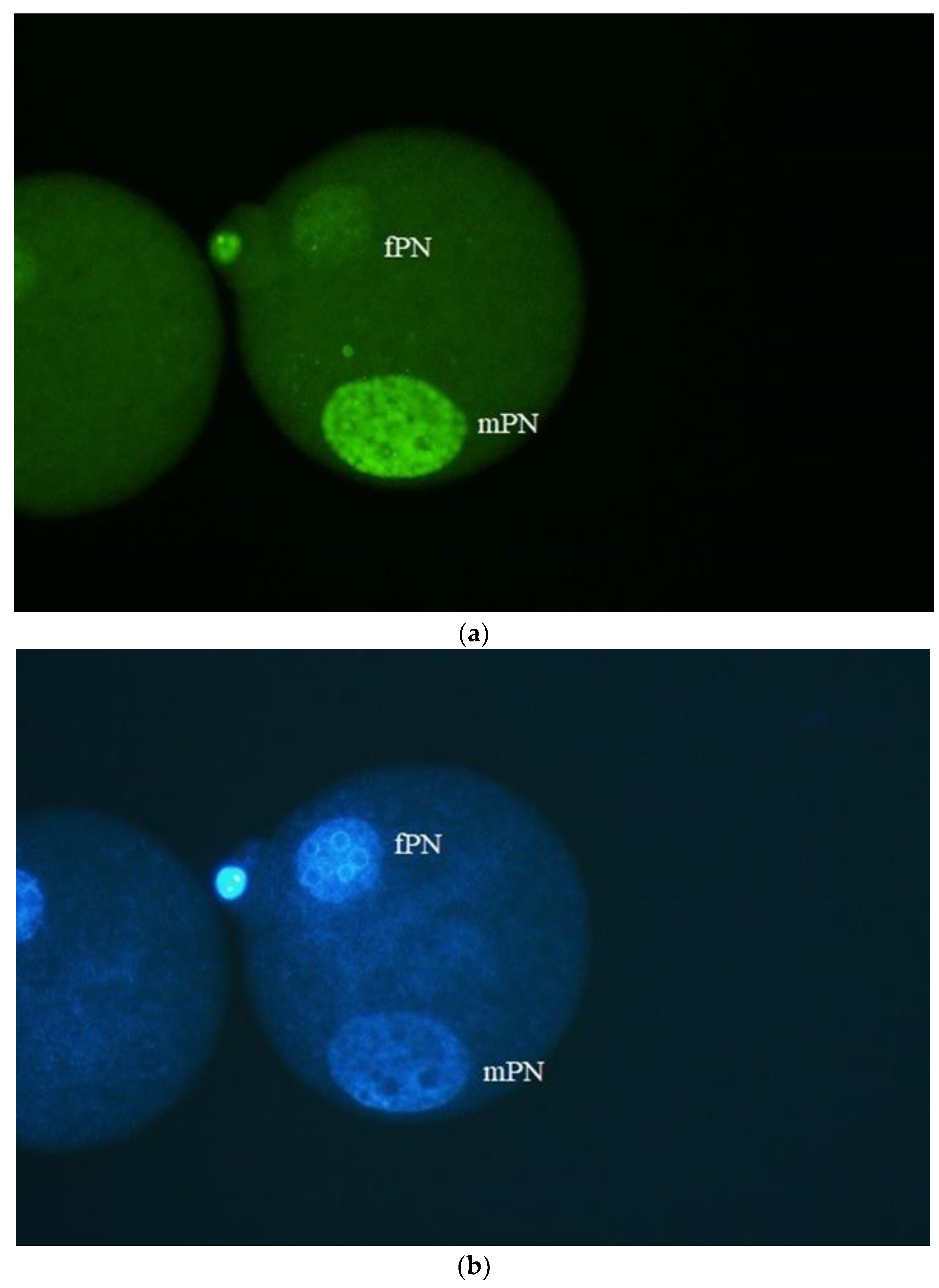
Figure 3a, b). Quite different situation we detected when heads from immotile spermatozoa were used for ICSI. With stallions II, III and IV (bad freezers) all paternal PNs were labelled with anti-γH2AX antibody. The most intensive fluorescence was evident for stallion IV (
Figure 4 a,b). Interestingly, even when immotile spermatozoa from stallion I (good freezer) were used for ICSI about 50% of mPNs showed no labelling whilst the other half was positively labelled. The results are summarized in
Table 1.
Abbreviations: PN -pronucleus, mPN – male, paternal pronucleus.
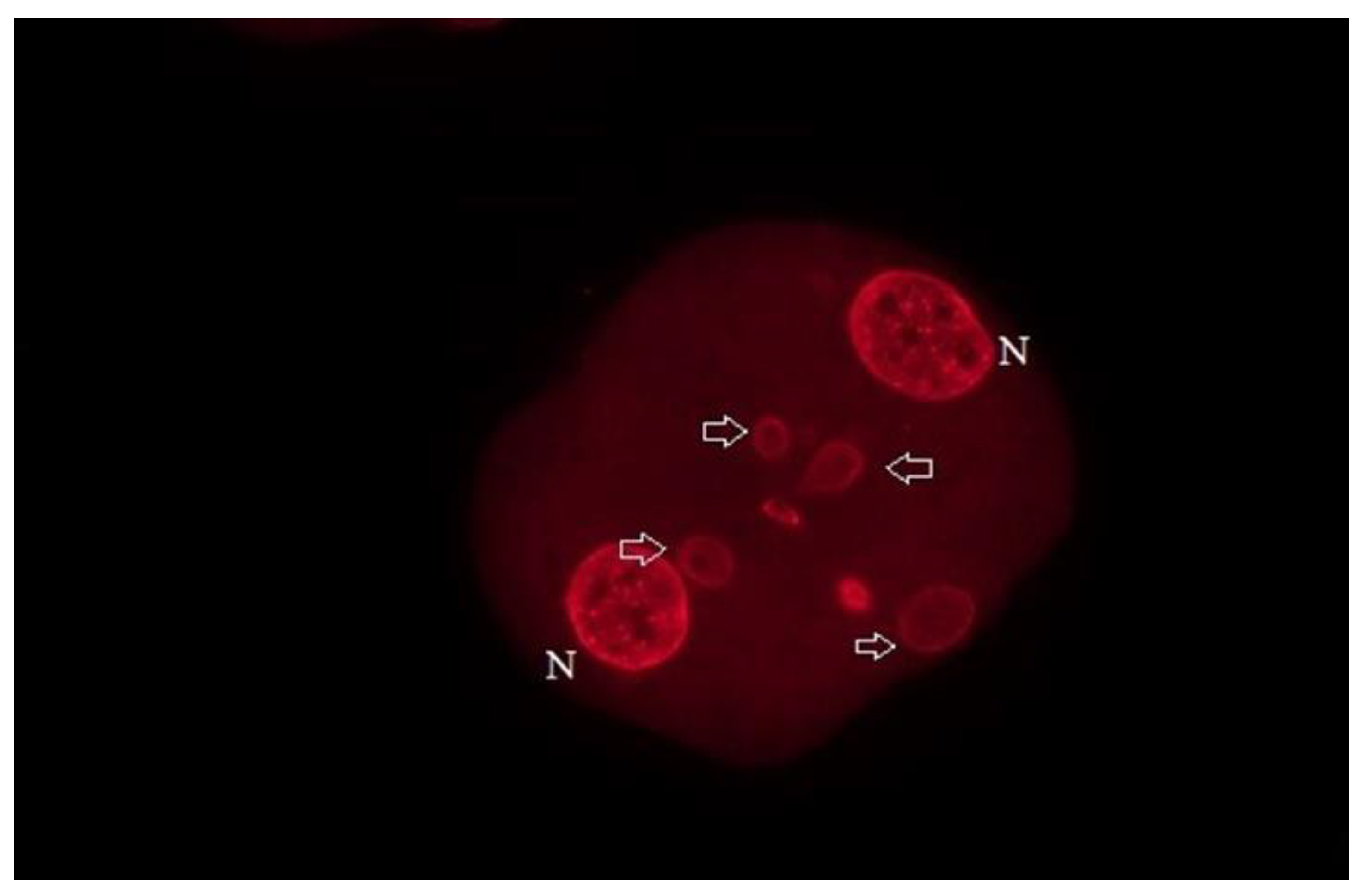
In the second part of experiments, we have evaluated the ability of interspecific embryos to cleave to the two-cell stage, and we have assessed the presence of micronuclei (MNs) in blastomeres as it is well documented that their presence is common in embryos with damaged DNA. As expected, MNs were only exceptionally present in intraspecific mouse two-cell stage embryos irrespective on the origin of spermatozoa that were used for ICSI. The same situation was detected in interspecific embryos where motile spermatozoa were used. Here, almost all embryos cleaved to the two-cell stage and blastomeres contained each a single nucleus with no MNs (
Figure 5). More dramatic situation was detected when immotile spermatozoa were used. In stallion I, where about half of mPNs showed DNA damage, the frequency of MNs presence in blastomeres essentially mirrored this pattern and approximately half of the embryos contained MNs. The other extreme was the stallion IV where mPNs were extremely heavily labelled. Here, the zygotes cleaved to the two-cell stage only exceptionally and remained arrested at the one-cell stage. In stallion II and III with moderate but evident mPNs labelling about two thirds of embryos contained MNs in blastomeres (
Figure 6). The results are summarized in
Table 2.
In conclusion, the (even sporadically) motile spermatozoa from frozen/thawed horse ejaculates can be used for ICSI, irrespective of whether the ejaculate originate from good or bad freezers. On the other hand, our results indicate that it is very risky to use the immotile spermatozoa for ICSI unless the damage of DNA is assessed before. The same pays true also when the cleavage of xenogenic zygotes into the two- cell stage is taken as an eventual indicator of sperm normality. We do believe that the interspecific ICSI may not only help us to evaluate the level of sperm head DNA damage but also to accelerate the progress in the development of horse sperm storage. With our approach, it is possible to very rapidly define the characteristics of individual ejaculates and thus the efficiency of a specific freezing method or its modification.
4. Discussion
The long-term storage of mammalian spermatozoa with different approaches used has been most extensively studied in the mouse as the main laboratory model. Here, the spermatozoa, albeit mostly epididymal, can be easily collected and what is also important their viability post thawing can be easily tested because high quality oocytes can be obtained by superovulation and in vitro fertilization and ICSI are routinely used. The situation is quite different for domestic and wild animals. The satisfactory situation is in bovine and humans where the long-term semen storage is used for many years [
6]. But even here the range of associated ART techniques where frozen/thawed spermatozoa can be used is incomparable to the mouse.
Very problematic situation is in the horse where ejaculates from some horses can be frozen well (good freezers) whilst the freezing of ejaculates from other horses gives very unsatisfactory results (bad freezers). The reason(s) for this inconsistency is not yet well defined. Although different extenders, cryoprotectants, freezing/thawing protocols and their combinations were tested the results are still rather disappointing [
16,
17,
18]. Moreover, the seasonal effects and the breed may also play an important role [
8,
19].
Achieving the success of horse sperm storage is important from a commercial point of view. First, successful freezing enables the use of a single ejaculate to inseminate numerous females. Second, transport of genetic material is possible without the transport of animals that may include the possibility of injury. Third, the transmission of disease can also be prevented [
8]. Next, with the use of ICSI or IVF in combination with transferring the resulting embryos to surrogate females, the sporting activities of oocyte donor mares would, in fact, not be interrupted. Finally, the sudden death of males and castration of stallions must also be considered [
17].
Our results demonstrate that motile spermatozoa from frozen/thawed ejaculates showed no sperm head DNA damage and can be theoretically used for the production of viable embryos. If the number of motile spermatozoa is low, the successful artificial insemination is logically impossible. Relatively high number of progressively motile spermatozoa is also needed for IVF. On the other hand, ICSI needs only minimum numbers of spermatozoa. Moreover, one straw can be cut into 10 – 15 pieces and thus the given frozen ejaculate can be used several times [
17]. The disadvantage here is that IVF and ICSI in this species still give rather suboptimal results and for ICSI not only that expensive equipment is necessary but also a certain skill is important.
We showed that the interspecific (xenogenic) ICSI can be a useful method how to test the suitability of frozen/thawed ejaculates for further use especially, as mentioned above, if the straw is cut into several pieces. However, this method has much wider potential. Watanabe et al. [
12] used this approach as the human sperm chromosomes (DNA damage) assay. Specifically, he evaluated the sperm karyotypes in the first mitotic metaphases and found large numbers of abnormalities that cannot be detected with other methods like TUNEL, Comet assay or acridine orange test. Logically, this can also be implemented for horse sperm should the situation require. Kaneko et al. [
11] showed that chimpanzee, giraffe, jaguar, weasel and longhaired rat sperm also decondense in mouse oocytes and form mPNs. He concluded that this means that these spermatozoa are fully viable. Our results however indicate that the simple formation of paternal PN formation cannot be used as an indicator of sperm normality because the spermatozoa with heavily damaged DNA also form PNs. Although the oocyte is able to repair the sperm DNA damage we can hardly expect that this will be possible in heavily damaged sperm DNA [
19,
20]. Indeed, our results show that when sperm DNA is very heavily damaged (stallion IV) the produced embryos remain arrested in one-cell stage and do not cleave at all. If DNA is less damaged, the cleavage occurs but MNs are present in two-cell staged embryos. Although occasionally MNs can be detected even in normal embryos [
21] their frequency is much higher in embryos originating from oocytes fertilized with spermatozoa with damaged DNA [
14]. The development of these embryos is typically arrested after few cleavages. Overall, this indicates that it advantageous to primarily evaluate the produced embryos at the pronuclear stage, with the additional assessment of MNs in two-cell stage embryos as an additional criterium.
5. Conclusions
In conclusion, we demonstrate that the interspecific ICSI may serve as a useful approach for evaluation of suitability of given ejaculate for further in vitro use. In our experiments we have used mouse ovulated oocytes but the use of oocytes of other species is also possible. Hildebrandt et al. [
7] injected the frozen/thawed spermatozoa of rhinoceros into porcine mature oocytes and based on the results obtained he selected the suitable sperm doses for the production of rhino embryos. The use of mouse oocytes is, according to our opinion, simpler but pig oocytes may be used in those cases when mouse oocytes are not available. Additionally, the xenogenic ICSI may not only be restricted to karyotype and DNA damage studies as it offers much more possibilities how to study and evaluate the processes occurring in mPNs that may also indicate the suitability of spermatozoa for further use [
22].
Author Contributions
Conceptualization of experiments, J.R., P.L. and J.F. Jr. Manipulations, J. F. Jr. and H.F. Microscopy, H.F. and J.F. Jr. Writing and preparing the original draft, including Figures: J.R., H.F., P.L. and J. F. Jr. All authors have read and agreed to the published version of the manuscript.
Funding
J.R. and J.F. Jr. supported from The Ministry of Agriculture of the Czech Republic (No. QK1910156), The Technology Agency of the Czech Republic (No.TN02000017) and MZE-RO 0723. HF is supported from The Czech Science Foundation GACR 21-42225L. PL acknowledges support from MUR Prin 2022CSHPAS and Prin P2022FA79R.
Institutional Review Board Statement
The use of animals and the experimental design were approved by the Institutional Animal Ethics Board 5/2018.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Ogonuki, N.; Mochida, K.; Miki, H.; Fray, M.; Iwaki, T.; Moriwaki, K.; Obata, Y.; Morozumi, K.; Yanagimachi, R.; Ogura, A. Spermatozoa and spermatids retrieved from frozen reproductive organs or frozen whole bodies of male mice can produce normal offspring. Proc. Natl. Acad. Sci. USA 2006, 103, 13098–13103. [Google Scholar] [CrossRef]
- Ohta, H.; Sakaide, Y.; Wakayama, T. Long-term preservation of mouse spermatozoa as frozen testicular sections. J. Reprod. Dev. 2008, 54, 295–298. [Google Scholar] [CrossRef]
- Wakayama, T.; Whittingham, D.G.; Yanagimachi, R. Production of normal offspring from mouse oocytes injected with spermatozoa cryopreserved with and without cryoprotection. J. Reprod. Fert. 1998, 112, 11–17. [Google Scholar] [CrossRef]
- Wakayama, T.; Yanagimachi, R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat. Biotechnol. 1998, 16, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mizutani, E.; Ono, T.; Terashita, Y. , Jia, X. ; Shi, H., Wakayama, T. Intracytoplasmic sperm injection with mouse spermatozoa preserved without freezing for six months can lead to full-term development. Biol. Reprod. 2011, 85, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Ortiz, I.; Catalan, J.; Rodriguez-Gil, J.E.; Miro, J.; Yeste, M. Advances in sperm cryopreservation in farm animals: cattle, horse, pig and sheep. Anim. Reprod. Sci. 2022 Nov:246:106904. [CrossRef]
- Hildebrandt, T.B.; Hermes, R.; Colleoni, S.; Diecke, S.; Holtze, S.; Renfree, M.B.; Stejskal, J.; Hayashi, K.; Drukker, M.; Loi, P.; Goritz, F.; Lazzari, G.; Galli, C. Embryos and embryonic stem cells from the white rhinoceros. Nat. Commun. 2018, 9, 2589. [Google Scholar] [CrossRef]
- Prien, S.; Iacovides, S. Cryoprotectants & cryopreservation of equine semen: a review of industry cryoprotectants and the effects of cryopreservation on equine semen membrane. J. Dairy Vet. Anim. Res. 2016, 3, 00063. [Google Scholar] [CrossRef]
- Shamsi, M.B.; Imam, S.N.; Dada, R. Sperm DNA integrity assays: diagnostic and prognostic challenges and implications in management of fertility. J. Assist. Reprod. Genet. 2011, 28, 1073–1085. [Google Scholar] [CrossRef]
- Rychtarova, J; Langerova, A. ; Fulka, H.; Loi, P.; Benc, M.; Fulka, Jr., J. Interspecific ICSI for the assessment of sperm DNA damage: technology report. Animals 2021, 11, 1250. [CrossRef]
- Kaneko, T.; Ito, H.; Sakamoto, H.; Onuma, M.; Inoue-Murayama, M. Sperm preservation by freeze-drying for the conservation of wild animals. PLoS ONE 2014, 9, e113381. [Google Scholar] [CrossRef]
- Watanabe, S. DNA damage in human sperm: the sperm chromosome assay. Reproductive Medicine and Biology 2022, 21, e12461. [Google Scholar] [CrossRef] [PubMed]
- Salamone, D.F.; Canel, N.G.; Rodriguez, M.B. Intracytoplasmic sperm injection in domestic and wild mammals. Reproduction 2017, 154, F111–124. [Google Scholar] [CrossRef] [PubMed]
- Grenier, L.; Robaire, B.; Hales, B.F. Paternal cyclophosphamide exposure induces the formation of functional micronuclei during the first mitotic division. PLoS ONE 2015, 6, e27600. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Perry, A.C.F. Piezo-actuated mouse intracytoplasmic sperm injection (ICSI). Nat. Protoc. 2007, 2, 296–304. [Google Scholar] [CrossRef]
- Wu, Z.; Zheng, X.; Luo, Y. , Huo, F. , Dong, H.; Zhang, G.; Yu, W., Tian, F.; He, L,; Chen, J. Cryopreservation of stallion spermatozoa using different cryoprotectants and combinations of cryoprotectants. Anim. Reprod. Sci. 2015, 163, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Gonzales/ Castro, R.A.; Trentin, J.M.; Carnevale, E.M. Effects of extender, cryoprotectants and thawing protocol on motility of frozen-thawed stallion sperm that were refrozen for intracytoplasmic sperm injection doses. Theriogenology 2019, 136, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Aurich, J.; Kuhl, J.; Tichy, A.; Aurich, C. Efficiency of semen cryopreservation in stallions. Animals 2020, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Musson, R.; Gasior, L.; Bisogno, S.; Ptak, G.E. DNA damage in preimplantation embryos and gametes: specification, clinical relevance and repair strategies. Hum. Reprod. Update 2022, 28, 376–399. [Google Scholar] [CrossRef] [PubMed]
- Newman, H.; Catt, S. , Vining, B. ; Vollenhoven, B., Horta, F. DNA repair and response to sperm DNA damage in oocytes and embryos, ant the potential consequences in ART: a systematic review. Mol. Hum. Reprod. 2022, 28, gaab071. [Google Scholar] [CrossRef]
- Vazquez-Diez, C.; FitzHarris, G. Causes and consequences of chromosome segregation error in preimplantation embryos. Reproduction 2018, 155, R63–R76. [Google Scholar] [CrossRef]
- Fulka, H.; Barnetova, I.; Mosko, T.; Fulka, Jr. J. Epigenetic analysis of human spermatozoa after their injection into ovulated mouse oocytes. Hum. Reprod. 2008, 23, 627–634. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).