Submitted:
15 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Hot Water and Ethanol Extracts from Ophiocordyceps Sinensis Submerged Culture
2.3. Determination of Bioactive Compounds
2.3.1. Measurement of Adenosine
2.3.2. Measurement of Ergosterol
2.3.3. Measurement of GABA
2.3.4. Measurement of TPC, TFC, and EPS Contents
2.4. Cytotoxicity Test of OMSC on BV2 Microglial
2.5. Measurement of Nitrite Production
2.6. Anti-Inflammatory Activities of 95% Ethanol Extract OMSC (EEOS-95)
2.6.1. Measurement of Pro-Inflammatory Cytokines Level
2.6.2. Western Blotting Analysis
2.7. Statistical Analysis
3. Results
3.1. Bioactive Compounds in Extract of OMSC
3.2. Cytotoxicity Evaluation of OMSC
3.3. Effect of Os Mycelia on Nitric Oxide
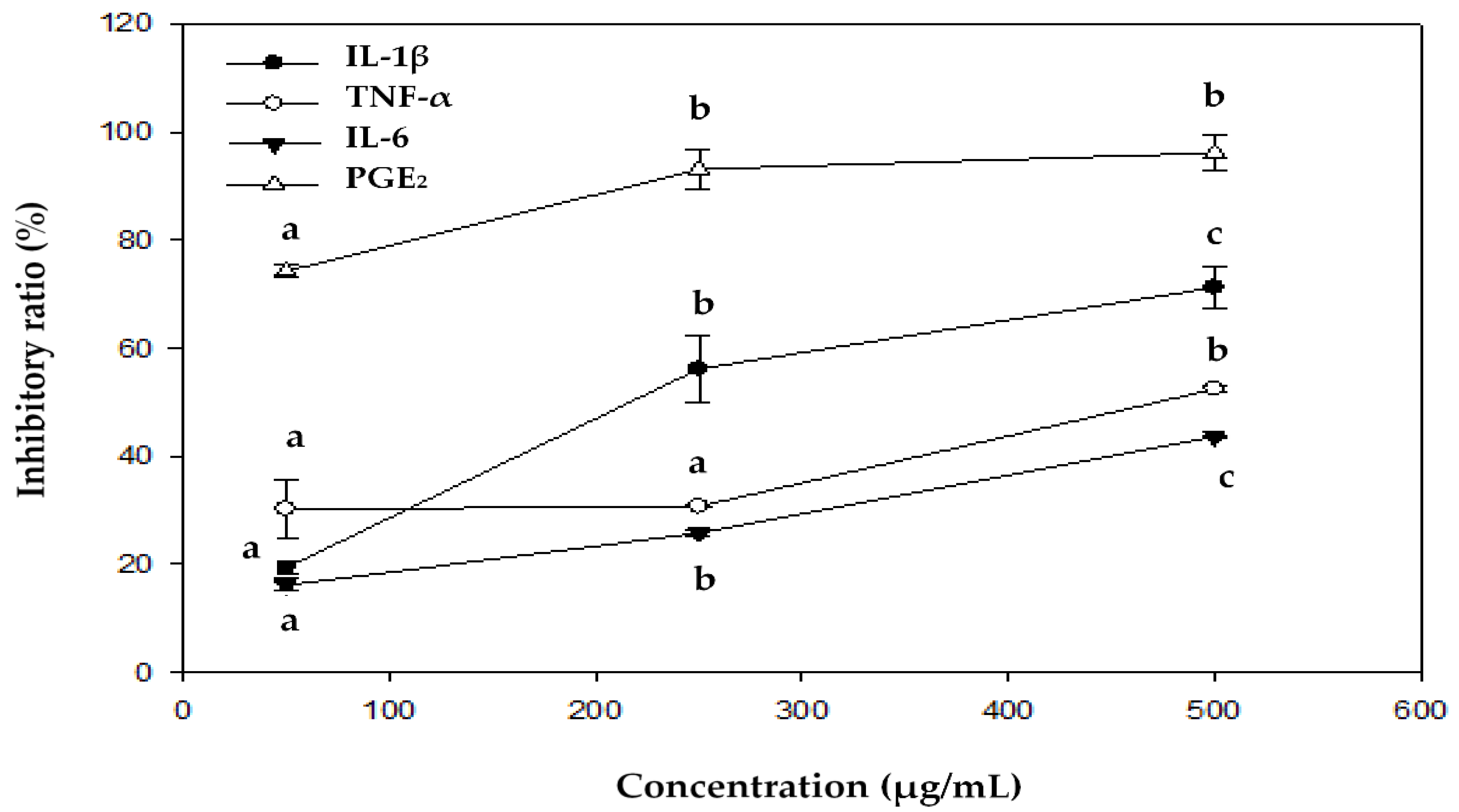
3.4. Anti-Inflammation Effect of EEOS-95 on Lipopolysaccharide-Induced BV2 Microglial Cells
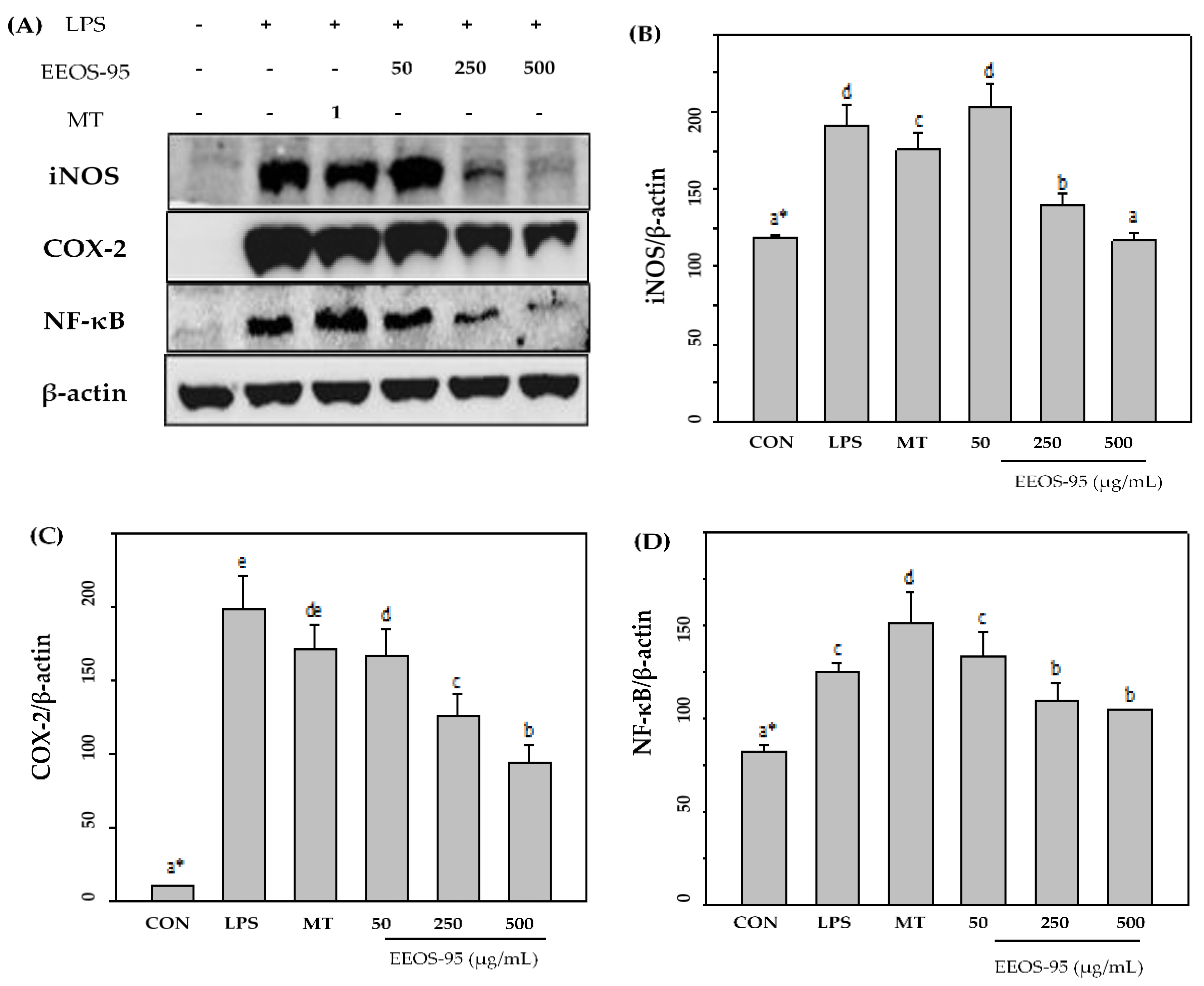
3.5. Effect of EEOS-95 on the Expression of Inflammatory Proteins in LPS-Induced BV2 Microglial
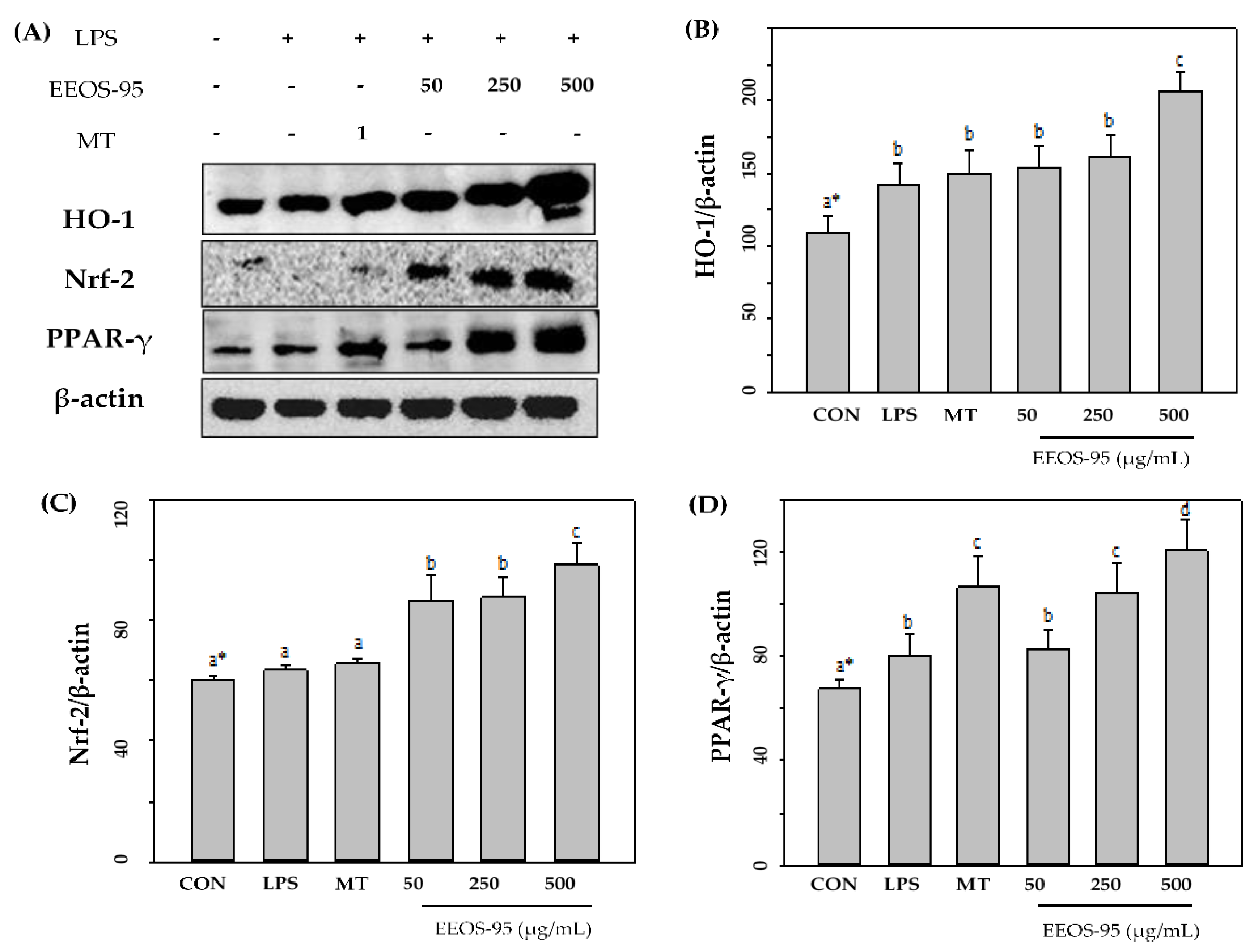
3.6. Effect of EEOS-95 on the Expression of Anti-Inflammatory Protein in LPS-Induced BV2 Microglial
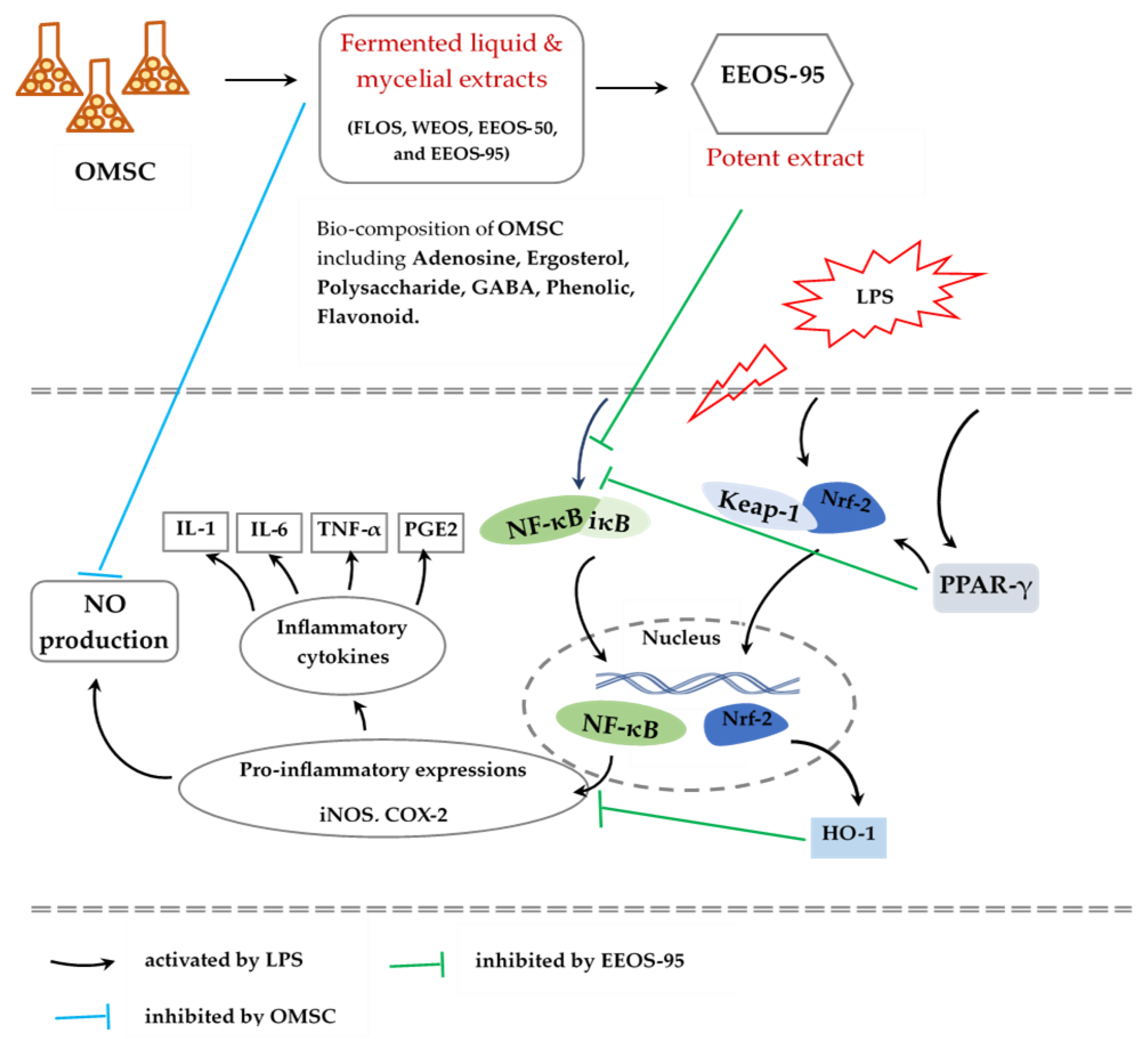
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chakraborty, S.; Chowdhury, S.; and Nandi, G. Review on Yarsagumba (Cordyceps sinensis)- an exotic medicinal mushroom. Int J PharmacognPhytochem Res 2014, 6(2), 339–346. [Google Scholar]
- Pandey, K. A review of the medicinal value of Ophiocordyceps sinensis (Yarshagumba). Asian J Pharmacognosy 2022, 6(1), 6–9. [Google Scholar]
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: a review. J Funct Foods 2014, 6, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Karati, D.; Priyadarshini, R.; Dua, T.K.; Paul, P.; Sahu, R.; Nandi, G. Cordyceps sinensis (yarsagumba): Pharmacological properties of a mushroom. Pharmacol Res Mod Chin Med 2023, 8, 100294.
- Wei, Y.; Zhang, L.; Wang, J.; Wang, W.; Niyati, N.; Guo, Y.; Wang, X. Chinese caterpillar fungus (Ophiocordyceps sinensis) in China: Current distribution, trading, and futures under climate change and overexploitation. Sci Total Environ 2021, 755, 142548. [Google Scholar] [CrossRef]
- Knopman, D.S.; Petersen, R.C. Mild cognitive impairment and mild dementia: a clinical perspective. Mayo Clin Proc 2014, 89(10), 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Hague, S.; Klaffke, S.; Bandmann, O. Neurodegenerative disorders: Parkinson’s disease and Huntington’s disease. J Neurol Neurosurg Psychiatry 2005, 76, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Teoh, H.L.; Carey, K.; Sampaio, H.; Mowat, D.; Roscioli, T.; Farrar, M. Inherited Paediatric Motor Neuron Disorders: Beyond Spinal Muscular Atrophy. Neural Plast 2017, 2017, 6509493. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.D.; Mao, S.S.; Lin, L.; Bai, G.N.; Liu, B.J.; Mao, J.H. Stress granules in the spinal muscular atrophy and amyotrophic lateral sclerosis: The correlation and promising therapy. Neurobiol Dis 2022, 170, 105749. [Google Scholar] [CrossRef] [PubMed]
- Klockgether, T; Mariotti, C.; Paulson, H.L. Spinocerebellar ataxia. Nat Rev Dis Primers 2019, 5(1), 24. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Machado, M.; Dias-Teixeira, M.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. The neuroprotective effect of traditional Chinese medicinal plants—A critical review. Acta Pharm Sin B 2023, 13(8), 3208–3237. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, J.; Wang, X.; Yang, P.; Zhao, Y.; Li, K.; Chen, Y.; Xu, Y. Herbal Compounds Play a Role in Neuroprotection through the Inhibition of Microglial Activation. J Immunol Res 2018, 2018, 9348046. [Google Scholar] [CrossRef]
- Gupta, D.P.; Park, S.H.; Lee, Y.S.; Lee, S.; Lim, S.; Byun, J.; Cho, I.H.; Song, G.J. Daphne genkwa flower extract promotes the neuroprotective effects of microglia. Phytomedicine 2023, 108, 154486. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.Q.; Wang, C.Y.; Zhao, R.; Li, H.H.; Zhang, Q.H.; Cai, D. Artificial cordyceps mycelium by submerged fermentation of Hirsutella sinensis HS 1201 using rice bran hydrolysate as substrate. Environ Qual Manag 2021, 31(1), 109–118. [Google Scholar] [CrossRef]
- Chatnarin, S. and Thirabunyanon, M. Potential bioactivities via anticancer, antioxidant, and immunomodulatory properties of cultured mycelial enriched β-D-glucan polysaccharides from a novel fungus Ophiocordyceps sinensis OS8. Front Immunol 2023, 14, 1150287. Front Immunol, 2023; 14, 1150287.
- Li, S.P.; Zhao, K.J.; Ji, Z.N.; Song, Z.H.; Dong, T.T.; Lo, C.K.; Cheung, J.K.; Zhu, S.Q.; Tsim, K.W. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci 2003, 73(19), 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Lue, M.Y.; and Pan, T.M. Determination of adenosine, cordycepin and ergosterol contents in cultivated Antrodia camphorata by HPLC method. J Food Drug Anal 2005, 13(4), 338–342. [Google Scholar] [CrossRef]
- Yuan, J.P.; Wang, J.H.; Liu, X.; Kuang, H.C.; and Zhao, S.Y. Simultaneous determination of free ergosterol and ergosteryl esters in Cordyceps sinensis by HPLC. Food Chem 2007, 105, 1755–1759. [Google Scholar] [CrossRef]
- Rogério da Silva Moraes, E.; Santos-Silva, M.; Grisólia, A.A.; Braga, D.V.; Reis Leão, L.K.; Bahia, C.P.; Soares de Moraes, S.A.; Passos, A.F.; de Jesus Oliveira Batista, E.; Herculano, A.M.; Matos Oliveira, K.R.H. High performance liquid chromatography-based method to analyze activity of GABA transporters in central nervous system. Neurochem Int 2022, 158, 105359. [Google Scholar] [CrossRef] [PubMed]
- Quero-Jiménez, P.C.; Montenegro, O.N.; Sosa, R.; de la Torre, J.B.; Valero Acosta, J.; López Pérez, D.; Santana Rodríguez, A.; Ramos Méndez, R.; Calvo Alonso, A.; Corrales, A.J.; Broche Hernández, N. Total carbohydrates concentration evaluation in products of microbial origin. Afinidad Journal of Chemical Engineering Theoretical and Applied Chemistry 2019, 76, 195–203. [Google Scholar]
- Yang, S.E.; Vo, T.L.T.; Chen, C.L.; Yang, N.C.; Chen, C.I.; Song, T.Y. Nutritional Composition, Bioactive Compounds and Functional Evaluation of Various Parts of Cajanus cajan (L.) Millsp. Agriculture 2020, 10, 55. [Google Scholar] [CrossRef]
- Vo, T.L.T.; Yang, N.C.; Yang, S.E.; Chen, C.L.; Wu, C.H.; Song, T.Y. Effects of Cajanus cajan (L.) millsp. Roots extracts on the antioxidant and anti-inflammatory activities. Chin J Physiol 2020, 63, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.D.; Wu, C.K.; Yuan, F.; Wang, Y.B.; Huang, L.D.; Chen, Z.H.; Zeng, W.B.; Wang, Y.; Yang, Z.L.; Zeng, P.S.; Lemetti, P.; Mo, X.X.; Yu, H. Evolutionary biogeography on Ophiocordyceps sinensis: An indicator of molecular phylogeny to geochronological and ecological exchanges. Geoscience Frontiers 2020, 11(3), 807–820. [Google Scholar] [CrossRef]
- Lo, H.C.; Hsieh, C.; Lin, F.Y.; Hsu, T.H. A systematic review of the mysterious caterpillar fungus Ophiocordyceps sinensis in DongChongXiaCao and related bioactive ingredients. J Tradit Complementary Med 2013, 3(1), 16–32. [Google Scholar] [CrossRef]
- Kim, H.O.; Yun, J.W. A comparative study on the production of exopolysaccharides between two entomopathogenic fungi Cordyceps militaris and Cordyceps sinensis in submerged mycelial cultures. J Appl Microbiol 2005, 99(4), 728–738. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.H.; Yao, Y.J. Nutritional requirements of mycelial growth of Cordyceps sinensis in submerged culture. J Appl Microbiol 2005, 99(3), 483–492. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Lim, J.S.; Yoon, C.S.; Koh, J.H.; Chang, H.I.; Kim, S.W. Production of mycelia and exo-biopolymer from molasses by Cordyceps sinensis 16 in submerged culture. Bioresour Technol 2007, 98(1), 165–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Liu, G.Q.; Zhu, C.Y.; Zhou, G.Y.; Kuang, S.M. Enhanced production of mycelial biomass and extracellular polysaccharides in caterpillar-shaped medicinal mushroom Cordyceps sinensis CS001 by the addition of palmitic acid. J Med Plant Res 2011, 5(13), 2873–2878. [Google Scholar]
- Zhang, Y.; Cao, H.; Qiu, X.; Xu, D.; Chen, Y.; Barnes, G.N.; Tu, Y.; Gyabaah, A.T.; Gharbal, A.H.A.A.; Peng, C.; Cai, J.; and Cai, X. Neuroprotective Effects of Adenosine A1 Receptor Signaling on Cognitive Impairment Induced by Chronic Intermittent Hypoxia in Mice. Front Cell Neurosci 2020, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, M.; Mujumdar, A.S. UV induced conversion during drying of ergosterol to vitamin D in various mushrooms: Effect of different drying conditions. Trends Food Sci Technol 2020, 105, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lin, C.; Wang, T.; Zhang, P.; Liu, Z.; Lu, C. Ergosterol attenuates LPS-induced myocardial injury by modulating oxidative stress and apoptosis in rats. Cell Physiol Biochem 2018, 48(2), 583–592. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, S.; Zhu, C.; Gao, Q.; Bai, J.; Si, J.; Chen, Y. Ergosterol ameliorates renal inflammatory responses in mice model of diabetic nephropathy. Biomed Pharmacother 2020, 128, 110252. [Google Scholar] [CrossRef] [PubMed]
- Kushairi, N.; Tarmizi, N.A.K.A.; Phan, C.W.; Macreadie, I.; Sabaratnam, V.; Naidu, M.; David, P. Modulation of neuroinflammatory pathways by medicinal mushrooms, with particular relevance to Alzheimer’s disease. Trends Food Sci Technol 2020, 104, 153–162. [Google Scholar] [CrossRef]
- Ano, Y.; Kutsukake, T.; Hoshi, A.; Yoshida, A.; Nakayama, H. Identification of a novel dehydroergosterol enhancing microglial anti-inflammatory activity in a dairy product fermented with Penicillium candidum. PLoS ONE 2015, 10(3), e0116598. [Google Scholar] [CrossRef]
- Nallathamby, N.; Guan-Serm, L.; Vidyadaran, S.; Abd Malek, S.N.; Raman, J.; Sabaratnam, V. Ergosterol of Cordyceps militaris attenuates LPS induced inflammation in BV2 microglia cells. Nat Prod Commun 2015, 10(6), 885–886. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Chuchawankul, S.; Nilkhet, S.; Moungkote, N.; Sarachana, T.; Ung, A.T.; Baek, S.J.; Tencomnao, T. Ergosterol isolated from cloud ear mushroom (Auricularia polytricha) attenuates bisphenol A-induced BV2 microglial cell inflammation. Food Res Int 2022, 157, 111433. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Mongkolpobsin, K.; Chuchawankul, S.; Tencomnao, T.; Baek, S.J. Neuroprotective effects of ergosterol against TNF-α-induced HT-22 hippocampal cell injury. Biomed Pharmacother 2022, 154, 113596. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tao, Y.; Wang, Q.; Shen, L.; Yang, T.; Liu, Z.; Liu, C. Ergosterol Is the Active Compound of Cultured Mycelium Cordyceps sinensis on Antiliver Fibrosis. Evid Based Complement Alternat Med 2014, 2014, 537234. [Google Scholar] [CrossRef]
- Xiong, M.; Huang, Y.; Liu, Y.; Huang, M.; Song, G.; Ming, Q.; Ma, X.; Yang, J.; Deng, S.; Wen, Y.; et al. Antidiabetic activity of ergosterol from Pleurotus ostreatus in KK-A(y) mice with spontaneous type 2 diabetes mellitus. Mol Nutr Food Res 2018, 62(3), 1700444. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as Food Supplements: The Effects of GABA on Brain and Behavior. Front Psychol 2015, 6, 1520. [Google Scholar] [CrossRef] [PubMed]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front Neurosci 2020, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Jędrejko, K.J.; Lazur, J.; Muszyńska, B. Cordyceps militaris: An Overview of Its Chemical Constituents in Relation to Biological Activity. Foods 2021, 10(11), 2634. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.; Ding, Z. Bioactive Mushroom Polysaccharides: A Review on Monosaccharide Composition, Biosynthesis and Regulation. Molecules 2017, 22(6), 955. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.H.; Xie, F.; Tan, J.; Yuan, Y.; Mei, H.; Zheng, Y.; Sheng, R. Extraction, structure and pharmacological effects of the polysaccharides from Cordyceps sinensis: A review. J Funct Foods 2022, 89, 104909. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Vu, T.T.; Tran, M.T.; Ho, P.T.T.; Le, T.H.T.; et al. Antioxidant activity and hepatoprotective effect of exopolysaccharides from cultivated ophiocordyceps sinensis against CCl4-induced liver damages. Nat Prod Commun 2021, 16(2), 1–9. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, L.; He, Y.; Wei, X. Extraction, characterization, utilization as wound dressing and drug delivery of Bletilla striata polysaccharide: A review. Int. J. Biol. Macromol. 2018, 120, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Sung, T.J.; Wang, Y.Y.; Liu, K.L.; Chou, C.H.; Lai, P.S.; Hsieh, C.W. Pholiota nameko polysaccharides promotes cell proliferation and migration and reduces ros content in h2o2-induced l929 cells. Antioxidants (Basel). 2020, 9(1), 65. [Google Scholar] [CrossRef] [PubMed]
- Cirigliano, A.; Macone, A.; Bianchi, M.M.; Oliaro-Bosso, S.; Balliano, G.; Negri, R.; Rinaldi, T. Ergosterol reduction impairs mitochondrial DNA maintenance in S. cerevisiae. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019, 1864, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Jordá, T. and Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes (Basel). 2020, 11(7), 795. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Fonseca, M.B.; Vidal-Limon, A.; Fernández-Pomares, C.; Rojas-Durán, F.; Hernández-Aguilar, M.E.; Espinoza, C.; Trigos, A.; Suárez-Medellín, J. Ergosterol exerts a differential effect on AR-dependent LNCaP and AR-independent DU-145 cancer cells. Natural Product Research 2021, 35(22), 4857–4860. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.L.T.; Cai, X.M.; Liao, J.W.; Huang, L.G.; Chen, C.L.; Wu, C.H.; Song, T.Y. Safety Assessment and Hepatic–Renal Protection of Cajanus cajan (L.) Millsp. Root and Its Soy Isoflavone Contents. Nutrients 2023, 15, 3963. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.T.; Tran, V.K.; Le, L.S.; Tran, T.M.; Nguyen, D.G.C.; Trinh, T.K.; Nguyen, Q.V.; Nguyen, C.C.; Le, T.H. Phenolic content and antioxidant activity of Ophiocordyceps Sobolifera extract for renal injury prevention. Process Biochemistry 2022, 121, 322–329. [Google Scholar]
- Shao, F.; Wang, X.; Wu, H.; Wu, Q.; Zhang, J. Microglia and Neuroinflammation: Crucial Pathological Mechanisms in Traumatic Brain Injury-Induced Neurodegeneration. Front Aging Neurosci 2022, 14, 825086. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, P.; Ge, H.; Qu, X.; Jin, X. Effects of cordycepin on the microglia-overactivation-induced impairments of growth and development of hippocampal cultured neurons. PLoS One 2015, 10(5), e0125902. [Google Scholar] [CrossRef]
- Ding, Y.; Kang, J.; Liu, S.; Xu, Y.; Shao, B. The Protective Effects of Peroxisome Proliferator-Activated Receptor Gamma in Cerebral Ischemia-Reperfusion Injury. Front Neurol 2020, 11, 588516. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tian, Z.; Wang, M.; Kou, J.; Wang, C.; Rong, X.; Li, J.; Xie, X. , Pang, X. Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARγ/Nrf2/NF-κB signaling pathway. Int Immunopharmacol 2019, 66, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Chuchawankul, S.; Nilkhet, S.; Moungkote, N.; Sarachana, T.; Ung, A.T.; Baek, S.J.; Tencomnao, T. Ergosterol isolated from cloud ear mushroom (Auricularia polytricha) attenuates bisphenol A-induced BV2 microglial cell inflammation. Food Research International 2022, 157, 111433. [Google Scholar] [CrossRef] [PubMed]
- Nallathamby, N.; Guan-Serm, L.; Vidyadaran, S.; Malek, S.N.A.; Raman, J.; Sabaratnam, V. Ergosterol of Cordyceps militaris Attenuates LPS Induced Inflammation in BV2 Microglia Cells. Nat Prod Commun. 2015, 10(6), 885–6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lu, X.; Yang, S.; Zou, Y.; Zeng, F.; Xiong, S.; Cao, Y.; Zhou, W. The anti-inflammatory activity of GABA-enriched Moringa oleifera leaves produced by fermentation with Lactobacillus plantarum LK-1. Front. Nutr. 2023, 10, 1–12. [Google Scholar]
- Zhao, X.R.; Gonzales, N.; Aronowski, J. Pleiotropic role of PPARγ in intracerebral hemorrhage: an intricate system involving Nrf2, RXR, and NF-κB. CNS Neurosci Ther 2015, 21(4), 357–366. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, T.; Xiao, H. The Implication of Oxidative Stress and AMPK-Nrf2 Antioxidative Signaling in Pneumonia Pathogenesis. Front Endocrinol (Lausanne) 2020, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Jiao, D.; Wang, H.; Wu, Q.; Men, W.; Yan, H.; Li, C. Activation of the PPARγ Prevents Ferroptosis-Induced Neuronal Loss in Response to Intracerebral Hemorrhage Through Synergistic Actions With the Nrf2. Front Pharmacol 2022, 13, 869300. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Kim, Y.M. Beneficial and Detrimental Roles of Heme Oxygenase-1 in the Neurovascular System. Int J Mol Sci 2022, 23, 7041. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.; Piras, S.; Brondolo, L.; Marinari, U.M.; Pronzato, M.A.; Furfaro, A.L. Heme Oxygenase 1 in the Nervous System: Does It Favor Neuronal Cell Survival or Induce Neurodegeneration? Int J Mol Sci 2018, 19, 2260. [Google Scholar] [CrossRef] [PubMed]
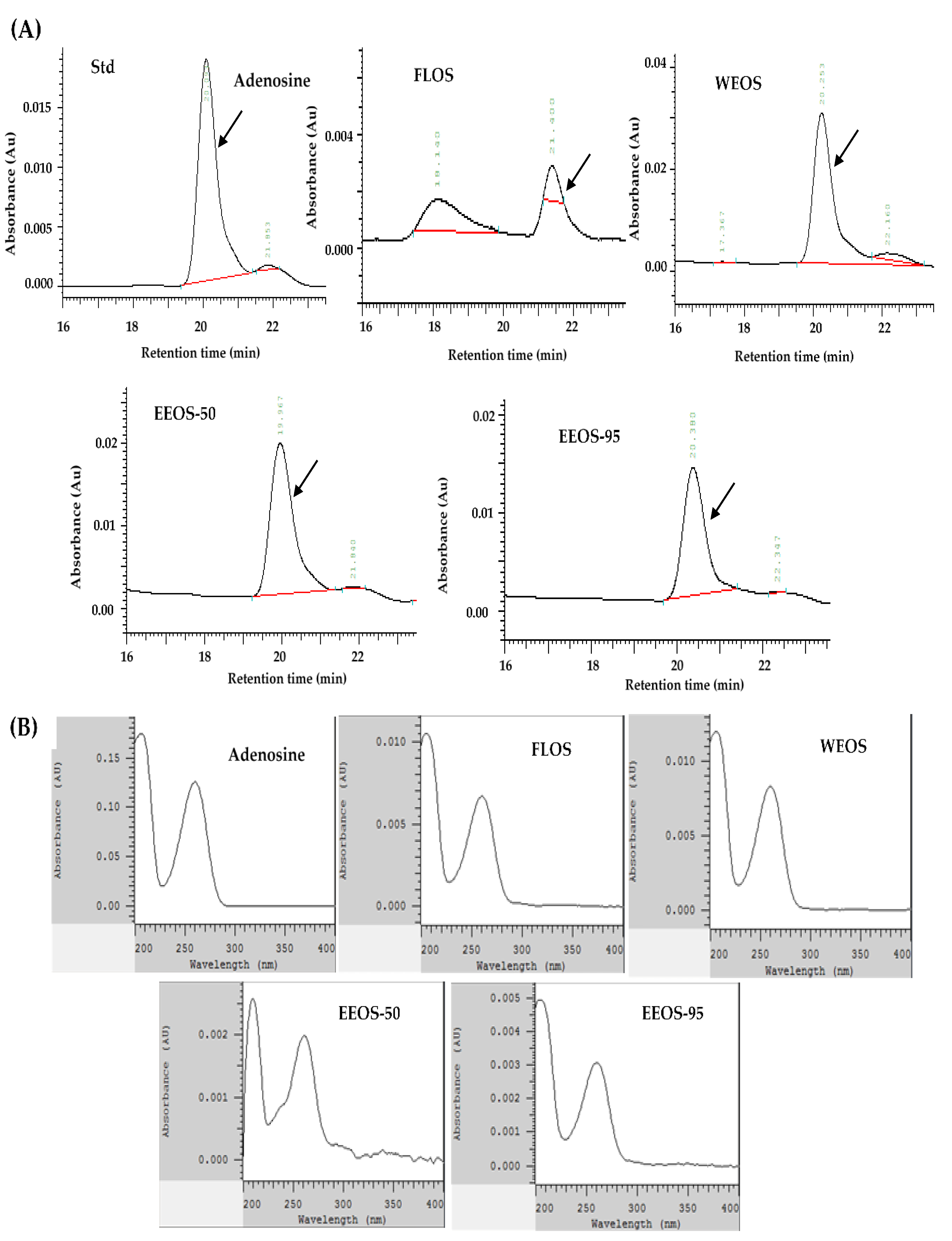
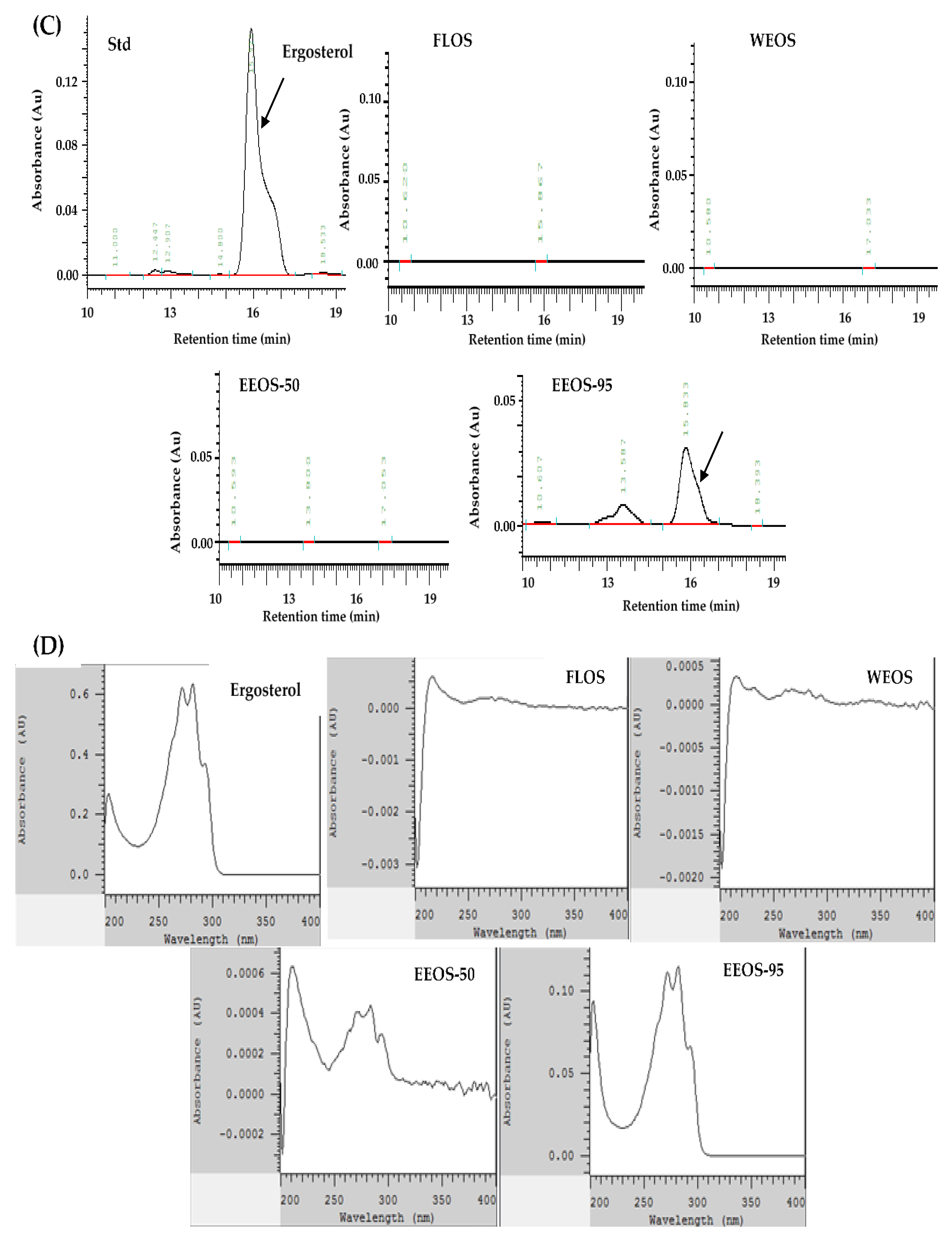
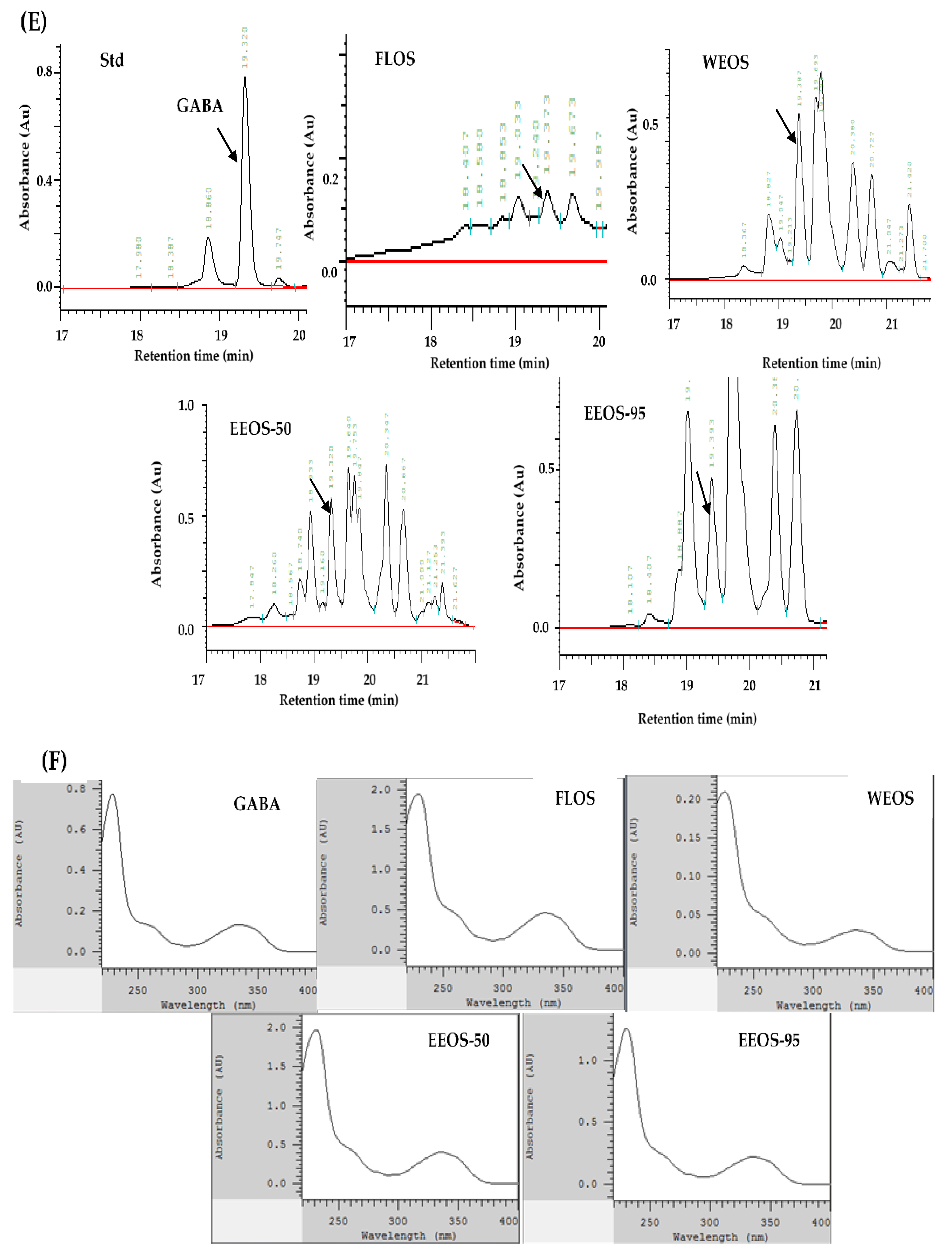
| Compounds (mg/g) | Extracts1 | |||
| FLOS | WEOS | EEOS-50 | EEOS-95 | |
| Adenosine | 2.12 ± 0.09b2 | 2.83 ± 0.05c | 3.29 ± 0.29d | 0.19 ± 0.01a |
| Ergosterol | ND | ND | ND | 18.60 ± 0.70 |
| Polysaccharide | 107.60 ± 10.20b | 156.30 ± 7.10c | 28.50 ± 7.70a | ND |
| Total Polyphenols | 1.57 ± 0.09a | 1.71 ± 0.07bc | 1.77 ± 0.15c | 2.28 ± 0.05d |
| Total Flavonoids | 1.34 ± 0.01b | 1.17 ± 0.02a | 1.65 ± 0.01c | 2.14 ± 0.06d |
| GABA | 3.70 ± 0.30a | 12.60 ± 0.80b | 13.20 ± 0.60b | 18.60 ± 0.50c |
| Treatments1 | Cell viability (% of CON) | |||
| FLOS2 | WEOS | EEOS-50 | EEOS-95 | |
| 50 | 89.80 ± 13.20a3 | 95.90 ± 7.20a | 94.30 ± 11.90a | 116.60 ± 8.60a |
| 100 | 106.40 ± 25.60a | 96.70 ± 1.40a | 102.20 ± 6.00a | 137.30 ± 12.60b |
| 250 | 119.40 ± 18.30ab | 108.80 ± 5.10a | 116.80 ± 6.20ab | 171.70 ± 12.90cd |
| 500 | 134.20 ± 11.30b | 123.70 ± 11.90b | 125.70 ± 4.40b | 196.50 ± 13.80d |
| 1000 | 114.00 ± 23.40ab | 116.50 ± 8.40ab | 123.00 ± 1.00b | 175.90 ± 3.00c |
| Treatments1 | Nitrite concentration (nmol/106 cells) | ||||
| 50 | 250 | 500 | |||
| CON | 0.33 ± 0.05a4 | - | - | - | |
| LPS (1 µg/mL) | 0.78 ± 0.12d | - | - | - | |
| MT (1mM)2 | + LPS | 0.45 ± 0.03b | - | - | - |
| FLOS3 | - | 0.55 ± 0.00c | 0.59 ± 0.07c | 0.59 ± 0.07c | |
| WEOS | - | 0.75 ± 0.02d | 0.74 ± 0.02d | 0.59 ± 0.05c | |
| EEOS-50 | - | 0.76 ± 0.11d | 0.66 ± 0.05cd | 0.58 ± 0.10c | |
| EEOS-95 | - | 0.64 ± 0.16cd | 0.53 ± 0.09c | 0.46 ± 0.02b | |
| Treatments1 | Cytokines | ||||
| IL-1β (pg/mL) | TNF-α (ng/mL) | IL-6 (ng/mL) | PGE2 (pg/mL) | ||
| CON | 4.29 ± 1.30a3 | 13.71 ± 0.69a | 0.00 ± 0.00a | 97.60 ± 5.50a | |
| LPS (1 µg/mL) | 76.66 ± 3.47f | 70.54 ± 4.89d | 608.22 ± 17.40e | 933.90 ± 12.30e | |
| MT (1 mM)2 | + LPS | 54.79 ± 0.94d | 35.53 ± 3.58b | 538.87 ± 28.22de | 877.90 ± 10.30d |
| 50 | 62.86 ± 3.90e | 49.58 ± 0.75c | 510.21 ± 7.09cd | 308.20 ± 9.80c | |
| 250 | 36.59 ± 1.54c | 53.10 ± 3.53c | 451.58 ± 2.73c | 155.40 ± 30.40b | |
| 500 | 25.76 ± 0.61b | 42.22 ± 3.22bc | 343.20 ± 1.86b | 126.00 ± 27.90a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





