Submitted:
12 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pathophysiology
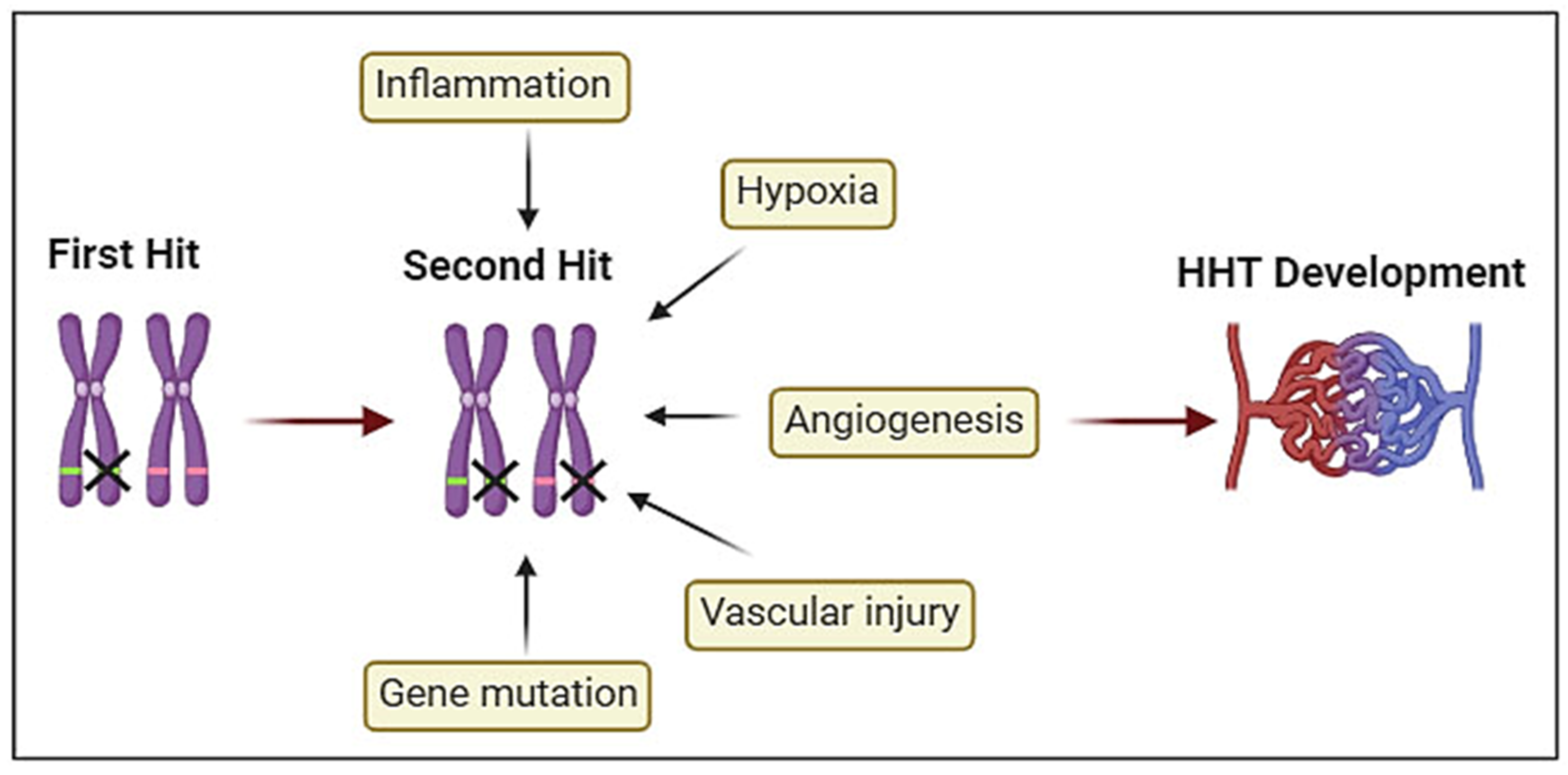
2.1. Second Hit Hypothesis
2.1.1. Genetic Two-Hit
2.1.2. Angiogenesis
2.1.3. Inflammation
2.2. Role of BMP9 and BMP10
3. Advancements in Developing New Therapies
3.1. Enhancing ALK1 or ENG Expression
3.2. Anti-Angiogenic Gene Therapy
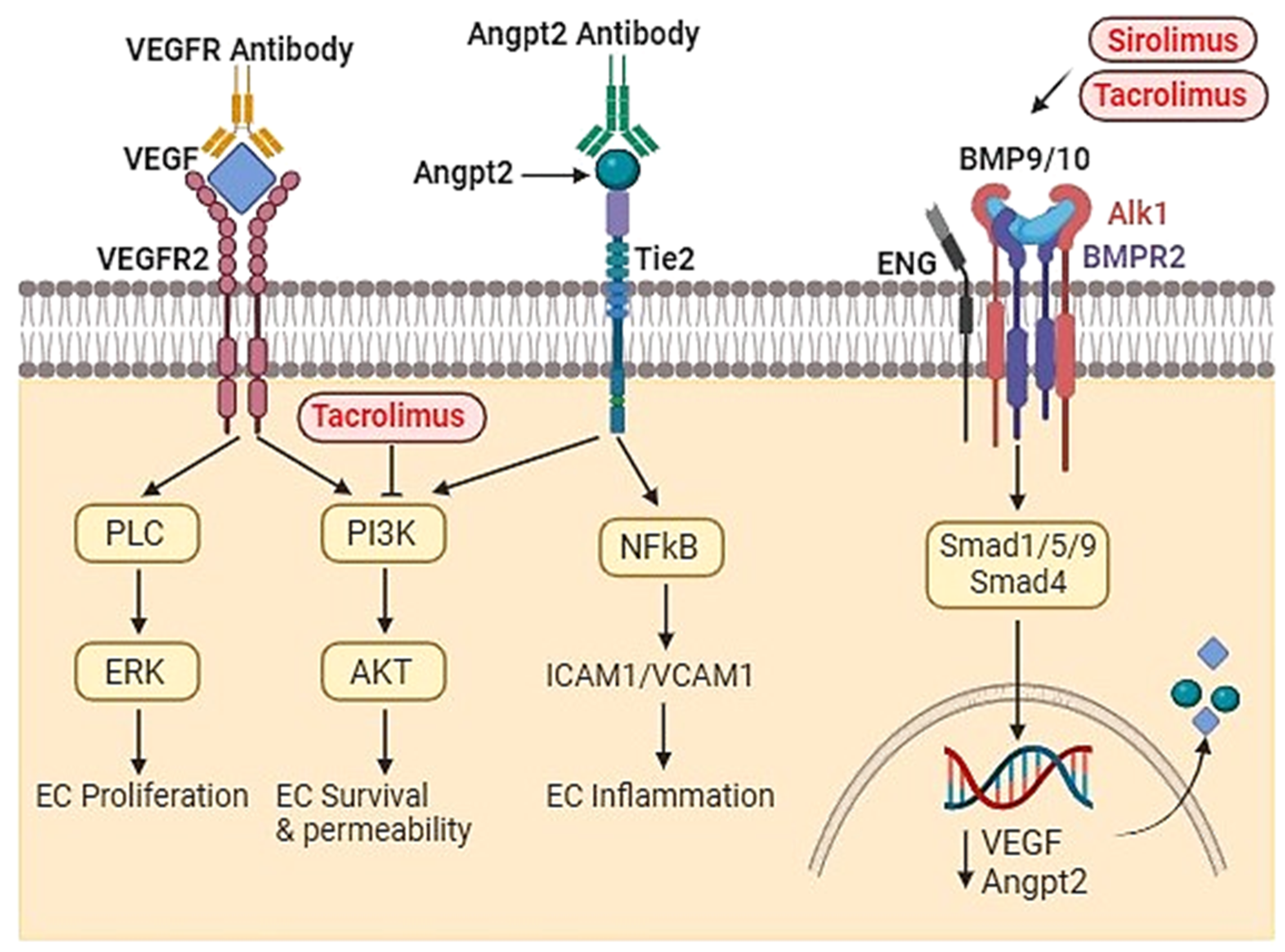
3.3. Angiopoetin-2 (Angpt2) Antibodies
3.4. PI3-Kinase Inhibitor and Other Agents
3.5. Modulation of BMP9-10/ENG/ALK1/SMAD Pathway as an Emerging Therapeutic Target
4. Summary and Future Perspective
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Labeyrie P-E, Courthéoux P, Babin E, Bergot E, Touzé E, Pelage J-P (2016) Neurological involvement in hereditary hemorrhagic telangiectasia. Journal of Neuroradiology 43 (4):236-245. [CrossRef]
- de Gussem EM, Kroon S, Hosman AE, Kelder JC, Post MC, Snijder RJ, Mager JJ (2020) Hereditary Hemorrhagic Telangiectasia (HHT) and Survival: The Importance of Systematic Screening and Treatment in HHT Centers of Excellence. Journal of Clinical Medicine 9 (11). [CrossRef]
- Arthur HM, Roman BL (2022) An update on preclinical models of hereditary haemorrhagic telangiectasia: Insights into disease mechanisms. Frontiers in Medicine 9. [CrossRef]
- Balachandar S, Graves TJ, Shimonty A, Kerr K, Kilner J, Xiao S, Slade R, Sroya M, Alikian M, Curetean E et al. (2021) Identification and validation of a novel pathogenic variant in GDF2 (BMP9) responsible for hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations. American Journal of Medical Genetics Part A 188 (3):959-964. [CrossRef]
- Farhan A, Yuan F, Partan E, Weiss CR (2022) Clinical manifestations of patients with GDF2 mutations associated with hereditary hemorrhagic telangiectasia type 5. Am J Med Genet A 188 (1):199-209. [CrossRef]
- Guilhem A, Dupuis-Girod S, Espitia O, Rivière S, Seguier J, Kerjouan M, Lavigne C, Maillard H, Magro P, Alric L, et al. (2023) Seven cases of hereditary haemorrhagic telangiectasia-like hepatic vascular abnormalities associated withEPHB4pathogenic variants. Journal of Medical Genetics 60 (9):905-909. [CrossRef]
- Jiang X, Wooderchak-Donahue WL, McDonald J, Ghatpande P, Baalbaki M, Sandoval M, Hart D, Clay H, Coughlin S, Lagna G et al. (2018) Inactivating mutations in Drosha mediate vascular abnormalities similar to hereditary hemorrhagic telangiectasia. Sci Signal 11 (513). [CrossRef]
- Hata A, Lagna G (2019) Deregulation of Drosha in the pathogenesis of hereditary hemorrhagic telangiectasia. Current Opinion in Hematology 26 (3):161-169. [CrossRef]
- Kritharis A, Al-Samkari H, Kuter DJ (2018) Hereditary hemorrhagic telangiectasia: diagnosis and management from the hematologist’s perspective. Haematologica 103 (9):1433-1443. [CrossRef]
- Bernabeu C, Bayrak-Toydemir P, McDonald J, Letarte M (2020) Potential Second-Hits in Hereditary Hemorrhagic Telangiectasia. Journal of Clinical Medicine 9 (11). [CrossRef]
- Karlsson T, Cherif H (2018) Mutations in the ENG, ACVRL1, and SMAD4 genes and clinical manifestations of hereditary haemorrhagic telangiectasia: experience from the Center for Osler's Disease, Uppsala University Hospital. Ups J Med Sci 123 (3):153-157. [CrossRef]
- Snellings DA, Gallione CJ, Clark DS, Vozoris NT, Faughnan ME, Marchuk DA (2019) Somatic Mutations in Vascular Malformations of Hereditary Hemorrhagic Telangiectasia Result in Bi-allelic Loss of ENG or ACVRL1. Am J Hum Genet 105 (5):894-906. [CrossRef]
- Weakley SM, Jiang J, Kougias P, Lin PH, Yao Q, Brunicardi FC, Gibbs RA, Chen C (2010) Role of somatic mutations in vascular disease formation. Expert Rev Mol Diagn 10 (2):173-185. [CrossRef]
- Mahmoud M, Allinson KR, Zhai Z, Oakenfull R, Ghandi P, Adams RH, Fruttiger M, Arthur HM (2010) Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ Res 106 (8):1425-1433. [CrossRef]
- Srinivasan S, Hanes MA, Dickens T, Porteous ME, Oh SP, Hale LP, Marchuk DA (2003) A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet 12 (5):473-482. [CrossRef]
- Bourdeau A, Faughnan ME, Letarte M (2000) Endoglin-deficient mice, a unique model to study hereditary hemorrhagic telangiectasia. Trends Cardiovasc Med 10 (7):279-285.
- Urness LD, Sorensen LK, Li DY (2000) Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet 26 (3):328-331. [CrossRef]
- Sorensen LK, Brooke BS, Li DY, Urness LD (2003) Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFbeta coreceptor. Dev Biol 261 (1):235-250.
- Milton I, Ouyang D, Allen CJ, Yanasak NE, Gossage JR, Alleyne CH, Jr., Seki T (2012) Age-dependent lethality in novel transgenic mouse models of central nervous system arteriovenous malformations. Stroke 43 (5):1432-1435. [CrossRef]
- Park SO, Wankhede M, Lee YJ, Choi EJ, Fliess N, Choe SW, Oh SH, Walter G, Raizada MK, Sorg BS et al. (2009) Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest 119 (11):3487-3496. [CrossRef]
- Shaligram SS, Zhang R, Zhu W, Ma L, Luo M, Li Q, Weiss M, Arnold T, Santander N, Liang R et al. (2022) Bone Marrow-Derived Alk1 Mutant Endothelial Cells and Clonally Expanded Somatic Alk1 Mutant Endothelial Cells Contribute to the Development of Brain Arteriovenous Malformations in Mice. Transl Stroke Res 13 (3):494-504. [CrossRef]
- Seki T, Yun J, Oh SP (2003) Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res 93 (7):682-689. [CrossRef]
- Morine KJ, Qiao X, Paruchuri V, Aronovitz MJ, Mackey EE, Buiten L, Levine J, Ughreja K, Nepali P, Blanton RM et al. (2017) Conditional knockout of activin like kinase-1 (ALK-1) leads to heart failure without maladaptive remodeling. Heart and Vessels 32 (5):628-636. [CrossRef]
- Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, Park A, Wu X, Kaartinen V, Roman BL et al. (2008) ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2 (HHT2). Blood 111 (2):633-642. [CrossRef]
- Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E (2000) Activin receptor-like kinase 1 modulates transforming growth factor- beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A 97 (6):2626-2631. [CrossRef]
- Walker EJ, Su H, Shen F, Degos V, Amend G, Jun K, Young WL (2012) Bevacizumab attenuates VEGF-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke 43 (7):1925-1930. [CrossRef]
- Choi EJ, Chen W, Jun K, Arthur HM, Young WL, Su H (2014) Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS One 9 (2):e88511. [CrossRef]
- Chen W, Sun Z, Han Z, Jun K, Camus M, Wankhede M, Mao L, Arnold T, Young WL, Su H (2014) De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke 45 (3):900-902. [CrossRef]
- Ma L, Zhu X, Tang C, Pan P, Yadav A, Liang R, Press K, Nelson J, Su H (2024) CNS resident macrophages enhance dysfunctional angiogenesis and circulating monocytes infiltration in brain arteriovenous malformation. Journal of Cerebral Blood Flow & Metabolism. [CrossRef]
- Rossi E, Sanz-Rodriguez F, Eleno N, Duwell A, Blanco FJ, Langa C, Botella LM, Cabanas C, Lopez-Novoa JM, Bernabeu C (2013) Endothelial endoglin is involved in inflammation: role in leukocyte adhesion and transmigration. Blood 121 (2):403-415. [CrossRef]
- Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, Hashimoto T, Yang GY, Young WL (2008) Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery 62 (6):1340-1349; discussion 1349-1350. [CrossRef]
- Guo Y, Tihan T, Kim H, Hess C, Lawton MT, Young WL, Zhao Y, Su H (2014) Distinctive distribution of lymphocytes in unruptured and previously untreated brain arteriovenous malformation. Neuroimmunol Neuroinflamm 1 (3):147-152. [CrossRef]
- Ma L, Guo Y, Zhao YL, Su H (2015) The Role of Macrophage in the Pathogenesis of Brain Arteriovenous Malformation. Int J Hematol Res 1 (2):52-56. [CrossRef]
- Chen W, Guo Y, Walker EJ, Shen F, Jun K, Oh SP, Degos V, Lawton MT, Tihan T, Davalos D et al. Akassoglou K, Nelson J, Pile-Spellman J, Su H, Young WL (2013) Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol 33 (2):305-310. [CrossRef]
- Zhang R, Han Z, Degos V, Shen F, Choi EJ, Sun Z, Kang S, Wong M, Zhu W, Zhan L et al. (2016) Persistent infiltration and pro-inflammatory differentiation of monocytes cause unresolved inflammation in brain arteriovenous malformation. Angiogenesis 19 (4):451-461. [CrossRef]
- van Laake LW, van den Driesche S, Post S, Feijen A, Jansen MA, Driessens MH, Mager JJ, Snijder RJ, Westermann CJ, Doevendans PA et al. (2006) Endoglin has a crucial role in blood cell-mediated vascular repair. Circulation 114 (21):2288-2297. [CrossRef]
- Post S, Smits AM, van den Broek AJ, Sluijter JP, Hoefer IE, Janssen BJ, Snijder RJ, Mager JJ, Pasterkamp G, Mummery CL et al. (2010) Impaired recruitment of HHT-1 mononuclear cells to the ischaemic heart is due to an altered CXCR4/CD26 balance. Cardiovasc Res 85 (3):494-502. [CrossRef]
- Shen F, Degos V, Chu PL, Han Z, Westbroek EM, Choi EJ, Marchuk D, Kim H, Lawton MT, Maze M et al. (2014) Endoglin deficiency impairs stroke recovery. Stroke 45 (7):2101-2106. [CrossRef]
- Han Z, Shaligram S, Faughnan ME, Clark D, Sun Z, Su H (2020) Reduction of endoglin receptor impairs mononuclear cell-migration. Explor Med 1:136-148. [CrossRef]
- Dingenouts CK, Goumans MJ, Bakker W (2015) Mononuclear cells and vascular repair in HHT. Front Genet 6:114. [CrossRef]
- Meurer SK, Weiskirchen R (2020) Endoglin: An 'Accessory' Receptor Regulating Blood Cell Development and Inflammation. Int J Mol Sci 21 (23). [CrossRef]
- Ojeda-Fernandez L, Recio-Poveda L, Aristorena M, Lastres P, Blanco FJ, Sanz-Rodriguez F, Gallardo-Vara E, de las Casas-Engel M, Corbi A, Arthur HM et al. (2016) Mice lacking endoglin in macrophages show an impaired immune response. PLoS Genet 12 (3):e1005935. [CrossRef]
- Park ES, Kim S, Yao DC, Savarraj JPJ, Choi HA, Chen PR, Kim E (2022) Soluble Endoglin Stimulates Inflammatory and Angiogenic Responses in Microglia That Are Associated with Endothelial Dysfunction. Int J Mol Sci 23 (3). [CrossRef]
- Germans MR, Sun W, Sebök M, Keller A, Regli L (2022) Molecular Signature of Brain Arteriovenous Malformation Hemorrhage: A Systematic Review. World Neurosurgery 157:143-151. [CrossRef]
- Nakamura Y, Sugita Y, Nakashima S, Okada Y, Yoshitomi M, Kimura Y, Miyoshi H, Morioka M, Ohshima K (2016) Alternatively Activated Macrophages Play an Important Role in Vascular Remodeling and Hemorrhaging in Patients with Brain Arteriovenous Malformation. Journal of Stroke and Cerebrovascular Diseases 25 (3):600-609. [CrossRef]
- Geisthoff U, Nguyen H-L, Lefering R, Maune S, Thangavelu K, Droege F (2020) Trauma Can Induce Telangiectases in Hereditary Hemorrhagic Telangiectasia. Journal of Clinical Medicine 9 (5). [CrossRef]
- Tillet E, Bailly S (2015) Emerging roles of BMP9 and BMP10 in hereditary hemorrhagic telangiectasia. Front Genet 5:456. [CrossRef]
- Wooderchak-Donahue WL, McDonald J, O'Fallon B, Upton PD, Li W, Roman BL, Young S, Plant P, Fülöp GT, Langa C et al. (2013) BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet 93 (3):530-537. [CrossRef]
- David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S (2007) Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 109 (5):1953-1961. [CrossRef]
- Chen D, Zhao M, Mundy GR (2004) Bone morphogenetic proteins. Growth Factors 22 (4):233-241. [CrossRef]
- Laux DW, Young S, Donovan JP, Mansfield CJ, Upton PD, Roman BL (2013) Circulating Bmp10 acts through endothelial Alk1 to mediate flow-dependent arterial quiescence. Development 140 (16):3403-3412. [CrossRef]
- Capasso TL, Li B, Volek HJ, Khalid W, Rochon ER, Anbalagan A, Herdman C, Yost HJ, Villanueva FS, Kim K et al. (2020) BMP10-mediated ALK1 signaling is continuously required for vascular development and maintenance. Angiogenesis 23 (2):203-220. [CrossRef]
- Hata A, Chen Y-G (2016) TGF-β Signaling from Receptors to Smads. Cold Spring Harbor Perspectives in Biology 8 (9). [CrossRef]
- Larrivee B, Prahst C, Gordon E, Del Toro R, Mathivet T, Duarte A, Simons M, Eichmann A (2012) ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell 22 (3):489-500. [CrossRef]
- Choi H, Kim B-G, Kim YH, Lee S-J, Lee YJ, Oh SP (2022) BMP10 functions independently from BMP9 for the development of a proper arteriovenous network. Angiogenesis 26 (1):167-186. [CrossRef]
- Bouvard C, Tu L, Rossi M, Desroches-Castan A, Berrebeh N, Helfer E, Roelants C, Liu H, Ouarné M, Chaumontel N et al. (2022) Different cardiovascular and pulmonary phenotypes for single- and double-knock-out mice deficient in BMP9 and BMP10. Cardiovascular Research 118 (7):1805-1820. [CrossRef]
- Chen H, Brady Ridgway J, Sai T, Lai J, Warming S, Chen H, Roose-Girma M, Zhang G, Shou W, Yan M (2013) Context-dependent signaling defines roles of BMP9 and BMP10 in embryonic and postnatal development. Proc Natl Acad Sci U S A 110 (29):11887-11892. [CrossRef]
- Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S (2012) BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood 119 (25):6162-6171. [CrossRef]
- Ruiz S, Zhao H, Chandakkar P, Chatterjee PK, Papoin J, Blanc L, Metz CN, Campagne F, Marambaud P (2016) A mouse model of hereditary hemorrhagic telangiectasia generated by transmammary-delivered immunoblocking of BMP9 and BMP10. Sci Rep 5:37366. [CrossRef]
- Al Tabosh T, Liu H, Koça D, Al Tarrass M, Tu L, Giraud S, Delagrange L, Beaudoin M, Rivière S, Grobost V et al. (2024) Impact of heterozygous ALK1 mutations on the transcriptomic response to BMP9 and BMP10 in endothelial cells from hereditary hemorrhagic telangiectasia and pulmonary arterial hypertension donors. Angiogenesis. [CrossRef]
- Al-Samkari H, Kasthuri RS, Parambil JG, Albitar HA, Almodallal YA, Vazquez C, Serra MM, Dupuis-Girod S, Wilsen CB, McWilliams JP et al. (2021) An international, multicenter study of intravenous bevacizumab for bleeding in hereditary hemorrhagic telangiectasia: the InHIBIT-Bleed study. Haematologica 106 (8):2161-2169. [CrossRef]
- Al Tabosh T, Al Tarrass M, Tourvieilhe L, Guilhem A, Dupuis-Girod S, Bailly S (2024) Hereditary hemorrhagic telangiectasia: from signaling insights to therapeutic advances. Journal of Clinical Investigation 134 (4). [CrossRef]
- Whitehead KJ, Sautter NB, McWilliams JP, Chakinala MM, Merlo CA, Johnson MH, James M, Everett EM, Clancy MS, Faughnan ME et al. (2016) Effect of topical intranasal therapy on epistaxis frequency in patients with Hereditary Hemorrhagic Telangiectasia: a randomized clinical trial. JAMA 316 (9):943-951. [CrossRef]
- Dupuis-Girod S, Ambrun A, Decullier E, Fargeton AE, Roux A, Breant V, Colombet B, Riviere S, Cartier C, Lacombe P et al. (2016) Effect of bevacizumab nasal spray on epistaxis duration in Hereditary Hemorrhagic Telangectasia: a randomized clinical trial. JAMA 316 (9):934-942. [CrossRef]
- Sadick H, Schäfer E, Weiss C, Rotter N, Müller C, Birk R, Sadick M, Häussler D (2022) An in vitro study on the effect of bevacizumab on endothelial cell proliferation and VEGF concentration level in patients with hereditary hemorrhagic telangiectasia. Experimental and Therapeutic Medicine 24 (3). [CrossRef]
- Galiatsatos P, Wilson C, O’Brien J, Gong AJ, Angiolillo D, Johnson J, Myers C, Strout S, Mathai S, Robinson G et al. (2022) A lack of race and ethnicity data in the treatment of hereditary hemorrhagic telangiectasia: a systematic review of intravenous bevacizumab efficacy. Orphanet Journal of Rare Diseases 17 (1). [CrossRef]
- Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, Al-Shahi Salman R, Vicaut E, Young WL, Houdart E et al. (2014) Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 383 (9917):614-621. [CrossRef]
- Snodgrass RO, Chico TJA, Arthur HM (2021) Hereditary Haemorrhagic Telangiectasia, an Inherited Vascular Disorder in Need of Improved Evidence-Based Pharmaceutical Interventions. Genes (Basel) 12 (2). [CrossRef]
- Kim YH, Phuong NV, Choe SW, Jeon CJ, Arthur HM, Vary CP, Lee YJ, Oh SP (2020) Overexpression of Activin Receptor-Like Kinase 1 in Endothelial Cells Suppresses Development of Arteriovenous Malformations in Mouse Models Of Hereditary Hemorrhagic Telangiectasia. Circ Res. [CrossRef]
- Schmid CD, Olsavszky V, Reinhart M, Weyer V, Trogisch FA, Sticht C, Winkler M, Kürschner SW, Hoffmann J, Ola R et al. (2023) ALK1 controls hepatic vessel formation, angiodiversity, and angiocrine functions in hereditary hemorrhagic telangiectasia of the liver. Hepatology 77 (4):1211-1227. [CrossRef]
- Ruiz S, Chandakkar P, Zhao H, Papoin J, Chatterjee PK, Christen E, Metz CN, Blanc L, Campagne F, Marambaud P (2017) Tacrolimus rescues the signaling and gene expression signature of endothelial ALK1 loss-of-function and improves HHT vascular pathology. Human Molecular Genetics 26 (24):4786-4798. [CrossRef]
- Sommer N, Droege F, Gamen KE, Geisthoff U, Gall H, Tello K, Richter MJ, Deubner LM, Schmiedel R, Hecker M et al. (2018) Treatment with low-dose tacrolimus inhibits bleeding complications in a patient with hereditary hemorrhagic telangiectasia and pulmonary arterial hypertension. Pulmonary Circulation 9 (2):1-3. [CrossRef]
- Hessels J, Kroon S, Boerman S, Nelissen RC, Grutters JC, Snijder RJ, Lebrin F, Post MC, Mummery CL, Mager JJ (2022) Efficacy and Safety of Tacrolimus as Treatment for Bleeding Caused by Hereditary Hemorrhagic Telangiectasia: An Open-Label, Pilot Study. J Clin Med 11 (18). [CrossRef]
- Álvarez-Hernández P, Patier JL, Marcos S, Gómez del Olmo V, Lorente-Herraiz L, Recio-Poveda L, Botella LM, Viteri-Noël A, Albiñana V (2023) Tacrolimus as a Promising Drug for Epistaxis and Gastrointestinal Bleeding in HHT. Journal of Clinical Medicine 12 (23). [CrossRef]
- Kilari S, Wang Y, Singh A, Graham RP, Iyer V, Thompson SM, Torbenson MS, Mukhopadhyay D, Misra S (2022) Neuropilin-1 deficiency in vascular smooth muscle cells is associated with hereditary hemorrhagic telangiectasia arteriovenous malformations. JCI Insight 7 (9). [CrossRef]
- Sullivan LA, Carbon JG, Roland CL, Toombs JE, Nyquist-Andersen M, Kavlie A, Schlunegger K, Richardson JA, Brekken RA (2010) r84, a novel therapeutic antibody against mouse and human VEGF with potent anti-tumor activity and limited toxicity induction. PLoS One 5 (8):e12031. doi:ARTN e1203110.1371/journal.pone.0012031.
- Han C, Choe SW, Kim YH, Acharya AP, Keselowsky BG, Sorg BS, Lee YJ, Oh SP (2014) VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis 17 (4):823-830. [CrossRef]
- Bose P, Holter JL, Selby GB (2009) Bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med 360 (20):2143-2144. [CrossRef]
- Oosting S, Nagengast W, de Vries E (2009) More on bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med 361 (9):931; author reply 931-932. [CrossRef]
- Brinkerhoff BT, Poetker DM, Choong NW (2011) Long-term therapy with bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med 364 (7):688-689. [CrossRef]
- Simons M, Eichmann A (2012) "On-target" cardiac effects of anticancer drugs: lessons from new biology. J Am Coll Cardiol 60 (7):626-627. [CrossRef]
- Levitzki A (2013) Tyrosine kinase inhibitors: views of selectivity, sensitivity, and clinical performance. Annu Rev Pharmacol Toxicol 53:161-185. [CrossRef]
- Faughnan ME, Gossage JR, Chakinala MM, Oh SP, Kasthuri R, Hughes CCW, McWilliams JP, Parambil JG, Vozoris N, Donaldson J et al. (2019) Pazopanib may reduce bleeding in hereditary hemorrhagic telangiectasia. Angiogenesis 22 (1):145-155. [CrossRef]
- Maestraggi Q, Bouattour M, Toquet S, Jaussaud R, Kianmanesh R, Durand F, Servettaz A (2015) Bevacizumab to treat cholangiopathy in hereditary hemorrhagic telangiectasia: Be cautious: a case report. Medicine (Baltimore) 94 (46):e1966. [CrossRef]
- Dupuis-Girod S, Ginon I, Saurin JC, Marion D, Guillot E, Decullier E, Roux A, Carette MF, Gilbert-Dussardier B, Hatron PY et al. (2012) Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA 307 (9):948-955. [CrossRef]
- Drabkin HA (2010) Pazopanib and anti-VEGF therapy. Open Access J Urol 2:35-40.
- Orphanos GS, Ioannidis GN, Ardavanis AG (2009) Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol 48 (7):964-970. [CrossRef]
- Kim YH, Kim MJ, Choe SW, Sprecher D, Lee YJ, Oh SP (2017) Selective effects of oral anti-angiogenic tyrosine kinase inhibitors on an animal model of hereditary hemorrhagic telangiectasia. J Thromb Haemost 15 (6):1095-1102. [CrossRef]
- Ola R, Dubrac A, Han J, Zhang F, Fang JS, Larrivee B, Lee M, Urarte AA, Kraehling JR, Genet G et al. (2016) PI3 kinase inhibition improves vascular malformations in mouse models of hereditary haemorrhagic telangiectasia. Nat Commun 7:13650. [CrossRef]
- Tanvetyanon T, Murtagh R, Bepler G (2009) Rupture of a cerebral arteriovenous malformation in a patient treated with bevacizumab. J Thorac Oncol 4 (2):268-269. [CrossRef]
- Tabouret T, Gregory T, Dhooge M, Brezault C, Mir O, Dreanic J, Chaussade S, Coriat R (2015) Long term exposure to antiangiogenic therapy, bevacizumab, induces osteonecrosis. Invest New Drugs 33 (5):1144-1147. [CrossRef]
- Kim H, Marchuk DA, Pawlikowska L, Chen Y, Su H, Yang GY, Young WL (2008) Genetic considerations relevant to intracranial hemorrhage and brain arteriovenous malformations. Acta Neurochir Suppl 105:199-206. [CrossRef]
- Chen W, Choi EJ, McDougall CM, Su H (2014) Brain arteriovenous malformation modeling, pathogenesis, and novel therapeutic targets. Transl Stroke Res 5 (3):316-329. [CrossRef]
- Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M (1998) Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A 95 (16):9349-9354. [CrossRef]
- Sawano A, Iwai S, Sakurai Y, Ito M, Shitara K, Nakahata T, Shibuya M (2001) Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 97 (3):785-791. [CrossRef]
- Niida S, Kondo T, Hiratsuka S, Hayashi S, Amizuka N, Noda T, Ikeda K, Shibuya M (2005) VEGF receptor 1 signaling is essential for osteoclast development and bone marrow formation in colony-stimulating factor 1-deficient mice. Proc Natl Acad Sci U S A 102 (39):14016-14021. [CrossRef]
- Zhu W, Shen F, Mao L, Zhan L, Kang S, Sun Z, Nelson J, Zhang R, Zou D, McDougall CM et al. (2017) Soluble FLT1 Gene Therapy Alleviates Brain Arteriovenous Malformation Severity. Stroke 48 (5):1420-1423. [CrossRef]
- Hadaczek P, Eberling JL, Pivirotto P, Bringas J, Forsayeth J, Bankiewicz KS (2010) Eight years of clinical improvement in MPTP-lesioned primates after gene therapy with AAV2-hAADC. Mol Ther 18 (8):1458-1461. [CrossRef]
- Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O'Malley KL, During MJ (1994) Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet 8 (2):148-154. [CrossRef]
- Riviere C, Danos O, Douar AM (2006) Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther 13 (17):1300-1308. [CrossRef]
- Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW, Clackson T, Wilson JM (2005) Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood 105 (4):1424-1430. [CrossRef]
- Rivera VM, Ye X, Courage NL, Sachar J, Cerasoli F, Jr., Wilson JM, Gilman M (1999) Long-term regulated expression of growth hormone in mice after intramuscular gene transfer. Proc Natl Acad Sci U S A 96 (15):8657-8662. [CrossRef]
- Koo T, Okada T, Athanasopoulos T, Foster H, Takeda S, Dickson G (2011) Long-term functional adeno-associated virus-microdystrophin expression in the dystrophic CXMDj dog. J Gene Med 13 (9):497-506. [CrossRef]
- Crist AM, Zhou X, Garai J, Lee AR, Thoele J, Ullmer C, Klein C, Zabaleta J, Meadows SM (2019) Angiopoietin-2 Inhibition Rescues Arteriovenous Malformation in a Smad4 Hereditary Hemorrhagic Telangiectasia Mouse Model. Circulation 139 (17):2049-2063. [CrossRef]
- Zhou X, Pucel JC, Nomura-Kitabayashi A, Chandakkar P, Guidroz AP, Jhangiani NL, Bao D, Fan J, Arthur HM, Ullmer C, Klein C, Marambaud P, Meadows SM (2023) ANG2 Blockade Diminishes Proangiogenic Cerebrovascular Defects Associated With Models of Hereditary Hemorrhagic Telangiectasia. Arteriosclerosis, Thrombosis, and Vascular Biology 43 (8):1384-1403. [CrossRef]
- Ojeda-Fernandez L, Barrios L, Rodriguez-Barbero A, Recio-Poveda L, Bernabeu C, Botella LM (2010) Reduced plasma levels of Ang-2 and sEng as novel biomarkers in hereditary hemorrhagic telangiectasia (HHT). Clin Chim Acta 411 (7-9):494-499. [CrossRef]
- Wetzel-Strong SE, Weinsheimer S, Nelson J, Pawlikowska L, Clark D, Starr MD, Liu Y, Kim H, Faughnan ME, Nixon AB et al. (2021) Pilot investigation of circulating angiogenic and inflammatory biomarkers associated with vascular malformations. Orphanet Journal of Rare Diseases 16 (1). [CrossRef]
- Ardelean DS, Jerkic M, Yin M, Peter M, Ngan B, Kerbel RS, Foster FS, Letarte M (2014) Endoglin and activin receptor-like kinase 1 heterozygous mice have a distinct pulmonary and hepatic angiogenic profile and response to anti-VEGF treatment. Angiogenesis 17 (1):129-146. [CrossRef]
- Fernandez-Lopez A, Garrido-Martin EM, Sanz-Rodriguez F, Pericacho M, Rodriguez-Barbero A, Eleno N, Lopez-Novoa JM, Duwell A, Vega MA, Bernabeu C et al. (2007) Gene expression fingerprinting for human hereditary hemorrhagic telangiectasia. Hum Mol Genet 16 (13):1515-1533. [CrossRef]
- Winkler EA, Kim CN, Ross JM, Garcia JH, Gil E, Oh I, Chen LQ, Wu D, Catapano JS, Raygor K et al. (2022) A single-cell atlas of the normal and malformed human brain vasculature. Science:eabi7377. [CrossRef]
- Wälchli T, Ghobrial M, Schwab M, Takada S, Zhong H, Suntharalingham S, Vetiska S, Rodrigues Rodrigues D, Rehrauer H, Wu R et al. (2021). [CrossRef]
- Geisthoff UW, Nguyen H-LP, Hess D (2013) Improvement in hereditary hemorrhagic telangiectasia after treatment with the phosphoinositide 3-kinase inhibitor BKM120. Annals of Hematology 93 (4):703-704. [CrossRef]
- Robert F, Desroches-Castan A, Bailly S, Dupuis-Girod S, Feige J-J (2020) Future treatments for hereditary hemorrhagic telangiectasia. Orphanet Journal of Rare Diseases 15 (1). [CrossRef]
- Piha-Paul SA, Taylor MH, Spitz D, Schwartzberg L, Beck JT, Bauer TM, Meric-Bernstam F, Purkayastha D, Karpiak L, Szpakowski S et al. (2019) Efficacy and safety of buparlisib, a PI3K inhibitor, in patients with malignancies harboring a PI3K pathway activation: a phase 2, open-label, single-arm study. Oncotarget 10 (60):6526-6535. [CrossRef]
- Ola R, Hessels J, Hammill A, Friday C, Clancy M, Al-Samkari H, Meadows S, Iyer V, Akhurst R (2023) Executive summary of the 14th HHT international scientific conference. Angiogenesis 26 (S1):27-37. [CrossRef]
- Viteri-Noël A, González-García A, Patier JL, Fabregate M, Bara-Ledesma N, López-Rodríguez M, Gómez del Olmo V, Manzano L (2022) Hereditary Hemorrhagic Telangiectasia: Genetics, Pathophysiology, Diagnosis, and Management. Journal of Clinical Medicine 11 (17). [CrossRef]
- Mei-Zahav M, Gendler Y, Bruckheimer E, Prais D, Birk E, Watad M, Goldschmidt N, Soudry E (2020) Topical Propranolol Improves Epistaxis Control in Hereditary Hemorrhagic Telangiectasia (HHT): A Randomized Double-Blind Placebo-Controlled Trial. Journal of Clinical Medicine 9 (10). [CrossRef]
- Albiñana V, Giménez-Gallego G, García-Mato A, Palacios P, Recio-Poveda L, Cuesta A-M, Patier J-L, Botella L-M (2019) Topically Applied Etamsylate: A New Orphan Drug for HHT-Derived Epistaxis (Antiangiogenesis through FGF Pathway Inhibition). TH Open 03 (03):e230-e243. [CrossRef]
- Cunha SI, Pietras K (2011) ALK1 as an emerging target for anti-angiogenic therapy of cancer. Blood 117 (26):6999-7006. [CrossRef]
- Ruiz S, Zhao H, Chandakkar P, Papoin J, Choi H, Nomura-Kitabayashi A, Patel R, Gillen M, Diao L, Chatterjee PK et al. (2019) Sirolimus plus nintedanib treatments vascular pathology in HHT mouse models. [CrossRef]
- Dupuis-Girod S, Bailly S, Plauchu H (2010) Hereditary hemorrhagic telangiectasia (HHT): from molecular biology to patient care. J Thromb Haemost 8 (7):1447-1456. [CrossRef]
| Subject | Title | Description | Ref. |
|---|---|---|---|
| Zebrafish | BMP10-mediated ALK1 signaling is continuously required for vascular development and maintenance. | The authors have found that loss of both bmp10 and bmp10-like genes leads to embryonic lethal cranial AVMs, which are distinguishable from alk1 mutants and concluded that BMP10 is vital for the maintenance of post-embryonic vascular development as a non-reductant ligand of Alk1. | [52] |
| Mice | Context-dependent signaling defines roles of BMP9 and BMP10 in embryonic and postnatal development. |
The study examined BMP9 and BMP10 in embryonic and postnatal development. The authors found that BMP9 is indispensable for postnatal vascular development in mice. BMP9 and BMP10 are ALK1's natural ligands. | [57] |
| BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. |
This study showed that administration of a neutralizing anti-BMP10 antibody to juvenile Bmp9-KO mice reduced retinal vascular expansion and vascular density. The data indicate that BMP9 and BMP10 are important in postnatal vascular remodeling of the retina and BMP10 can be a substitute for BMP9. | [58] | |
| A mouse model of HHT generated by trans-mammary-delivery of anti-BMP9/10 antibodies . | This study induced AVMs in postnatal retina through trans-mammary delivery of anti-BMP9/10 antibodies. This could be a practical and non-invasive method for the induction of HHT vascular pathology in the retina of postnatal mice. | [59] | |
| Human cell lines | Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in ECs . | The authors demonstrated that BMP9/BMP10 activate SMAD1/5/8 pathways and concluded that BMP9 and BMP10 serve as distinct ALK1 ligands and potentially elicit ALK1-mediated angiogenic effects. | [49] |
| Human | Impact of Heterozygous ALK1 mutations on the transcriptomic response to BMP9 and BMP10 in ECs from hereditary hemorrhagic telangiectasia and pulmonary arterial hypertension donors | Endothelial colony-forming cells (ECFCs) and microvascular ECs (HMVECs) were isolated from new-born HHT and adult PAH donors, and the impact of ALK1 mutations on BMP9 and BMP19 transcriptomic responses in ECs was consequently observed. RNA-sequencing was performed on these cells following an 18h stimulation with BMP9 or BMP10. The data showed that ALK1 heterozygosity modified a few of the BMP9/BMP19 regulated genes which are comparable to the controls. | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





