Submitted:
15 April 2024
Posted:
17 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Evolution of Endoscopic Techniques for Spine Surgery
| Year | Author | Technique described |
|---|---|---|
| 1973 | Kambin (10) | Percutaneous nucleotomy with Craig cannula (5mm) Fluoroscopy guided without visualization |
| 1975 | Hijikata (11) | Percutaneous nucleotomy (2.6mm cannula) Fluoroscopy guided without visualization |
| 1983 | Kambin (12) | Percutaneous arthroscopic discectomy |
| 1997 | Foley (14) | Microendoscopic discectomy |
| 1999 | Yeung (18) | YESS - inside out technique |
| 1999 | Destandau (16) | Destandau’s Endospine technique |
| 2005 | Hoogland (21) | Transforaminal Endoscopy - Outside in technique |
| 2006 | Choi (24) | Interlaminar approach for L5-S1 level |
| 2016 | Eum et al (26) | Unilateral Biportal Endoscopy (PBED) |
3. Lumbar Spine Endoscopy
3.1. Microendoscopic Discectomy and Decompression
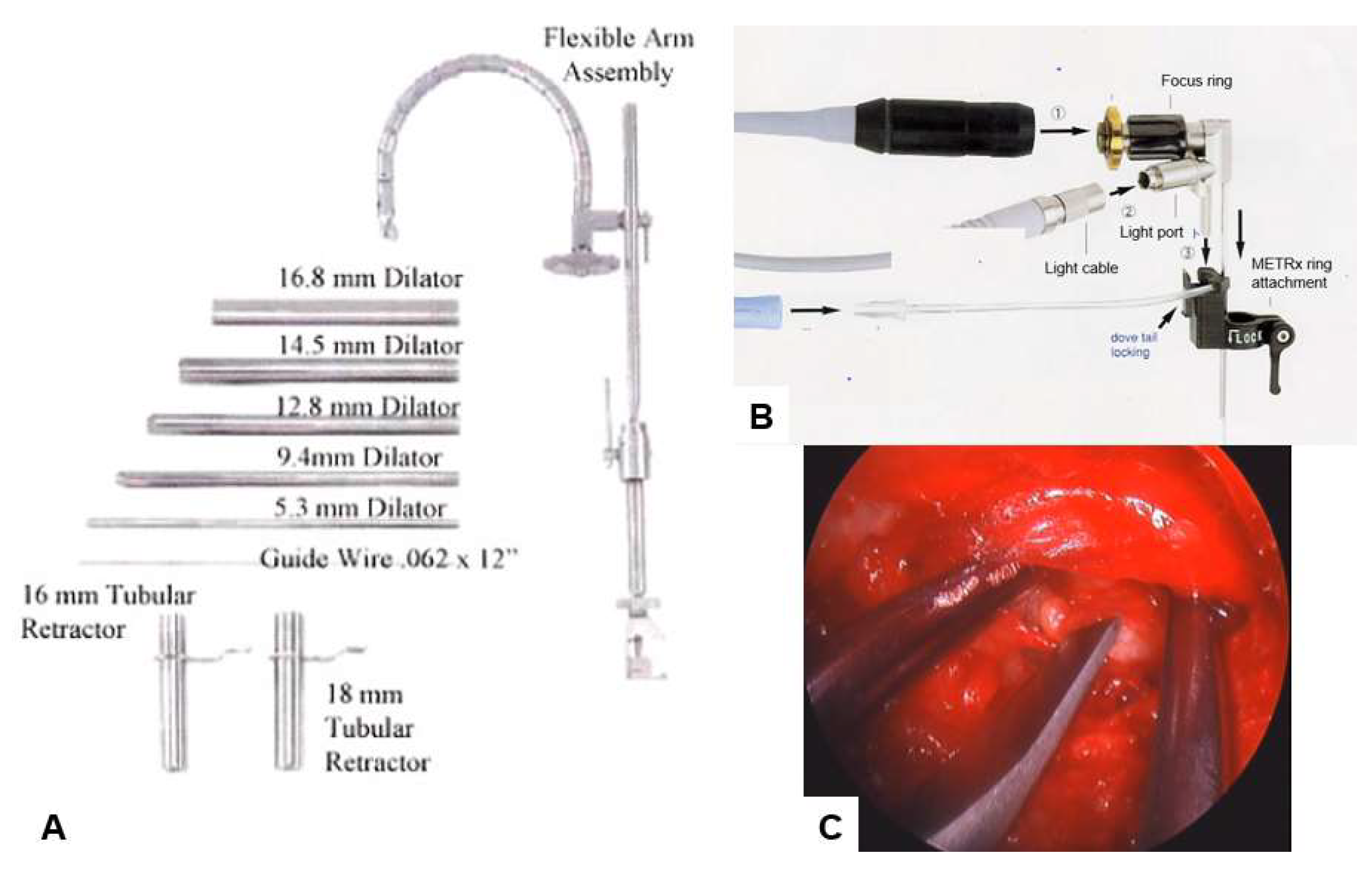
| Author | Sample | Approach | Follow up | Outcomes | Complication |
|---|---|---|---|---|---|
| Perez-Cruet 2002 [27] |
150 | Paramedian | 12 months | Mean operative time (min) 97 Mean hospital stay (hours) 7.7 (range, 2–24) Mean time to return to work (days) 17 |
Dural tears 8/150 (5%) recurrence 4/150 Pseudomeningocoele 1/150 (0.7%) Surgical site infection 1/150 (0.7%) |
| Khoo 2002 [30] | 25 vs 25 open | Paramedian | 12 months | Lesser blood loss 44ml for med vs 193 ml Shorter hospital stay 42 hours vs 94 hours |
Additional fusion surgery 0% vs. 12% Transfusion 0% vs. 8% Dural tear 16% vs. 8% |
| Ikuta 2005 [31] | 47 vs 29 microdiscectony |
Paramedian | 22 months | Rate of recovery 72% (improvement in JOA score) (38/47) | Higher complication rate compared to microdiscectomy |
| Wu 2006 [32] | 873 Vs 358 open | Not specified | 28 months vs 31 months for open | Earlier return to work
Shorter hospital stay Shorter operation time Lesser blood loss Lesser analgesic need |
35/873 for MED vs 19/358 |
| Fukushi 2015 [33] | 58 vs 39 open |
Midline | 42 months | Similar improvement in JOA and similar patient satisfaction | Higher rate of infection, |
| Wu 2020 [34] | 82 vs. 52 Full endoscopy |
Paramedian | 20 months | Similar improvement in VAS for leg pain Higher VAS for low back pain and ODI |
Dysesthesia 0% vs. 1.9%, Dural tear 2.4%vs. 1.9%, Urinary retention 1.2% vs. 0%, Total 3.85% vs. 3.66% |
| Iwai 2020 [35] | 60 vs. 54Microendoscopic | Paramedian | 3 months | Shorter operating time but longer hospital stay compared to biportal endoscopy. Similar VAS/NRS in both groups |
Dural tear 5.6% vs. 1.8% Hematoma 3.3% vs. 13.0% |
| Ito 2021 [36] | 139 vs. 42 Biportal Endoscopy | Paramedian | 6 months | Similar improvement in VAS for low back pain and leg pain, OD | Dural tear 5.8% vs. 4.7%, Hematoma 3.6% vs. 0%, Re-operation1.4% vs. 0% |
3.2. Destandau’s Endospine Technique:
3.3. Transforaminal Endoscopy
3.4. Interlaminar Endoscopy :
3.4.1. Interlaminar Endoscopic Lumbar Discectomy (IELD)
3.4.2. Percutaneous Biportal Endoscopic Discectomy and Decompression (PBED)
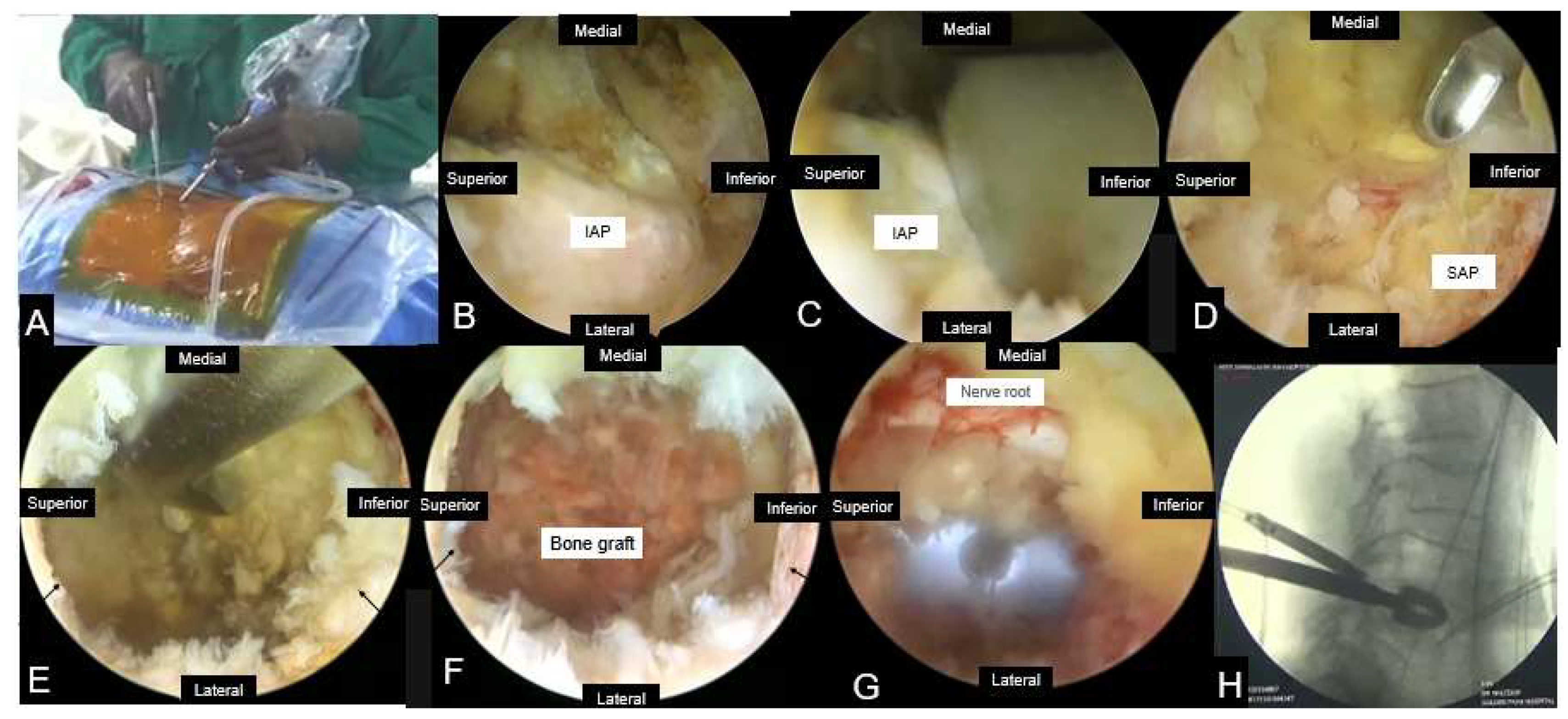
| Author | Study type | Sample | Diagnosis | Follow up(Months) | Operation time (minutes) | Complications |
|---|---|---|---|---|---|---|
| Eum et al(2016) [26] | Retrospective | 58 | LSS | 13.8 | 68.9 ± 16.1 | 13.8%; post-op headache(3),dural tear (2),transient numbness (2), epidural hepatoma (1) |
| Choi et al (2016) [63] | Retrospective | 68 | LDH(25),revision(3) stenosis(39),synovial cyst(1) | NR | 68.2±23.7 | 10.3%;dural tear(2),nerve root injury(1),incomplete decompression(4) |
| Kim et al(2018)[64] | Retrospective | 60 | LDH | 12.6 | 70.15 ± 22.0 | 5%, incomplete decompression (3) |
| Ahn et al (2018)[55] | Retrospective | 21 | Foraminal stenosis (11), foraminal LDH(9),ASD(1) | 14.8 | 96.7 | 4.8%;dural tear (1) |
| Kim and Choi (2018)[65] | Retrospective | 105 | LSS | 14 | 53 ± 13.5 | 2.9%;dural tear (2), epidural hematoma(1) |
| Akbary et al (2018)[66] | Retrospective | 30 | Lateral recess+foraminal stenosis | 5.67 | 102.5 ± 43.66 | 0% |
| Pao et al (2019)[67] | Retrospective | 81 | LSS | 8.6 | NR | 8.6%;dural tear(4), transient motor weakness (1), inadequate decompression (1),epidural hematoma(1) |
| Wang et al (2023)[68] | Prospective | 70 | LDH | 24 | NR | 0% |
3.4.3. Endoscopic Lumbar Interbody Fusion
4. Thoracic Spine Endoscopy
4.1. Full Endoscopy
4.2. Micro-Endoscopy
4.3. Bi-Portal Endoscopy (UBE)
5. Cervical Spine Endoscopy
5.2. Endoscopic Aterior Cevical Dscectomy and Fusion
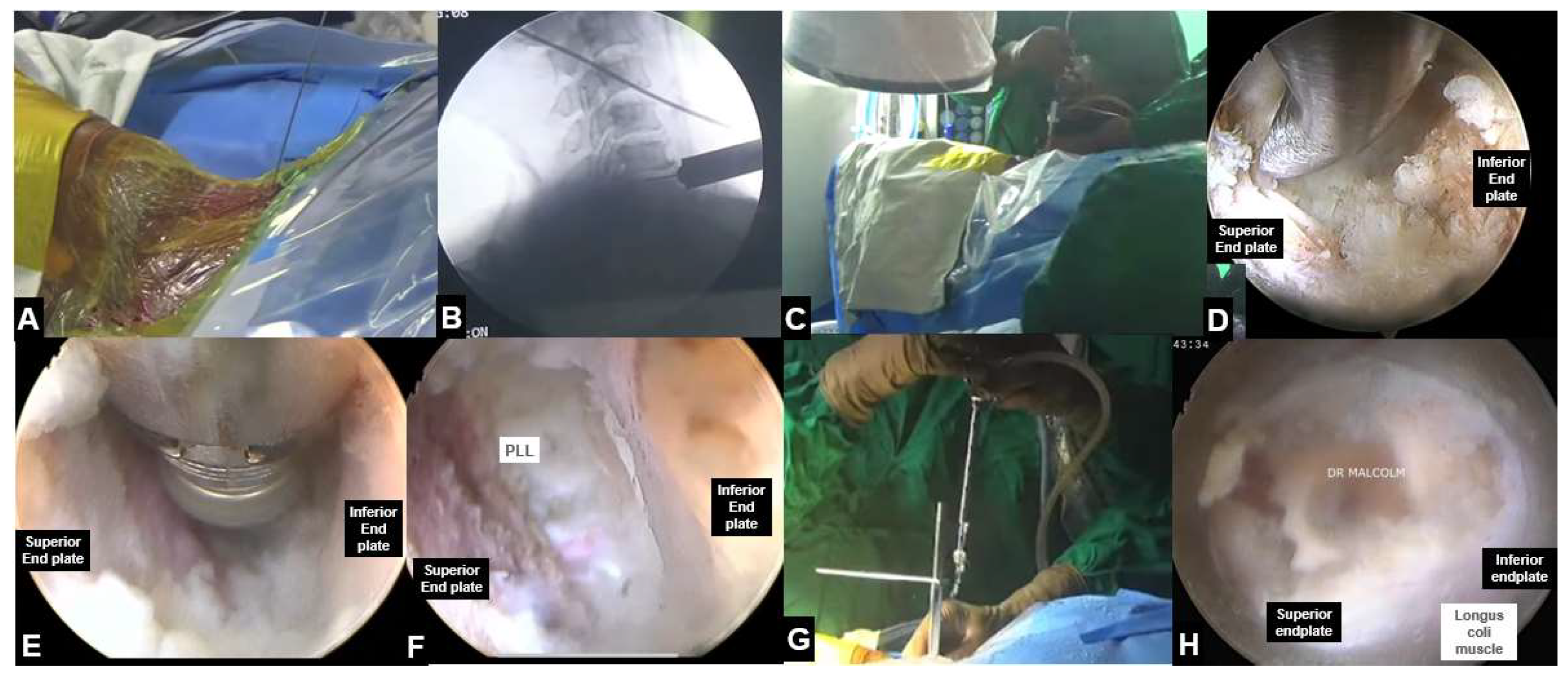
5.3. UBE Cervical Foraminotomy and Laminectomy
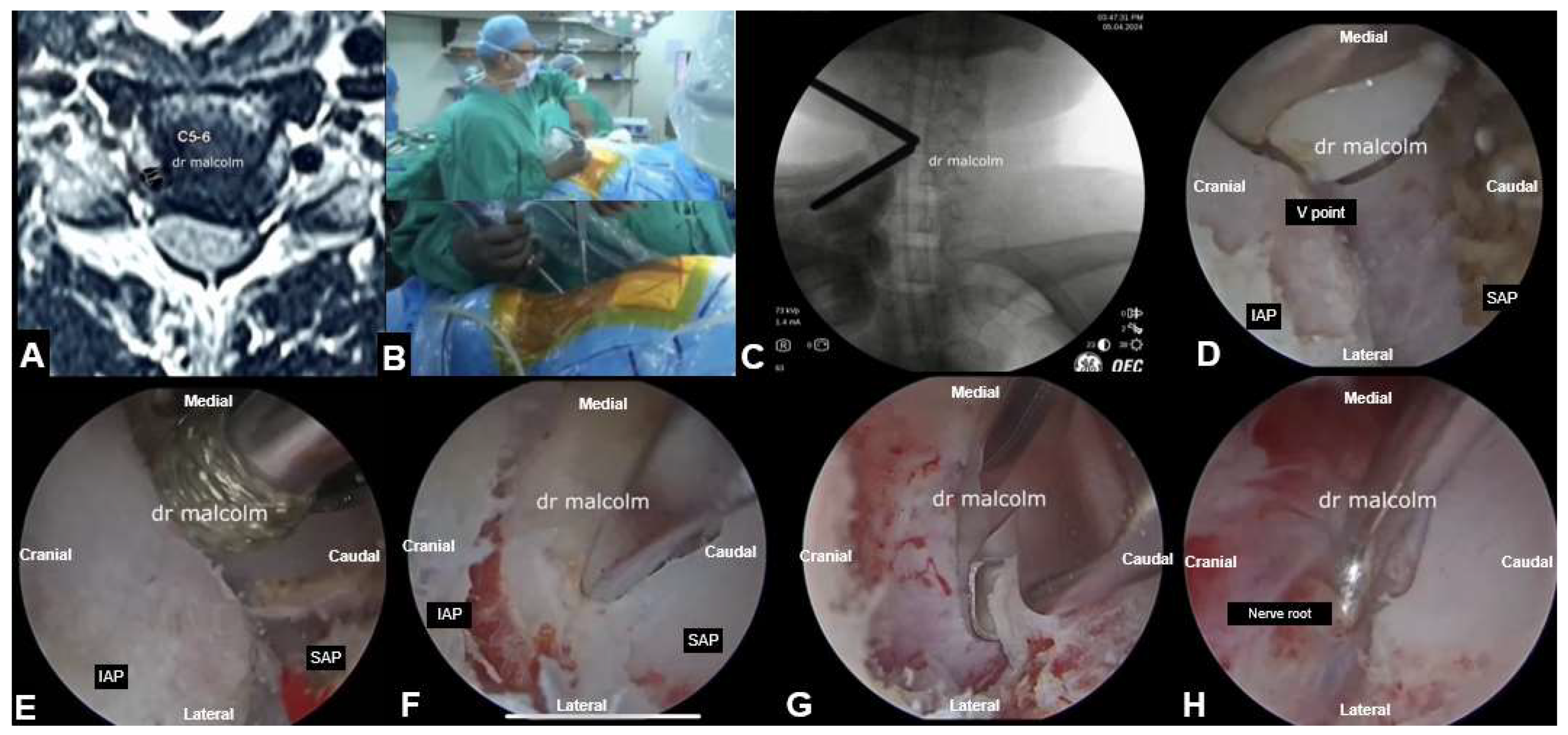
| Author | Sample | Approach | Follow up | Outcome | Complication |
|---|---|---|---|---|---|
| Fontanella (1999)[96] |
296 (273 anterior,23 posterior) |
Anterior, posterior | 12 months | 97% success at 1 year; average surgical time 25 min | Nil |
| Ahn (2005)[97] |
111 | Anterior | 49 months | Excellent/ good outcome in 80% | Persistent radicular pain in 1 patient 1 patient required ACDF |
| Lee (2007)[98] |
116 | Anterior | 36 months | 87 % had good outcomes | 2 patients ACDF 2 patients repeat endoscopy 1 patient - posterior fixation |
| Reutten (2009)[99] |
60 | Anterior | 24 months | 96% - good clinical outcome | 2 patients - progressive neck pain 2 patients - radicular pain 4 patients - ACDF |
| Yang (2014)[100] |
84 | Anterior 42 Posterior 42 |
18 months | Shorter operating time for anterior approach Shorter hospital stay for posterior approach |
1 Neurological deterioration 1 Hematoma - 1 Re-operation -1 post-operative headache - |
| Oertel (2016)[101] |
43 | Posterior | 6 months | 82% regained full arm strength; mean one level operation time 77 min | 1 haematoma, 1 triceps paresis |
6. Latest Advances
6.1. Navigation
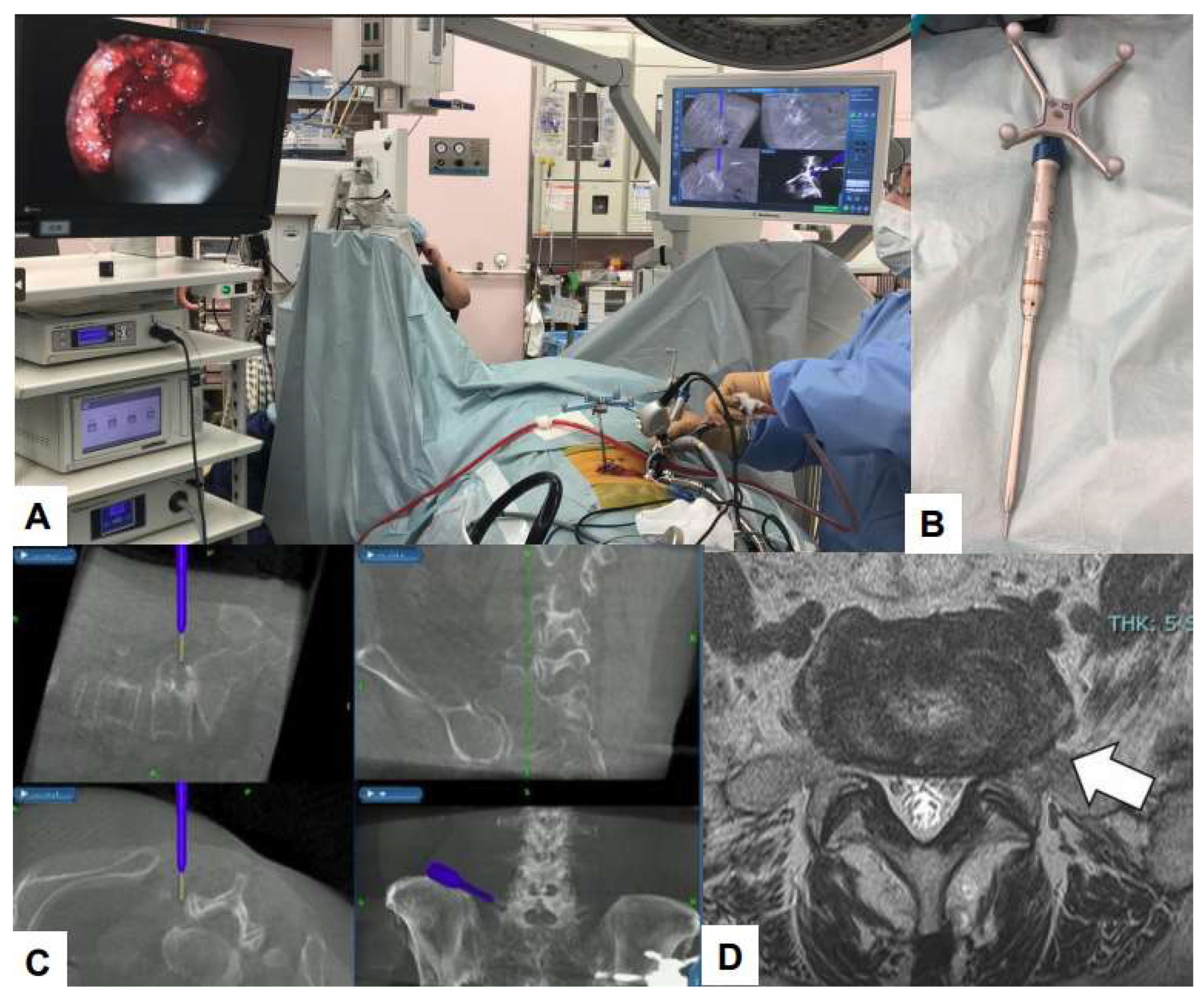
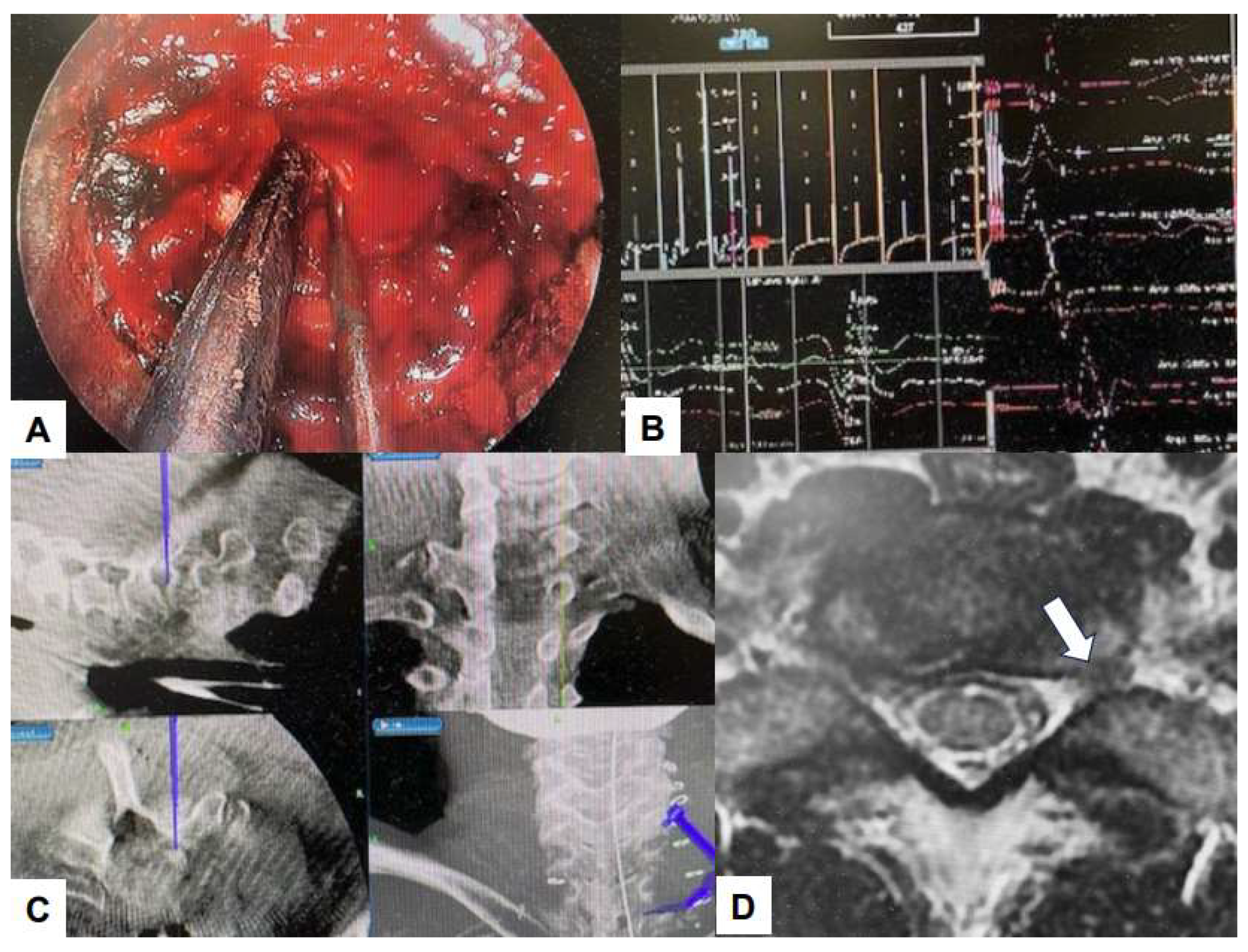
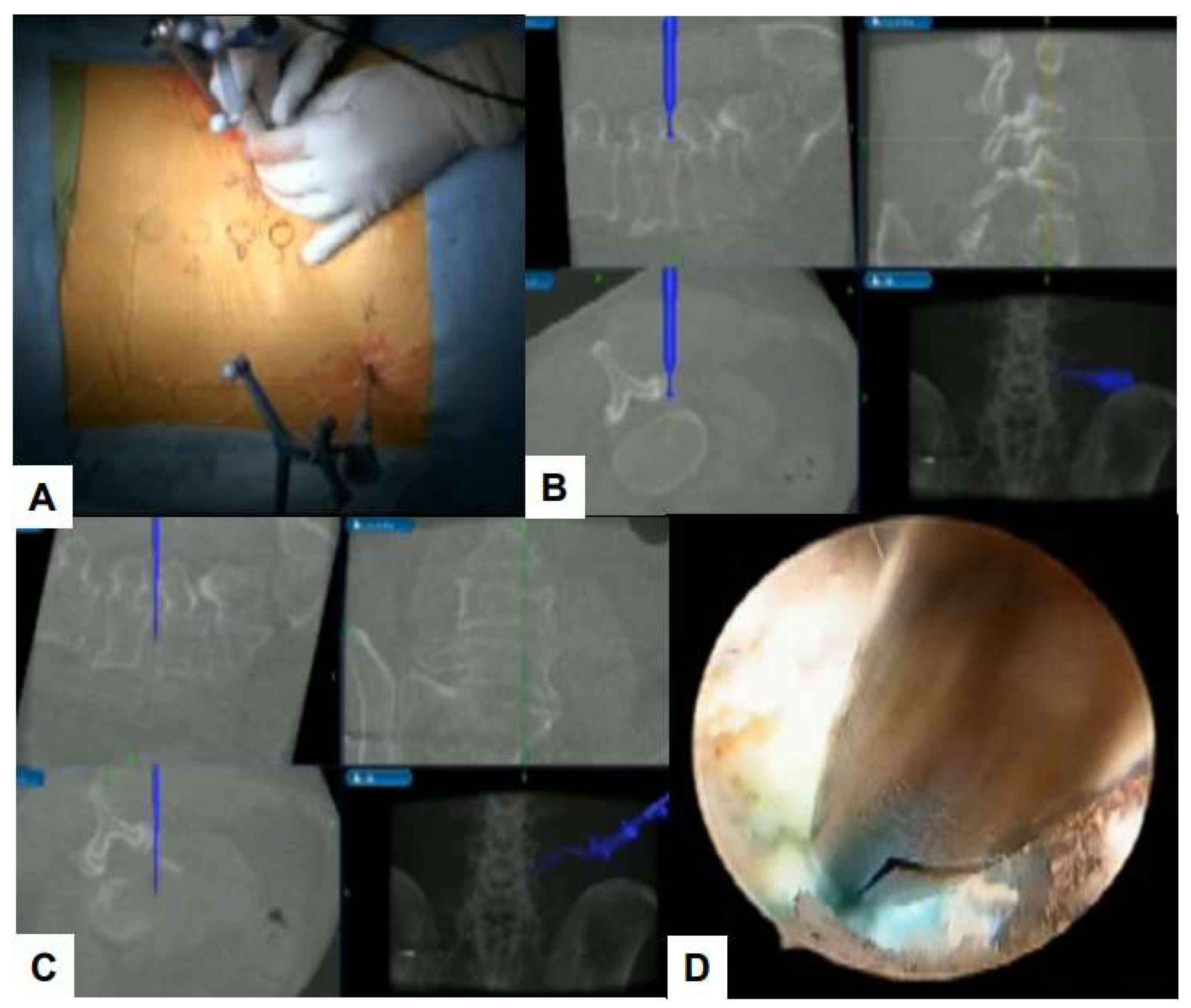
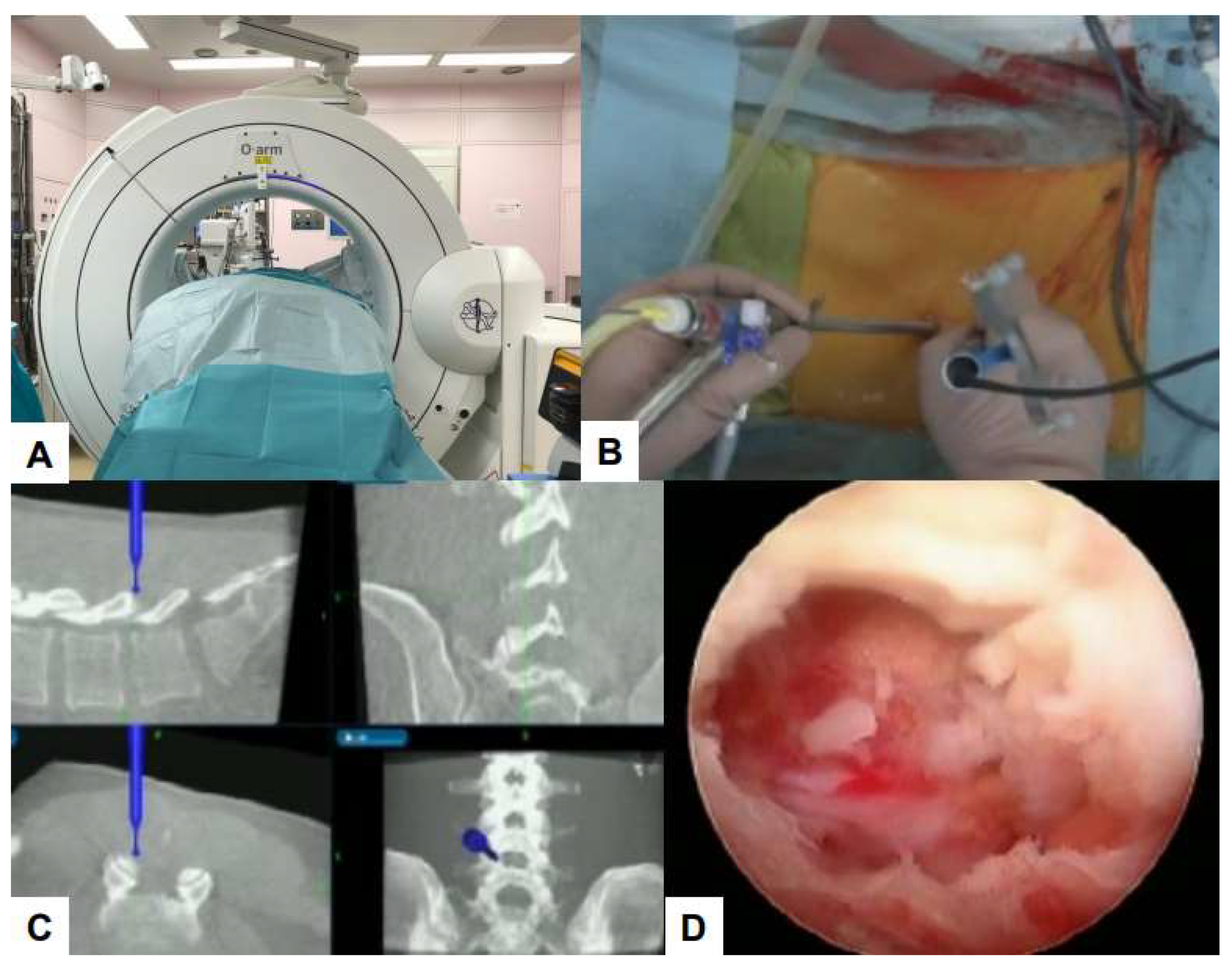
6.2. Ultraresolution and Three Dimensional Endoscopes
6.3. Robot-Assisted Endoscopic Surgery
7. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
References
- Fehlings, M.G.; Tetreault, L.; Nater, A.; Choma, T.; Harrop, J.; Mroz, T.; Santaguida, C.; Smith, J.S. The aging of the global population: the changing epidemiology of disease and spinal disorders. Neurosurgery 2015, 77, S1–S5. [Google Scholar] [CrossRef]
- Hirai, T.; Uehara, M.; Miyagi, M.; Takahashi, S.; Nakashima, H. Current advances in spinal diseases of the elderly: introduction to the special issue. Journal of Clinical Medicine 2021, 10, 3298. [Google Scholar] [CrossRef]
- Campbell, G.; Yadla, S.; Nasser, R.; Malone, J.; Maltenfort, M.G.; Ratliff, J.K. Patient comorbidity score predicting the incidence of perioperative complications: assessing the impact of comorbidities on complications in spine surgery. Journal of Neurosurgery: Spine 2012, 16, 37–43. [Google Scholar] [CrossRef]
- Katz, J.N.; Lipson, S.J.; Larson, M.G.; McInnes, J.M.; Fossel, A.H.; Liang, M.H. The outcome of decompressive laminectomy for degenerative lumbar stenosis. The Journal of Bone & Joint Surgery 1991, 73, 809–816. [Google Scholar]
- Sharif, S.; Shaikh, Y.; Peev, N. Minimally invasive spinal surgery: how to keep out of trouble. World Neurosurgery 2018, 119, 517–526. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.S.; Oh, S.W.; Adsul, N.M.; Singh, R.; Kashlan, O.N.; Noh, J.H.; Jang, I.T.; Oh, S.H. Evolution of spinal endoscopic surgery. Neurospine 2019, 16, 6. [Google Scholar] [CrossRef]
- Kim, H.S.; Wu, H.; Jang, I.T. Current and future of endoscopic spine surgery: what are the common procedures we have now and what lies ahead? World Neurosurgery 2020, 140, 642–653. [Google Scholar] [CrossRef]
- Fu, T.S.; Wang, Y.C.; Lin, T.Y.; Chang, C.W.; Wong, C.B.; Su, J.Y. Comparison of percutaneous endoscopic surgery and traditional anterior open surgery for treating lumbar infectious spondylitis. Journal of Clinical Medicine 2019, 8, 1356. [Google Scholar] [CrossRef]
- Şentürk, S.; Ünsal, Ü.Ü. Percutaneous full-endoscopic removal of lumbar intradural extramedullary tumor via translaminar approach. World Neurosurgery 2019, 125, 146–149. [Google Scholar] [CrossRef]
- Telfeian, A.E.; Veeravagu, A.; Oyelese, A.A.; Gokaslan, Z.L. A brief history of endoscopic spine surgery. Neurosurgical focus 2016, 40, E2. [Google Scholar] [CrossRef]
- HIJIKATA, S. Percutaneous Nucleotomy: A New Concept Technique and 12 Years' Experience. Clinical Orthopaedics and Related Research (1976-2007) 1989, 238, 9–23. [Google Scholar]
- Kambin, P.; Zhou, L. ▪ History and Current Status of Percutaneous Arthroscopic Disc Surgery. Spine 1996, 21, 57S–61S. [Google Scholar] [CrossRef]
- Kambin, P. Arthroscopic microdiskectomy. The Mount Sinai journal of medicine, New York 1991, 58, 159–164. [Google Scholar]
- KT, F. Microendoscopic discectomy. Techniques in neurosurgery 1997, 3, 301–307. [Google Scholar]
- Foley, K.T.; Smith, M.M.; Rampersaud, Y.R. Microendoscopic approach to far-lateral lumbar disc herniation. Neurosurgical focus 1999, 7, E7. [Google Scholar] [CrossRef]
- Destandau, J. A special device for endoscopic surgery of lumbar disc herniation. Neurological Research 1999, 21, 39–42. [Google Scholar] [CrossRef]
- Kaushal, M.; Kaushal, M. Destandau’s Approach to the Cervical and Thoracic Spine. Journal of Minimally Invasive Spine Surgery and Technique 2023, 8, 89–96. [Google Scholar] [CrossRef]
- Yeung, A.T. Minimally Invasive Disc Surgery with the Yeung Endoscopic Spine System (YESS). Surgical technology international 1999, 8, 267–277. [Google Scholar]
- Yeung, A.T.; Yeung, C.A. Advances in endoscopic disc and spine surgery: foraminal approach. Surg Technol Int 2003, 11, 255–263. [Google Scholar]
- Gore, S.; Yeung, A. The “inside out” transforaminal technique to treat lumbar spinal pain in an awake and aware patient under local anesthesia: results and a review of the literature. International journal of spine surgery 2014, 8. [Google Scholar] [CrossRef]
- Schubert, M.; Hoogland, T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper Orthop Traumatol. 2005, 17, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, S.R.; Schwartz, D.G.; Glazier, K.D. Anatomic considerations in lumbar posterolateral percutaneous procedures. Spine 1995, 20, 1965–1971. [Google Scholar] [CrossRef]
- Reulen, H.J.; Müller, A.; Ebeling, U. Microsurgical anatomy of the lateral approach to extraforaminal lumbar disc herniations. Neurosurgery 1996, 39, 345–351. [Google Scholar] [CrossRef]
- Choi, G.; Lee, S.H.; Raiturker, P.; Lee, S.; Chae, Y.S. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5–S1 using a rigid working channel endoscope. Operative Neurosurgery 2006, 58, ONS-59. [Google Scholar] [CrossRef]
- Heo, D.H.; Lee, D.C.; Park, C.K. Comparative analysis of three types of minimally invasive decompressive surgery for lumbar central stenosis: biportal endoscopy, uniportal endoscopy, and microsurgery. Neurosurgical focus 2019, 46, E9. [Google Scholar] [CrossRef]
- Eum, J.H.; Heo, D.H.; Son, S.K.; Park, C.K. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. Journal of neurosurgery: Spine 2016, 24, 602–607. [Google Scholar]
- Perez-Cruet, M.J.; Foley, K.T.; Isaacs, R.E.; Rice-Wyllie, L.; Wellington, R.; Smith, M.M.; Fessler, R.G. Microendoscopic lumbar discectomy. Neurosurgery 2002, 51, S2–S129. [Google Scholar] [CrossRef]
- Issacs, R.E.; Podichetty, V.; Fessler, R.G. Microendoscopic disccetomy for recurrent disc herniations. Neurosurg Focus 2003, 15, 11. [Google Scholar]
- Jhala, A.; Mistry, M. Endoscopic lumbar disccetomy; Experience of first 100 cases. Indian J orthop 2010, 44, 184–190. [Google Scholar] [CrossRef]
- Khoo, L.T.; Fessler, R.G. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery 2002, 51, S2–S146. [Google Scholar] [CrossRef]
- Ikuta, K.; Arima, J.; Tanaka, T.; Oga, M.; Nakano, S.; Sasaki, K.; Goshi, K.; Yo, M.; Fukagawa, S. Short-term results of microendo- scopic posterior decompression for lumbar spinal stenosis. J. Neurosurg. Spine 2005, 2, 624–633. [Google Scholar] [CrossRef]
- Wu, X.; Zhuang, S.; Mao, Z.; Chen, H. Microendoscopic discectomy for lumbar disc herniation: surgical technique and outcome in 873 consecutive cases. Spine 2006, 31, 2689–2694. [Google Scholar] [CrossRef]
- Fukushi, R.; Yoshimoto, M.; Iesato, N.; Terashima, Y.; Takebayashi, T.; Yamashita, T.; Fukushi, R. Short-term results of microendoscopic muscle-preserving interlaminar decompression versus spinal process splitting laminectomy. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2018, 79, 511–517. [Google Scholar]
- Wu, B.; Xiong, C.; Tan, L.; Zhao, D.; Xu, F.; Kang, H. Clinical outcomes of MED and iLESSYS® delta for the treatment of lumbar central spinal stenosis and lateral recess stenosis: A comparison study. Exp. Ther. Med. 2020, 20, 252. [Google Scholar] [CrossRef]
- Iwai, H.; Inanami, H.; Koga, H. Comparative study between full-endoscopic laminectomy and microendoscopic laminectomy for the treatment of lumbar spinal canal stenosis. J. Spine Surg. 2020, 6, E3–E11. [Google Scholar] [CrossRef]
- Ito Shibayama, M.; Nakamura, S.; Yamada, M.; Kawai, M.; Takeuchi, M.; Yoshimatsu, H.; Kuraishi, K.; Hoshi, N.; Miura, Y.; et al. Clinical comparison of unilateral biportal endoscopic laminectomy versus microendoscopic laminectomy for single-level laminectomy: A single-center, retrospective analysis. World Neurosurg. 2021, 148, e581–e588. [Google Scholar]
- Dey, P.C.; Nanda, N. Functional outcome after endoscopic lumbar discectomy by destandau’s technique: a prospective study of 614 patients. Asian Spine Journal 2019, 13, 786. [Google Scholar] [CrossRef]
- KimHSWu, P.H.; Jang, I.T. Current and future of endoscopic spine surgery: what are the common procedures we have now and what lies ahead? World Neurosurgery 2020, 140, 642–653. [Google Scholar]
- Kim, H.S.; Paudel, B.; Jang, J.S.; Lee, K.; Oh, S.H.; Jang, I.T. Percutaneous endoscopic lumbar discectomy for all types of lumbar disc herniations (LDH) including severely difficult and extremely difficult LDH cases. Pain physician 2018, 21, E401. [Google Scholar]
- Knight, M.T.; Jago, I.; Norris, C.; Midwinter, L.; Boynes, C. Transforaminal endoscopic lumbar decompression & foraminoplasty: a 10 year prospective survivability outcome study of the treatment of foraminal stenosis and failed back surgery. International journal of spine surgery 2014, 8. [Google Scholar] [CrossRef]
- Kim HS, Yudoyono F, Paudel B, et al. Suprapedicular circumferential opening technique of percutaneous endoscopic transforaminal lumbar discectomy for high grade inferiorly migrated lumbar disc herniation. Biomed Res Int.
- Lewandrowski, K.U. Incidence, management, and cost of complications after transforaminal endoscopic decompression surgery for lumbar foraminal and lateral recess stenosis: a value proposition for outpatient ambulatory surgery. International Journal of SpineSurgery 2019, 13, 53–67. [Google Scholar] [CrossRef]
- Osman, S.G.; Nibu, K.; Panjabi, M.M.; et al. Transforaminal and posterior decompressions of the lumbar spine. A comparative study of stability and intervertebral foramen area. Spine (Phila Pa 1976) 1997, 22, 1690–1695. [Google Scholar] [CrossRef]
- Sairyo, K.; Higashino, K.; Yamashita, K.; et al. A new concept of transforaminal ventral facetectomy including simultaneous decompression of foraminal and lateral recess stenosis: technical considerations in a fresh cadaver model and a literature review. J Med Invest. 2017, 64, 1–6. [Google Scholar] [CrossRef]
- Yagi, K.; Kishima, K.; Tezuka, F.; et al. Advantages of revision transforaminal full-endoscopic spine surgery in patients who have previously undergone posterior spine surgery. J Neurol Surg A Cent Eur Neurosurg 2022. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.U. Endoscopic transforaminal and lateral recess decompression after previous spinal surgery. Int J Spine Surg. 2018, 12, 98–111. [Google Scholar] [CrossRef]
- Jadczak, C.N.; Vanjani, N.N.; Pawlowski, H.; Cha, E.D.; Lynch, C.P.; Prabhu, M.C.; Hartman, T.J.; Nie, J.W.; MacGregor, K.R.; Zheng, E.; Oyetayo, O.O. The Current Status of Awake Endoscopic Surgery: A Systematic Review and Meta-Analysis. World Neurosurgery, 2023. [Google Scholar]
- Telfeian AE, Sastry R, Oyelese A, et al. Awake, transforaminal endoscopic lumbar spine surgery in octogenarians: case series. Pain Physician. 2022, 25, E255–62.
- Song, S.K.; Son, S.; Choi, S.W.; Kim, H.K. Comparison of the outcomes of percutaneous endoscopic interlaminar lumbar discectomy and open lumbar microdiscectomy at the L5-S1 level. Pain physician 2021, 24, E467. [Google Scholar]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. 2008. [Google Scholar]
- Chen, K.T.; Choi, K.C.; Shim, H.K.; Lee, D.C.; Kim, J.S. Full-endoscopic versus microscopic unilateral laminotomy for bilateral decompression of lumbar spinal stenosis at L4–L5: comparative study. International Orthopaedics 2022, 46, 2887–2895. [Google Scholar] [CrossRef]
- Lee, C.H.; Choi, M.; Choi, I.; Kim, C.H.; Kim, H.S.; Sohn, M.J. Efficacy and safety of full-endoscopic decompression via interlaminar approach for central or lateral recess spinal stenosis of the lumbar spine: a meta-analysis. Spine 2018, 43, 1756–1764. [Google Scholar] [CrossRef]
- McGrath, L.B.; White-Dzuro, G.A.; Hofstetter, C.P. Comparison of clinical outcomes following minimally invasive or lumbar endoscopic unilateral laminotomy for bilateral decompression. Journal of Neurosurgery: Spine 2019, 30, 491–499. [Google Scholar] [CrossRef]
- Heo, D.H.; Son, S.K.; Eum, J.H.; Park, C.K. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: technical note and preliminary clinical results. Neurosurg Focus. 2017, 43, E8. [Google Scholar] [CrossRef]
- Ahn, J.S.; Lee, H.J.; Choi, D.J.; Lee, K.Y.; Hwang, S.J. Extraforaminal approach of biportal endoscopic spinal surgery: a new endoscopic technique for transforaminal decompression and discectomy. J Neurosurg Spine. 2018, 28, 492–498. [Google Scholar] [CrossRef]
- Heo, D.H.; Quillo-Olvera, J.; Park, C.K. Can percutaneous biportal endoscopic surgery achieve enough canal decompression for degenerative lumbar stenosis? prospective case-control study. World Neurosurg. 2018, 120, e684–9. [Google Scholar] [CrossRef]
- Pao, J.L. A review of unilateral biportal endoscopic decompression for degenerative lumbar canal stenosis. Int J Spine Surg. 2021, 15, S65–S71. [Google Scholar] [CrossRef]
- Li, Y.S.; Chen, C.M.; Hsu, C.J.; Yao, Z.K. Complications of unilateral biportal endoscopic lumbar discectomy: a systematic review. World Neurosurgery 2022, 168, 359–368. [Google Scholar] [CrossRef]
- Kwon, O.; Yoo, S.J.; Park, J.Y. Comparison of unilateral biportal endoscopic discectomy with other surgical technics: a systemic review of indications and outcomes of unilateral biportal endoscopic discectomy from the current literature. World Neurosurgery 2022, 168, 349–358. [Google Scholar] [CrossRef]
- Hua, W.; Liao, Z.; Chen, C.; Feng, X.; Ke, W.; Wang, B.; Li, S.; Wang, K.; Zeng, X.; Wu, X.; Zhang, Y. Clinical outcomes of uniportal and biportal lumbar endoscopic unilateral laminotomy for bilateral decompression in patients with lumbar spinal stenosis: a retrospective pair-matched case-control study. World Neurosurgery 2022, 161, e134–e145. [Google Scholar] [CrossRef]
- Heo, D.H.; Lee, D.C.; Park, C.K. Comparative analysis of three types of minimally invasive decompressive surgery for lumbar central stenosis: biportal endoscopy, uniportal endoscopy, and microsurgery. Neurosurg Focus. 2019, 46, E9. [Google Scholar] [CrossRef]
- Kang, M.S.; Park, H.J.; You, K.H.; Choi, D.J.; Park, C.W.; Chung, H.J. Comparison of Primary Versus Revision Lumbar Discectomy Using a Biportal Endoscopic Technique. Global Spine Journal 2023, 13, 1918–1925. [Google Scholar] [CrossRef]
- Choi, D.J.; Choi, C.M.; Jung, J.T.; Lee, S.J.; Kim, Y.S. Learning curve associated with complications in biportal endoscopic spinal surgery: challenges and strategies. Asian Spine J. 2016, 10, 624–629. [Google Scholar] [CrossRef]
- Kim, S.K.; Kang, S.S.; Hong, Y.H.; Park, S.W.; Lee, S.C. Clinical comparison of unilateral biportal endoscopic technique versus open microdiscectomy for single-level lumbar discectomy: a multicenter, retrospective analysis. J Orthop Surg Res. 2018, 13, 22. [Google Scholar] [CrossRef]
- Kim, J.E.; Choi, D.J. Unilateral biportal endoscopic decompression by 30 endoscopy in lumbar spinal stenosis: technical note and preliminary report. J Orthop. 2018, 15, 366–371. [Google Scholar] [CrossRef]
- Akbary, K.; Kim, J.S.; Park, C.W.; Jun, S.G.; Hwang, J.H. Biportal endoscopic decompression of exiting and traversing nerve roots through a single interlaminar window by a contralateral approach: technical feasibilities and morphometric changes of the lumbar canal and foramen. World Neurosurg. 2018, 117, 153–161. [Google Scholar] [CrossRef]
- Pao, J.L.; Lin, S.M.; Chen, W.C.; Chang, C.H. Unilateral biportal endoscopic decompression for degenerative lumbar canal stenosis. Journal of Spine Surgery 2020, 6, 438. [Google Scholar] [CrossRef]
- Wang, J.C.; Li, Z.Z.; Cao, Z.; Zhu, J.L.; Zhao, H.L.; Hou, S.X. Modified unilateral biportal endoscopic lumbar discectomy results in improved clinical outcomes. World Neurosurgery 2023, 169, e235–e244. [Google Scholar] [CrossRef]
- Morimoto, M.; Sairyo, K. Full-endoscopic trans-Kambin’s triangle lumbar interbody fusion (Fullendo-KLIF). Transforaminal Full-Endoscopic Lumbar Surgery Under the Local Anesthesia: State of the Art 2021, 87–95. [Google Scholar]
- Pholprajug, P.; Kotheeranurak, V.; Liu, Y.; Kim, J.S. The endoscopic lumbar interbody fusion: a narrative review, and future perspective. Neurospine 2023, 20, 1224. [Google Scholar] [CrossRef]
- Li, Y.; Dai, Y.; Wang, B.; Li, L.; Li, P.; Xu, J.; Jiang, B.; Lü, G. Full-endoscopic posterior lumbar interbody fusion via an interlaminar approach versus minimally invasive transforaminal lumbar interbody fusion: a preliminary retrospective study. World neurosurgery 2020, 144, e475–e482. [Google Scholar] [CrossRef]
- Han, H.; Song, Y.; Li, Y.; Zhou, H.; Fu, Y.; Li, J. Short-term clinical efficacy and safety of unilateral biportal endoscopic transforaminal lumbar interbody fusion versus minimally invasive transforaminal lumbar interbody fusion in the treatment of lumbar degenerative diseases: a systematic review and meta-analysis. Journal of Orthopaedic Surgery and Research 2023, 18, 656. [Google Scholar]
- Simpson, A.K.; Lightsey, H.M., 4th; Xiong, G.X.; Crawford, A.M.; Minamide, A.; Schoenfeld, A.J. Spinal endoscopy: evidence, techniques, global trends, and future projections. Spine J. 2022, 22, 64. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Ahn, Y.; Choi, G.; et al. AO Spine consensus paper on nomenclature for working channel endoscopic spinal procedures. Global Spine J 2020, 10, 111S–121. [Google Scholar] [CrossRef]
- Gibson, R. D. S.; Wagner, R.; Gibson, J. N. A. Full endoscopic surgery for thoracic pathology: an assessment of supportive evidence. In EFORT Open Reviews; Retrieved Apr 10, 2024, from; 2021; Volume 6, pp. 50–60. [Google Scholar]
- Cho, J.Y.; Lee, S.H.; Jang, S.H.; et al. Oblique paraspinal approach for thoracic disc herniations using tubular retractor with robotic holder: a technical note. Eur Spine J 2012, 21, 2620–5. [Google Scholar] [CrossRef]
- Bae, J.; Chachan, S.; Shin, S.-H.; Lee, S.-H. Percutaneous Endoscopic Thoracic Discectomy in the Upper and Midthoracic Spine: A Technical Note. Neurospine 2019, 16, 148–153. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chang, C.-C.; Chen, Y.-J.; et al. New strategy for minimally invasive endoscopic surgery to treat infectious spondylodiscitis in the thoracolumbar spine. Pain Physician 2019, 22, 281–293. [Google Scholar]
- Yang, J.-S.; Chu, L.; Deng, R.; et al. Treatment of single level thoracic tuberculosis by percutaneous endoscopic debridement and allograft via the transforaminal approach combined with percutaneous pedicle screw fixation: a multicenter study with a median follow-up of 36 months. World Neurosurg 2019, 122, e1472–e1481. [Google Scholar] [CrossRef]
- Eichholz, K.M.; O&, *!!! REPLACE !!!*; #39, *!!! REPLACE !!!*; Toole, J.E.; Fessler, R.G. Thoracic microendoscopic discectomy. Neurosurg Clin N Am. 2006, 17, 441–6. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cruet, M.J.; Kim, B.S.; Sandhu, F.; Samartzis, D.; Fessler, R.G. Thoracic microendoscopic discectomy. J Neurosurg Spine. 2004, 1, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, R.E.; Podichetty, V.K.; Sandhu, F.A.; Santiago, P.; Spears, J.D.; Aaronson, O.; Kelly, K.; Hrubes, M.; Fessler, R.G. Thoracic microendoscopic discectomy: a human cadaver study. Spine (Phila Pa 1976). 2005, 30, 1226–31. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.S.; Chen, X.S.; Zhou, S.Y.; Shao, J.; Zhu, W. En Bloc Resection of Lamina and Ossified Ligamentum Flavum in the Treatment of Thoracic Ossification of the Ligamentum Flavum. Neurosurgery 2010, 66, 1181–1186. [Google Scholar] [CrossRef]
- Kang, M.S.; Chung, H.J.; You, K.H.; et al. How i do it: biportal endoscopic thoracic decompression for ossification of the ligamentum flavum. Acta Neurochir 2022, 164, 43–47. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, M.; Xia, C.; et al. Unilateral biportal endoscopic decompression for symptomatic thoracic ossification of the ligamentum flavum: a case control study. International Orthopaedics (SICOT) 2022, 46, 2071–2080. [Google Scholar] [CrossRef]
- Man-Kyu Park, Daewon Park, Sang-Kyu Son, Journal of Minimally Invasive Spine Surgery and Technique 8 (1), 82-88, 202.
- Ahn, Y. The current state of cervical endoscopic spine surgery: an updated literature review and technical considerations. Expert Review of Medical Devices 2020, 17, 1285–1292. [Google Scholar] [CrossRef]
- Gao, F.; Mao, T.; Sun, W.; Guo, W.; Wang, Y.; Li, Z.; Abhinav, P. An updated meta- analysis comparing artificial cervical disc arthroplasty (CDA) versus anterior cervical discectomy and fusion (ACDF) for the treatment of cervical degenerative disc disease (CDDD). Spine 2015, 40, 1816–1823. [Google Scholar] [CrossRef]
- Ruetten, S.; Komp, M.; Merk, H.; et al. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008, 33, 940–948. [Google Scholar] [CrossRef]
- Ma, W.; Peng, Y.; Zhang, S.; et al. Comparison of percutaneous endoscopic cervical keyhole foraminotomy versus microscopic anterior cervical discectomy and fusion for single level unilateral cervical radiculopathy. Int J Gen Med. 2022, 15, 6897–6907. [Google Scholar] [CrossRef]
- Clark, J.G.; Abdullah, K.G.; Steinmetz, M.P.; et al. Minimally invasive versus open cervical foraminotomy: a systematic review. Global Spine J. 2011, 1, 9–14. [Google Scholar] [CrossRef]
- Wang, D.; Xu, J.; Zhu, C.; Zhang, W.; Pan, H. Comparison of Outcomes between Unilateral Biportal Endoscopic and Percutaneous Posterior Endoscopic Cervical Keyhole Surgeries. Medicin(Kaunas). 2023, 59, 437. [Google Scholar] [CrossRef]
- Ahn Y, Keum HJ, Shin SH. Percutaneous endoscopic cervical discectomy versus anterior cervical discectomy and fusion: a comparative cohort study with a five-year follow-up.
- Zhu, C.; Zhou, X.; Ge, G.; Wang, C.; Zhuang, X.; Cheng, W.; Wang, D.; Zhu, H.; Pan, H.; Zhang, W. Unilateral Biportal Endoscopic Laminectomy for Treating Cervical Stenosis: A Technical Note and Preliminary Results. Medicina (Kaunas). 2023, 59, 305. [Google Scholar] [CrossRef]
- Ahn Y, Keum HJ, Shin SH. Percutaneous endoscopic cervical discectomy versus anterior cervical discectomy and fusion: a comparative cohort study with a five-year follow-up.
- Fontanella, A. Endoscopic microsurgery in herniated cervical discs. Neurol Res 1999, 21, 31–38. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, S.H.; Lee, S.C.; et al. Factors predicting excellent outcome of percutaneous cervical discectomy: analysis of 111 consecutive cases. Neuroradiol 2004, 46, 378–384. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Choi, W.C.; et al. Anterior minimally invasive approaches for the cervical spine. Orthop Clin N Am 2007, 38, 327–337. [Google Scholar] [CrossRef]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic anterior decompression versus conventional anterior decompression and fusion in cervical disc herniations. Int Orth 2009, 33, 1677–1682. [CrossRef]
- Yang JS, Chu L, Chen L, et al. Anterior or posterior approach of full-endoscopic cervical discectomy for cervical intervertebral disc herniation? A comparative cohort study. Spine 2014, 39, 1743–1750. [CrossRef]
- Oertel, J.M.; Philipps, M.; Burkhardt, B.W. Endoscopic foraminotomy as a treatment for osseous foraminal stenosis. World Neurosurg 2016, 91, 50–57. [Google Scholar] [CrossRef]
- Dusad, T.; Kundnani, V.; Dutta, S.; Patel, A.; Mehta, G.; Singh, M. Comparative prospective study reporting intraoperative parameters, pedicle screw perforation, and radiation exposure in navigation-guided versus non-navigated fluoroscopy-assisted minimal invasive transforaminal lumbar interbody fusion. Asian Spine J. 2018, 12, 309–316. [Google Scholar] [CrossRef]
- Kaliya-Perumal, A.K.; Limthongkul, W.; Oh, J.Y. Utilization of spinal navigation to facilitate hassle-free rod placement during minimally-invasive long-construct posterior instrumentation. Asian Spine J. 2019, 13, 511–514. [Google Scholar] [CrossRef]
- Ransom, N.A.; Gollogly, S.; Lewandrowski, K.U.; Yeung, A. Navigating the learning curve of spinal endoscopy as an established traditionally trained spine surgeon. J Spine Surg. 2020; 6, (Suppl 1), S197–207. [Google Scholar]
- Hanna, G.; Kim, T.T.; Uddin, S.A.; Ross, L.; Johnson, J.P. Video-assisted thoracoscopic image-guided spine surgery: evolution of 19 years of experience, from endoscopy to fully integrated 3D navigation. Neurosurg Focus. 2021, 50, E8. [Google Scholar] [CrossRef]
- Yuh, W.T.; Lee, Y.S.; Jeon, J.H.; Choi, I. Future of Endoscopic Spine Surgery: Insights from Cutting-Edge Technology in the Industrial Field. Bioengineering 2023, 10, 1363. [Google Scholar] [CrossRef]
- Kwon, H.; Park, J.Y. The Role and Future of Endoscopic Spine Surgery: A Narrative Review. Neurospine. 2023, 20, 43–55. [Google Scholar] [CrossRef]
- Heo DH, Kim JY, Park JY, et al. Clinical experiences of 3-dimensional biportal endoscopic spine surgery for lumbar degenerative disease. Oper Neurosurg (Hagerstown) 2022, 22, 231–238. [CrossRef]
- Hahn, B.S.; Park, J.Y. Incorporating New Technologies to Overcome the Limitations of Endoscopic Spine Surgery: Navigation, Robotics, and Visualization. World Neurosurgery. 2021, 145, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.J.; Kim, K.J.; Jahng, T.A.; Kim, H.J. Minimally invasive robotic versus open fluoroscopic- guided spinal instrumented fusions: a randomized controlled trial. Spine. 2017, 42, 353–358. [Google Scholar] [CrossRef]
- Kantelhardt, S.R.; Martinez, R.; Baerwinkel, S.; Burger, R.; Giese, A.; Rohde, V. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic- guided, pedicle screw placement. Eur Spine J. 2011, 20, 860–868. [Google Scholar] [CrossRef]
- Overley, S.C.; Cho, S.K.; Mehta, A.I.; Arnold, P.M. Navigation and robotics in spinal surgery: where are we now? Neurosurgery. 2017, 80, S86–S99. [Google Scholar] [CrossRef]
- Choi, G.; Uniyal, P.; Hassan, Z.; Patel, B.; Kim, W.J.J.S. A new progression towards a safer anterior percutaneous endoscopic cervical discectomy: a technical report. J Spine. 2016, 5, 2. [Google Scholar] [CrossRef]
- Ahn, Y.J. Transforaminal percutaneous endoscopic lumbar discectomy: technical tips to prevent complications. Expert Rev Med Devices. 2012, 9, 361–366. [Google Scholar] [CrossRef]
- Wang Z, Tan Y, Fu K, et al. Minimally invasive trans-superior articular process percutaneous endoscopic lumbar discectomy with robot assistance. BMC Musculoskelet Disord. 2022, 23, 1144.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





