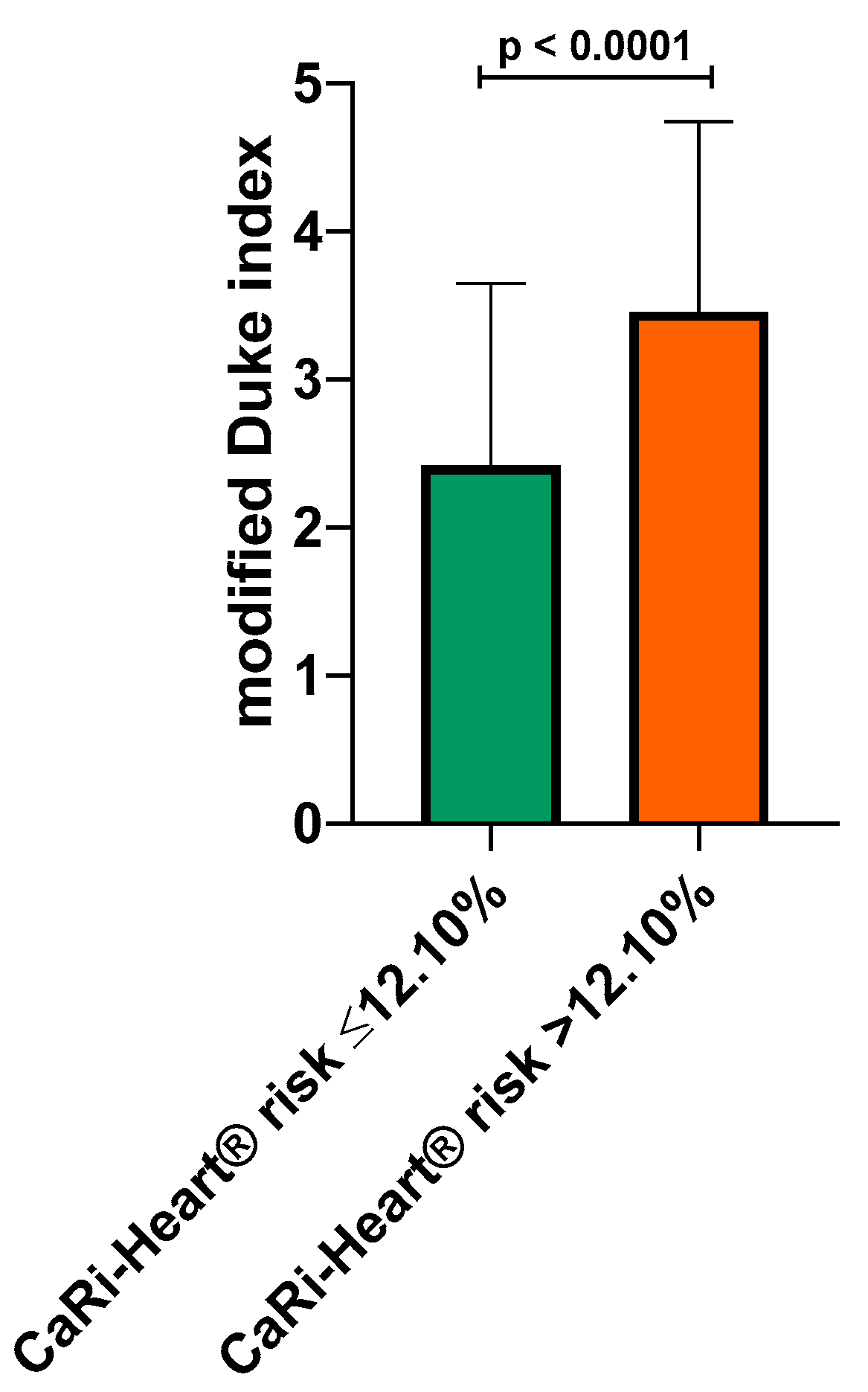1. Introduction
Cardiac Computed Tomography Angiography (CCTA) has emerged in the latest years as a first-line option to investigate coronary artery disease (CAD) [
1]. Besides providing accurate anatomic information regarding coronary arteries
via a non-invasive route, it can reliably identify features of increased vulnerability in the coronary circulation. Various studies investigated the association between these features and different cardiovascular pathologies, such as acute coronary syndromes (ACSs), atrial fibrillation or heart failure [
2,
3].
Several CCTA-derived scores have been proposed to characterize the severity of the coronary stenoses, in an attempt to better stratify the risk of cardiovascular events and restrict the indication for invasive coronary imaging to those who really need it. For instance, the modified Duke index, derived from CCTA, was designed to predict cardiovascular outcome based on the severity of coronary stenoses. An increased Duke index was proved to be associated with a higher risk of fatal events, validating the role of CCTA as a reliable tool for predicting cardiovascular risk [
4].
However, the Duke CAD index does not take into consideration the presence or severity of peri-coronary inflammation. An increased level of inflammation at this level may trigger plaque vulnerabilisation, ultimately leading to plaque rupture and acute myocardial infarction [
5].
It is well-known that not all unstable plaque progress to rupture. However, persistently high inflammation at the level of the epicardial fat may lead to plaque progression with high potential to vulnerabilization and rupture in a close future [
6,
7]. Therefore, assessment of coronary inflammation may have a strong clinical impact, contributing to a superior evaluation of plaque-associated risk. It has been suggested that inflammation may be different at various levels of coronary circulation, underlining the role of regional flow hemodynamic and shear stress in the complex process of plaque vulnerabilisation [
8]. In this light, in addition to the classic risk factors of CAD, pathologies such as periodontal disease, and COVID19 infection have recently been highlighted,
via inflammation, as risk factors of CAD and, implicitly, progression to ACS [
9,
10]. Thus, increased attention should be paid to patients whose associated comorbidities imply an increased inflammatory syndrome.
Nowadays, inflammation may be measured at the level of epicardial fat surrounding coronary arteries, using the novel technology patented by Caristo (Oxford, UK), which measures the computed tomography attenuation gradient which is in direct relation with the inflammatory-mediated change in adipocyte composition and phenotype. This technique measures the peri-coronary fat attenuation index (FAI), validated as a novel imaging marker determined by CCTA which reflects the degree of inflammation in the coronary tree in patients with CAD [
11]. A recent analysis, published in 2022, indicated that the FAI is associated not only with coronary stenosis, a stenosis with a diameter ≥50% had significantly higher FAI values compared to those with a diameter <50%, but also with vulnerable plaque features [
12].
While the association between severity of coronary lesions and cardiovascular risk is well-known, there are very few data so far regarding the role of inflammation in progression of coronary lesions towards a higher severity.
The aim of this study was to investigate the association between two CCTA-derived score associated with increased cardiovascular risk: peri-coronary inflammation at the level of epicardial fat expressed CaRi-Heart® analysis and severity of coronary lesions expressed by Duke CAD index, in patients with CAD.
2. Materials and Methods
2.1. Study Population
This single-center retrospective analysis included 172 adult patients aged over 18 who underwent CCTA at the Center of Advanced Research in Multimodality Cardiac Imaging, Cardiomed Târgu Mureș, Romania, for typical chest pain and a low to intermediate likelihood of CAD. Patients with a history of coronary artery disease or non-cardiac chest pain were excluded. Demographic data and risk factors related to coronary artery disease (hypertension, hyperlipidemia, smoking, and diabetes) were obtained prior to the CCTA examination.
Based on the modified Duke index assessed on CCTA, the study population was divided into two groups as follows: group 1 – patients with a modified Duke index ≤ 3 (n = 107) and group 2 – patients with a modified Duke index > 3 (n = 65).
All study procedures were carried out in compliance with the Declaration of Helsinki, with the prior approval of the local ethics committee of the Emergency Clinical County Hospital Târgu Mureș (Ad. 26884/10.11.2021) and the George Emil Palade University of Medicine, Pharmacy, Science and Technology Târgu Mureș (1515/09.12.2021).
2.2. Coronary Computed Tomography Angiography Acquisition
Image acquisition was performed using a 128-slice high-definition scanner (Siemens Healthcare, Erlangen, Germany) before and after intravenous administration of iodinated contrast according to the patient's weight (60 to 100 mL). The images were obtained under continuous ECG monitoring in the three bipolar limb leads and after prior determination of blood pressure, heart rate and O2 saturation. Oral metoprolol tartrate was previously administered when needed to achieve a target heart rate below 65 bpm.
2.3. Image Analyses
The obtained images were analyzed independently by two randomly selected CCTA interpreters, blinded to the clinical data, in order to obtain the modified Duke index, using a dedicated software (Syngo.via Frontier, Siemens Healthcare, Erlangen, Germany). Based on the atherosclerotic burden, the patients were classified according to the modified Duke index, a coronary plaque severity score for predicting 5-year cardiovascular survival. Calculation of the Duke index has been described previously [
4] (
Table 1).
All images were subsequently transferred to the Centre of CARISTO Diagnostics, Oxford, United Kingdom, for assessment of perivascular adipose tissue (PVAT) and related inflammation. Using artificial intelligence algorithms, the PVAT - fat attenuation index (PVAT-FAI) reckons attenuation measurements of perivascular adipose tissue surrounding the coronary arterial wall with high accuracy [
13,
14]. The FAI-HU, FAI score and FAI -score centile were assessed for each of the major coronary arteries. Further, the CaRi-Heart® risk, a score validated to predict fatal cardiac event within the next 8 years was assesed for each patient.
2.4. Statistical Analysis
GraphPad InStat 3.10 software (GraphPad Software Inc., San Diego, CA, USA) was used to conduct the statistical analysis. Prior to statistical analysis, all data underwent normality tests. All data are reported as absolute value or percentage, or median and standard deviation. For numerical data, unpaired t-test or ANOVA test were used for between-group comparisons, and chi-squared test was used for categorical data. Pearson correlation analysis was performed to investigate the association between modified Duke index and PVAT-FAI parameters. Afterwards, the receiver operating characteristic (ROC) analysis was employed to evaluate CaRi-Heart® risk ability to predict high modified Duke index. The α value was set at 0.05 for statistical significance.
3. Results
3.1. Baseline Characteristics of the Study Population
The baseline characteristics of the study population and the differences between the low and high modified Duke index groups are presented in
Table 2. Patients with Duke index > 3 were significantly older (64.78 y.o. versus 60.03 y.o.; p= 0.002) and were more frequently diabetic (36.96 % versus 21.50 %; p= 0.02). There were no significant differences between the two groups in terms of gender distribution, hypertension, hyperlipidemia and smoking status (all p> 0.05).
3.2. Coronary Inflammation and CT-Derived Duke Index
Table 3 summarizes the differences between the two groups in terms of peri-coronary inflammation magnitude and derived risk, using standard adipose tissue CT density and the percentile curves for the FAI score for each coronary artery analyzed. Based on the standard adipose tissue Hounsfield unit (HU), which ranged between −190 to −30 HU, the coronary FAI index values did not differ significantly between the patients with low versus high modified Duke index in any of the 3 coronary arteries analyzed: left anterior descending (LAD), left circumflex (LCX) and right coronary artery (RCA).
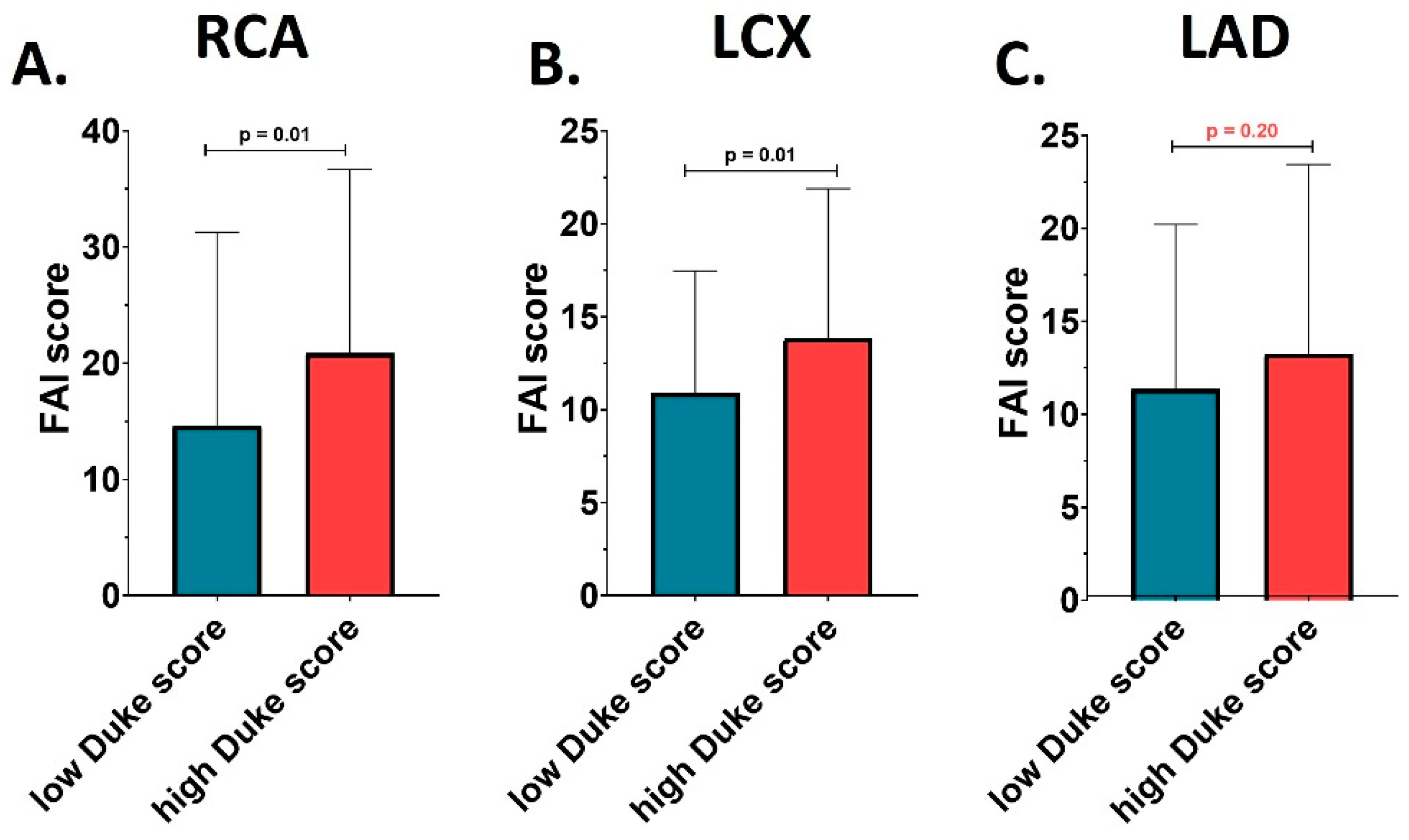
Interestingly, FAI scores of coronary inflammation at the level of the RCA and LCX were significantly different between the two studied groups, while this difference was not observed for LAD. Patients with a Duke index > 3 presented a significantly higher FAI-score at level of the RCA (20.85 ± 15.83 versus 14.61 ± 16.66; p= 0.02), and LCX (13.85 ± 8.04 versus 10.91 ± 6.56; p= 0.02), compared to patients with a Duke index ≤ 3, as showed by
Figure 1. However, this was not reflected in an increased risk per coronary artery compared with reference standards for the same age group. FAI score percentiles were not significantly different between the two analyzed groups at any of the 3 coronary arteries analyzed (all p> 0.05).
3.3. Coronary Inflammation and Risk of Fatal Cardiac Events as Assessed by CaRi-Heart® Risk Score
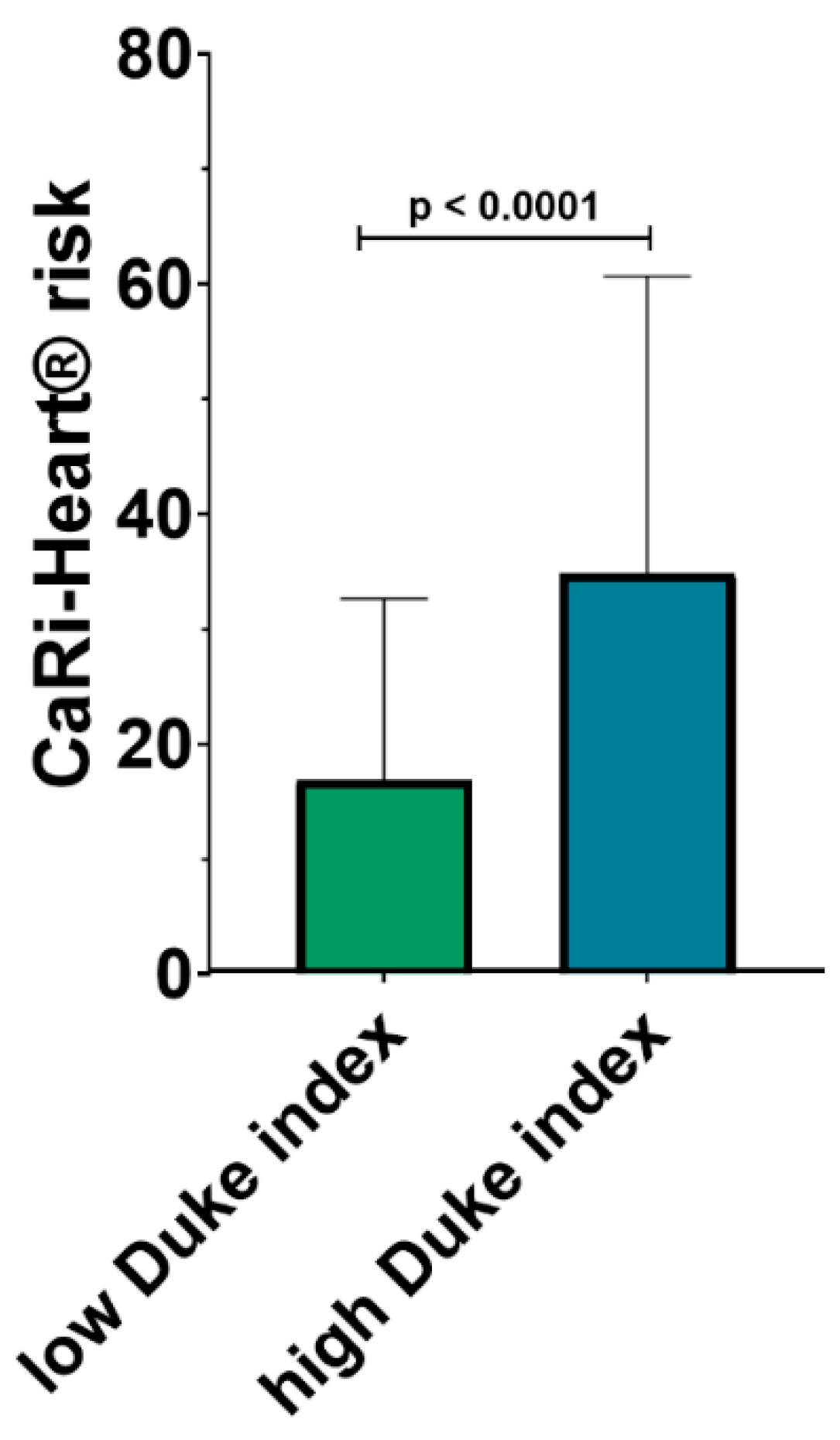
The risk of future fatal cardiac events was determined using the CaRi-Heart® risk score, integrating in a global risk, the individual risks related to inflammation at the level of the 3 coronary arteries and the risk factors of the patients (smoker status, hypertension, diabetes and hypercholesterolemia). CaRi-Heart® analysis identified a significantly higher risk of future events among patients with a high modified Duke index (34.84 % ± 25.86 % versus 16.87 % ± 15.80 %; p <0.0001) as shown in
Figure 2.
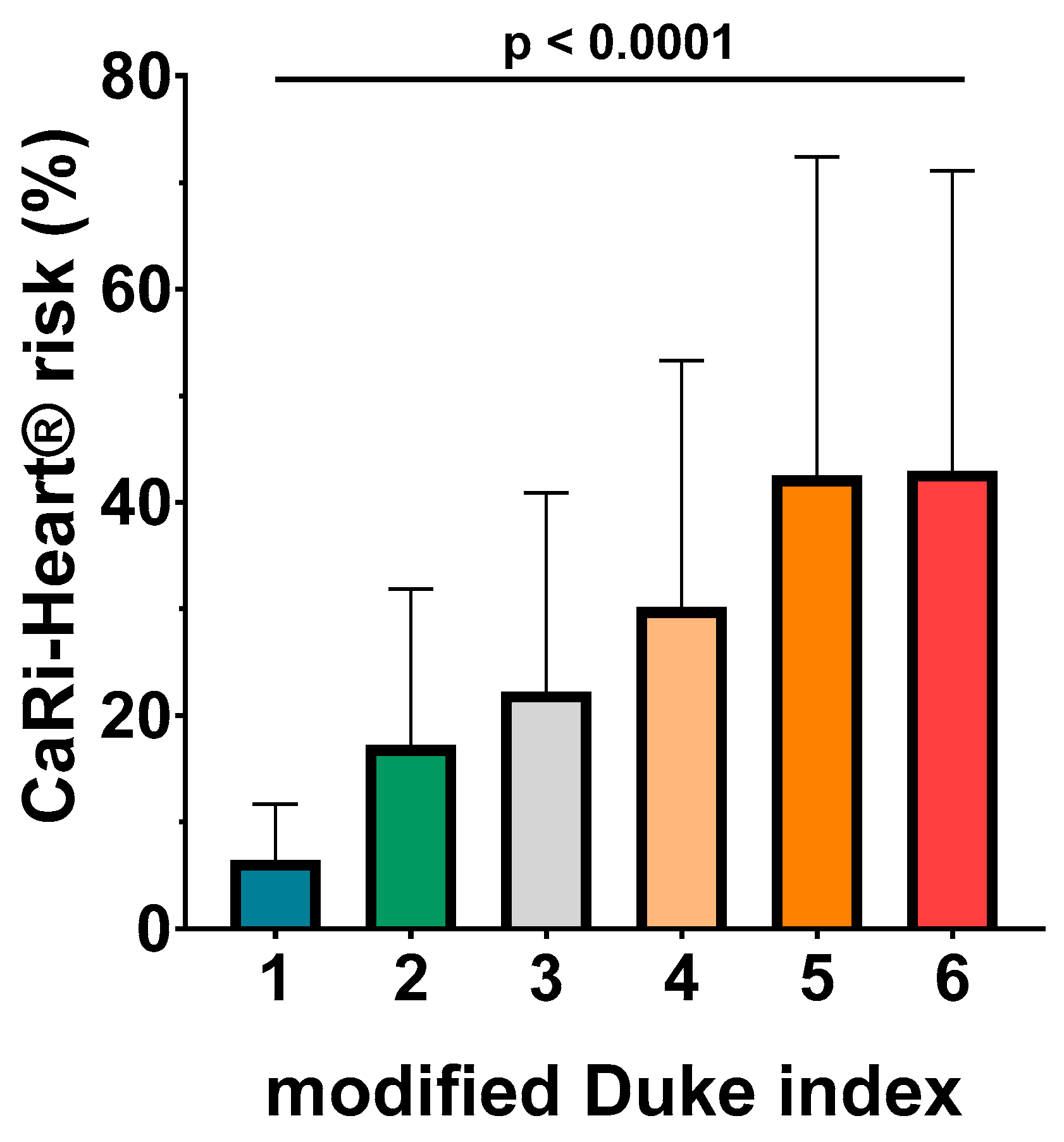
The ANOVA analysis identified a very good association between CaRi-Heart® risk and modified Duke index, with higher levels of CaRi-Heart® score for each superior level of Duke index (6.46 % ± 5.26 % for Duke 1; 7.26 % ± 14.64 % for Duke 2; 22.24 % ± 18.65 % for Duke 3; 30.15 % ± 23.16 % for Duke 4; 42.47 % ± 29.93 % for Duke 5; and 42.95 % ± 28.13 % for Duke 6; p< 0.0001) (
Figure 3.).
3.4. Correlation between Coronary Inflammation, Cardiac Computed Tomography Lesion Severity and CaRi-Heart® Risk
The correlation between the modified Duke index, coronary inflammation and CaRi-Heart® risk respectively, is presented in
Table 4. A high positive correlation was observed between the modified Duke index and CaRi-Heart® risk, revealed by the Pearson correlation coefficient of 0.75. Weak but significant correlations were also observed between the modified Duke index and the FAI-score, at the level of all three coronary vessels (all p< 0.05).
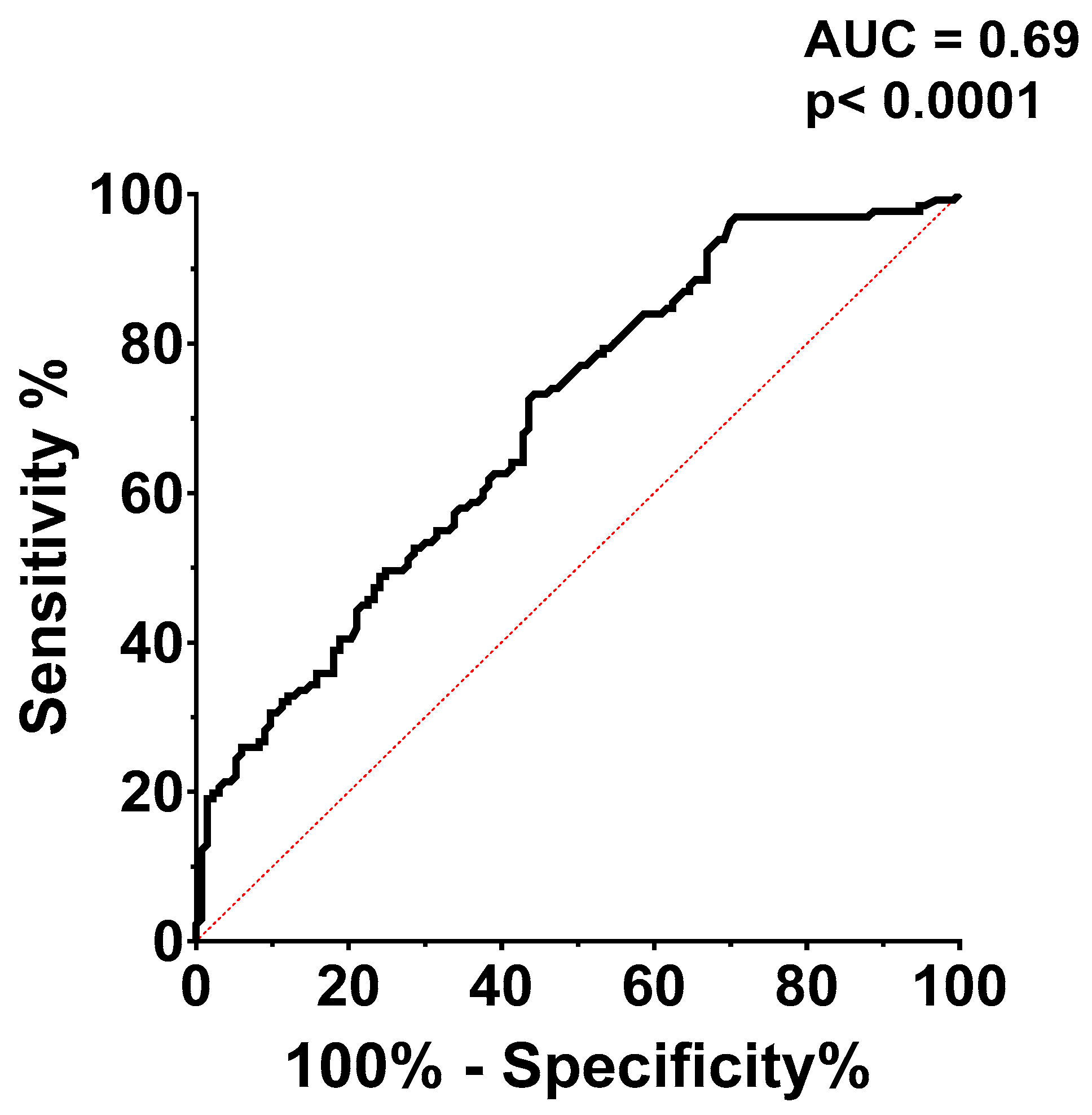
Receiver operating characteristic (ROC) curve analysis (
Figure 4.) was used to assess CaRi-Heart® risk's ability to predict a modified Duke index above 3. The CaRi-Heart® risk optimal cut-off value for predicting a high Duke index was set at 12.10%, with a sensitivity of 61.83% and a specificity of 61.65%, with an AUC of 0.69.
Based on this cut-off value, mean modified Duke index was calculated in patients with high CaRi-Heart® risk (below 12.10%) and in patients with high CaRi-Heart® risk > 12.10%. Mean modified Duke index was significantly higher in the group with CaRi-Heart® risk > 12.10% (2.42 ± 1.22 versus 3.46 ± 1.28; p< 0.0001), indicating a very strong association between high inflammation, lesion severity and future risk of fatal cardiovascular events (
Figure 5.).
4. Discussion
In the present study, we report the results of a CCTA study in patients with low to intermediate likelihood of CAD. Our study is focused on the importance of coronary inflammation in predicting the severity of CAD and indicates that, both high inflammatory burden expressed by FAI index and high CaRi-Heart® risk, are associated with coronary lesion severity as revealed by the modified Duke index assessed on the CCTA.
4.1. Modified Duke Coronary Artery Disease Index Assessed at the Coronary Computed Tomography Angiography
Over the past ten years, CCTA established itself in clinical medicine more than any other non-invasive imaging technique [
15]. To identify patients who are at high risk for adverse events, a number of composite risk scores based on CCTA have been proposed lately. The previously reported modified Duke index and prognostic CAD index are used to assess the atherosclerotic burden on CCTA. According to the original Duke index, patients are given a risk score ranging from 0 to 100, which takes into account the severity of the stenoses and both proximal LAD stenosis and left main stenosis [
16]. In a study of 1127 people with a low-intermediate risk of CAD, the Duke index was highly correlated with the risk of all-cause mortality [
4]. According to data from the ISCHEMIA trial, which included over 5000 patients, the severity of ischemia determined by functional tests was not associated with an increased risk of adverse outcomes, while the severity of coronary lesions assessed by the CCTA Duke CAD index was associated with an increased risk of mortality, underlining the importance of this score in the prediction of a poor outcome [
17]. A study conducted in China on a population of over 9500 patients with CAD, which reported a mortality rate of more than 5% after 4 years, found that the risk of death from any cause was proportional to the Duke CAD index [
18].
However, the Duke CAD index only includes CCTA-derived information and no information on patients' risk factors or peri-coronary inflammation. For a better clinical decision and for improved outcomes, developing risk scores in combination with the patient’s history and data regarding peri-coronary inflammation are of great importance.
4.2. Coronary Inflammation and the Severity of Lesions Expressed by the Duke Index
In the present study, several important findings arise regarding the association between coronary inflammation and the severity of coronary lesions, as indicated by the modified Duke index. Demographic characteristics reveal that individuals with a higher Duke index, indicating more severe coronary lesions, tend to be of older age and have a higher prevalence of diabetes. This aligns with existing literature, which suggests that both age and diabetes are risk factors for more severe CAD [
19,
20].
The study's analysis of peri-coronary inflammation, as measured by the FAI using CT imaging, reveals nuanced insights. The FAI scores, indicative of the degree of inflammation, were significantly higher in the RCA and LCX in patients with a Duke index > 3, compared to those with a lower score. This suggests a correlation between the severity of coronary lesions and the level of inflammation in these specific arteries. However, the LAD did not show this trend, indicating that the relationship between coronary inflammation and lesion severity might be artery-specific as suggested by previous studies. [
21,
22].
The lack of a significant difference in FAI score percentiles between the two groups across all three coronary arteries, despite the observed differences in FAI scores, suggests that while inflammation is indeed higher in patients with more severe lesions, it may not exceed the expected range for their age group. This could imply that the extent of inflammation in CAD might be more related to individual patient characteristics rather than the severity of the disease itself [
23].
The CaRi-Heart® risk score, which integrates the risks associated with the coronary arteries and patient-specific risk factors, showed a significantly higher risk of future fatal cardiac events in patients with a high Duke index. This finding underscores the importance of comprehensive risk assessment in patients with CAD, considering not just the anatomical severity of the disease but also the inflammatory milieu and other patient-related factors.
The ROC curve analysis for the CaRi-Heart® risk in predicting a high Duke index establishes a specific cut-off value with moderate sensitivity and specificity. This indicates the potential utility of the CaRi-Heart® risk score in clinical settings for predicting lesion severity. Moreover, the significantly higher mean Duke index in patients with a CaRi-Heart® risk above the cut-off point solidifies the link between higher inflammation, more severe coronary lesions, and an increased risk of fatal cardiac events.
4.3. Coronary Inflammation and Cardiovascular Risk
The CAD, recognized as the primary contributor to global morbidity and mortality rates [
24], is now comprehensively perceived as a process characterized by multiple steps and factors, with chronic inflammation playing a crucial role at each phase [
25]. The diverse clinical presentations of CAD, spanning from initial atherosclerotic changes to the advancement of plaques and sudden coronary incidents, highlight the disease's intricate nature. This underscores the necessity for all-encompassing evaluation methods that prioritize inflammation as a central element, especially in the onset of ACSs
via triggering cardioembolic events [
26,
27].
Recent progress in precision medicine for cardiovascular disease has been marked by significant enhancements in risk assessment methodologies. Contemporary models integrating a range of biomarkers, including those related to inflammation, have improved the accuracy and subtlety of cardiovascular risk predictions. Notably, a link has been established between various inflammatory biomarkers - such as fibrinogen, interleukin-6 (IL-6), C-reactive protein (CRP), and galectin-3 and the onset of CVD [
28]. Furthermore, several inflammatory protein biomarkers were strongly linked to poor outcomes after ACS. In a study that included almost 400 patients, inflammatory biomarkers, such as IL-6, IL-10, and IL-8 demonstrated adequate predictive ability for short term (28 days) mortality after ST-elevation myocardial infarction [
29]. In a recent analysis which included postmenopausal women, complete blood count derived inflammatory markers, neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio, were found to be significantly higher in women with ACS compared to those with stable CAD, emphasizing the role of inflammation in the occurrence of ACS [
30].
A separate meta-analysis investigating the role of vascular inflammation biomarkers in cardiovascular risk prediction further supports this trend. The analysis found that incorporating these biomarkers alongside traditional clinical risk factors significantly improves the prediction of cardiovascular events. This finding accentuates the critical role of inflammation markers in both the evaluation and management of CVD risk [
31]. Additionally, contemporary research is reshaping the understanding of atherosclerosis. New findings challenge established beliefs and bring to light a range of non-conventional risk factors and intricate biological mechanisms underlying the disease. This evolving perspective emphasizes the complexity of atherosclerosis and the necessity for advanced diagnostic and management strategies in cardiovascular medicine [
32].
An emerging focus in cardiovascular research is the measurement of the FAI, which carries important clinical implications. Recent investigations have underscored the FAI's significance as an innovative biomarker for evaluating cardiovascular risk [
33,
34]. The FAI Score, recognized as a proprietary biomarker and a key component of the CaRi-Heart® report, plays a pivotal role in quantifying coronary inflammation. It also serves to gauge an individual's risk in comparison to a similar demographic group [
13].
The development of the peri-coronary FAI as a biomarker for imaging coronary inflammation represents a significant advance in the field. Utilized in conjunction with standard CTA, the FAI plays a crucial role in detecting vulnerable plaques, key indicators for predicting and managing cardiovascular risks [
35]. Beyond plaque identification, the FAI's standardized application in CTA allows for non-invasive monitoring of coronary artery inflammation. This method involves analyzing spatial variations in the composition of perivascular fat, thus offering deeper insights into the inflammatory processes central to CAD [
11]. Additionally, this approach enhances prognostic accuracy beyond what traditional risk factors provide, highlighting the critical role of inflammation in comprehensive cardiovascular risk evaluation [
36]. Beside inflammation, we should also take into account that the CAD displays some differences regarding the risk factors and pharmacological treatment, which may influence the outcome [
37,
38].
The results of the current study must also be seen from the perspective of some limitations. The present study is an observational one; while it can identify associations, establishing a direct cause-and-effect relationship is challenging due to the potential influence of confounding variables. Another limitation of the study is the lack of follow-up data, which would have been of great importance in monitoring the relationship between the imaging markers studied and cardiovascular outcome.
The present study indicates a correlation between the modified Duke index and perilesional inflammation, as assessed by the FAI score. The more pronounced inflammation observed in patients with a modified Duke score > 3, should lead to a more aggressive strategy regarding statin treatment as a secondary prevention measure and to reduce mortality in this category of patients. Quantifying both, the modified Duke score and pericoronary inflammation using the FAI could guide the decision of revascularization strategy for some borderline lesions, but which present a high degree of inflammation and consequently constitute lesions with an increased vulnerability. The current study emphasizes the additive role of FAI at the modified Duke score in establishing the optimal therapeutic decision.
5. Conclusions
The CT-derived modified Duke index correlates well with local perilesional inflammation, as assessed by the FAI score at different levels of the coronary circulation. The CCTA assessment of coronary inflammation may identify dangerous plaques and reveal increased inflammation in the coronary tree of patients with low to intermediate likelihood of CAD, and consequently to guide the therapeutic decision.
Author Contributions
Conceptualization, V.-B.H., I.B. and T.B.; methodology, V.-B.H., I.B. and T.B.; software, I.-P.R., B.-B.M., E.B. and R.G.; validation, F.B., T.B. and I.B.; formal analysis, L.-O.C., T.M., A.R. and R.I.-P.; investigation, V.-B.H., L.-O.C., T.M., E.B., A.R., B.-B.M., R.G., F.B., I.-P.R., I.B. and T.B.; resources I.B. and T.B.; data curation, V.-B.H. and T.B.; writing—original draft preparation, V.-B.H, I.-P.R., L.-O.C., E.B. and B.-B.M.; writing—review and editing, I.B., F.B. and T.B.; visualization, V.-B.H., L.-O.C., T.M., E.B., A.R., B.-B.M., R.G., F.B. and I.-P.R.; supervision, I.B., F.B. and T.B.; project administration, V.-B.H., I.B. and T.B.; funding acquisition, T.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All study procedures were conducted in accordance with
good clinical practice guidelines and the Declaration of Helsinki and were reviewed and approved
by the institution’s ethics committee.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in
the study.
Data Availability Statement
Archived datasets are available upon request by any interested third party.
Acknowledgments
This work was supported by the research grant Intel-FAT, proposal registration code PN-III-P4-ID-PCE-2020-2861, contract number PCE 206/2021, Project funded by the Romanian Ministry of Education - UEFISCDI. The initial results of this research were presented at the European Society of Cardiology’s 2023 EACVI Congress in Barcelona.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DISCHARGE Trial Group, Maurovich-Horvat, P., Bosserdt, M., Kofoed, K. F., Rieckmann, N., Benedek, T., Donnelly, P., et al. CT or Invasive Coronary Angiography in Stable Chest Pain. The New England journal of medicine, 2022, 386(17), 1591–1602. [CrossRef]
- Yuan, D., Chu, J., Qian, J., Lin, H., Zhu, G., Chen, F., Liu, X.. New Concepts on the Pathophysiology of Acute Coronary Syndrome. Rev. Cardiovasc. Med. 2023, 24(4), 112. [CrossRef]
- Bordi, L., Opincariu, D., Benedek, T., Kovács, I., Parajkó, Z., Márton, E., Gerculy,R., Benedek,I. Left Atrial Volume Quantified by MSCT Predicts Emergency Hospitalizations for AF and Arrhythmia Recurrence after Catheter Ablation. Journal of Cardiovascular Emergencies 2023,9(2), 24-31.
- Min, J. K., Shaw, L. J., Devereux, R. B., Okin, P. M., Weinsaft, J. W., Russo, D. J., Lippolis, N. J., Berman, D. S., Callister, T. Q. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J. Am. Coll. Cardiol. 2007, 50(12), 1161–1170.
- Rodean, I. P., Lazăr, L., Halațiu, V. B., Biriș, C., Benedek, I., Benedek, T. Periodontal Disease Is Associated with Increased Vulnerability of Coronary Atheromatous Plaques in Patients Undergoing Coronary Computed Tomography Angiography-Results from the Atherodent Study. J. Clin. Med., 2021, 10(6), 1290. [CrossRef]
- Chistiakov, D. A., Kashirskikh, D. A., Khotina, V. A., Grechko, A. V., & Orekhov, A. N. Immune-Inflammatory Responses in Atherosclerosis: The Role of Myeloid Cells. Journal of clinical medicine, 2019, 8(11), 1798. [CrossRef]
- Rodean, I.P.; Biris,, C.I.; Halatiu, V.B.; Modiga, A.; Lazar, L.; Benedek, I.; Benedek, T. Is there a link between COVID-19 infection, periodontal disease and acute myocardial infarction? Life 2021, 11, 1050. [CrossRef]
- Blîndu, E., Benedek, I., Rodean, I.P., Halațiu, V.B., Raț, N., Țolescu, C., Mihăilă, T., Roșca, A., Matyas, B.B., Zsabo, E., et al. Regional Differences in the Level of Inflammation Between the Right and Left Coronary Arteries – a Coronary Computed Tomography Angiography Study of Epicardial Fat Attenuation Index in Four Scenarios of Cardiovascular Emergencies. Journal of Cardiovascular Emergencies 2023, 9(4), 111-119. [CrossRef]
- Wojtkowska, A., Zapolski, T., Wysokińska-Miszczuk, J., & Wysokiński, A. P. The inflammation link between periodontal disease and coronary atherosclerosis in patients with acute coronary syndromes: case-control study. BMC oral health, 2021, 21(1), 5. [CrossRef]
- Esposito, L., Cancro, F. P., Silverio, A., Di Maio, M., Iannece, P., Damato, A., Alfano, C., De Luca, G., Vecchione, C., & Galasso, G. COVID-19 and Acute Coronary Syndromes: From Pathophysiology to Clinical Perspectives. Oxidative medicine and cellular longevity 2021, 4936571. [CrossRef]
- Oikonomou, E. K., Antonopoulos, A. S., Schottlander, D., Marwan, M., Mathers, C., Tomlins, P., Siddique, M., Klüner, L. V., Shirodaria, C., Mavrogiannis, M. C., et al. Standardized measurement of coronary inflammation using cardiovascular computed tomography: integration in clinical care as a prognostic medical device. Cardiovascular research 2021, 117(13), 2677–2690.
- Zhang, R., Ju, Z., Li, Y., Gao, Y., Gu, H., & Wang, X. Pericoronary fat attenuation index is associated with plaque parameters and stenosis severity in patients with acute coronary syndrome: a cross-sectional study. Journal of thoracic disease, 2022, 14(12), 4865–4876. [CrossRef]
- Antonopoulos, A.S., Angelopoulos, A., Papanikolaou, P., Simantiris, S., Oikonomou, E.K., Vamvakaris,K., Koumpoura, A., Farmaki, M., Trivella, M., Vlachopoulos, C., et al. Biomarkers of Vascular Inflammation for Cardiovascular Risk Prognostication: A Meta-Analysis. JACC 2022, 15(3), 460-271.
- Antoniades, C., Kotanidis, C. P., Berman, D. S. State-of-the-art review article. Atherosclerosis affecting fat: What can we learn by imaging perivascular adipose tissue?. JCCT 2019, 13(5), 288–296.
- Jaltotage, B., Sukudom, S., Ihdayhid, A.R., Dwivedi, R. Enhancing Risk Stratification on Coronary Computed Tomography Angiography: The Role of Artificial Intelligence. Clinical Therapeutics. 2023, 45(11), 1023-1028. [CrossRef]
- Kolossváry, M., Szilveszter, B., Merkely, B., & Maurovich-Horvat, P. Plaque imaging with CT-a comprehensive review on coronary CT angiography-based risk assessment. Cardiovascular diagnosis and therapy 2017, 7(5), 489–506.
- Reynolds, H. R., Shaw, L. J., Min, J. K., Page, C. B., Berman, D. S., Chaitman, B. R., Picard, M. H., Kwong, R. Y., O'Brien, S. M., Huang, Z., et al. Outcomes in the ISCHEMIA Trial Based on Coronary Artery Disease and Ischemia Severity. Circulation 2021, 144(13), 1024–1038.
- Huang, Z., Zhang, S., Jin, N., Hu, Y., Xiao, J., Li, Z., Yang, Y., Sun, R., Wang, Z., Li, X., et al. Prognostic value of CAD-RADS classification by coronary CTA in patients with suspected CAD. BMC cardiovascular disorders 2021, 21(1), 476.
- Jing, M., Xi, H., Zhu, H., Zhang, X., Xu, Z., Wu, S., Sun, J., Deng, L., Han, T., Zhang, B., Zhou, J. Is there an association between coronary artery inflammation and coronary atherosclerotic burden?. QIMS 2023, 13(9), 6048–6058.
- Tam, D. Y., Dharma, C., Rocha, R., Farkouh, M. E., Abdel-Qadir, H., Sun, L. Y., Wijeysundera, H. C., Austin, P. C., Udell, J. A., Gaudino, M., et al. Long-Term Survival After Surgical or Percutaneous Revascularization in Patients With Diabetes and Multivessel Coronary Disease. J Am Coll Cardiol 2020, 76(10), 1153–1164.
- Henein, M. Y., Vancheri, S., Longo, G., Vancheri, F. The Role of Inflammation in Cardiovascular Disease. IJMS 2022, 23(21), 12906.
- Zhao, Y. W., Yan, K. X., Sun, M. Z., Wang, Y. H., Chen, Y. D., Hu, S. Y. Inflammation-based different association between anatomical severity of coronary artery disease and lung cancer. JGC, 2022, 19(8), 575–582. [CrossRef]
- Manoochehri, H., Gheitasi, R., Pourjafar, M., Amini, R., & Yazdi, A. Investigating the relationship between the severity of coronary artery disease and inflammatory factors of MHR, PHR, NHR, and IL-25. MJIRI 2021, 35, 85.
- Tsao, C. W., Aday, A. W., Almarzooq, Z. I., Alonso, A., Beaton, A. Z., Bittencourt, M. S., Boehme, A. K., Buxton, A. E., Carson, A. P., Commodore-Mensah, Y., et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145(8), e153–e639.
- Knuuti, J., Wijns, W., Saraste, A., Capodanno, D., Barbato, E., Funck-Brentano, C., Prescott, E., Storey, R. F., Deaton, C., Cuisset, T., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020, 41(3), 407–477.
- Libby, P., Ridker, P. M., Hansson, G. K. Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009, 54(23), 2129–2138.
- Sorriento, D., Iaccarino, G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. IJMS 2019, 20(16), 3879.
- Liu, Y., Guan, S., Xu, H., Zhang, N., Huang, M., Liu, Z. Inflammation biomarkers are associated with the incidence of cardiovascular disease: a meta-analysis. Front Cardiovasc 2023, 10, 1175174.
- Schmitz, T., Harmel, E., Heier, M., Peters, A., Linseisen, J., & Meisinger, C. Inflammatory plasma proteins predict short-term mortality in patients with an acute myocardial infarction. Journal of translational medicine, 2022, 20(1), 457. [CrossRef]
- Dziedzic, E.A.; Gąsior, J.S.; Tuzimek, A.; Kochman, W. Blood Count-Derived Inflammatory Markers and Acute Complications of Ischemic Heart Disease in Elderly Women. J. Clin. Med. 2023, 12, 1369. [CrossRef]
- Wang, W., Wang, C. Y., Wang, S. I., & Wei, J. C. Long-term cardiovascular outcomes in COVID-19 survivors among non-vacinated population: A retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine 2022, 53, 101619. [CrossRef]
- Libby P. The changing landscape of atherosclerosis. Nature 2021, 592(7855), 524–533.
- Antoniades, C., Tousoulis, D., Vavlukis, M., Fleming, I., Duncker, D. J., Eringa, E., Manfrini, O., Antonopoulos, A. S., Oikonomou, E., Padró, T., et al. Perivascular adipose tissue as a source of therapeutic targets and clinical biomarkers. Eur Heart J 2023, 44(38), 3827–3844.
- Yan, H., Zhao, N., Geng, W., Hou, Z., Gao, Y., Lu, B. The Perivascular Fat Attenuation Index Improves the Diagnostic Performance for Functional Coronary Stenosis. J. Cardiovasc. Dev. Dis. 2022, 9, 128. [CrossRef]
- Sun, J. T., Sheng, X. C., Feng, Q., Yin, Y., Li, Z., Ding, S., Pu, J. Pericoronary Fat Attenuation Index Is Associated With Vulnerable Plaque Components and Local Immune-Inflammatory Activation in Patients With Non-ST Elevation Acute Coronary Syndrome. JAHA 2022, 11(2), e022879.
- Klüner, L. V., Oikonomou, E. K., Antoniades, C. Assessing Cardiovascular Risk by Using the Fat Attenuation Index in Coronary CT Angiography. Radiology. Cardiothoracic imaging 2021, 3(1), e200563.
- Zeinali-Nezhad, N., Najafipour, H., Shadkam, M., Pourhamidi, R. Prevalence and trend of multiple coronary artery disease risk factors and their 5-year incidence rate among adult population of Kerman: results from KERCADR study. BMC public health, 2024, 24(1), 25. [CrossRef]
- Nita, D., Ionescu, M., Mazilu, L., Suceveanu, A.I., Munteanu, A., Ionescu, P., et al. Statins and the risk for coronary in-stent restenosis in diabetic patients. Farmacia, 2021, 69(3), 576. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).