Submitted:
10 April 2024
Posted:
11 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Obtaining Leaves from the Cultivation by Cuttings
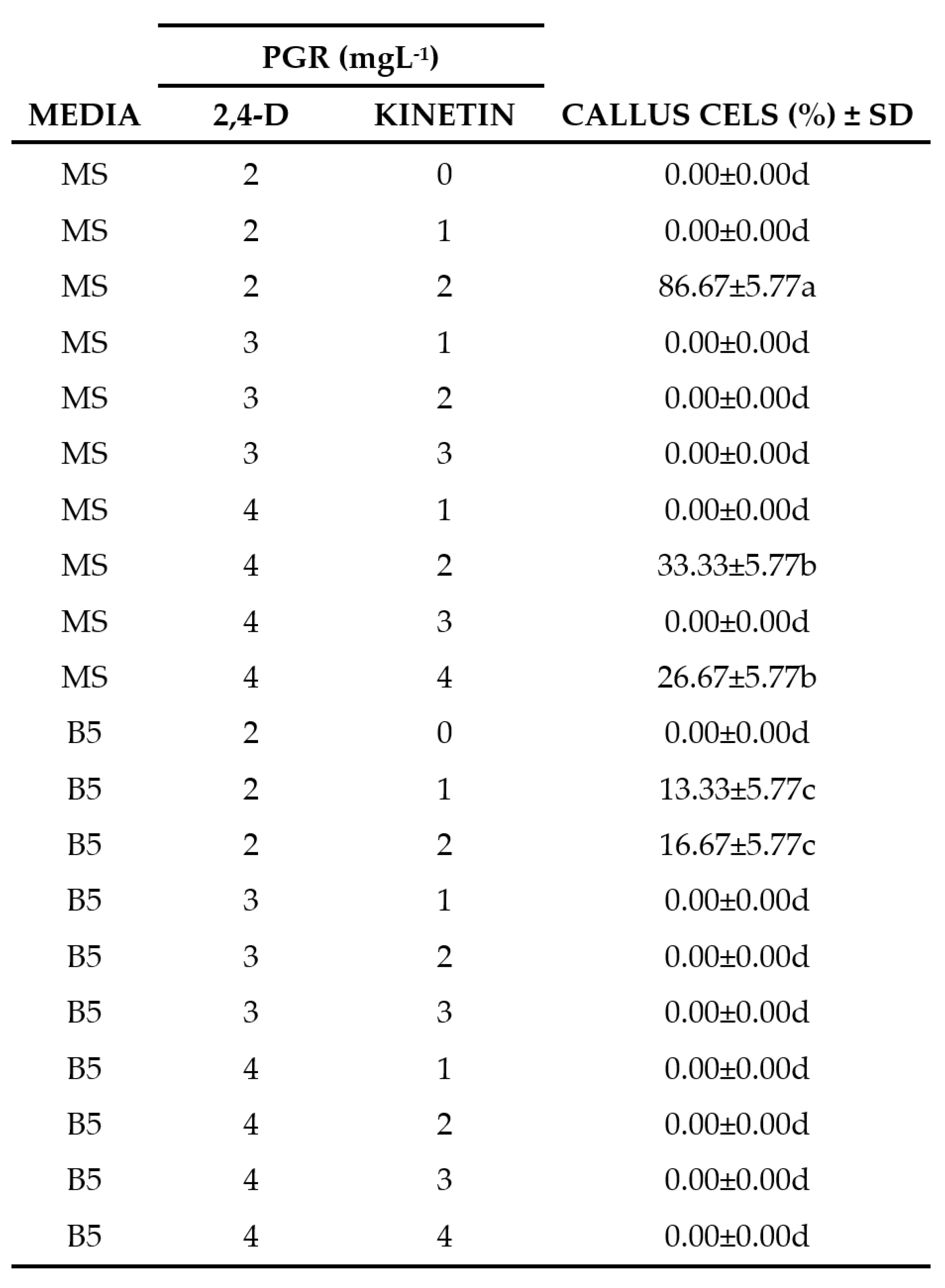
2.2. Callus Induction
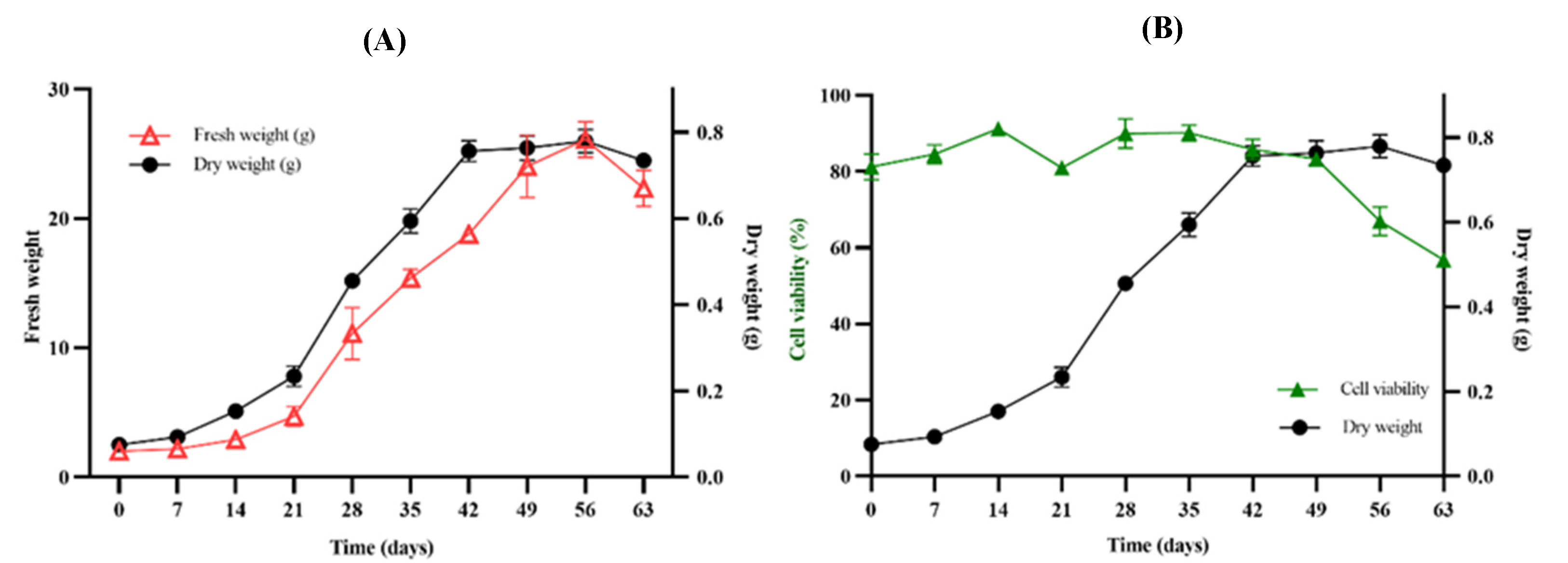
2.3. Callus Culture Growth Kinetics
2.4. Appearance and Percentage of Viability of the Callus Culture
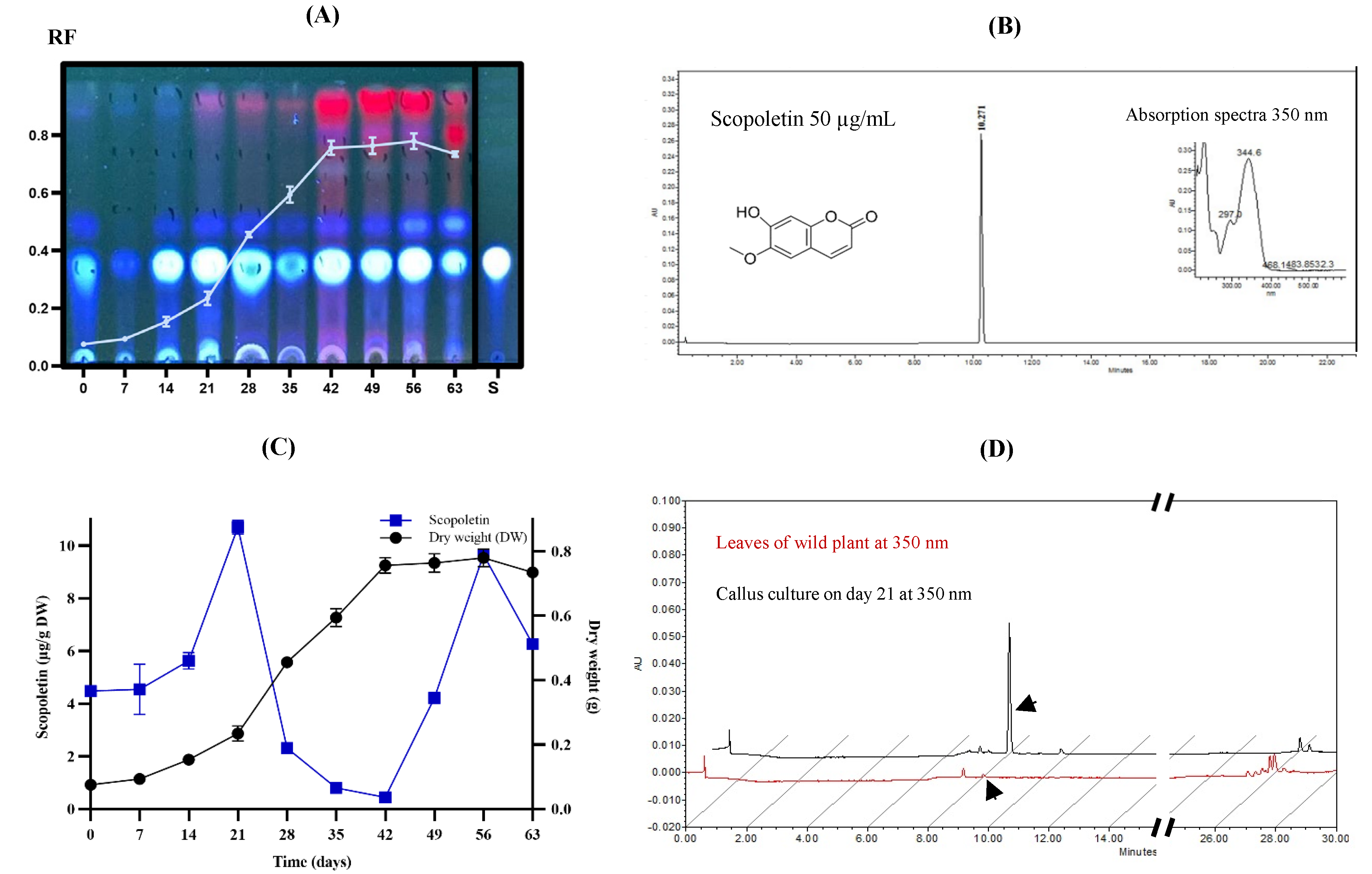
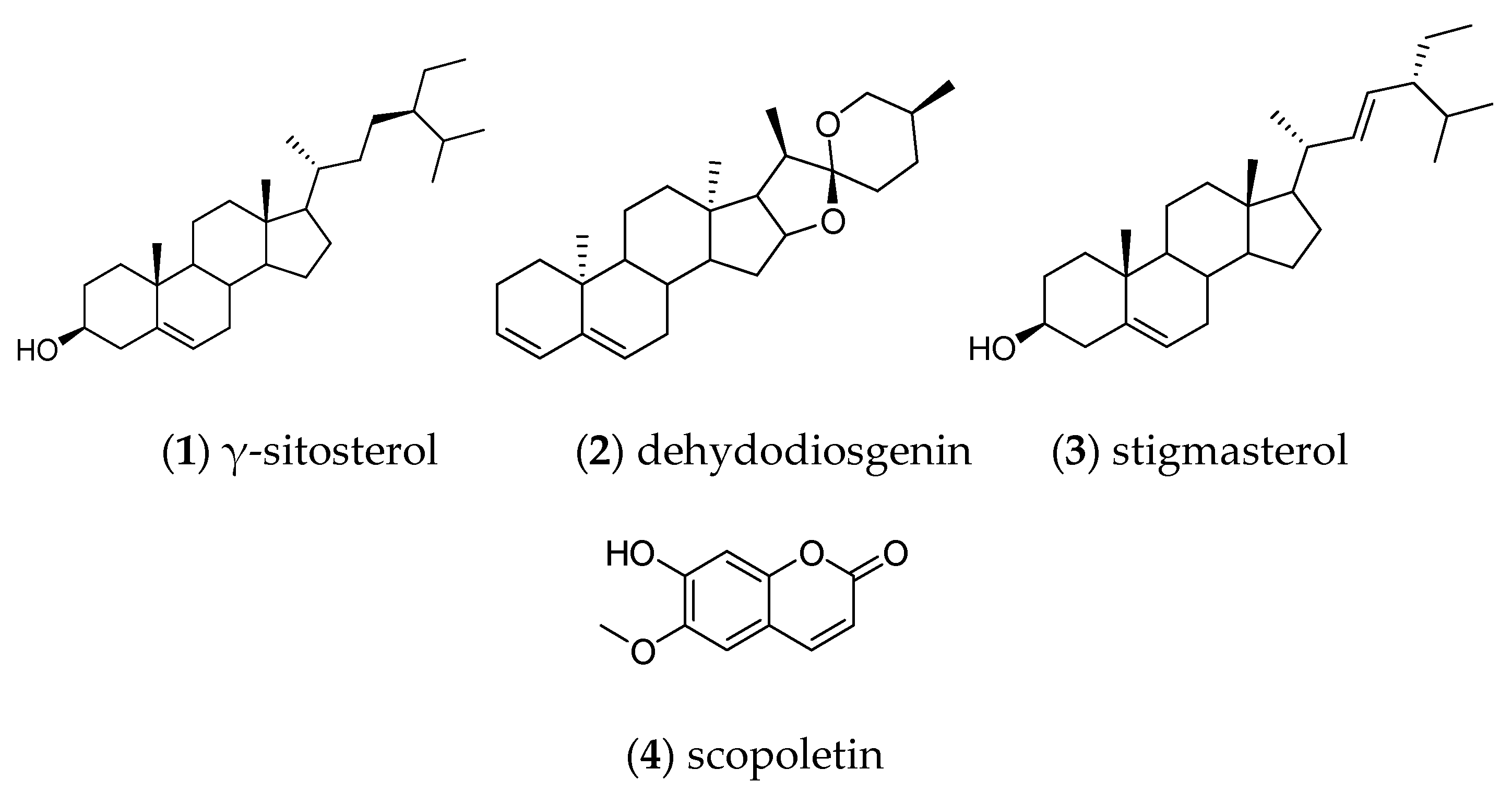
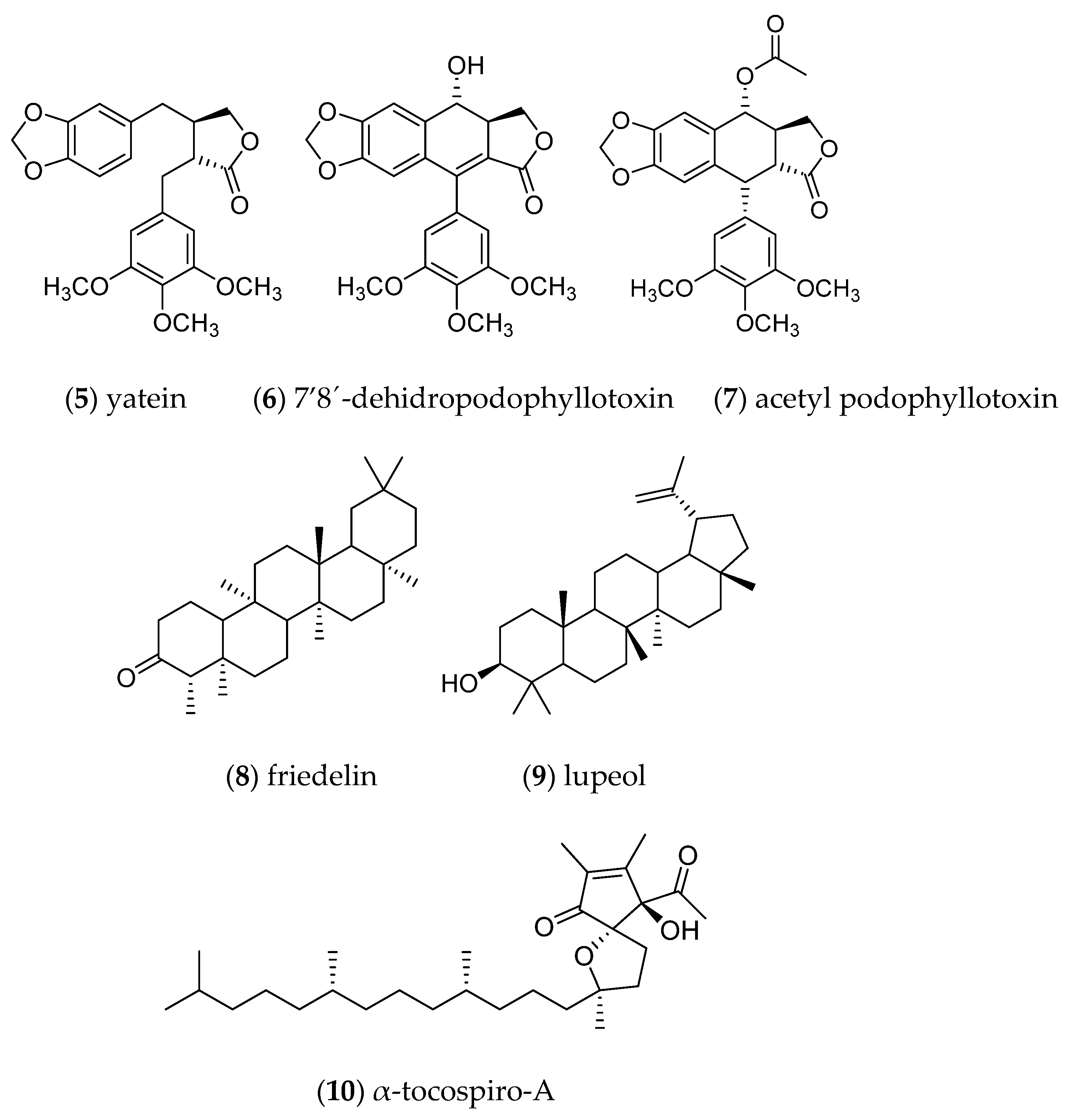
2.5. Chemical Profile of B. fagaroides Callus Culture
2.6. Chemical Profile of wild B. fagaroides Leaves
2.7. Evaluation of Cytotoxic Activity
3. Discussion
4. Materials and Methods
4.1. Identification of Plant Material
4.2. Obtaining of Plant Material
4.3. Callus Induction
4.4. Growth Kinetics of Callus Culture
4.5. Cell Morphology and Viability
4.6. Phytochemical Analysis of the Callus Culture
4.6.1. Obtaining the Extracts
4.6.2. Chemical Profile and Identification of Major Compounds
4.7. Phytochemical Analysis of Wild Plant Leaves
4.7.1. Obtaining the Extracts
4.7.2. Chemical Profile and Identification of Major Compounds
4.8. Analysis of Callus and Leaf Extracts by Gas Chromatography Coupled to Mass Spectrometry (GC/MS)
4.9. Cytotoxicity Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noge, K. and Becerra, J.X. Germacrene D, a common sesquiterpene in the genus Bursera (Burseraceae). Molecules 2009, 14, 5289–5297. [Google Scholar] [CrossRef] [PubMed]
- Rzedowski, J.; Medina, L.R.; and Calderón, de R. G. Las especies de Bursera (Burseraceae) en la cuenca superior del río Papaloapan (México). Acta Bot. Mex. 2004, 66, 23–151. [Google Scholar] [CrossRef]
- Rzedowski, J.; Medina, L.R.; and Calderón, de R. G. Inventario del conocimiento taxonómico, así como de la diversidad y del endemismo regionales de las especies mexicanas de Bursera (Burseraceae). Acta Bot Mex. 2005, 70, 85–111. [Google Scholar] [CrossRef]
- Marcotullio, M.C.; Curini, M.; and Becerra, J.X. An Ethnopharmacological, Phytochemical and Pharmacological Review on Lignans from Mexican Bursera spp. Molecules. 2018, 23, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Sepúlveda, A.M.; Mendieta-Serrano, M.; Antúnez, M.M. Y.; Salas-Vidal, E.; Marquina, S.; Villarreal, M.L.; Puebla, A.M.; Delgado, J.; and Alvarez, L. Cytotoxic podophyllotoxin type-lignans from the steam bark of Bursera fagaroides var. fagaroides. Molecules. 2012, 17, 9506–9519. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, M.M.; León, A.; Rojas-Sepúlveda, A.M.; Marquina, S.; Mendieta-Serrano, M.A.; Salas-Vidal, E.; Villarreal, M.L.; and Alvarez, L. Aryldihydronaphthalene-type lignans from Bursera fagaroides var. fagaroides and their antimitotic mechanism of action. RSC. 2016, 6, 4950–4959. [Google Scholar] [CrossRef]
- Antúnez-Mojica, M.; Romero-Estrada, A.; Hurtado-Díaz, I.; Miranda-Molina, A.; and Alvarez, L. Lignans from Bursera fagaroides: Chemistry, Pharmacological Effects and Molecular Mechanism. A Current Review. Life. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Bianchi, E.; Sheth, K.; and Cole, J.R. Antitumor agents from Bursera fagaroides (Burseraceae). (β -peltatin-a-methylether and 5′-desmethoxy-β-peltatin-a-methylether). Tetrahedron Lett. 1969, 10, 2759–2762. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Jiménez, R. , Torres-Valencia, J.M.; Cerda-García-Rojas, C.M.; Hernández-Hernández, J.D.; Román-Marín, L.U.; Manríquez-Torres, J.J.; Gómez-Hurtado, M.A.; Valdez-Calderón, A.; Motilva, V.; García-Mauriño, S.; Telero, E.; Ávila, J. and Joseph-Nathan, P. Absolute configuration of podophyllotoxin related lignans from Bursera fagaroides using vibrational circular dichroism. Phytochem. 2011, 72, 2237–2243. [Google Scholar] [CrossRef]
- Acevedo, M.; Nuñez, P.; González-Maya, L.; Cardoso, T.A. and Villarreal, M.L. Cytotoxic and Anti-inflamatory activities of Bursera species from Mexico. J. Clin. Toxicol. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Antúnez-Mojica, M.; Rojas-Sepúlveda, A.M.; Mendieta-Serrano, M.A.; Gonzalez-Maya, L; Marquina, S. ; Salas-Vidal, E. and Alvarez, L. Lignans from Bursera fagaroides affect in vivo cell behavior by disturbing the tubulin cytoskeleton in zebrafish embryos. Molecules. 2019, 24, 1–13. [Google Scholar] [CrossRef]
- Ceballos, G.; Martínez, L., García, A., Espinoza, E.; Bezaury, C.J. and Dirzo, R. Diversidad, amenazas y áreas prioritarias para la conservación de las selvas secas del Pacífico de México. 1st ed. Fondo de Cultura Económica, CONABIO, México D.F. 2010. 9-11.
- Bonfil-Sanders, C.; Mendoza-Hernández-P. E., and Ulloa-Nieto, J.A. Root and callus development in cuttings of seven species of the genus Bursera. Agrociencia. 2007, 41, 103–109. [Google Scholar]
- Satyanarayana, B.N. and Varghese, D.B. Plant tissue culture: practices and new experimental protocols. I. K. International Pub. House. Pvt. Ltd., New Delhi. 2007; 9-54. ISBN: 978-81-89866-11-2.
- Verpoorte, R. and Alfermann, A. W. Metabolic Engineering of Plant Secondary Metabolism. Kluger Academic Publishers. Springer Netherlands, 2013, 1-46. ISBN: 978-94-015-9423-3.
- Rodríguez, B.M.M.; Latsague, V.M.I.; Chacón, F.M.A. and Astorga, B.P.K. In vitro induction of callogenesis and indirect organogenesis from explants of cotyledon, hypocotyl and leaf in Ugni molinae. Bosque. 2014, 35, 21–22. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Sugimoto, K. and Iwase, A. Plant Callus: Mechanisms of Induction and Repression. Plant Cell. 2013, 25, 3159–3173. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.R.; Reyes, J. and Loyola, V.M. Biosíntes y bioconversión de metabolitos secundarios por células cultivadas in vitro. Cultivo de tejidos en la agricultura. 9th Chapter, 212-231.
- Mishra, S.K. and Kumar, A. Biosynthesis of guggulsterone in the callus culture of Commiphora wightii. Arnott. bhandari (Burseraceae). RJPBCS. 2010, 1, 35–41. [Google Scholar]
- Al-Abdallat, A.M.; Adayileh, B.K.; Sawwan, J.S.; Shibli, R.; Al-Qudah, T.S.; Abu-Irmaileh, B.; Albdaiwi, R.N.; Almaliti, J. and Bustanji, Y. Secondary Metabolites Profiling, Antimicrobial and Cytotoxic Properties of Commiphora gileadensis L. Leaves, Seeds, Callus, and Cell Suspension Extracts. Metabolites. 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Nikam, T.D.; Ghorpade, R.P. Nitnaware K.M.; Ahire, M.L.; Lokhande, V.H. and Chopra, A. Micropropagation and non-steroidal anti-inflammatory and anti-arthritic agent boswellic acid production in callus cultures of Boswellia serrata Roxb. Physiol Mol Biol Plants. 2013, 19, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, I.I.; Dogan, I.; Hocaoglu-Ozyigit, A.; Yalcin, B.; Erdogan, A.; Yalcin, I.E. , Cabi, E. and Kaya, Y. Production of secondary metabolites using tissue culture-based biotechnological applications. Front Plant Sci. 2023, 14, 1–28. [Google Scholar] [CrossRef]
- Trejo-Tapia, G.; Rodríguez-Monroy, M. La agregación celular en la producción de metabolitos secundarios en cultivos vegetales in vitro. Interciencia. 2007, 32, 669–674. [Google Scholar]
- Larson, C.G.; Gómez, C.; Sánchez-Olate, M. and Ríos, D. Induction of indirect callogenesis in Eucalyptus globulus. Bosque. 2006, 27, 250–257. [Google Scholar] [CrossRef]
- Feeney, M.; Bhagwat, B.; Mitchell, J.S. and Lane, W.D. Shoot regeneration from organogenic callus of sweet cherry (Prunus avium L.). Plant Cell Tissue Organ Cult. 2007, 90, 201–214. [Google Scholar] [CrossRef]
- Rashmi, R. and Trivedi, M.P. Effect of Various Growth Hormone Concentration and Combination on Callus Induction, Nature of Callus and Callogenic Response of Nerium odorum. Appl Biochem Biotechnol. 2014, 172, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Bonfil-Sanders, C.; Cajero-Lázaro, I. and Evans, R. Y. Seed germination of six Bursera species from central México. Agrociencia. 2008, 42, 827–834. [Google Scholar]
- Cultid-Medina, C.A. and Rico, Y. Los aliados emplumados de los Copales y Cuajiotes de México: aves y la dispersión de semillas de Bursera. RDU. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Rzedowski, J. y Guevara-Férer, F. Burseraceae. Flora del Bajío y de regiones adyacentes, fascículo 3. 1992, 1-46.
- Lodish, H.; Berk, A.; Kaiser, C.A.; Krieger, M.; Bretscher, A.; Ploegh, H.; Amon, A. and Scott, M.P. Biología celular y molecular. 7th. Buenos Aires: Panamericana, 2016; Volume 20, pp.967-71.
- Mc-Caughey-Espinoza, D.; Ayala-Astorga, G.; García-Baldenegro, C.; Buitimea-Cantúa, N.; Buitimea-Cantúa, G. and Ochoa-Meza, A. In vitro germination and induction of callus and root in Bursera laxiflora S. Watson. Abanico Agrofor. 2020, 2, 1–14. [Google Scholar]
- Pavón-Reyes, L.; Evangelista-Lozano, S.; Sepúlveda-Jiménez, G.; Chávez, A.V. and Rodríguez-Monroy, M. Cell Culture of Bursera linanoe in a Stirred Tank Bioreactor for Production of Linalool and Linalyl Acetate. Nat Prod Commun. 2017, 12, 319–322. [Google Scholar] [PubMed]
- Malik, S.I.; Rashid, H.; Yasmin, T. and Minhas, N. M. Effect of 2,4-dichlorophenoxyacetic Acid on Callus Induction from Mature Wheat (Triticum aestivum L.) Seeds. Int J Agric Biol. 2003, 6, 156–159. [Google Scholar]
- Zheng, M.Y. and Konzak, C.F. Effect of 2,4-dichlorophenoxyacetic acid on callus induction and plant regeneration in anther culture of wheat ( Triticum aestivum L.). Plant Cell Rep. 1999, 19, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Carsono, N.; Juwendah, E.; Liberty, L.; Sari, S.; Damayanti, F. and Rachmadi, M. Optimize 2,4-D concentration and callus induction time enhance callus proliferation and plant regeneration of three rice genotypes. Biodiversitas. 2021, 22, 2555–2560. [Google Scholar] [CrossRef]
- Ozias-Akins, P. and Vasil, I.K. Callus induction and growth from the mature embryo of Triticum aestivum (Wheat). Protoplasma. 1983, 115, 104–113. [Google Scholar] [CrossRef]
- Pádua, M.S.; Paiva, L.V.; Silva, L.C.D.; Livramento, K.G.D.; Alves, E. and Castro, A.H.F. Morphological characteristics and cell viability of coffee plants calli. Cienc. Rural. 2014, 44, 660–665. [Google Scholar] [CrossRef]
- Taguchi, G.; Fujikawa, S.; Yazawa, T.; Kodaira, R.; Hayashida, N.; Shimosaka, M. and Okazaki, M. Scopoletin uptake from culture medium and accumulation in the vacuoles after conversion to scopolin in 2,4-D-treated tobacco cells. Plant Sci. 2000, 151, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Hino, F.; Kominami, K. and Miura, Y. Effects of Plant Hormones on Formation of Scopoletin and Scopolin in Tobacco Tissue Cultures. Agric Biol Chem. 1982, 46, 609–614. [Google Scholar] [CrossRef]
- Cisneros-Torres, D.; Cruz-Sosa, F.; González-Cortazar, M.; Martínez-Trujillo, A. and Nicasio-Torres, P. Enhancing the production of scopoletin and quercetin 3-O-β-d-glucoside from cell suspension cultures of Tilia americana var. mexicana by modulating the copper and nitrate concentrations. Plant Cell Tiss Organ Cult. 2019, 139, 305–316. [Google Scholar] [CrossRef]
- Siatka, T.; Chlebekb, J. and Hoštálková, A. Copper (II) Sulfate Stimulates Scopoletin Production in Cell Suspension Cultures of Angelica archangelica. Nat Prod Commun. 2017, 12, 1779–1780. [Google Scholar] [CrossRef]
- Gnonlonfin, G.J.B.; Sanni, A. and Brimer, L. Review Scopoletin – A Coumarin Phytoalexin with Medicinal Properties. Critical Reviews in Plant Sciences. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Bagni, N. and Fracassini, D.S. The effect of coumarin derivatives on organogenesis and callus growth of Cichorium Intybus roots and Helianthus tuberosus tubers in vitro. Experientia. 1971, 27, 1239–1241. [Google Scholar] [CrossRef]
- Ekiert, H. and Gomółka, E. Coumarin compounds in Ammi majus L. callus cultures. Die Pharmazie. 2000, 55, 684–687. [Google Scholar] [PubMed]
- Fritig, B.; Hirth, L. and Ourisson, G. Biosynthesis of the coumarins: Scopoletin formation in tobacco tissue cultures. Phytochem. 1970, 9, 1963–1975. [Google Scholar] [CrossRef]
- Modafar, C.E.; Clerivet, A.; Fleuriet, A. and Macheix, J.J. Inoculation of Platanus acerifolia with Ceratocystis fimbriata F. Sp. Platani induces scopoletin and umbelliferone accumulation. Phytochem. 1993, 34, 1271–1276. [Google Scholar]
- Habibullah, M.; Al-Mansur, M.A.; Mahboob, M.; Siddiqi, A.; Chowdhury, A.M.S.; Sohrab, M. and Hasan, C.M. Scopoletin Isolated from Stem Bark of Bursera serrata Wall. With Antimicrobial and Cytotoxic Activities of the Crude Extract. Dhaka Univ. J. Sci. 2010, 58, 287–289. [Google Scholar]
- Ara, K.; Rahman, M.S.; Rahman, A.H.M.; Hasan, C.M. and Rashid, M.A. Terpenoids and Coumarin from Bursera serrata Wall. Dhaka Univ J Pharm Sci. 2009, 8, 107–110. [Google Scholar] [CrossRef]
- Aguilar, S.L.; Romero, C.O.; González, C.M. and Tortoriello, J. Efecto de Bursera grandiflora sobre el peso corporal y lipemia en ratones obesos. BLACPMA. 2012, 11, 138–146. [Google Scholar]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E. and Yordi, E.G. Coumarins — An Important Class of Phytochemicals. Phytochemicals - Isolation, Characterization. in Human Health, Rao, A.V., Rao, L.G., Eds.; InTech: 2015; Volume 20, pp. 113–40. [CrossRef]
- Rojas-Sepúlveda, A.M. Búsqueda de metabolitos con actividad citotóxica y antitumoral en Bursera fagaroides var. fagaroides y Bursera morelensis, y evaluación de su efecto como inhibidores del ciclo celular en el modelo de pez cebra. Doctorado, Universidad Autónoma del Estado de Morelos, Cuernavaca, Morelos, junio 2012.
- Infante-Rodríguez, D.A.; Landa-Cansigno, C.; Gutiérrez-Sánchez, A.; Murrieta-León, D.L.; Reyes-López, C.; Castillejos-Pérez, A.B.; Pucheta-Fiscal, J.E.; Velázquez-Narváez, A.C.; Monribot-Villanueva, J.L. and Guerrero-Analco, J.A. Análisis fitoquímico y actividad antidiabética, antibacteriana y antifúngica de hojas de Bursera simaruba (Burseraceae). Acta Bot Mex. 2022, 129, 1–15. [Google Scholar] [CrossRef]
- Barry, L.N. Use of Fluorescein Diacetate in Citrus Tissue cultures for the determination of cell viability and the selection of mutants. Sci. Hortic. 1989, 39, 15–21. [Google Scholar]
- Jones, K.H. and Senft, J.A. An improved method to determine cell viability by viability by simultaneous staining with fluorescein diacetate-propidium Iodide. JHC. 1985, 33, 77–79. [Google Scholar]
- Ocampo-Bautista, F.; Mussali-Galante, P.; Alvarez, L.; Marquina-Bahena, S.; Valencia-Cuevas, L.; Valencia-A, S. and Tovar Sánchez, E. Natural Hybridization between Bursera bicolor × B. glabrifolia (Burseraceae). Forests. 2023, 14, 1–16. [Google Scholar] [CrossRef]
- Bork, P.M. , Schmitz, M.L.; Kuhnt, M.; Escher, C. and Heinrich, M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett. 1997, 402, 85–90. [Google Scholar] [CrossRef] [PubMed]


| Extract | HepG2 | HeLa | PC3 | H1299 | HFF-1 |
|---|---|---|---|---|---|
| Leavesb Callusb Paclitaxela |
20.3±3.38 155.4±4.95 10.12±2.5 |
18.6±5.2 72±5 40.23 ±7.2 |
12.6±4.6 141±5.8 17.5 ±2.8 |
69.1±6.2 210.4±5 45.25 ±7 |
79.6±5.2 95.4±6.2 989 ±21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





