Submitted:
11 April 2024
Posted:
12 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
- Blank control group (UT) served as the reference without any form of stabilization;
- Cement-treated groups (CM-4, CM-6, CM-8) employed various cement contents to modify and stabilize the loess;
- DAP-treated groups (DT-0.5, DT-1.0, DT-1.5, DT-2.0, DT-3.0) employed different concentrations of DAP solution.
2.2.1. Unconfined Uniaxial Compressive Test
- The sieved loess was subsequently dried in an oven over 24 hours at a temperature of 105 °C for. After allowing the loess to cool to room temperature, an appropriate amount of cement and water, or an equal volume of DAP solution, were added into the loess according to the optimal moisture content ratio and the mix proportions in Table 3. The mixture was thoroughly blended with water for at least 5-10 minutes to ensure a uniform blend.
- The mixed loess was then placed into a steel mold with dimensions of 50 mm by 100mm. The loess within the mold was compacted using an electric compactor, and the specimens were removed after their top and bottom surfaces were leveled. The number of compactions for the remaining groups of loess specimens was based on this standard to maintain the degree of compaction and ensure uniformity in the preparation of the specimens. After compaction, the dry density of the untreated loess specimens should achieve 1.72 g/cm³.
- To simulate the curing environment of a roadbed, the demolded cylindrical loess specimens were kept inside a curing chamber set to 23 degrees Celsius and 96% humidity. Five specimens were cured as a group for curing periods of 3 d, 7 d, 14 d, and 28 d. After curing, they were transferred to an oven and heated at 105 degrees Celsius until the weight remained constant before removal.
2.2.2. Permeability Test
- Both untreated loess (control group) and loess treated with cement/DAP were compressed using an electric compactor to achieve maximum dry density. Subsequently, ring samples were extracted from the compressed specimens. These ring samples were subsequently stored in a conditioning room for a specified age.
- The ring samples were inserted into the container of saturation permeameter, sealed, and connected to a water head. The water was drained until no bubbles were observed in the overflow water. The permeability coefficient test was conducted when the sample saturation exceeded 0.95.
- At ambient room temperature, the time interval was recorded for the water level’s descent from 90 cm to 70 cm. After each measurement, the water head was raised back to the specified height for another measurement. This process was repeated no less than five times. When the inflow and outflow rates stabilized and showed consistency, the calculated permeability coefficient was determined to represent the saturated permeability coefficient.
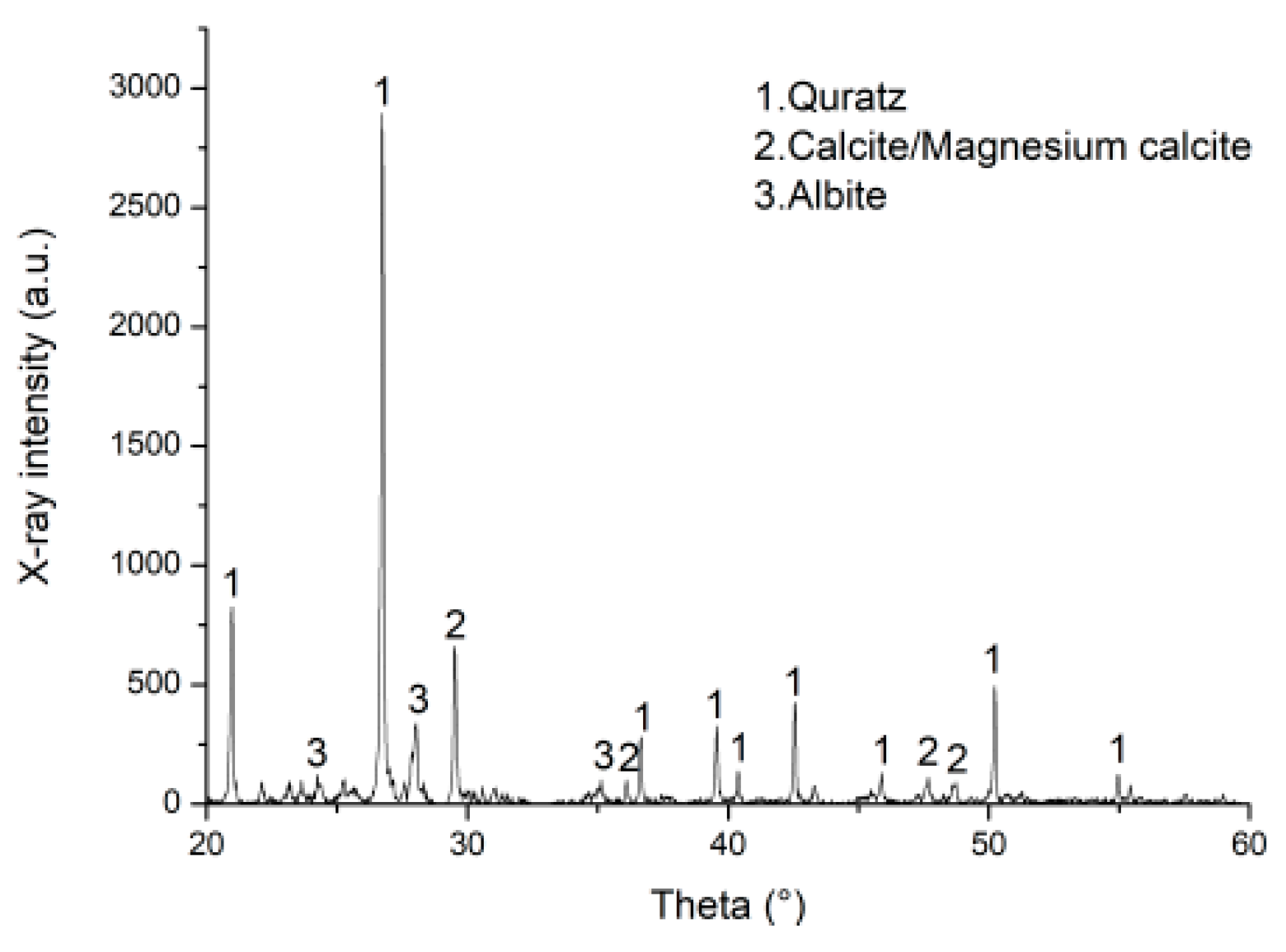
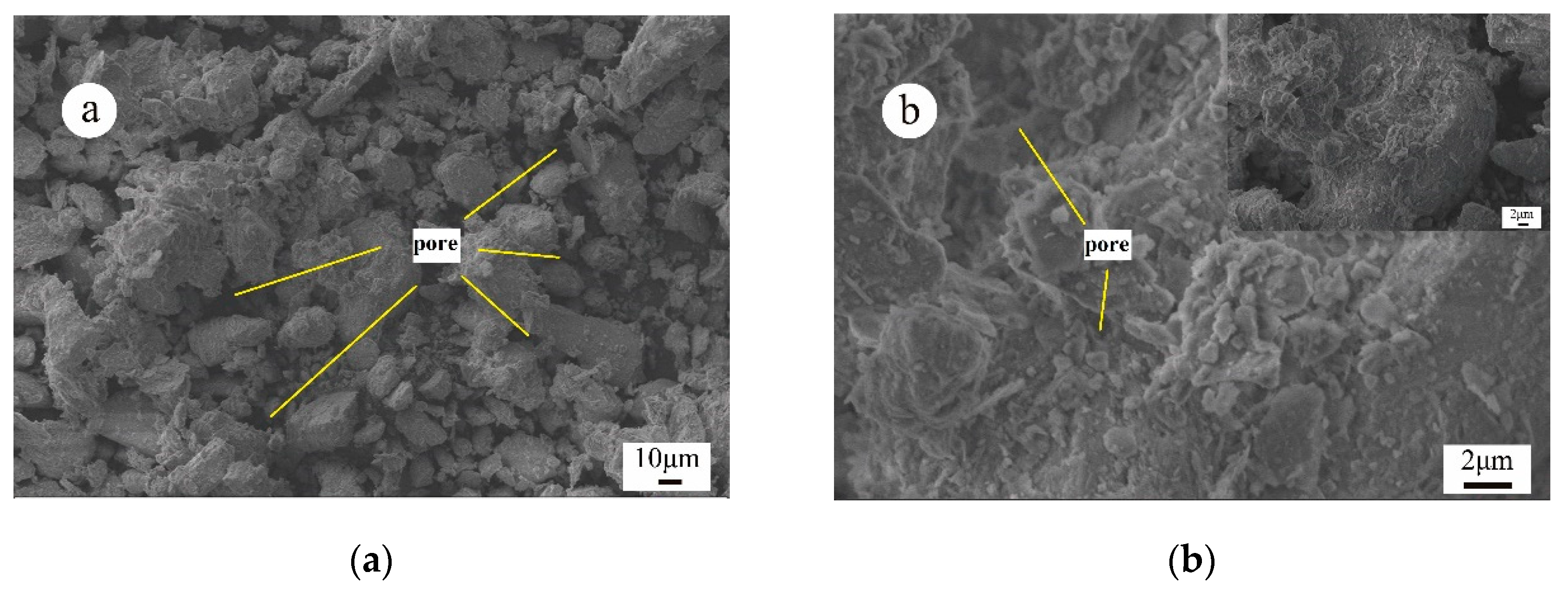
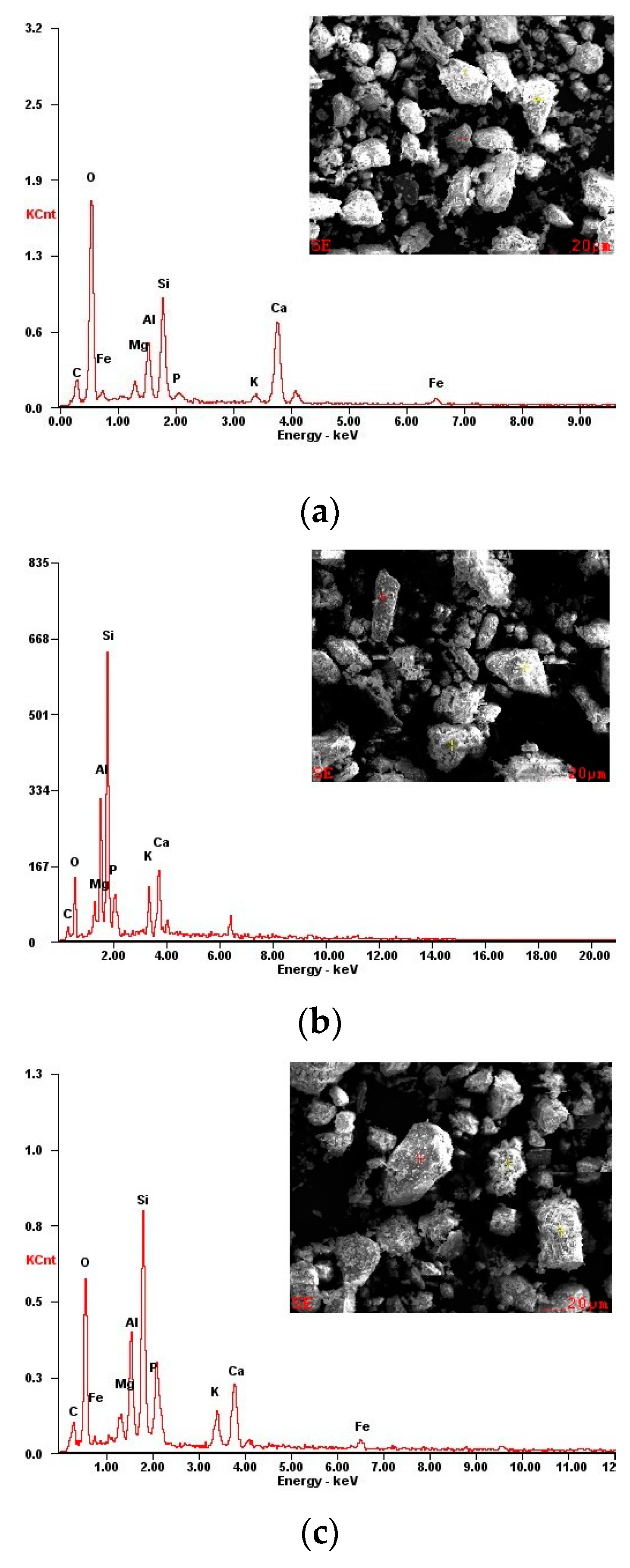
2.2.3. Characterization Analysis
3. Results and Discussion
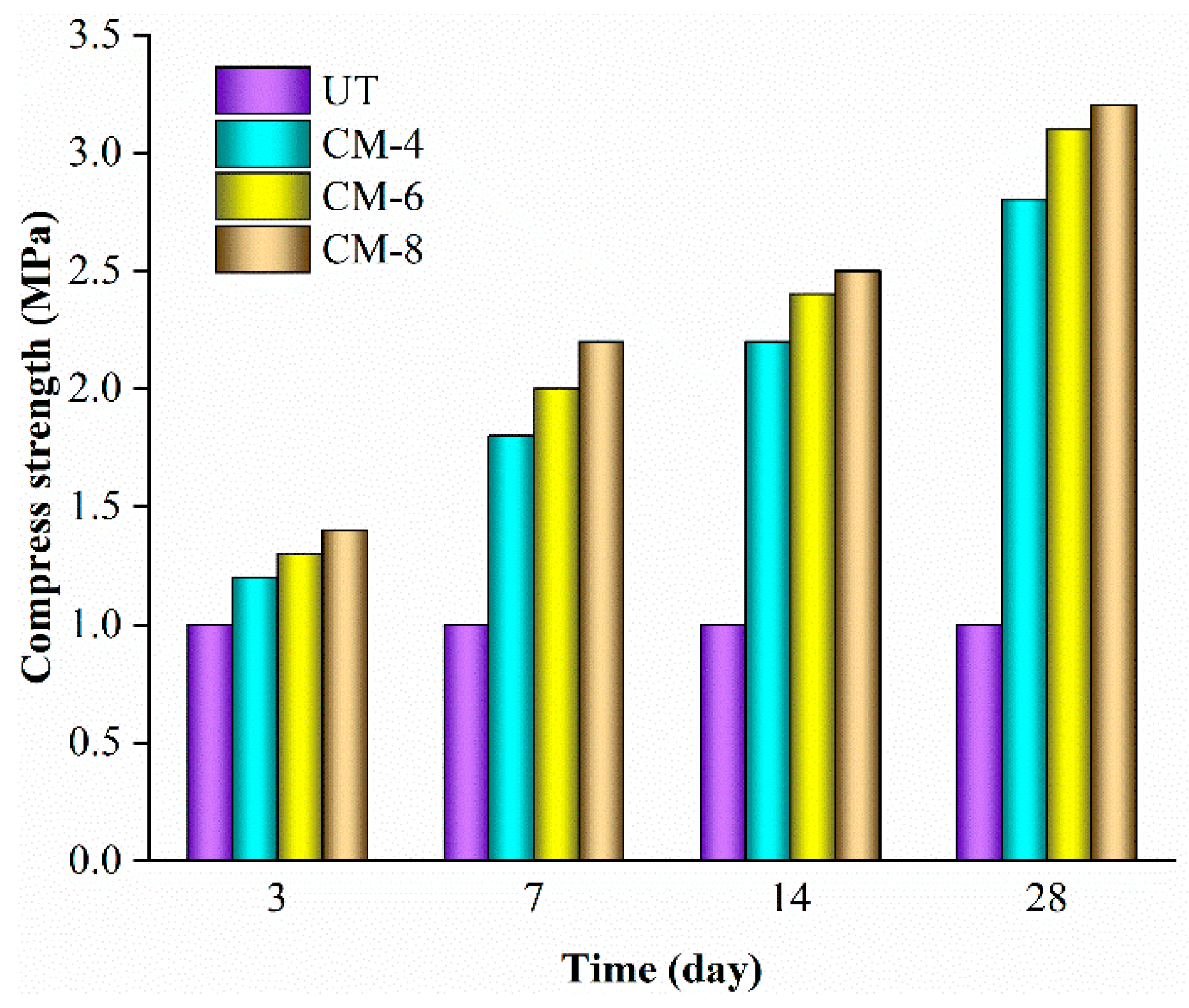
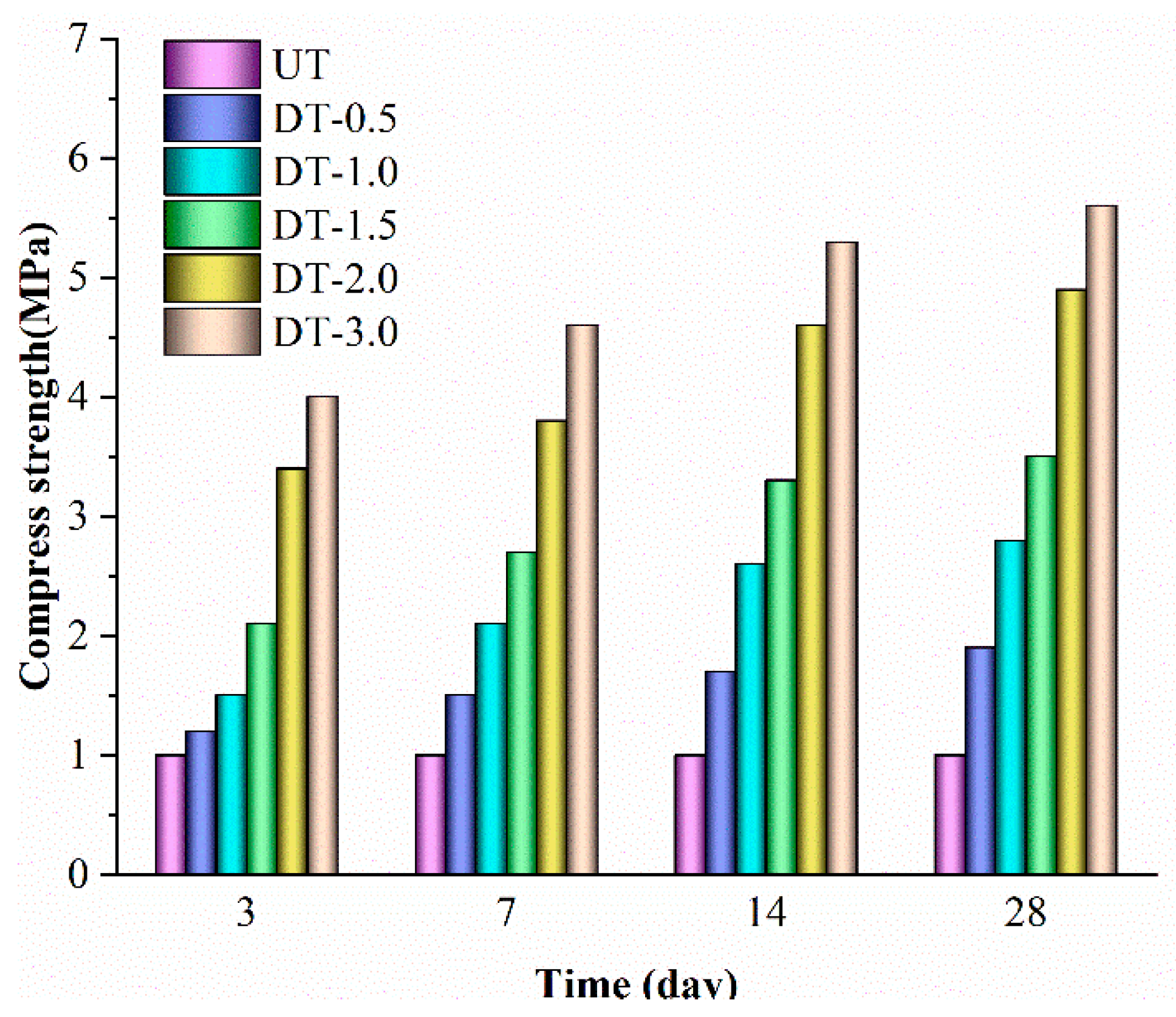
3.1. Compressive Strength
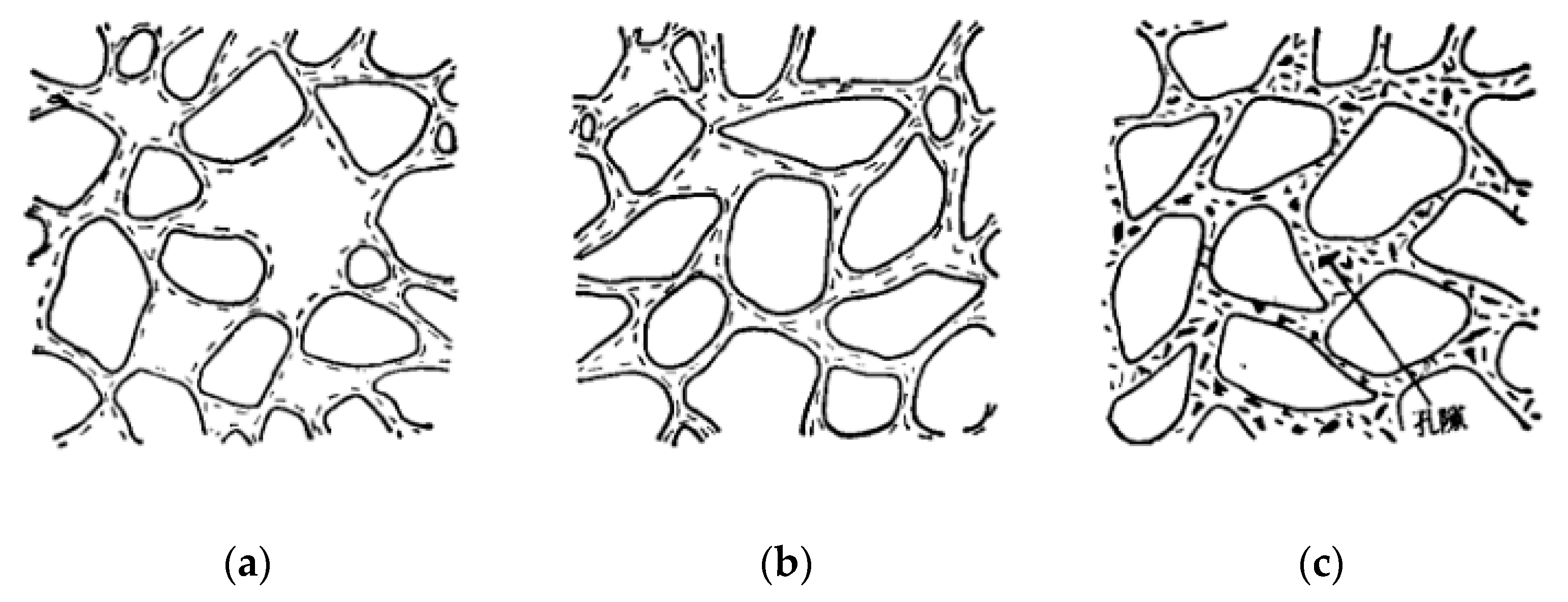
3.2. Permeability and Porosity
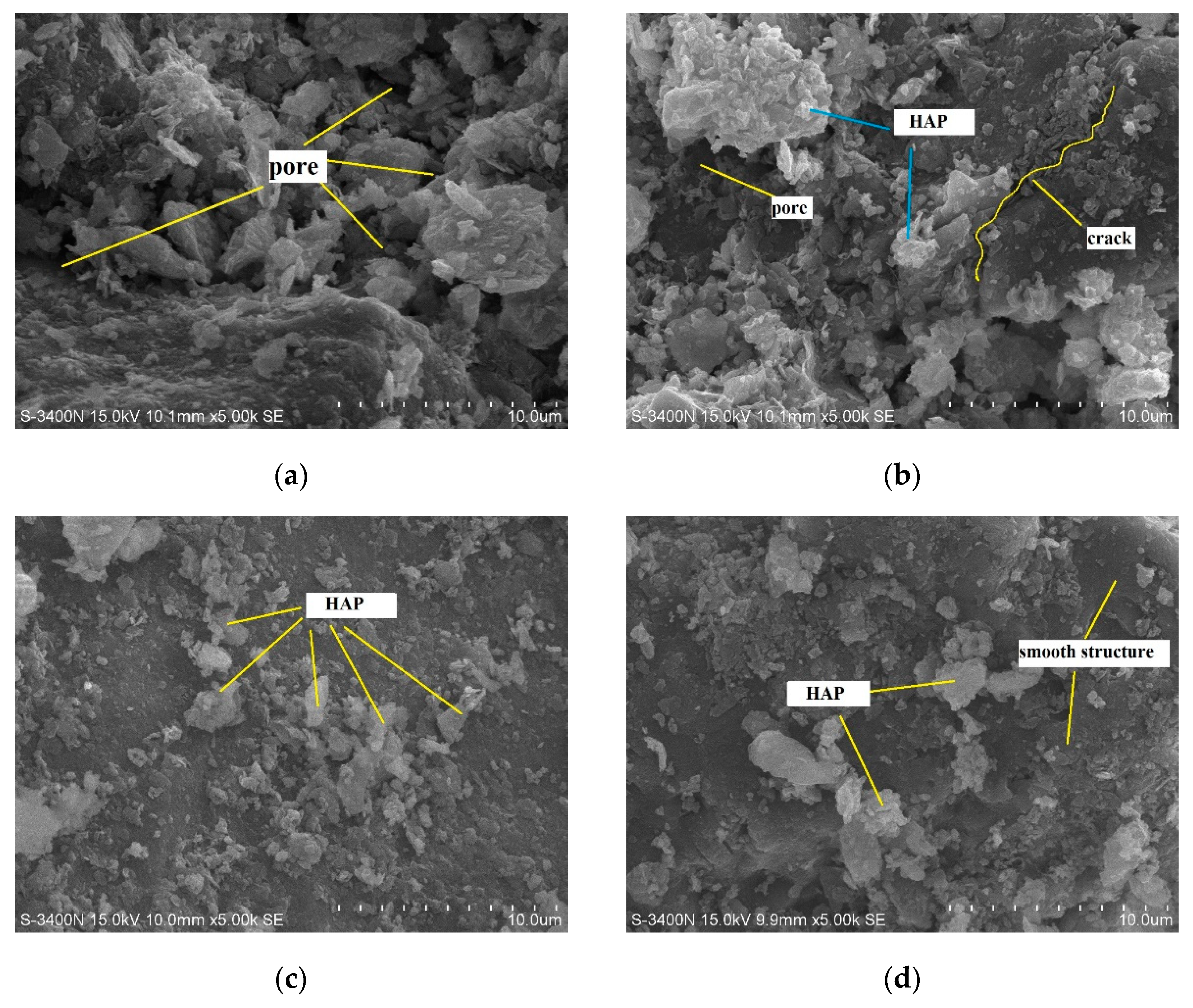
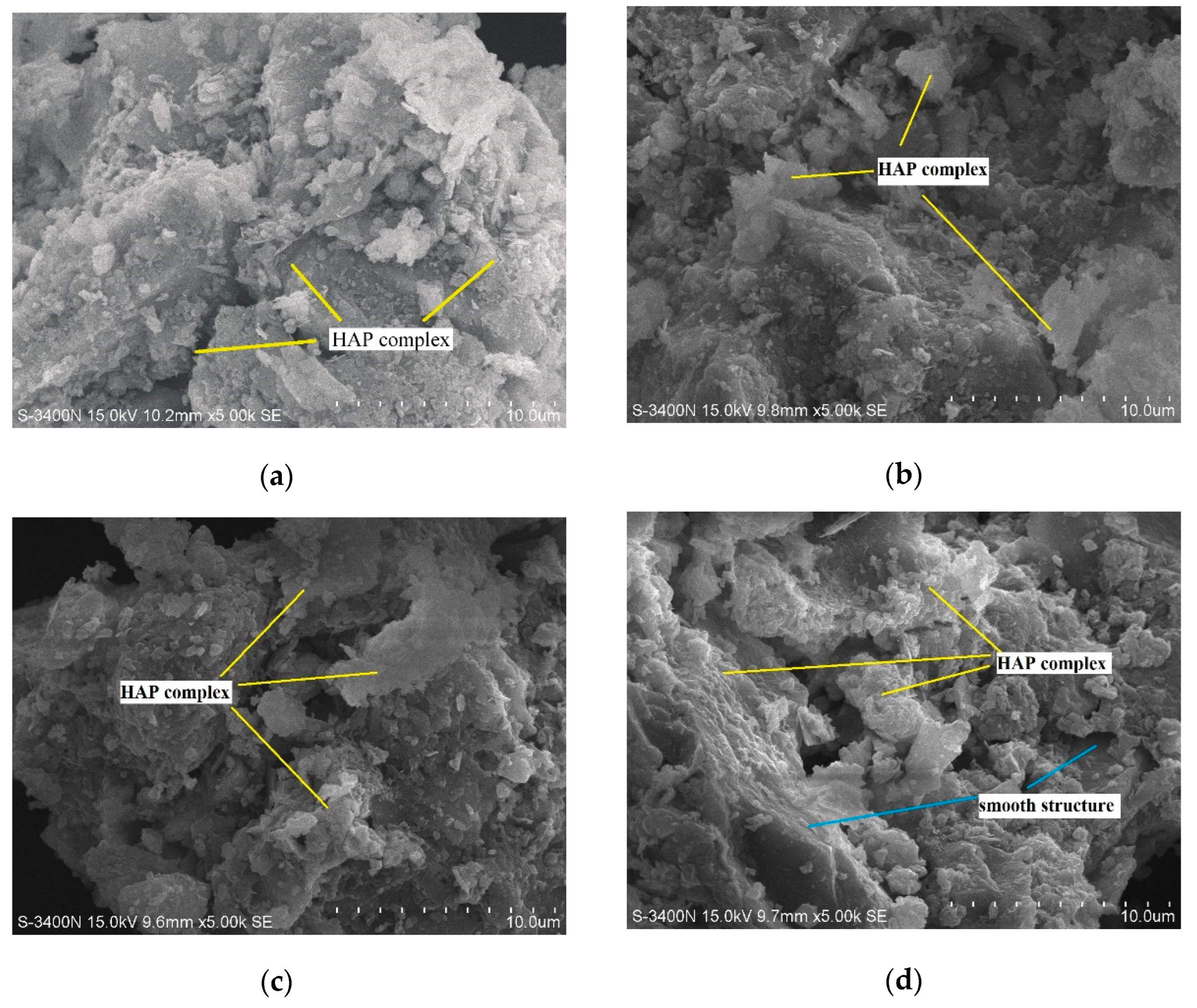
3.3. Micro-Mechanism of DAP Stabilization
3.4. Carbon Emissions and Cost Analysis
4. Conclusions
- Unconfined compressive tests suggest that DAP has a better stabilization effect on loess than Portland cement. Loess treated with a 3.0 mol/L DAP solution showed a significant in maximum compressive strength by 297% after a 28-day curing period. Holding the solid content constant, DAP-treated loess exhibited a 14-29% greater compressive strength than that of cement-treated loess.
- HAP complexes are also more effective than cement in reducing interconnected pores within loess. The permeability coefficient of DT-3.0 was 0.31×10-4 cm/s at 3 days, and decreased to 0.13×10-4 cm/s at 28 days. As the curing age increases, there is a notable decrease of 58% in permeability coefficient of DAP-treated loess. Under the same solid content, the permeability coefficient of DAP-treated loess is 52.5% lower than that of cement-treated loess.
- SEM/EDX analysis showed that DAP reacted with the calcium carbonate in loess, leading to the formation of HAP during curing. This reaction improved the interparticle bonding and filled the pores within loess, strengthening its structure and significantly increasing its compressive strength. The curing time is pivotal for effectively promoting the development and bonding strength of HAP complexes within the treated loess. After 28 days, a large amount of distinct nanoscale reticular structures of HAP complexes can be observed between the soil particles, enveloping both the particles and clay debris.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, D. S. Loess and environment. Journal of Xi’an Jiaotong University(Social Sciences Edition) 2002.
- Li, P. Y.; Qian, H.; Wu, J. H. Environment: accelerate research on land creation. Nature 2014, 510(7503), 29. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. J.; Pan, C. F.; Yu, J.; Fan, J. Y. Study on micro-characteristics of microbe-induced calcium carbonate solidified loess. Crystals 2021, 11(12), 12. [Google Scholar] [CrossRef]
- Li, Z. X.; Wang, J. D.; Yang, S.; Liu, S. H.; Li, Y. W. Characteristics of microstructural changes of malan loess in yan’an area during creep test. Water 2022, 14(3), 22. [Google Scholar] [CrossRef]
- Zheng, Z. Y.; Li, X. A.; Wang, L.; Li, L. C.; Shi, J. F.; Bi, M. L. A new approach to evaluation of loess collapsibility based on quantitative analyses of colloid-clay coating with statistical methods. Engineering Geology 2021, 288, 12. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, F. Y.; Ma, F. L.; Wang, M.; Bai, X. H.; Zheng, Y. L.; Yin, H.; Zhang, G. P. Collapsibility, composition, and microstructure of loess in China. Canadian Geotechnical Journal 2015, 53(4), 673. [Google Scholar] [CrossRef]
- Gao, C. H.; Du, G. Y.; Liu, S. Y.; He, H.; Zhang, D. W. The microscopic mechanisms of treating collapsible loess with vibratory probe compaction method. Transportation Geotechnics 2021, 27. [Google Scholar] [CrossRef]
- Ge, M. M.; Pineda, J. A.; Sheng, D. C.; Burton, G. J.; Li, N. Microstructural effects on the wetting-induced collapse in compacted loess. Computers and Geotechnics 2021, 138, 14. [Google Scholar] [CrossRef]
- Ikeagwuani, C. C.; Obeta, I. N.; Agunwamba, J. C. Stabilization of black cotton soil subgrade using sawdust ash and lime. Soils and Foundations 2019, 59(1), 162. [Google Scholar] [CrossRef]
- Mahedi, M.; Cetin, B.; White, D. J. Cement, lime, and fly ashes in stabilizing expansive soils: performance evaluation and comparison. Journal of Materials in Civil Engineering 2020, 32(7), 16. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Rachan, R.; Suddeepong, A. Assessment of strength development in blended cement admixed Bangkok clay. Construction and Building Materials 2011, 25(4), 1521. [Google Scholar] [CrossRef]
- Lemaire, K.; Deneele, D.; Bonnet, S.; Legret, M. Effects of lime and cement treatment on the physicochemical, microstructural and mechanical characteristics of a plastic silt. Engineering Geology 2013, 166, 255. [Google Scholar] [CrossRef]
- Hou, Y. F.; Li, P.; Xiao, T. ; H., H. R. Review on strengthening loess with curing agents; Journal of Engineering Geology, 2019.
- Possenti, E.; Colombo, C.; Conti, C.; Gigli, L.; Merlini, M.; Plaisier, J. R.; Realini, M.; Sali, D.; Gatta, G. D. Diammonium hydrogenphosphate for the consolidation of building materials. Investigation of newly-formed calcium phosphates. Construction and Building Materials 2019, 195, 557.
- Wang, Y.; Guo, C. H.; Chen, X. J.; Jia, L. Q.; Guo, X., N.; Chen, R. S.; Zhang, M. S.; Chen, Z. Y.; Wang, H. D. Carbon peak and carbon neutrality in China: Goals, implementation path and prospects. China Geology 2021, 4(4), 720. [Google Scholar] [CrossRef]
- Zhang, F. Y.; Pei, X. J.; Yan, X. D. Physicochemical and mechanical properties of lime-treated loess. Geotechnical and Geological Engineering 2017, 36(1), 685. [Google Scholar] [CrossRef]
- Ravindran, G.; Bahrami, A.; Mahesh, V.; Katman, H. Y. B.; Srihitha, K.; Sushmashree, A.; Kumar, A. N.; Far, H. Global research trends in engineered soil development through stabilisation: scientific production and thematic breakthrough analysis. Buildings 2023, 13(10), 17. [Google Scholar] [CrossRef]
- Ikeagwuani, C. C.; Nwonu, D. C. Emerging trends in expansive soil stabilisation: A review. Journal of Rock Mechanics and Geotechnical Engineering 2019, 11(2), 423. [Google Scholar] [CrossRef]
- Yang, F. W.; Zhang, B. J.; Liu, Y.; Wei, G. F.; Zhang, H.; Chen, W. X.; Xu, Z. Biomimic conservation of weathered calcareous stones by apatite. New Journal of Chemistry 2011, 35(4), 887. [Google Scholar] [CrossRef]
- Song, J.; Ma, J. X.; Li, F. Y.; Chai, L. N.; Chen, W. F.; Dong, S.; Li, X. J. Study on fractal characteristics of mineral particles in undisturbed loess and lime-treated loess. Materials 2021, 14 (21).
- Li, X. A.; Sun, J. Q.; Ren, H. Y.; Lu, T.; Ren, Y. B.; Pang, T. The effect of particle size distribution and shape on the microscopic behaviour of loess via the DEM. Environmental Earth Sciences 2022, 81(10), 290. [Google Scholar] [CrossRef]
- Nan, Y. L.; Wei, Y. N.; Liu, K.; Cao, Y. B. Quantitative 3D characterization of pore structure in malan loess from different regions of the loess plateau. Water 2023, 15(17), 14. [Google Scholar] [CrossRef]
- Jiang, M. J.; Zhang, F. G.; Hu, H. J.; Cui, Y. J.; Peng, J. B. Structural characterization of natural loess and remolded loess under triaxial tests. Engineering Geology 2014, 181, 249. [Google Scholar] [CrossRef]
- Matteini, M.; Rescic, S.; Fratini, F.; Botticelli, G. Ammonium phosphates as consolidating agents for carbonatic stone materials used in architecture and cultural heritage: preliminary research. International Journal of Architectural Heritage 2011, 5(6), 717. [Google Scholar] [CrossRef]
- Sassoni, E.; Naidu, S.; Scherer, G. W. The use of hydroxyapatite as a new inorganic consolidant for damaged carbonate stones. Journal of Cultural Heritage 2011, 12(4), 346. [Google Scholar] [CrossRef]
- Sassoni, E.; D’Amen, E.; Roveri, N.; Scherer, G. W.; Franzoni, E. Durable self-cleaning coatings for architectural surfaces by incorporation of Tio2 nano-particles into hydroxyapatite films. Materials 2018, 11(2), 16. [Google Scholar] [CrossRef] [PubMed]
- Eliassi, M. D.; Zhao, W.; Tan, W. F. Effect of carbonate and phosphate ratios on the transformation of calcium orthophosphates. Materials Research Bulletin 2014, 55, 114. [Google Scholar] [CrossRef]
- Uskokovic, V.; Odsinada, R.; Djordjevic, S.; Habelitz, S. Dynamic light scattering and zeta potential of colloidal mixtures of amelogenin and hydroxyapatite in calcium and phosphate rich ionic milieus. Archives of Oral Biology 2011, 56(6), 521. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.-M.; Turcanu-Caruţiu, D.; Fierăscu, R.-C.; Fierăscu, I.; Bunghez, I.-R.; Ion, M.-L.; Teodorescu, S.; Vasilievici, G.; Rădiţoiu, V. Caoxite-hydroxyapatite composition as consolidating material for the chalk stone from Basarabi–Murfatlar churches ensemble. Applied Surface Science 2015, 358, 612. [Google Scholar] [CrossRef]
- Yang, F. W.; Liu, Y. Artificial hydroxyapatite film for the conservation of outdoor marble artworks. Materials Letters 2014, 124, 201. [Google Scholar] [CrossRef]
- Sena da Fonseca, B.; Ferreira Pinto, A. P.; Piçarra, S.; Caldeira, B.; Montemor, M. F. Consolidating efficacy of diammonium hydrogen phosphate on artificially aged and naturally weathered coarse-grained marble. Journal of Cultural Heritage 2021, 51, 145. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E. An innovative phosphate-based consolidant for limestone. Part 1: Effectiveness and compatibility in comparison With ethyl silicate. Construction and Building Materials 2016, 102, 918.
- Wang, L.; Nancollas, G. H. Calcium Orthophosphates: Crystallization and Dissolution. Chemical Reviews 2008, 108(11), 4628. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, W. Q.; Parise, J. B.; Phillips, B. L. Formation of hydroxylapatite from co-sorption of phosphate and calcium by boehmite. Geochimica Et Cosmochimica Acta 2012, 85, 289. [Google Scholar] [CrossRef]
- Wang, L. J.; Nancollas, G. H. Calcium orthophosphates: crystallization and dissolution. Chemical Reviews 2009, 108(11), 4628. [Google Scholar] [CrossRef]
- GB/T 50123-2019. Ministry of Housing and Urban Rural Development of the People’s Republic of China. Geotechnical test methods standard. State Administration for Market Regulation, 2019. Beijing, China.
- Nie, S.; Zhou, J.; Yang, F.; Lan, M.; Li, J.; Zhang, Z.; Chen, Z.; Xu, M.; Li, H.; Sanjayan, J. G. Analysis of theoretical carbon dioxide emissions from cement production: Methodology and application. Journal of Cleaner Production 2022, 334, 130270. [Google Scholar] [CrossRef]
| Grain-Size Fraction | Silty-Fine Sand | Coarse Silt | Fine Silt | Clay |
|---|---|---|---|---|
| Grain diameter (μm) | >50 | 10-50 | 5-10 | <5 |
| Content (%) | 12.4 | 61.9 | 17.3 | 7.9 |
| Composition | CaO | MgO | Al2O3 | SiO2 | CO2 | Fe2O3 |
|---|---|---|---|---|---|---|
| Content (%) | 13.0 | 2.93 | 12.9 | 48.0 | 10.3 | 6.23 |
| Groups | Cement Content (wt%) | Molar Concentration of DAP (mol/L) | Water Content (wt%) |
|---|---|---|---|
| UT | 0 | - | 16 |
| CM-4 | 4 | - | |
| CM-6 | 6 | - | |
| CM-8 | 8 | - | |
| DT-0.5 | - | 0.5 | |
| DT-1.0 | - | 1.0 | |
| DT-1.5 | - | 1.5 | |
| DT-2.0 | - | 2.0 | |
| DT-3.0 | - | 3.0 |
| Groups | UT | CM-4 | CM-6 | CM-8 | DT-1.5 | DT-2.0 | DT-3.0 |
|---|---|---|---|---|---|---|---|
| Void ratio | 0.542 | 0.525 | 0.518 | 0.505 | 0.502 | 0.493 | 0.489 |
| Time (s) | 18 | 40 | 74 | 105 | 98 | 126 | 189 |
| Permeability coefficient (10-4cm/s) | 2.42 | 1.08 | 0.59 | 0.41 | 0.44 | 0.34 | 0.28 |
| Groups | CM-6 3d | CM-6 14d | CM-6 28d | DT-3.0 3d | DT-3.0 14d | DT-3.0 28d |
|---|---|---|---|---|---|---|
| Void ratio | 0.523 | 0.518 | 0.519 | 0.496 | 0.489 | 0.485 |
| Time (s) | 78 | 74 | 84 | 143 | 189 | 327 |
| Permeability coefficient (10-4cm/s) | 0.56 | 0.59 | 0.52 | 0.31 | 0.28 | 0.13 |
| Raw Materials | Carbon Emission Factor (kg CO2/kg) | Market Price (RMB/kg) |
|---|---|---|
| OPC | 0.84 | 0.64 |
| DAP | 0.50 | 3.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





